Abstract
A new era of osteomyelitis treatment has been taking strides towards efficient, local administration of antibiotics at the site of infection. By having them localized to the site of infection, this toxicity is no longer an issue and actually has shown to be a more productive treatment for osteomyelitis. Researchers have focused the production of non-biodegradable, antibiotic, infused bone cements specifically designed for proficient osteocyte binding, useful antibiotic release over a desirable period of time, and promotion of bone regeneration. These cements are then surgically placed on the infected site following debridement and irrigation. The problem, however, is that the use of ineffective cements and the overuse of antibiotics has led to the development of resistant bacteria. Due to this, further research is being done in the field of antibiotic discovery and delivery. Specifically, the development of biodegradable materials capable of efficiently delivering antibiotics and also eliminating the need for follow-up surgery to remove the delivery material is being done, thus reducing exposure risk. Nanoparticles have been developed in the forms of scaffolds and injections to deliver a higher degree and longer lasting duration of antibiotic release, while promoting bone regeneration.
Keywords: osteomyelitis, bone infection, biodegradable, non-biodegradable, PLGA, injections, scaffolds
1. Introduction
Since the invention of prosthetic joints, some engineers and physicians have spent their careers trying to not only improve the materials which make up these prosthetics, but the manner they are utilized in order to make sure their use is as beneficial, comfortable, and long lasting as possible. One problem with this fact is the possibility of bone infection or osteomyelitis. It was concluded as recently as 2015 that infection following knee or hip arthroplasty has an incidence rate of 2 to 2.4 percent, with the infection burden for each (knee or hip) being slightly under one percent.1,2
Clinically, physicians are able to classify bone infection in to two different categories including chronic and acute cases. The most common form of acute cases is due to blood vessel weakness or damage more commonly known as hematogenous infection. Due to the high vascularization of bone in children, they are the most commonly infected individuals from this mechanism. Chronic cases are less common but when diagnosed have been shown to present for over 80 years, in some cases.3 Additionally, chronic cases are becoming much more prevalent now than previous years due to the more frequent use of prosthetics for joint replacements and fracture repair. During surgery or, in many cases, a fracture, the periosteum of the bone is disturbed allowing for increased access to the osteocytes by bacteria resulting in a more severe form of infection.4
However, the increased frequency of these types of surgeries is not the only reason for the increased prevalence of bone infections. The major influence is due to the damage inflicted upon the vascular and skeletal system by diabetes and cardiovascular diseases. Now, more than ever, the prevalence and incidence of such diseases are escalating. It has been stated by the Center for Disease Control (CDC)5 that 9.3 percent of the United States population is affected by diabetes whether they are diagnosed or not. Additionally, the American Heart Association (AHA)6 estimates that 35 percent of the United States population expresses greater than or equal to three out of the seven heart disease risk factors, which is the most up to date standard in recognition of susceptibility. Reviews published by Namba et al7,8 also found that men and people suffering from obesity or arthritis also have an increased prevalence of osteomyelitis, but they were unable to find a connection among people suffering from rheumatoid arthritis like other reviews.9–12
In order to combat the reality of bone infections it is important to first understand what is going on biologically during an infection before it is possible to find ways to prevent, treat, or even cure such manifestations. During an infection, the body’s first immunological response is to signal the collection and focus of immunologic cells to the site of injury or infection. This begins with the function of the non-specific system involving macrophages, neutrophils, and other phagocytic cells at the site, and the recognition of the infection by the body signals associated T-cells and B-cells of the specific immune system to produce the necessary antibodies.13 This concentration of cells and immunologic agents like histamine trigger an immune response resulting in pain, swelling, redness, heat, and loss of function. Though this is generally a beneficial response, if the infection is substantial or persistent, this response can eventually lead to cell and tissue necrosis and the most severe forms of osteomyelitis.3 During an immune response, there is a transition of cells between certain stages, by utilizing this fact, researchers are able to monitor the impact of the materials being tested for effectiveness and completion of treatment. One example of this is the transference of macrophage from a type 1 form to type 2. Each of which has a different impact favoring type 1 for less severe infection cases.14
Antibiotics have been proven to work but problems have been found to exist due to the bacteria’s own defenses against the immune cells. Its shown that once the bacteria imbed themselves in the osteocytes, they are able to produce a fibrinogen layer similar to the fibrinogen present in normal tissue and even lower its metabolic rate, both of which help to disguise the bacteria from immune cells and antibiotics.3 Additionally, some antibiotics proven to be affective have shown to be toxic in other tissues or organs of the body especially when in the concentrations needed for eradication to be successful. These two problems thus bring up two other problems including: the need for a way to localize the antibiotics necessary to the point of infection and to avoid toxicity.
The focus for this review is to provide information on new methods being developed in the field of osteomyelitis treatment and improved developments in the methods already adopted by the medical field both primary and surgical. These include a number of biological and non-biological methods with the latter being of older relevance and the prior consisting of the most recent research focuses.
2. Current Status for Bone Infections
Since the discovery, acceptance, and eventual mass production of antibiotics, there existed a state of mind that antibiotics were “wonder drugs” that finally gave the human race a leg up on the problems that bacterial disease had created over generations. The fact of the matter though is that this way of thinking has led the human race in to a period of overuse (frequency) of prescribing and an under use (duration) during treatment that has led to a new precaution in medicine due to antibiotic resistant bacteria. In the topic of osteomyelitis, the bacteria of biggest concern include staphylococcus aureus and staphylococcus epidermidis.1,3,15 In a study analyzing the United States incidence of infection caused by these bacteria in 2013, it was concluded that between 1.6 and 29.7 cases per 100,000, and of those cases, 2.8 to 43 percent of them were infections of the bone, depending on the location, and it has been shown these numbers are only increasing due to the increased frequency of knee and hip arthroplasty in the United States.2,11,15–17
A large amount of research has been dedicated to the discovery of the exact mechanisms of osteomyelitis infection and its subsequent treatment but problems exist. In vitro studies have been able to find fairly predictable patterns of infection and colonization among these infections, but once these circumstances are duplicated in vivo the results do not replicate in the same manner. The reason for this being that animal models have a much larger number of variables both environmental and genetic that must be taken in to account. This makes it difficult for researchers to test new antibiotics.18
Currently, the gold standard antibiotic for the treatment of osteomyelitis is gentamycin, but it is having the potential to be ineffective to resistant bacteria resulting in the need for new affective antibiotics to be discovered.1 Some of the most popular antibiotic investigations include: doxycycline, tigecycline, levofloxacin, nafcillin, vancomycin, minocycline, amoxicillin, and even silver particles.
In order to test new or already existing antibiotics for their effectiveness with bone infection, there must first be a delivery method. The most traditional method for delivery is through oral intake. The problem, however, is that this method can result in cytotoxicity and/or allergic reactions within body systems beyond the site of infection. This exact problem has been apparent in research measuring the efficiency of the antibiotic bortezomib, which showed potential in cell death and bone regeneration except it has the potential to cause peripheral neuropathy, and a fungal infection treatment method known as fungizone, which, if not isolated, can result in side effects such as fever, chills, hemolysis, and vomiting.19,20 Ironically, even a derivative of gentamycin (gentamycin sulphate) has been shown to present systemic toxicity when administered orally rather than locally.21
An answer to decrease toxicity and possibly increase the effectiveness of a treatment is to develop a way in order to localize the administration of a drug or antibiotic to the site of infection. The basis for this is to either directly apply them to an area surgically with the use of scaffolds either biological or non-biological or the use of compounds that demonstrate a high affinity for the site of infection or bone in this case. The detailed information on how this is being done will discussed later on.
By being directly administered to the location of infection, the drug is already at an advantage, but the antibiotic must be released in an efficient manner. Ideally, the delivery method should allow the antibiotic to release in large concentration at the start and then at a lower but still affective concentration over an extended period of time (days to weeks).20,22 This allows for efficient prevention of bacterial growth and adaptation to the treatment. Additionally, researchers are trying to find methods that will not only accomplish these goals but to also be able to promote bone growth following the elimination of infection by incorporating agonistic materials in to the delivery systems.
3. Non-biodegradable Delivery Systems
3.1.Cements
The use of non-biodegradable materials more commonly known as bone cements have been infused with antibiotics in order to effectively localize the administration of the drugs to a target site, with the most widely used composition being polymethylmethacrylate (PMMA).16,23 The reasons for this being that PMMA’s have shown to have ideal antibiotic release characteristics along with ideal compatibility characteristics with bone.
When taking in to account the risk verse reward possibilities from both the financial and health standpoint, a majority of public health researchers are split on the subject. There are a number of factors that can lead to osteomyelitis as mentioned earlier. Many publishers believe that due to the inconsistencies among the cements currently being used, the prevalence of antibiotic resistant bacteria will only increase faster rather than being halted. Thus, they believe that this method should only be used with high risk patients.7,24,25 On the other hand, there are publishers whom believe that these same viewpoints are the exact reason as to why these cements should be more regularly administered. Through citing of previous research, these authors state that infection is easier to prevent than it is to eliminate, in most cases. By preventing infection, less financial burden will be placed on patients and hospitals because of post-surgical treatments and other complications that can arise due to infection.26 This leads to the research that is currently being completed.
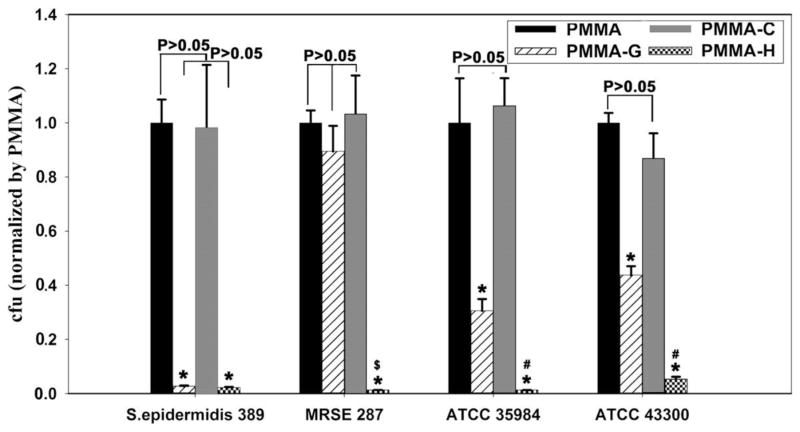
Tan et al.27 examined different compositions of PMMA cements in order to test the reliability and efficiency of each for osteomyelitis treatment. The compositions in question were a control version of PMMA, a gentamycin infused PMMA (PMMA-G), a chitosan infused PMMA (PMMA-C), and a chitosan derivative (hydroxypropyltrimethyl ammonium chloride chitosan, HACC) (PMMA-H), designed by the research team and shown to be affective against bacterial growth in a previous study, infused in PMMA.28 The reason for the development of the new derivative being that chitosan has been found to be effective as an antibacterial, but unlike many others, it is not very soluble in water making it difficult to infuse in to cement and also can lead to the development of biofilms. The parameters for analyses included the curing time and temperature, bone compatibility, proliferation rate, and bone growth/healing. Among all parameters, PMMA-H excelled especially as an antibacterial (Fig. 1). The reason being that its small particle size allowed it to create pores in the cement that ultimately allowed it to better comply with the bone tissue and provide areas for proliferation. Consequently, this advantage led to its ability to stimulate bone growth through an enzyme agonistic mechanism on bone’s alkaline phosphatase (ALP).
Fig. 1.
The live bacterial number of the four bacterial strains in suspension after contact with four PMMA-based bone cements for 24 h. The number of cells is expressed relative to that after contact with PMMA. The data are representative of the results from three independent experiments and are expressed as the means ± SD. Asterisks (*) denote a significant difference compared to PMMA and PMMA-C for all tested strains (p < 0.01), (#) denotes a significant difference compared to PMMA-G for ATCC 35984 and ATCC 43300 (p < 0.05), and ($) denotes a significant difference compared with PMMA-G for MRSE287 (p < 0.01)28.
A similar study was completed on the effectiveness of chitosan when integrated in to cement and was able to provide additional information. This includes the finding that its integration as a nanoparticle was able to preserve the mechanical properties of the cement better than when compared to its integration as a powder. The nanoparticles were produced through an ionic gelation technique verse other known techniques (micro-emulsion) due to its ability to produce nanoparticles with high protein binding efficiency.29,30 This led to greater antibacterial properties by the nanoparticle derivative than the powder derivative. In one case, the release lasted for 3 weeks and was believed to be the result of an increased bone compatibility and a greater negatively charged surface against the bacterial membranes.31
This same type of research method was used in order to test the efficiency of silver nanoparticles in PMMA verse the traditional gentamycin. The difference in this case however is that rather than testing all the previous parameters, the impact on MRSA (methicillin resistant staphylococcus aureus) alone was being studied. This resulted in gentamycin showing little antibacterial activity, while the silver nanoparticles completely inhibited proliferation of the bacteria.32
Previously, it was stated how an important characteristic of these cements is to have an efficient release of antibiotic over a period of time. This characteristic, in addition to MRSA inhibition and cement stability, was what Matos et al.33 were examining in their study of an acrylic bone cement. The antibiotic of focus was minocycline due to its believed influence on MRSA, but unlike the previously mentioned studies, commercial acrylic bone cement was used. Additionally, just as was discovered by the study completed by Tan et al.27, it was understood that the porosity of the cement is important for efficient minocycline release and bone attachment. In order to assure that the cement being used would express this characteristic, lactose was mixed in to the solution because of recent discoveries in the field.34 In the end they studied a control group consisting of plain cement and then two cements with consistent minocycline content (2.5%) but varying lactose content (10% or 20%). For all three cements, the initial release of antibiotic was consistent and ideal. The differences started to appear once the duration of release, porosity, and biomechanical properties were evaluated. It was concluded that porosity and duration of release were directly related to the concentration of lactose present in the cement, but the 10% lactose cement was still able to perform at an effective release characteristic. The problem with the 20% lactose cement was that the increased porosity resulted in a compromised mechanical structure not only for bone compatibility but also for stability. This led to the conclusion that bone cement with 2.5% minocycline and 10% lactose is a successful modification to the traditionally used cements.
3.2.Stabilizers
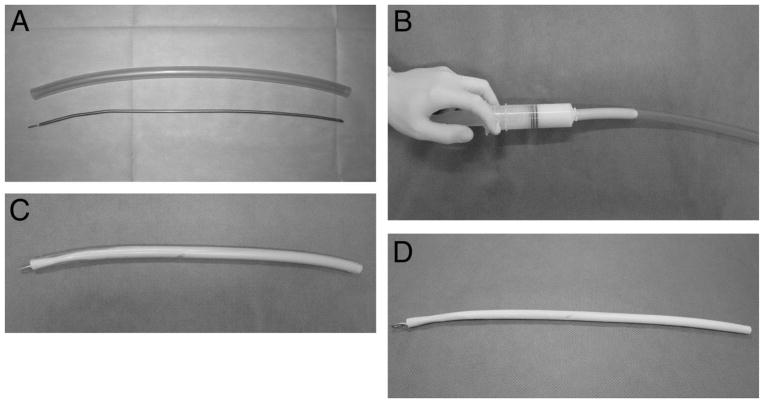
In addition to bone cements, some researchers have been experimenting with the use of antibiotic-cement-coated stabilizing devices to be used in surgery. The idea for this came from the impact that these stabilizing devices have for fracture repair, and by coating either the rods or plates with antibiotic cement, one is able to administer infection treatment, while also providing a source of stability to the location of injury. An example of one method from Sancineto and Barla35 for coating surgical rods with these cements is displayed in Fig. 2.
Fig. 2.
(A) Ender nail and tube length selection, (B) Cement introduction using a 60-mL syringe, (C) Once the nail is inserted, the cement needs to get hard, (D) Intramedullary antibiotic dispenser35.
A benefit to these studies compared to most of the cement studies is that they are completed in vivo rather than in vitro. By doing so, the researchers are actually able to observe and analyze the diffusion properties of the cements being used along with being able to find out whether antibiotics used successfully in a laboratory setting will in fact work when the additional variables provided by a living organism are presented. A downfall, however, is that these studies are non-randomized studies, but considering the fact that these methods are designed to treat a small population of people with certain, specific ailments, this bias will not falsify the statistics quite as much as it would traditionally.
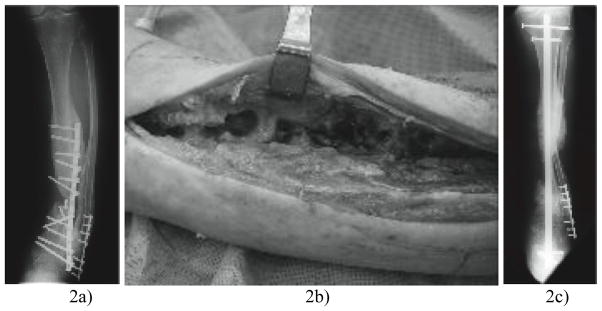
The most popular application is the use of antibiotic infused cement-coated rods in to the infected area. In a study completed by Conway et al.36 this method was examined on patients suffering from an infected arthrodesis (group A) and patients with an infected nonunion (group B, Fig. 3). All in all the method caused 105 of the 110 patients to recover on all aspects, but no matter the ailments being treated, only slightly over 50 percent of the subjects (57 and 60, respectively) were cured with only one treatment, while the remaining individuals needing further surgery or treatment in order to repair the nonunion or fully clear the infection. Additionally, 27% and 30% of the individuals in group A and group B, respectively, had a recurrent infection. This may have been due to the mixture of antibiotics used, which were vancomycin and tobramycin, and how the individuals reacted to them. These were also administered orally.
Fig. 3.
Anteroposterior (AP) radiograph taken a) pre-operatively showing a chronically infected nonunion of the tibia b), intra-operative photograph of tibial osteomyelitis and c) post-operative AP radiograph showing a fibular osteotomy and custom antibiotic-coated hindfoot fusion rod spanning a defect36. Note the correction of varus.
In a similar study, but different for how the implantation of the rods was prepared by using a Reamer-Irrigator-Aspirator (RIA) system rather than a traditional implantation by drilling out the area, similar results were obtained. 37% of the patients required further treatment after an initial surgery. This is in fact lower than the previously mentioned study, but rather than administering antibiotics solely by oral medication and through the implanted cement, this study also placed each of the patients on venous antibiotics, which may have impacted the results. The antibiotics included gentamycin and vancomycin. This also led to 96% of the subjects continuing their lives without recurrence of infection.37
Sancineto and Barla,35 using a very similar reaming and irrigation method were able to obtain much more positive results. Just as Kanakaris et al.37 a period of systemic (oral and venous) antibiotic use including gentamycin, vancomycin, and tobramycin were included in addition to the antibiotic cement following surgery. The three groups studied, though small (18 total), included infected nonunion (group A), infected nonunion repair (group B), and recurrent osteomyelitis (group C). The group distributions, infection sites, and antibiotics used are displayed in Table 135. At the completion of the study there weren’t any signs of recurrent infections and every subject recovered with a complete union demonstrating that complete debridement, reaming, and irrigation of the infected site in addition to stability and local administration of antibiotics can be a very effective treatment.
Table 135.
Patients characteristics for 18 total patients, the three groups included infected nonunion (group A), infected nonunion repair (group B), and recurrent osteomyelitis (group C)
| Patient | Age | Segment | Open Fracture | Bacteria | Local Antibiotic | |
|---|---|---|---|---|---|---|
| Group A | 1 | 34 | Tibia | GIIIB | MRSA/Enterobacter cloacae | Vancomycin/gentamycin |
| 2 | 20 | Tibia | GIIIB | MRSA/Serratia spp | Vancomycin/tobramycin | |
| 3 | 26 | Tibia | GIIIB | MRSA | Vancomycin/gentamycin | |
| 4 | 44 | Femur | No | MRSA | Vancomycin | |
| 5 | 36 | Tibia | GIIIB | MRSA | Vancomycin/tobramycin | |
| 6 | 52 | Tibia | GIIIA | MRSA | Vancomycin/gentamycin | |
| 7 | 45 | Tibia | GIII A | MRSA | Vancomycin/gentamycin | |
| 8 | 29 | Tibia | GIIIB | MRSA | Vancomycin/gentamycin | |
| 9 | 18 | Femur | No | MRSA | Vancomycin/gentamycin | |
| Tibia | No | MRSA | Vancomycin/gentamycin | |||
| 10 | 42 | Tibia | GIIIA | MSSA*/pseudom | Vancomycin/gentamycin/imipenem | |
| 11 | 41 | Tibia | GIIIB | Pseudomona/MRSA | Vancomycin/gentamycin/colis | |
| Group B | 12 | 20 | Tibia | No | MSSA | Vancomycin/gentamycin |
| 13 | 34 | Tibia | GIIIA | MRSA | Vancomycin | |
| 14 | 50 | Tibia | GII | MRSA | Vancomycin/gentamycin | |
| 15 | 33 | Femur | GIIIA | MRSA | Vancomycin/gentamycin | |
| 16 | 41 | Femur | No | MRSA | Vancomycin/tobramycin | |
| Group C | 17 | 38 | Tibia | No | MRSA† | Vancomycin/gentamycin |
| 18 | 65 | TIBIA | No | MRSA/Enterobacter cloacae | Vancomycin/gentamycin |
Staphylococcus sensitive to methicillin and Pseudomona aureoginosa.
Staphylococcus resistent to methicillin.
It is important to know that in every one of these studies, the bacteria being treated was cultured and MRSA was the most abundant cause of infection in every case resulting in the wide diversity of antibiotics being used. Additionally, there is some discrimination between how the surgeries should be completed. In each study a different number of surgeons are used ranging from one to five. This leads to the conclusion that the method for implantation might have a large impact on the effectiveness of such treatments.
Conway et al.,38 in addition to studying the effectiveness of internal fixation and antibiotic delivery have also been involved in the use of external fixation studies. In one particular study, they used the very same technique (method, cement, and antibiotics) for coating rods but in this case applied it to plates. The reason for this is to provide a just as effective method for antibiotic administration while providing stability but to have a less invasive surgery. In the 4 cases mentioned every application after debridement and irrigation resulted in union of the bone along with infection eradication without recurrence, demonstrating plausibility for the treatment.
Ultimately, the goal is to be able to administer these treatments with the expectation that they will be affective after a single application. As mentioned and demonstrated, however, this is not yet a reality. In many cases, there is still a large amount of second surgeries needed in order to clear an infection. Additionally, a number of methods being used are designed in a way that requires a patient to take part in a number of procedures.38,39 Not to mention, a second surgery is needed to remove these cements and stabilizers no matter the result. Due to this fact a number of researchers are starting to develop cements and beads able to be dissolved or degraded without the need for a follow-up surgery, thus decreasing cost and energy toward treatment.
4. Biodegradable Delivery Systems
4.1. Nanoparticles as Scaffolds
There are a number of mechanisms being tested in this field including scaffolds and injectable materials. The most important aspect is the nanoparticles making up the “backbone” of all of these materials. As mentioned in the research completed by Shi et al.,31 by developing and separating the antibiotic materials in to these nanoparticles there exists an increased surface area, porosity, and bone compatibility, which, together, allow for an increased concentration and duration for release of treatment. The problem is that researchers now must find methods for producing biodegradable nanoparticles and compounds that can be incorporated successfully. By doing so, antibiotics can be localized to a site of infection for treatment and possibly promote bone regeneration all without requiring a second surgery for release.40,41
In terms of these scaffolds, it is relatively understood through a number of studies that the mechanism of delivery for any material whether antibiotic, metal, or ionic loaded in to the particle is delivered to its location site by a degradation, diffusion mechanism. This involves the degradation of the compound stabilizing the particles and the particles themselves, which then allows for an imbalance between water, ions, and particles in the location resulting in natural diffusion and uptake by the infected cells. This however, in most cases, only results in a diffusion of 60% of the material loaded in to the nanoparticles.42,43
One of the most popular materials in this field is calcium phosphate and its derivatives. The reasons for this being its ability to promote bone compatibility (adhesion, binding, and growth), and its ability to be drug loaded. One of the main reasons for these abilities is due to the fact that it is a natural component of bone.16 However, on its own, it has shown to have a high burst release. This brings forth its possible incorporation in to a polymer. Bastari et al.44 did just this by incorporating varying amounts of nafcillin and levofloxacin in to poly(lactide-co-glycolic acid) (PLGA) and encapsulating it with calcium phosphate using an emulsion method. By using multiple antibiotics, Bastari et al. were able to not only examine how affective the use of calcium phosphate was on an efficient profile, but whether or not there was a different profile for each antibiotic. The results found that the decreased solubility of levofloxacin actually led to a decreased initial release whether coated or uncoated with calcium phosphate unlike nafcillin. This led to more efficient bacterial inhibition of 100% by levofloxacin within 7 days.
Ignjatovic et al.,45 using this knowledge of PLGAs, decided to also analyze exactly how they can be modified to promote bone regeneration to a greater degree. In order to study this, the research team developed combinations of calcium phosphate and PLGA in both microparticulate and nanoparticle forms. They then added a third variable of autologous plasma from the particular patient that would be receiving the treatment. This allowed for the addition of growth factors and cytokines that would normally not be present. In total, there were 5 treatment groups in the study including: control, 2 calcium phosphate microparticle, and 2 calcium phosphate nanoparticle groups (half receiving autologous plasma). As expected, the nanoparticulate calcium phosphate/PLGA mixed with the autologous plasma produced the greatest amount of bone regeneration and growth after infection. This was apparent due to the presence of a large quantity of mature bone cells, blood cells, and a dense bone cell network, progressively replaced by lamellar bone and, further, by mature cortical bone (Fig. 4).
Fig. 4.
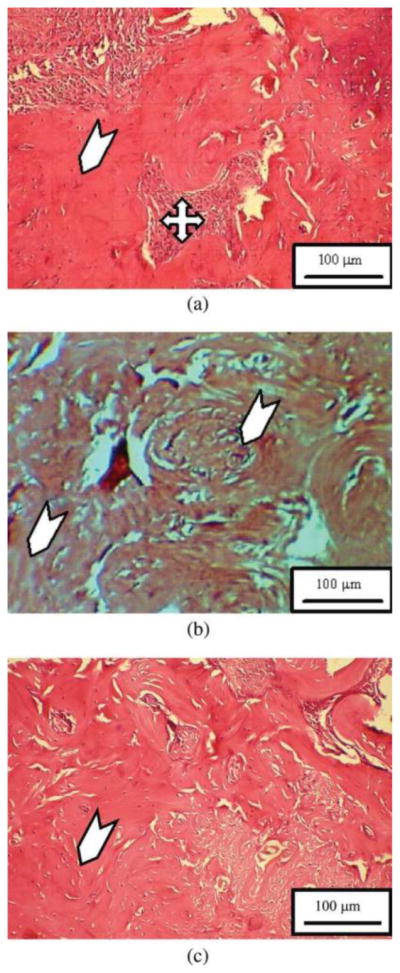
Healing of arti cial defect of the experimental group of animals. (a) 6 weeks, (b) 12 weeks, and (c) 24 weeks after the implantation of NPs CP/PLGA composite with autologous plasma46.
Ignjatovic et al.,46 then expanded on their research in order to evaluate what impact the concentration of antibiotic had on treatment delivery and infection clearing. The antibiotic of choice was tigecycline and was loaded in to the calcium phosphate/PLGA particles at concentrations of 0.6%, 3% and 5% by weight through a chemical bottom-up, dissolving procedure. Through microscopy, it was then found that the average particle sizes were 65 nm, 80 nm, and 95 nm respectively. This categorizes them as nanoparticles as they are all below 100 nm. At the completion of the study, it was found that just as there was a direct relationship between the percent weights of tigecycline to the sizes of the particles, there was also a direct relationship with the total quantity of tigecycline released to the weight percent. After statistical analyses, it was found that the 0.6% tigecycline nanoparticle was able to release a satisfactory amount (1700 ng/g) of antibiotic to the local area (500 ng/g is required), while also still being able to allow bone regeneration. This was not when systemic administration is also needed, however. It was discovered that the 3% or 5% particles would be needed as long as the antibiotic of choice is not cytotoxic to the body out of the bone tissue environment.
In one in vivo study, the use of calcium sulfate nanoparticles has shown some promising results not only for antibiotic release but also bone regeneration. In this study involving 25 patients requiring infection irrigation and bone grafting, and 16 also requiring fracture repair, 23 of the 25 recovered completely from infection, and 14 of 16 recovered from both infection and fracture repair (Table 2). These results demonstrated that results found in vitro can be transferred to a clinical setting even though much of the in vitro environment does not correlate with in vivo. The only downfalls to this study were that there was not a control group and even though the graft used was in fact biodegradable, some of the participants did in fact require a second surgery due to the use of stabilizers for the fracture repair.47
Table 247.
Patient Complications
| Patient | Complication | Treatment | Outcome | Comments |
|---|---|---|---|---|
| 3 | Hypertrophic nonunion | Brace | Functional | Elderly patient |
| 4 | Refracture | Cast × 8 weeks | Healed | No reoperation |
| 21 | Deep infection, persistent nonunion | Extensive reconstruction | Unresolved | Currently under treatment |
| 18 | Refracture | Repeat ORIF | Healed | No further infection |
| 8 | Refracture above old nonunion | ORIF failed, circular fixator applied | Healed | Psychiatric disorder, third fall/jump |
| 7 | Superficial infection | Dressing changes | Healed | Different organism |
| 11 | Sterile draining sinus | None | Healed | Tibia |
| 25 | Sterile draining sinus | None | Healed | Tibia |
| 10 | Sterile draining sinus | None | Healed | Tibia |
| 24 | Sterile draining sinus | None | Healed | Tibia |
| 2 | Sterile draining sinus | None | Healed | Ulna |
| 17 | Sterile draining sinus | None | Healed | Tibia |
| 14 | Sterile draining sinus | None | Healed | Tibia |
| 19 | Sterile draining sinus | None | Healed | Tibia |
| 12 | Persistent infection | Repeat debridement | Healed | Missed area on initial procedure |
ORIF, open reduction and internal fixation.
One downfall that has been presented with the use of PLGAs is that their naturally hydrophobic surface can cause for a slight decrease in its biocompatibility and antibiotic loading. In order to counteract this, the addition of mono-methoxypoly(ethylene glycol) (mPEG), a hydrophilic polymer, has been a popular and successful way to reduce this characteristic. Peng et al.42 used these findings in order to study the impact of different concentrations of mPEG to PLGA on antibiotic release profiles. Their end results not only showed that their antibiotic of choice (teicoplanin) was affective at bacterial inhibition, but the amphipathic mixture showed a more efficient release profile. The most efficient ratio being 550/1405 (mPEG/PLGA by weight percent) due to its physical state profile at varying temperatures, its water solubility, and its ability to sustain a high viscosity in normal body temperatures.
In research completed by Wilberforce at el.,40 tricalcium phosphate (TCP) has also been studied by its combination in a crystalline polymer consisting of poly-L-lactide acid (PLLA) through a method known as twin screw extrusion. This method involves the melting and cycling of the PLLA and TCP for a period of time (in this case 15 min) before they are extruded through a strand die. This led to the development of both quenched and annealed sample groups consisting of four nominal weight fraction subgroups of pure PLLA and 10%, 20%, and 30% nanoparticle composition. The particle sizes of these nanoparticulate compounds included: 150 nm, 100nm, and 200 nm, respectively. These samples were then examined for their cold crystallization temperature (Tcc), crystallinity, glass transition temperature (Tg), energy values (E’), and dampening factor. 40
They discovered that there was not a statistically significant difference between the different subgroup compositions in terms of the temperature measurements, but there was in fact a statistical difference between the E’ measurements favoring the nanocomposites. Additionally, a large statistical difference existed between the quenched and annealed groups within each subgroup, favoring the annealed. It was then interpreted that if this type of composite were implicated for in vivo use, an annealed material would thus be much more suitable for bone sites requiring stiffness and high resistance, while the quenched composites would be more suitable for bone sites allowing for higher mobility and flexibility due to a higher dampening factor. The problem, however, is that this research was completed in dry environments unlike the type of environment that the composites would encounter in vivo, thus further research needs to be completed.
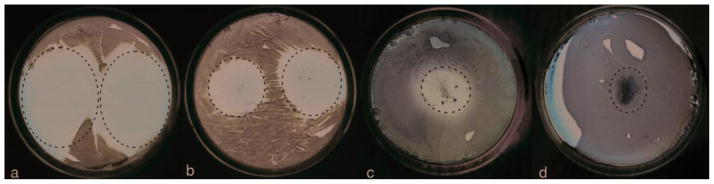
Hydroxyapatite (HA) is also being studied due to its sufficient ability for bone compatibility, drug loading and delivery, and chemical activity. One problem however is that when used by itself, HA tends to crystallize and coagulate very quickly in vivo. In order to mitigate this characteristic, its incorporation with other compounds has been studied. One of these is chitosan, which was mentioned before because of its use in PMMAs. Not surprisingly, the coating of HA with chitosan not only helped alleviate the crystallization, but it also minimized the initial, burst release of antibiotic to the infection site, and with the external positive charge provided by chitosan it is expected that it would benefit infection treatment by increasing bone compatibility.48,49 However, this was not exactly the case. During comparisons of inhibition zone by clindamycin, HA/clindamycin, HA/clindamycin/chitosan, and HA/chitosan, there was a respective decrease as the chitosan reduced the burst too drastically (Fig. 5). There was also some decrease is in bone regeneration.41,50 Because of this, HA incorporation with PLGA nanoparticles was examined in order to analyze whether it could minimize the burst release that is normally present with the use of PLGAs alone.
Fig. 5.
Inhibition zones formed around 1 mg of clindamycin (a), 1 mg of clindamycin-loaded Hap particles (b), 5 mg of clindamycin-loaded HAp/chitosan particles (c), and 50 mg of HAp/chitosan particles (d) on sheep blood agar plates seeded with 7 × 103 S aureus bacteria per mm2 following an overnight incubation. The area around the deposited powders is encircled, with a dashed line.41
In order to prepare the compounds, an emulsive, electrospinning method was adapted, which has the ability to produce very fine nano-fibers that would act as the backbone for the antibiotic material. The antibiotic of choice was amoxicillin and was then examined for its loading, release kinetics, antimicrobial activity, and cytocompatibility. It was discovered that amoxicillin loading was directly related to the amount of antibiotic exposed to the PLGA fibers, but indirectly related to the amount of HA. This same relationship was related to the release kinetics. Meaning, the nanomaterials with a higher concentration of amoxicillin but a lower concentration of HA were able to release a higher percentage antibiotic initially. The problem however is that this characteristic is not efficient for bacterial inhibition. By combining PLGA, HA, and amoxicillin, the antibiotics could be released over a sufficient concentration and a desired period of time allowing for considerable decline in bacterial growth no matter the concentration of amoxicillin in the compound.51
A more recent substance for incorporating these nanoparticles or microspheres is gelatin or hydrogels because they are naturally occurring, biodegradable, permeable to nutrients and other substances, and easy for researchers to manipulate or with which to work.52,53
Im et al.13 set out to create a hydrogel-based scaffold that not only included the use of nanomaterials (in this case nanotubes) but also HA and chitosan. Similar to the other studies mentioned, the use of HA in coordination with chitosan was to increase not only the osteoconductive properties of the scaffold but to also increase the overall strength. The use of nanotubes was due to relatively recent research showing their ability to promote the bone extracellular matrix in part by their structure (elongated).54 In addition, they were analyzing the impact of magnetically oriented nanotubes in comparison to a non-magnetized control group. Once again, it was concluded that the combination of HA and chitosan did improve the structural integrity and stability of the scaffold, however the release kinetics were not analyzed in this study. Additionally, it was discovered that there was actually more of an increase in osteoblast proliferation when around the magnetized nanotubes, which was expected due to their tighter and more ordered structure because of the added polarity.
Similar research conducted by Qi et al. also found that the incorporation of halloysitebes in to PLGAs was also affective at reducing the burst release of antibiotics, while also having significant bacterial inihibition.55
Mentioned in the research by Mckee, MD47, the use of these biodegradable materials has been implemented in coordination with stabilizers during fracture repairs and joint replacements. A series of studies completed by Neut et al.56,57 discussed the success of this kind of treatment. During which, they were testing the efficacy of a gentamycin-releasing coating that could be sprayed on to any implant, and in order to limit the burst release of gentamycin, the group implemented the use of a PLGA coating that would be applied around the outside of the spray, which is in fact biodegradable. They found that their new gentamycin sulfate spray (applied in either a porous-coating or a grit-blasting method) was just as, if not more (comparing to gentamycin release alone) affective at bacterial growth inhibition, especially when at a concentration of 1 mg/cm2 (the highest concentration measured). The team, with bio-optical imaging, then further evaluated these results in order to reinforce their findings.
In a follow-up study, Neut et al.58, in order to increase the overall strength of their coating and to possibly increase the bacterial inhibition of the polymer, they decided to modify their gentamycin/PLGA coating with HA due to its ability to add these features in another analyses.54 By doing so, the team not only measured the ability of the compound to cause efficient bacterial release and growth inhibition in vitro, but to also apply it to an animal model (white albino rabbits). As expected, the in vitro results were similar to their previous study as the same concentration of gentamycin (1 mg/cm2) was used and the same application methods (grit blasted and spray) were employed. The animal models not only integrated the gentamycin/PLGA/HA compound, but it also administered a HA/gentamycin compound for comparison. The results found that of while the HA/gentamycin treated rabbits did not show any infection clearing, the HA/gentamycin/PLGA treated rabbits showed a 100 percent clearing due to the release profile and inhibition duration (Fig. 6). This result concluded that HA is only beneficial for binding and strength for it had too high of a burst release on its own in order to inhibit the bacterial growth, along with decreased bone regeneration properties. Another method of using calcium phosphate rather than PLGA was mentioned but the antibiotic loading is drastically diminished in this case.59 A number of other studies have also implemented the use of HA on implants, but a majority of them have not adopted the use of PLGAs in coordination resulting in lower infection clearing rates.60
Fig. 6.
Width of the zones of inhibition (mm) around gentamicin-HA-coated titanium coupons with a protective PLGA overlayer on TSA plates as a function of time for six different staphylococcal strains56. Growth inhibition zones were measured 24 h after inoculation, after which coupons were transferred to a freshly inoculated agar plate and incubated for another 24 h. This procedure was repeated every 24 h to determine the longevity of the antibiotic release up to 4 days. Data represent averages over three separate experiments with error bars indicating the standard deviation. For each strain, significant differences (p < 0.05) within a time series with respect to the day before is indicated by *.
In a study by Zhu et al.,61 the efficiency of gentamycin sulfate was analyzed again, along with the implementation of calcium phosphate as a scaffold background. The innovative part of this study was to try to adopt the use of liposomes as an additional drug carrier. The results of the study did in fact show a desired release kinetic, but the problem with this method is that the release mechanism has two stages that can delay the antibiotic release. Meaning, it was discovered that the calcium phosphate scaffold, after attaching to the infection site, releases the liposomes in a vacuole form, which then move on to releasing the gentamycin. This release mechanism had a greater anti-biofilm activity than a control group (pure gentamycin), but that is expected considering the direct interaction. The study would need to be compared to other delivery methods for statistical significance.
Feng et al.62 also employed the study of a similar release mechanism. The difference however is that they adopted the success of PLGAs as their drug (doxycycline) carrier and a prefabricated PLLA scaffold as the binding backbone. Unlike what Zhu et al. discovered, the results showed a desired release profile and anti-biofilm activity. Additionally, they compared the doxycycline/PLGA/PLLA scaffolds to a doxycycline/PLLA scaffold rather than pure antibiotic in order to demonstrate a more statistically significant relationship. And in doing so, they discovered that by manipulating the PLGA’s components ratio and consequently its molecular weight and size they could inversely change the initial burst release and duration of release. This led to the development of scaffolds that have the ability of releasing doxycycline for a period of two to six weeks, and thus have the ability to be used in a variety of infections. The problem, once again, is that many of these results are published following in vitro studies.
4.2. Nanoparticles as Injections
Some researchers are trying to take these biodegradable materials one step further by finding ways to implant these polymers through injection and thus eliminate the need for invasive surgery. One popular method for doing this is through hydrogels and gelatins, which were mentioned in some of the research, and a prevalent research focus is on adding thermoregulative properties to these polymers in order to increase the water solubility and manipulating their physical state (solid, gel, liquid) temperatures.42,63,64 This simply means that the polymer has specifically engineered solid-gel-liquid transition temperatures that favor the gel phase around body temperature.
A study conducted by Lin et al.,65 applied this method to a composite of PLGA- poly(ethylene-glycol) (PLGA-g-PEG). In addition, they were studying the affects that HA would have on the compound. The HA integrated compound demonstrated many beneficial characteristics in comparison to the PLGA-g-PEG alone including: increased strength, increased storage capabilities, and sustained release of stored components (a dye in this case). The HA was also able to preserve the originally designed thermoregulative properties and even neutralize the pH of the final compound allowing for greater compatibility during in vitro or in vivo trials of the future.
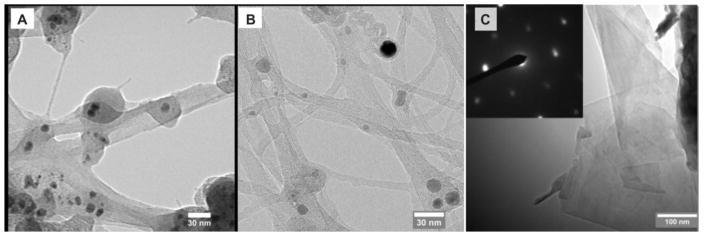
Similarly, a number of studies have been able to adopt the use of chitosan with nanomaterials eg., carbon nanotubes53 (Fig. 7) for scaffold production in to the development of injectable materials. The main reason for the success is because of believed chemical cross-linking (shown through infrared spectroscopy and x-ray diffraction) that occurs and allows for a strong, interlinked scaffold, while still displaying the desired thermoregulative properties.53,66,67
Fig. 7.
TEM images of (A) single-walled carbon nanotubes without magnetic field (N-SWCNT); (B) single-walled carbon nanotubes with magnetic field of 0.06 Tesla (B-SWCNT); and (C) graphene flakes with magnetic field of 0.06 (B-SWCNT). Inset of figure (C) is the selected area electron diffraction pattern showing the crystalline structure of graphene.53
Chitosan has also shown to be affective when in combination with bioactive, inorganic compounds such as glass. This is a relatively new idea in the field, but has merit due to its ability for thermoregulation, particle size regulation, structural support, and bone compatibility/binding abilities.68 The problem however is that in at least one study, the gelation points become very sensitive to increasing amounts of these compounds.69 Thus, more research is needed on the subject.
The adaptation of calcium phosphate compounds as injectable materials is also taking place. A study completed by Ignjatovic et al.,70 by using a composite of calcium phosphate and poly(dl-lactide-co-glycolide) (DLPLG) were able to produce both micro (150–200nm) and nano (40–50nm) particles. After surface analyses of the particles through atomic force microscopy, it was predicted that the nanoparticles would provide the most efficient bone filling capabilities due to their small size, adhesion, absorption, and release kinetics, which was backed up by previous research.19,31 This was the result, and in fact, the nanoparticles were able to produce the most well distributed solutions once placed in to an injectable matrix. One reason for this being that the saturation could not be any less that 50 percent or greater than 65 percent in order for the desired viscosity/gelation to exist. By having such a small size, the concentration in any one area of the solution was still high enough to be affective.
McLaren et al.22 were only one of a few studies that actually transferred these findings in to an in vivo study. In their study, they surgically injected a PLGA-PEG matrix in to a group of English sheep varying in the amounts of antibiotic and bacteria (S. aureus) received (Table 3). Through in vitro analyses before surgical intervention, it was calculated that the created PLGA-PEG matrix had a traditional fast initial release followed by a slower, stable release for a little over a week. Within the 13-day trial study, it was found that the combination of gentamycin (4%) and clindamycin (2.5%) did in fact inhibit bacterial growth, plus the use of the PLGA-PEG matrix did in fact promote bone regeneration. This demonstrates that the results found in vitro are capable of being transmitted to in vivo scenarios.
Table 3.
Results from in vivo study22
| Group 1 (n = 6) Bacteria and control scaffold (2 week sacrifice) |
Group 2 (n = 6) Bacteria and antibiotic impregnated scaffold (2 week sacrifice) |
Group 3 (n = 6) Scaffold only control (2 week sacrifice) |
Group 4 (n = 6) Scaffold only control (13 week sacrifice) |
Group 5 (n = 6) Bacteria and antibiotic impregnated scaffold (13 week sacrifice) |
|
|---|---|---|---|---|---|
| Pain relief post-operatively | 2 weeks | 3 days | 3 days | 3 days | 3 days |
| Lameness, swelling and increased respiration rate | Yes | No | No | No | No |
| Weight change at sacrifice | − 7.8 kg | + 6.2 kg | + 5.8 kg | + 8.8 kg | +14.3 kg |
| Bacteria present at sacrifice | S aureus (F2789) | None | None | None | None |
4.3. New Methods
The majority of this review has focused on the use of PLGA and HA for the production of nanoparticles, but over the years there have been new innovations that incorporate other materials into nanoparticles, and in most cases they are still very effective. The materials to be discussed include: silver and gold nanoparticles, diamond nanoparticles, carbon nanotubes, and nitric oxide and zinc oxide incorporated nanoparticles.
A number of studies have been able to demonstrate the antibacterial properties of silver in nanoparticles due to its believed ability to disrupt bacterial cell membranes.1,32,71,72 Juan et al.73 adopted the use of silver nanoparticles for their implantation onto titanium devices such as joint replacements and then tested the efficiency of the nanoparticles with s. aureus. It was found that the small (100 nm) nanoparticles, produced through a salinization method, showed very high antibacterial properties in a film applicator coating (FAC) assay. The problem that existed however is that the nanoparticles had the tendency to aggregate on the surface of the titanium rather than distribute themselves evenly, which could lead to problematic antibacterial properties in the future. This has led to the more common incorporation of silver into inorganic compounds for stable delivery and strong antibacterial properties as mentioned earlier by the research by Alt et al.32,72 A very similar study by DeGiglio et al.74 except the silver particles were produced through a “green” synthesis, found that a very dispersed number of silver nanoparticles were able to be applied to the surface of titanium implants when a hydrogel method was additionally used.
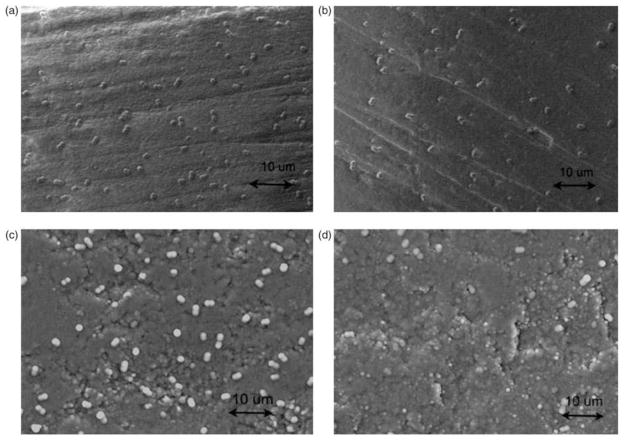
A more recent study completed by Afzal et al.75 has been able to improve the distribution problem of silver nanoparticles as well by applying them to the surface of biocompatible carbon nanotubes (CNT), which have a more lattice structure for the nanoparticles to attach. Consequently, this was able to improve the antibacterial properties (Fig. 8). In the study, there were four different test groups of which two were pure CNTs and HA and the remaining two consisted of CNT and HA with 5% by weight silver nanoparticles.
Fig. 8.
Afzal et al. Journal of Biomaterials Applications. 2013. SEM images of Staphylococcus epidermidis cell adhesion on (a) HA(950), (b) HA-Ag(950), (c) CNT(1700), and (d) CNT-Ag(950) pellets after bacteria culture for 4 h. Ag: silver; CNT: carbon nanotube; HA: hydroxyapatite; SEM: Scanning Electron Microscope75.
The use of CNTs was mentioned previously for their strong biocompatibility and their ability to be incorporated in to hydrogel materials.53 Just as nanoparticles, nanotube size is able to be manipulated and a variety of material can be combined to the surface of them to create a more complex structure and to provide the greatest amount of antibacterial properties (the smaller the better). One benefit of CNTs to nanoparticles, however, is their cylindrical structure. This allows researchers to design antibiotic loaded scaffolds and materials with these CNTs in similar orientation to bone osteocytes and osteons. This is the reason for the strong biocompatibility and in some cases even promotes bone regeneration.75 It has also been demonstrated that single-walled CNTs can be more effective for antibacterial properties, and are even more effective when insolated from each other but still at a high concentration due to an increased mobilization and bacterial interaction.76 A recent article by Zhang et al.77 demonstrates how important CNTs can be to future medical treatments due to their stable structure, biocompatibility, and easy drug loading. These characteristics are what allow them to be used in a very wide variety of disease treatments including bone infections.
The relatively recent use of nitric oxide (NO) in to nanoparticles and other materials is due to its antibacterial properties as a result of its oxygen radical formation after it interacts with peroxides and superoxides. This disrupts metabolic mechanisms and DNA transcription mechanisms in bacteria.72,78 After producing NO loaded nanoparticles by a combination of tetramethylorthosilicate, PEG, chitosan, glucose, and sodium nitrite in a 0.5 M sodium phosphate buffer (pH 7), Martinez et al.79 were able to efficiently demonstrate that NO is able have strong release kinetics for the treatment of infections through a period of 24 hours. Rothrock et al.,80 were able to show similar release effectiveness with the use of gold nanoparticles, which were produced through a method of hydrogen tetrachloroaurate salt with hexanethiol in the presence of sodium borohydride. The reason gold nanoparticles were chosen was because they are easily modifiable allowing for easy loading of any substance and the very small size in which they are able to remain stable (1–5 nm). This allows for a large surface area for infection contact and NO release. In an antibacterial mechanism evaluation study conducted by Cui et al.81 it was found that the surface modifications on gold nanoparticles by thiol groups actually causes a decrease the in the expression of tRNA in bacteria and an increase in the expression of chemotaxic genes in E coli and s. aureus of infection sites.
Similar to gold nanoparticles, the use of diamond nanoparticles have also shown some interest in the minds of researchers due to their ability to be formed into strong, stable nanoparticles of small size (2–8nm), while also being able to release antibacterials for even as long as a month in some instances.72 Huang et al.,82 were able to show through in vitro studies (mitochondrial assays) that diamond nanoparticles are able to increase the activity genes that promote regeneration while also inhibiting the production of interleukin factors, and cytokines that promote the characteristics of inflammation and further damage. The problem, however, is that the production of diamond nanofilms is a very tedious and expensive process at this current time, so a more efficient method is needed.
The analysis of zinc oxide for its incorporation in to nanoparticles has also shown to be effective as an antibacterial agent and a bone regeneration enhancer. This has been evident for the inhibition of a number of problematic bacteria including MRSA (Table 4). From the table, it can be seen that three different concentrations (100, 200, and 500 ppm) were tested for their impact on inhibition zone size, and it was discovered that the inhibition zone actually decreased with increasing concentration of zinc oxide. After analysis, it was discovered that above 100 ppm, the particles settled in the agar plates resulting in inefficient diffusion.83 This efficiency was analyzed through light microscopy and scanning electron microscopy for the antibacterial efficiency and osteoblast activity respectively. This demonstrated that both the bacterial and osteoblast species have strong compatibly with the zinc oxide nanoparticles (40–50 nm) through a period of 2, 24, and 48 hours. The exact mechanism of zinc oxide as an antibacterial is currently being studied however.84,85 This shows much more research needs to be completed in order to further understand these results.
Table 483.
Zone of inhibition of ZnO NPS against MSSA, MRSA and MRSE at different concentration of ZnO NPs
| (a)
|
(b)
|
(c)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ZOI (mm) | MSSA (n = 54) | MRSA (n = 50) | MRSE (n=10) | ZOI (mm) | MSSA (n = 54) | MRSA (n = 50) | MRSE(n=10) | ZOI (mm) | MSSA (n = 54) | MRSA (n = 50) | MRSE (n = 10) |
| 24 | 30 (55.5)* | 20 (40)* | 4 (40)* | 20 | 24 (44.4)* | 18 (36)* | 2 (20)* | 10 | 2 (3.7)* | 2 (4)* | 0 |
| 22 | 10 (18.5)* | 16 (32)* | 3 (30)* | 16 | 12 (22.2)* | 15 (30)* | 2 (20)* | 8 | 7 (12.9)* | 8 (16)* | 2 (20)* |
| 18 | 8 (14.8)* | 12 (22.2)* | 1 (10)* | 13 | 10 (18.5)* | 12 (24)* | 4 (40)* | 6 | 25 (46.3)* | 18 (36)* | 4 (40)* |
| 14 | 6 (11.1)* | 2 (3.7)* | 2 (20)* | 10 | 8 (14.8)* | 5 (10)* | 2 (20)* | 4 | 20 (37)* | 22 (44)* | 4 (40)* |
The value given in parenthesis is the % of strains of MSSA, MRSA and MRSE showing Zone of inhibition (ZOI, in mm) at 100 (a), 200 (b) and 500 (c) ppm of ZnO NPs
The last few methods to be mentioned were slightly mentioned in previous sections of this review as additives to an original study or as an accessory method being analyzed but deserve to be reiterated for their importance. These include the use of magnetically charged nanomaterials and the integration of growth factors and non-specific immunity factors such as macrophages in to the composites. It has been discovered that the by adding a magnetic charge to nanomaterials there exists a polarity that is favored by osteoblasts. This promotes bone regeneration and biocompatibility, while also promoting further antibacterial properties.54,85,86 The addition of growth factors and macrophages has shown to important for bone regeneration purposes. When growth factors and blood plasma from an infected patient are included in a infection treatment complex it has shown to have a dramatic effect on improving the rate and amount of bone regeneration that occurs following the infection due to their ability to enhance the activity of the bodies natural immune and growth mechanisms.45,78,87
Additionally, the use of bioactive glass particles was also mentioned in an earlier section and deserves further analyses. These glasses are produced through a sol-gel method allowing the glasses to be formed to nanoparticle size thus allowing for an increase in exposed surface area and increased pore size.88,89 It has even been noted that the degradation products of bioactive glasses actually promote bone regeneration.90 The problem however is finding a stable compound that will efficiently utilize these properties. One possible solution is the use of sodium alginate as a mixing liquid in combination with HA. This has shown to conserve the beneficial properties of bioactive glass, but only to a certain point. It was found that if the concentration of sodium alginate became to high, the stability of the compound became to great. This results in a decreased amount of natural degradation of the product at the site of infection, which is necessary for efficient antibiotic delivery. Consequently, this resulted in decreased migration of necessary degradation products to the osteoblasts and diminished results.91
Mesoporous silica nanospheres are also a prominent area of investigation. Produced through a poor expansion strategy involving a pore expansion agent, they possess the capability of being formed in to nanoparticles, they thus provide a large surface area and a variable pore size.92 Additionally, they are easily modifiable in terms of loading other desired particles for delivery to the site of infection and altering the outer charge on the nanoparticles for specifications of the specific tissue being targeted.14,92–94 Similar to bioactive glass materials, they have also shown to encourage bone regeneration, but more importantly, they are one of a few methods mentioned in this article that actually have shown to promote angiogenesis and pro-inflammatory and macrophage functions within the immune system when combined with copper ions. This was analyzed with the use of alizarin red S and a combination of buffer solutions to track their degradation. Except, once again, there is a limit to the amount of copper added to the compound. The reason being that too high of a copper concentration actually manipulates the shape of the nanoparticles, which can actually decrease the efficiency of antibiotic release from the particles. .14
5. Conclusion
With an increasing prevalence of osteomyelitis, whether following surgery or not has brought on a new era of treatments. Traditionally, the use of oral antibiotics was used but over treatment and lack of efficiency has lead to antibiotic resistant bacteria resulting in the demand for a more localized treatment method along with the use of a wider range of antibiotics. To this day, the most widely used treatment involves the use of non-biodegradable cements that require surgical implantation along with surgical removal following their effective period. This can lead to infection clearance, but the need for a second surgery has shown to drastically increase the patient’s likelihood of developing a post-surgical infection. In order to combat this truth, many physicians and scientists are working to develop new biodegradable materials that cannot only be implanted through traditional surgical methods but also through relatively new injection methods from the creation of hydrogels. Of the number of methods being developed, the most important or promising are the polymers like PLGAs and their incorporation with chitosan, HA, and calcium phosphates, which have all shown to be beneficial towards structural integrity, bone compatibility, antibiotic release kinetics, and promotion of bone regeneration. A couple in vivo studies were mentioned, but with further in vitro knowledge, more studies can be completed and eventually transferred to surgical departments.
Highlights.
Osteomyelities or bone infection is significant challenge for the patients.
Over treatment and lack of efficiency has lead to antibiotic resistant bacteria.
Antibiotics can be delivered using non biodegradable and biodegradable systems.
Nanoparticle carriers seem to be deliver longer period of antibiotic release.
Acknowledgments
This work is partially supported by the National Institutes of Health (NIH) grant number R01DE023356.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Slane J, Vivanco J, Rose W, Ploeg HL, Squire M. Mechanical, material, and antimicrobial properties of acrylic bone cement impregnated with silver nanoparticles. Materials Science and Engineering: C. 2015;48:188–196. doi: 10.1016/j.msec.2014.11.068. [DOI] [PubMed] [Google Scholar]
- 2.Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. The Journal of arthroplasty. 2008;23(7):984–991. doi: 10.1016/j.arth.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 3.Ciampolini J. Pathophysiology of chronic bacterial osteomyelitis. Why do antibiotics fail so often? Postgraduate medical journal. 2000;76(898):479–483. doi: 10.1136/pmj.76.898.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Physicians AAoF. [Accessed March 12, 2015];Diagnosis and Management of Osteomyelitis. 2011 http://www.aafp.org/afp/2011/1101/p1027.html-ref-list-1.
- 5.(CDC) CfDC. [Accessed March 12, 2015];2014 National Diabetes Statistics Report. 2014 http://www.cdc.gov/diabetes/data/statistics/2014statisticsreport.html.
- 6.(AHA) AHA. Heart Disease and Stroke Statistics - 2014 Update. 2014 http://circ.ahajournals.org/content/early/2013/12/18/01.cir.0000441139.02102.80.
- 7.Namba RS, Inacio MC, Paxton EW. Risk factors associated with deep surgical site infections after primary total knee arthroplasty: an analysis of 56,216 knees. The Journal of bone and joint surgery. American volume. 2013;95(9):775–782. doi: 10.2106/JBJS.L.00211. [DOI] [PubMed] [Google Scholar]
- 8.Namba RS, Paxton L, Fithian DC, Stone ML. Obesity and perioperative morbidity in total hip and total knee arthroplasty patients. The Journal of arthroplasty. 2005;20(7 Suppl 3):46–50. doi: 10.1016/j.arth.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 9.Jamsen E, Huhtala H, Puolakka T, Moilanen T. Risk factors for infection after knee arthroplasty. A register-based analysis of 43,149 cases. The Journal of bone and joint surgery. American volume. 2009;91(1):38–47. doi: 10.2106/JBJS.G.01686. [DOI] [PubMed] [Google Scholar]
- 10.Gandhi R, Razak F, Pathy R, Davey JR, Syed K, Mahomed NN. Antibiotic bone cement and the incidence of deep infection after total knee arthroplasty. The Journal of arthroplasty. 2009;24(7):1015–1018. doi: 10.1016/j.arth.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Kurtz SM, Ong KL, Lau E, Bozic KJ, Berry D, Parvizi J. Prosthetic joint infection risk after TKA in the Medicare population. Clinical orthopaedics and related research. 2010;468(1):52–56. doi: 10.1007/s11999-009-1013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hatzenbuehler J, Pulling TJ. Diagnosis and management of osteomyelitis. American family physician. 2011;84(9):1027–1033. [PubMed] [Google Scholar]
- 13.Philadelphia TCoPo. The Human Immune System and Infectious Disease. 2014 http://www.historyofvaccines.org/content/articles/human-immune-system-and-infectious-disease.
- 14.Shi M, Chen Z, Farnaghi S, et al. Copper-doped mesoporous silica nanospheres, a promising immunomodulatory agent for inducing osteogenesis. Acta biomaterialia. 2015 doi: 10.1016/j.actbio.2015.11.033. [DOI] [PubMed] [Google Scholar]
- 15.Vardakas KZ, Kontopidis I, Gkegkes ID, Rafailidis PI, Falagas ME. Incidence, characteristics, and outcomes of patients with bone and joint infections due to community-associated methicillin-resistant Staphylococcus aureus: a systematic review. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology. 2013;32(6):711–721. doi: 10.1007/s10096-012-1807-3. [DOI] [PubMed] [Google Scholar]
- 16.Desai TA. Calcium phosphate nanoparticles: a future therapeutic platform for the treatment of osteomyelitis? Therapeutic delivery. 2013;4(6):643–645. doi: 10.4155/tde.13.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Del Pozo JL, Patel R. Clinical practice. Infection associated with prosthetic joints. The New England journal of medicine. 2009;361(8):787–794. doi: 10.1056/NEJMcp0905029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monzón M. A simple infection model using pre-colonized implants to reproduce rat chronic Staphylococcus aureus osteomyelitis and study antibiotic treatment. Journal of orthopaedic research. 2001;19(5):820–826. doi: 10.1016/S0736-0266(00)00076-0. [DOI] [PubMed] [Google Scholar]
- 19.Swami A. Engineered nanomedicine for myeloma and bone microenvironment targeting. Proceedings of the National Academy of Sciences - PNAS. 2014;111(28):10287–10292. doi: 10.1073/pnas.1401337111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Venugopal J, Prabhakaran MP, Low S, et al. Continuous nanostructures for the controlled release of drugs. Current Pharmaceutical Design. 2009;15(15):1799–1808. doi: 10.2174/138161209788186344. [DOI] [PubMed] [Google Scholar]
- 21.Gitelis S, Brebach GT. The treatment of chronic osteomyelitis with a biodegradable antibiotic-impregnated implant. Journal of orthopaedic surgery (Hong Kong) 2002;10(1):53–60. doi: 10.1177/230949900201000110. [DOI] [PubMed] [Google Scholar]
- 22.McLaren JS, White LJ, Cox HC, et al. A biodegradable antibiotic-impregnated scaffold to prevent osteomyelitis in a contaminated in vivo bone defect model. European Cells and Materials. 2014;27:332–349. doi: 10.22203/ecm.v027a24. [DOI] [PubMed] [Google Scholar]
- 23.Gogia JS, Meehan JP, Di Cesare PE, Jamali AA. Local Antibiotic Therapy in Osteomyelitis. Seminars in Plastic Surgery. 2009;23(2):100–107. doi: 10.1055/s-0029-1214162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanssen AD. Prophylactic use of antibiotic bone cement: an emerging standard--in opposition. The Journal of arthroplasty. 2004;19(4 Suppl 1):73–77. doi: 10.1016/j.arth.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Jiranek WA, Hanssen AD, Greenwald AS. Antibiotic-loaded bone cement for infection prophylaxis in total joint replacement. The Journal of bone and joint surgery. American volume. 2006;88(11):2487–2500. doi: 10.2106/JBJS.E.01126. [DOI] [PubMed] [Google Scholar]
- 26.Bourne RB. Prophylactic use of antibiotic bone cement: an emerging standard--in the affirmative. The Journal of arthroplasty. 2004;19(4 Suppl 1):69–72. doi: 10.1016/j.arth.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Tan H, Guo S, Yang S, Xu X, Tang T. Physical characterization and osteogenic activity of the quaternized chitosan-loaded PMMA bone cement. Acta biomaterialia. 2012;8(6):2166–2174. doi: 10.1016/j.actbio.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 28.Tan H, Peng Z, Li Q, Xu X, Guo S, Tang T. The use of quaternised chitosan-loaded PMMA to inhibit biofilm formation and downregulate the virulence-associated gene expression of antibiotic-resistant staphylococcus. Biomaterials. 2012;33(2):365–377. doi: 10.1016/j.biomaterials.2011.09.084. [DOI] [PubMed] [Google Scholar]
- 29.Calvo P, Remuñán-López C, Vila-Jato JL, Alonso MJ. Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. Journal of Applied Polymer Science. 1997;63(1):125–132. [Google Scholar]
- 30.Kunjachan S, Jose S, Lammers T. Understanding the mechanism of ionic gelation for synthesis of chitosan nanoparticles using qualitative techniques. Asian Journal of Pharmaceutics. 2010;4(2):148. [Google Scholar]
- 31.Shi Z, Neoh KG, Kang ET, Wang W. Antibacterial and mechanical properties of bone cement impregnated with chitosan nanoparticles. Biomaterials. 2006;27(11):2440–2449. doi: 10.1016/j.biomaterials.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 32.Alt V, Bechert T, Steinrücke P, et al. Nanoparticulate silver. A new antimicrobial substance for bone cement. Der Orthopäde. 2004;33(8):885–892. doi: 10.1007/s00132-004-0690-8. [DOI] [PubMed] [Google Scholar]
- 33.Matos AC, Gonçalves LM, Rijo P, Vaz MA, Almeida AJ, Bettencourt AF. A novel modified acrylic bone cement matrix. A step forward on antibiotic delivery against multiresistant bacteria responsible for prosthetic joint infections. Materials Science and Engineering: C. 2014;38:218–226. doi: 10.1016/j.msec.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Frutos G, Pastor JY, Martinez N, Virto MR, Torrado S. Influence of lactose addition to gentamicin-loaded acrylic bone cement on the kinetics of release of the antibiotic and the cement properties. Acta Biomater. 2010;6(3):804–811. doi: 10.1016/j.actbio.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 35.Sancineto CF, Barla JD. Treatment of long bone osteomyelitis with a mechanically stable intramedullar antibiotic dispenser: nineteen consecutive cases with a minimum of 12 months follow-up. Journal of Trauma-Injury Infection & Critical Care. 2008;65(6):1416–1420. doi: 10.1097/TA.0b013e31818c6a09. [DOI] [PubMed] [Google Scholar]
- 36.Conway J, Mansour J, Kotze K, Specht S, Shabtai L. Antibiotic cement-coated rods: an effective treatment for infected long bones and prosthetic joint nonunions. The Bone & Joint Journal. 2014;96-B(10):1349–1354. doi: 10.1302/0301-620X.96B10.33799. [DOI] [PubMed] [Google Scholar]
- 37.Kanakaris N, Gudipati S, Tosounidis T, Harwood P, Britten S, Giannoudis PV. The treatment of intramedullary osteomyelitis of the femur and tibia using the Reamer-Irrigator-Aspirator system and antibiotic cement rods. The Bone & Joint Journal. 2014;96-B(6):783–788. doi: 10.1302/0301-620X.96B6.32244. [DOI] [PubMed] [Google Scholar]
- 38.Conway JD, Hlad LM, Bark SE. Antibiotic Cement-Coated Plates for Management of Infected Fractures. The American Journal of Orthopedics. 2015;44(2):E49–E53. [PubMed] [Google Scholar]
- 39.Berkes M, Obremskey WT, Scannell B, Ellington JK, Hymes RA, Bosse M. Maintenance of hardware after early postoperative infection following fracture internal fixation. The Journal of bone and joint surgery. American volume. 2010;92(4):823–828. doi: 10.2106/JBJS.I.00470. [DOI] [PubMed] [Google Scholar]
- 40.Wilberforce SI, Finlayson CE, Best SM, Cameron RE. A comparative study of the thermal and dynamic mechanical behaviour of quenched and annealed bioresorbable poly-L-lactide/alpha-tricalcium phosphate nanocomposites. Acta Biomater. 2011;7(5):2176–2184. doi: 10.1016/j.actbio.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 41.Uskoković V. In vitro analysis of nanoparticulate hydroxyapatite/chitosan composites as potential drug delivery platforms for the sustained release of antibiotics in the treatment of osteomyelitis. Journal of pharmaceutical sciences. 2014;103(2):567–579. doi: 10.1002/jps.23824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peng KT, Chen CF, Chu IM, et al. Treatment of osteomyelitis with teicoplanin-encapsulated biodegradable thermosensitive hydrogel nanoparticles. Biomaterials. 2010;31(19):5227–5236. doi: 10.1016/j.biomaterials.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 43.Noukrati H, Cazalbou S, Demnati I, Rey C, Barroug A, Combes C. Injectability, microstructure and release properties of sodium fusidate-loaded apatitic cement as a local drug-delivery system. Materials science & engineering. C, Materials for biological applications. 2016;59:177–184. doi: 10.1016/j.msec.2015.09.070. [DOI] [PubMed] [Google Scholar]
- 44.Bastari K. A controlled release of antibiotics from calcium phosphate-coated poly(lactic-co-glycolic acid) particles and their in vitro efficacy against Staphylococcus aureus biofilm. Journal of materials science. Materials in medicine. 2014;25(3):747–757. doi: 10.1007/s10856-013-5125-9. [DOI] [PubMed] [Google Scholar]
- 45.Ignjatovic NL, Ajdukovic ZR, Savic VP, Uskoković DP. Size effect of calcium phosphate coated with poly-DL-lactide- co-glycolide on healing processes in bone reconstruction. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2010;94(1):108–117. doi: 10.1002/jbm.b.31630. [DOI] [PubMed] [Google Scholar]
- 46.Ignjatovi NL, Ninkov P, Sabetrasekh R, Uskoković DP. A novel nano drug delivery system based on tigecycline-loaded calciumphosphate coated with poly-DL-lactide-co-glycolide. Journal of Materials Science: Materials in Medicine. 2010;21(1):231–239. doi: 10.1007/s10856-009-3854-6. [DOI] [PubMed] [Google Scholar]
- 47.McKee MD. The use of an antibiotic-impregnated, osteoconductive, bioabsorbable bone substitute in the treatment of infected long bone defects: early results of a prospective trial. Journal of orthopaedic trauma. 2002;16(9):622–627. doi: 10.1097/00005131-200210000-00002. [DOI] [PubMed] [Google Scholar]
- 48.Bernkop-Schnurch A, Dunnhaupt S. chitosan-based drug delivery systems. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e. V. 2012;81(3):463–469. doi: 10.1016/j.ejpb.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 49.Yeh TH, Hsu LW, Tseng MT, et al. Mechanism and consequence of chitosan-mediated reversible epithelial tight junction opening. Biomaterials. 2011;32(26):6164–6173. doi: 10.1016/j.biomaterials.2011.03.056. [DOI] [PubMed] [Google Scholar]
- 50.Loh JW, Saunders M, Lim LY. Cytotoxicity of monodispersed chitosan nanoparticles against the Caco-2 cells. Toxicology and applied pharmacology. 2012;262(3):273–282. doi: 10.1016/j.taap.2012.04.037. [DOI] [PubMed] [Google Scholar]
- 51.Zheng F, Wang S, Wen S, Shen M, Zhu M, Shi X. Characterization and antibacterial activity of amoxicillin-loaded electrospun nano-hydroxyapatite/poly(lactic-co-glycolic acid) composite nanofibers. Biomaterials. 2013;34(4):1402–1412. doi: 10.1016/j.biomaterials.2012.10.071. [DOI] [PubMed] [Google Scholar]
- 52.Di Silvio L, Bonfield W. Biodegradable drug delivery system for the treatment of bone infection and repair. Journal of Materials Science: Materials in Medicine. 1999;10(10/11):653–658. doi: 10.1023/a:1008995926566. [DOI] [PubMed] [Google Scholar]
- 53.Im O, Li J, Wang M, Zhang LG, Keidar M. Biomimetic three-dimensional nanocrystalline hydroxyapatite and magnetically synthesized single-walled carbon nanotube chitosan nanocomposite for bone regeneration. Int J Nanomedicine. 2012;7:2087–2099. doi: 10.2147/IJN.S29743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harrison BS, Atala A. Carbon nanotube applications for tissue engineering. Biomaterials. 2007;28(2):344–353. doi: 10.1016/j.biomaterials.2006.07.044. [DOI] [PubMed] [Google Scholar]
- 55.Qi R, Cao X, Shen M, Guo R, Yu J, Shi X. Biocompatibility of electrospun halloysite nanotube-doped poly(lactic-co-glycolic acid) composite nanofibers. Journal of biomaterials science. Polymer edition. 2012;23(1–4):299–313. doi: 10.1163/092050610X550340. [DOI] [PubMed] [Google Scholar]
- 56.Neut D, Dijkstra RJ, Thompson JI, van der Mei HC, Busscher HJ. Antibacterial efficacy of a new gentamicin-coating for cementless prostheses compared to gentamicin-loaded bone cement. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2011;29(11):1654–1661. doi: 10.1002/jor.21433. [DOI] [PubMed] [Google Scholar]
- 57.Neut D, Dijkstra RJ, Thompson JI, van der Mei HC, Busscher HJ. A gentamicin-releasing coating for cementless hip prostheses-Longitudinal evaluation of efficacy using in vitro bio-optical imaging and its wide-spectrum antibacterial efficacy. Journal of biomedical materials research. Part A. 2012;100(12):3220–3226. doi: 10.1002/jbm.a.34258. [DOI] [PubMed] [Google Scholar]
- 58.Neut D, Dijkstra RJ, Thompson JI, Kavanagh C, van der Mei HC, Busscher HJ. A biodegradable gentamicin-hydroxyapatite-coating for infection prophylaxis in cementless hip prostheses. European cells & materials. 2015;29:42–56. doi: 10.22203/ecm.v029a04. [DOI] [PubMed] [Google Scholar]
- 59.Stigter M, Bezemer J, de Groot K, Layrolle P. Incorporation of different antibiotics into carbonated hydroxyapatite coatings on titanium implants, release and antibiotic efficacy. Journal of controlled release : official journal of the Controlled Release Society. 2004;99(1):127–137. doi: 10.1016/j.jconrel.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 60.Moojen DJ, Vogely HC, Fleer A, et al. Prophylaxis of infection and effects on osseointegration using a tobramycin-periapatite coating on titanium implants--an experimental study in the rabbit. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2009;27(6):710–716. doi: 10.1002/jor.20808. [DOI] [PubMed] [Google Scholar]
- 61.Zhu CT. Liposome combined porous beta-TCP scaffold: preparation, characterization, and anti-biofilm activity. Drug delivery. 2010;17(6):391–398. doi: 10.3109/10717541003762870. [DOI] [PubMed] [Google Scholar]
- 62.Feng K, Sun H, Bradley MA, Dupler EJ, Giannobile WV, Ma PX. Novel antibacterial nanofibrous PLLA scaffolds. Journal of controlled release : official journal of the Controlled Release Society. 2010;146(3):363–369. doi: 10.1016/j.jconrel.2010.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qiao M, Chen D, Ma X, Liu Y. Injectable biodegradable temperature-responsive PLGA-PEG-PLGA copolymers: synthesis and effect of copolymer composition on the drug release from the copolymer-based hydrogels. Int J Pharm. 2005;294(1–2):103–112. doi: 10.1016/j.ijpharm.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 64.Chen JP, Cheng TH. Thermo-responsive chitosan-graft-poly(N-isopropylacrylamide) injectable hydrogel for cultivation of chondrocytes and meniscus cells. Macromolecular bioscience. 2006;6(12):1026–1039. doi: 10.1002/mabi.200600142. [DOI] [PubMed] [Google Scholar]
- 65.Lin G, Cosimbescu L, Karin NJ, Tarasevich BJ. Injectable and thermosensitive PLGA-g-PEG hydrogels containing hydroxyapatite: preparation, characterization and in vitro release behavior. Biomedical Materials. 2012;7(2):024107. doi: 10.1088/1748-6041/7/2/024107. [DOI] [PubMed] [Google Scholar]
- 66.Yasmeen S, Lo MK, Bajracharya S, Roldo M. Injectable scaffolds for bone regeneration. Langmuir. 2014;30(43):12977–12985. doi: 10.1021/la503057w. [DOI] [PubMed] [Google Scholar]
- 67.Tang Y, Du Y, Li Y, Wang X, Hu X. A thermosensitive chitosan/poly(vinyl alcohol) hydrogel containing hydroxyapatite for protein delivery. Journal of biomedical materials research. Part A. 2009;91(4):953–963. doi: 10.1002/jbm.a.32240. [DOI] [PubMed] [Google Scholar]
- 68.Sohrabi M, Hesaraki S, Kazemzadeh A. The influence of polymeric component of bioactive glass-based nanocomposite paste on its rheological behaviors and in vitro responses: Hyaluronic acid versus sodium alginate. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2014;102(3):561–573. doi: 10.1002/jbm.b.33035. [DOI] [PubMed] [Google Scholar]
- 69.Couto DS, Hong Z, Mano JF. Development of bioactive and biodegradable chitosan-based injectable systems containing bioactive glass nanoparticles. Acta biomaterialia. 2009;5(1):115–123. doi: 10.1016/j.actbio.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 70.Ignjatović NL, Liu CZ, Czernuszka JT, Uskoković DP. Micro- and nano-injectable composite biomaterials containing calcium phosphate coated with poly(DL-lactide-co-glycolide) Acta biomaterialia. 2007;3(6):927–935. doi: 10.1016/j.actbio.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 71.Brennan SA, Ni Fhoghlu C, Devitt BM, O'Mahony FJ, Brabazon D, Walsh A. Silver nanoparticles and their orthopaedic applications. Bone Joint J. 2015;97-b(5):582–589. doi: 10.1302/0301-620X.97B5.33336. [DOI] [PubMed] [Google Scholar]
- 72.Simchi A, Tamjid E, Pishbin F, Boccaccini AR. Recent progress in inorganic and composite coatings with bactericidal capability for orthopaedic applications. Nanomedicine : nanotechnology, biology, and medicine. 2011;7(1):22–39. doi: 10.1016/j.nano.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 73.Juan L, Zhimin Z, Anchun M, Lei L, Jingchao Z. Deposition of silver nanoparticles on titanium surface for antibacterial effect. Int J Nanomedicine. 2010;5:261–267. doi: 10.2147/ijn.s8810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.De Giglio E, Cafagna D, Cometa S, et al. An innovative, easily fabricated, silver nanoparticle-based titanium implant coating: development and analytical characterization. Analytical and bioanalytical chemistry. 2013;405(2–3):805–816. doi: 10.1007/s00216-012-6293-z. [DOI] [PubMed] [Google Scholar]
- 75.Afzal MA, Kalmodia S, Kesarwani P, Basu B, Balani K. Bactericidal effect of silver-reinforced carbon nanotube and hydroxyapatite composites. Journal of biomaterials applications. 2013;27(8):967–978. doi: 10.1177/0885328211431856. [DOI] [PubMed] [Google Scholar]
- 76.Liu S, Wei L, Hao L, et al. Sharper and faster "nano darts" kill more bacteria: a study of antibacterial activity of individually dispersed pristine single-walled carbon nanotube. ACS nano. 2009;3(12):3891–3902. doi: 10.1021/nn901252r. [DOI] [PubMed] [Google Scholar]
- 77.Zhang Y, Bai Y, Yan B. Functionalized carbon nanotubes for potential medicinal applications. Drug discovery today. 2010;15(11–12):428–435. doi: 10.1016/j.drudis.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang BG, Myers DE, Wallace GG, Brandt M, Choong PF. Bioactive coatings for orthopaedic implants-recent trends in development of implant coatings. Int J Mol Sci. 2014;15(7):11878–11921. doi: 10.3390/ijms150711878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Martinez LR, Han G, Chacko M, et al. Antimicrobial and healing efficacy of sustained release nitric oxide nanoparticles against Staphylococcus aureus skin infection. The Journal of investigative dermatology. 2009;129(10):2463–2469. doi: 10.1038/jid.2009.95. [DOI] [PubMed] [Google Scholar]
- 80.Rothrock AR, Donkers RL, Schoenfisch MH. Synthesis of nitric oxide-releasing gold nanoparticles. Journal of the American Chemical Society. 2005;127(26):9362–9363. doi: 10.1021/ja052027u. [DOI] [PubMed] [Google Scholar]
- 81.Cui Y, Zhao Y, Tian Y, Zhang W, Lu X, Jiang X. The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli. Biomaterials. 2012;33(7):2327–2333. doi: 10.1016/j.biomaterials.2011.11.057. [DOI] [PubMed] [Google Scholar]
- 82.Huang H, Pierstorff E, Osawa E, Ho D. Protein-mediated assembly of nanodiamond hydrogels into a biocompatible and biofunctional multilayer nanofilm. ACS nano. 2008;2(2):203–212. doi: 10.1021/nn7000867. [DOI] [PubMed] [Google Scholar]
- 83.Ansari MA, Khan HM, Khan AA, Sultan A, Azam A. Characterization of clinical strains of MSSA, MRSA and MRSE isolated from skin and soft tissue infections and the antibacterial activity of ZnO nanoparticles. World journal of microbiology & biotechnology. 2012;28(4):1605–1613. doi: 10.1007/s11274-011-0966-1. [DOI] [PubMed] [Google Scholar]
- 84.Memarzadeh K, Sharili AS, Huang J, Rawlinson SC, Allaker RP. Nanoparticulate zinc oxide as a coating material for orthopedic and dental implants. Journal of biomedical materials research. Part A. 2015;103(3):981–989. doi: 10.1002/jbm.a.35241. [DOI] [PubMed] [Google Scholar]
- 85.Taylor EN, Webster TJ. The use of superparamagnetic nanoparticles for prosthetic biofilm prevention. Int J Nanomedicine. 2009;4:145–152. [PMC free article] [PubMed] [Google Scholar]
- 86.Durmus NG, Webster TJ. Eradicating antibiotic-resistant biofilms with silver-conjugated superparamagnetic iron oxide nanoparticles. Advanced healthcare materials. 2013;2(1):165–171. doi: 10.1002/adhm.201200215. [DOI] [PubMed] [Google Scholar]
- 87.Goodman SB, Yao Z, Keeney M, Yang F. The Future of Biologic Coatings for Orthopaedic Implants. Biomaterials. 2013;34(13):3174–3183. doi: 10.1016/j.biomaterials.2013.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. Specific proteins mediate enhanced osteoblast adhesion on nanophase ceramics. Journal of biomedical materials research. 2000;51(3):475–483. doi: 10.1002/1097-4636(20000905)51:3<475::aid-jbm23>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 89.Vallet-Regí M, Ragel CV, Salinas Antonio J. Glasses with Medical Applications. European Journal of Inorganic Chemistry. 2003;2003(6):1029–1042. [Google Scholar]
- 90.Hench LL, Xynos ID, Polak JM. Bioactive glasses for in situ tissue regeneration. Journal of biomaterials science. Polymer edition. 2004;15(4):543–562. doi: 10.1163/156856204323005352. [DOI] [PubMed] [Google Scholar]
- 91.Sohrabi M, Hesaraki S, Kazemzadeh A. The influence of polymeric component of bioactive glass-based nanocomposite paste on its rheological behaviors and in vitro responses: hyaluronic acid versus sodium alginate. Journal of biomedical materials research. Part B, Applied biomaterials. 2014;102(3):561–573. doi: 10.1002/jbm.b.33035. [DOI] [PubMed] [Google Scholar]
- 92.Hoffmann F, Cornelius M, Morell J, Froba M. Silica-based mesoporous organic-inorganic hybrid materials. Angewandte Chemie (International ed. in English) 2006;45(20):3216–3251. doi: 10.1002/anie.200503075. [DOI] [PubMed] [Google Scholar]
- 93.Luo XJ, Yang HY, Niu LN, et al. Translation of a solution-based biomineralization concept into a carrier-based delivery system via the use of expanded-pore mesoporous silica. Acta biomaterialia. 2015 doi: 10.1016/j.actbio.2015.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Beck GR, Jr, Ha SW, Camalier CE, et al. Bioactive silica-based nanoparticles stimulate bone-forming osteoblasts, suppress bone-resorbing osteoclasts, and enhance bone mineral density in vivo. Nanomedicine : nanotechnology, biology, and medicine. 2012;8(6):793–803. doi: 10.1016/j.nano.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]