Abstract
Animal models have long been used to study the mechanisms underlying the complex association between alcohol and stress. Female mice prevented from running on a home-cage activity wheel increase voluntary ethanol consumption. β-endorphin is an endogenous opioid involved in negatively regulating the stress response and has also been implicated in the risk for excessive drinking. The present study investigates the role of β-endorphin in moderating free-choice consumption of ethanol in response to a blocked activity wheel. Female, transgenic mice with varying levels of the opioid peptide were given daily 2-h access to 20% ethanol with rotations on a running wheel blocked on alternate days. Subjects with low β-endorphin exhibited enhanced stress sensitivity by self-administering larger quantities of ethanol on days when wheel running was prevented. β-endorphin levels did not influence voluntary activity on the running wheel. There were genotypic differences in plasma corticosterone levels as well as corticotropin-releasing hormone mRNA content in multiple brain regions associated with the stress response in these free drinking and running subjects. Susceptibility to stress is enhanced in female mice with low levels of β-endorphin, and better understanding of the role for this opioid in mitigating the response to stressors may aid in the development of interventions and treatments for excessive use of alcohol in women.
Keywords: opioid, stress, ethanol, Crh, corticosterone, voluntary activity, exercise, self-administration, sex differences
Introduction
Alcohol is a physiological stressor, potently activating the neuroendocrine stress response, yet people often consume the drug as a way to cope with stress. In part because both stress and alcohol impact and recruit a multitude of factors, the mechanisms underlying their relationship remain largely unclear (Phillips, Reed, & Pastor, 2015; Stephens & Wand, 2012). Attempts to better understand the complex interactions between stress and ethanol have frequently employed animal models (Crabbe, 2014), and these strategies have helped to identify some of the specific mechanisms contributing to aspects of the paradoxical relationship, especially as they pertain to the dependent state (Becker, Lopez, & Doremus-Fitzwater, 2011; Crabbe, Phillips, & Belknap, 2010). Our lab has employed intermittent interruptions of access to a running wheel as a model of stress (Ehringer, Hoft, & Zunhammer, 2009; Piza-Palma et al., 2014). We have argued that blocking access to a running wheel in the home cage induces frustration stress, and that this manipulation may be relevant to the human condition where stressors often involve loss of something desired, such as a loved one, health, or job (Thoits, 2010).
Individual differences in drug use and abuse as well as stress susceptibility are impacted by a vast number of factors. These factors include environmental challenges, genetic background, family history, and gender. Restricting rotations of an appetitive running wheel results in significant increases in voluntary ethanol consumption in female, but not male, C57BL/6J mice (Piza-Palma et al., 2014). Because women are generally more sensitive to stress (Burk et al., 2011; Randall et al., 1999; Young-Wolff, Kendler, & Prescott, 2012) and prone to disproportionately escalating their use and abuse of the drug compared to men (Becker et al., 2005; Greenfield, Back, Lawson, & Brady, 2010; Keyes, Grant, & Hasin, 2008), this sex-dependent effect may be a useful tool in addressing the general shortage of research on sex-dependent factors (Beery & Zucker, 2011) and may help shed light on the general relationship between stress and alcohol in non-dependent subjects.
Stress is a multifaceted adaptation to environmental perturbation that can evoke a wide range of physiological and behavioral changes. One such consequence is the increased synthesis and secretion of corticotropin-releasing hormone (CRH), also implicated as a key player in chronic ethanol exposure and dependence (Phillips et al., 2015). In response to stressors (including ethanol), CRH stimulates transcription of the proopiomelanocortin gene, which produces precursors for adrenocorticotropin hormone (ACTH) and β-endorphin. While ACTH is carried in the blood to help coordinate the peripheral stress response, β-endorphin, an endogenous opioid peptide, modulates the hypothalamic-pituitary-adrenal (HPA) axis (Charmandari, Tsigos, & Chrousos, 2005; Pechnick, 1993) by inhibiting secretion of CRH (Buckingham, 1986; Plotsky, 1991; Sarkar, Kuhn, Marano, Chen, & Boyadjieva, 2007). β-endorphin also contributes to behavioral stress responses in a number of ways (Amir, 1982; Ribeiro, Kennedy, Smith, Stohler, & Zubieta, 2005; Yamada & Nabeshima, 1995). Our lab has shown that mice lacking this peptide exhibit exaggerated behavioral responses to stressors (Barfield et al., 2010; Barfield, Moser, Hand, & Grisel, 2013; Grisel, Bartels, Allen, & Turgeon, 2008; Grisel et al., 1999). Moreover, a clinical correlation has been established between heritable levels of β-endorphin and risk for excessive drinking (Froehlich, Harts, Lumeng, & Li, 1990; Gianoulakis, 2009; Wand, Mangold, El Deiry, McCaul, & Hoover, 1998). This body of research supports the contention that β-endorphin modulates the relationship between stress and alcohol.
Using the transgenic model for β-endorphin deficit developed by Rubinstein and colleagues in 1996, we have shown that heterozygous mice (βE-HT), with reduced levels of the opioid peptide, consume slightly but significantly more ethanol in standard two-bottle choice experiments than either wild-type controls or mice entirely lacking the opioid (βE-KO) (Grisel et al., 1999; Williams, Holloway, Karwan, Allen, & Grisel, 2007). We have previously argued that βE-HT mice find ethanol especially rewarding since the drug can stimulate production of the peptide and ameliorate a deficient state. Because β-endorphin deficiency also results in increased stress sensitivity, it is possible that the tendency to self-medicate would be even higher under stressful conditions.
The purpose of our study was to investigate voluntary drinking in mice with varying levels of β-endorphin in the context of external stress from a blocked running wheel. In order to begin exploring the effects of constitutive β-endorphin deficiency on endocrine responses to stress, we also evaluated plasma ACTH, corticosterone (CORT), and mRNA for the CRH peptide and its type 1 receptor (CRH-R1) in brain areas implicated in the stress response, including the ventral hippocampus (VH), amygdala and bed nucleus stria terminalis (BNST), and dorsomedial prefrontal cortex (dmPFC) (Silberman & Winder, 2013; Stamatakis et al., 2014). A modified drinking-in-the-dark paradigm was employed with locked running wheels acting as an external stressor in female C57BL/6J, βE-HT, and βE-KO mice (Ehringer et al., 2009; Piza-Palma et al., 2014). We hypothesized the finding of increased drinking in β-endorphin-deficient mice in response to stress, and speculated that the differences in behavioral sensitivity to stress would be reflected in heightened ACTH and/or CORT levels and alterations in Crh and/or Crh-R1.
Methods
Subjects
The β-endorphin-deficient model was developed about 20 years ago in the laboratory of Malcolm Low (Rubinstein et al., 1996) by insertion of a premature stop codon into the Pomc gene. The gene mutation has been fully backcrossed to the C57BL/6J strain (>20 generations). Homozygotes (KO) cannot synthesize β-E, though all other products of the POMC protein show normal expression. Opioid receptor expression also remains unchanged (Rubinstein et al., 1996). The model has been used in studies of metabolism, as KO males (but not females, which were used here) show an altered growth curve resulting in increased body mass and white fat (Low, Hayward, Appleyard, & Rubinstein, 2003). We previously suggested hypersensitivity to stress in β-E deficient mice (e.g., Barfield et al., 2013) but no overt alterations in the HPA axis have been reported, and homozygous mutant mice appear otherwise normal in terms of development and behavior. HT mice produce 50% of B6 levels of β-E.
Thirty-six adult naïve female mice between the ages of 55 and 81 days at the start of the experiment were used. Mice for these studies were bred in-house from stock purchased from Jackson Laboratories (Bar Harbor, ME). βE-HT mice were bred from βE-KO males and B6 females; others were bred under identical conditions from genotype-matched pairs. Subjects included 13 B6, 13 βE-HT, and 10 βE-KO mice. Mice were weaned at 21 days and group-housed by sex and genotype in Plexiglas® cages filled with corn cob bedding in a colony with a 12-h reverse light:dark cycle (lights off at 0930) maintained at 21 ± 2 °C. Subjects were given free access to standard mouse chow and tap water at all times before and during the study.
During the experimental period subjects were moved to an experimental room across the hall from the colony that was maintained with the same temperature and light conditions. However, during a 4-day habituation period and throughout the 10-day experimental period, subjects were housed individually in TSE Phenomaster Plexiglas® cages that contained a running wheel (11 cm in diameter; TSE Systems, Bad Homburg, Germany) in addition to ad libitum access to food and water and limited access to ethanol (see below). The corn cob bedding was changed once during the experiment, between habituation and the beginning of the experimental period.
Drinking procedure
A modified drinking-in-the-dark procedure (Ehringer et al., 2009; Rhodes et al., 2007) was used throughout this study. Thus, from the time subjects were individually housed in the experimental room, they were allowed access to 20% ethanol (v:v in tap water) for 2 h each day, beginning 3 h into their dark cycle, always with food and tap water freely available. Ethanol presentation was switched every 2 days to prevent the development of a side preference. During the 10-day experimental period, wheel rotations were limited every other day. On unlocked days (1, 3, 5, 7, and 9) the wheel freely rotated for active animals as during habituation, but on locked days (2, 4, 6, 8, and 10) a brake was remotely engaged so that the wheel could not rotate beginning 1 h before, and continuing throughout the ethanol-access period. The TSE PhenoMaster program was intended as our method for collecting both drinking data and running data, but the program generated unreliable and incorrect fluid consumption data. Therefore, the TSE PhenoMaster program measured only running data, and drinking was assessed manually by reading gradations on a 13-mL tube with a ball-bearing sipper. Each day we calculated the dose administered by each mouse (g ethanol/kg of body weight) as well as ethanol preference (the percentage of total fluid consumed that was from the ethanol-filled tube) during the 2 h of ethanol access. Twelve mice could be tested at a time in our facility, so subjects were tested in three runs, with efforts to counterbalance genotype within and between runs, and with individuals randomly assigned to cages in the testing room.
Blood ethanol content (BEC) analysis
Immediately following the final experimental manipulation on Day 10 (locked running wheel for 1 h before and 2 h during ethanol availability), subjects were removed from their cages one at a time, carried in individual cages to a nearby room, exposed to isoflurane anesthesia, and sacrificed via rapid decapitation. The time between removing the subject from her cage and decapitation was 1–2 min. Trunk blood was collected into EDTA-treated vacutainer tubes (Becton Dickinson, Rutherford, NJ) and brains were immediately removed and frozen on dry ice and stored at −80 °C. Blood was centrifuged at 3000 rpm for 30 min at 4 °C, and was then analyzed for BEC using an Analox BEC Analyzer (Analox Instruments Ltd., London, UK). The test uses an alcohol oxidase enzyme, which oxidizes ethanol in the presence of molecular oxygen. The rate of oxygen consumption is measured and is directly proportional to the alcohol concentration in the plasma. Sensitivity of the analyzer is 0.1 mg/dL. Plasma not used for BEC analysis was stored at −20 °C and subsequently used to measure hormone levels (CORT and ACTH) with enzyme-linked immunosorbent assay (ELISA).
RNA isolation and real-time quantitative RT-PCR
Frozen brains were dissected on ice into three regions of interest using the mouse brain matrix (Kent Scientific). The regions of interest included the ventral hippocampus (−2.5 to −4 mm relative to bregma, 2 mm lateral of the midline and 2 mm from the ventral border) and dorsomedial prefrontal cortex (3.0 to 1.0 mm relative to bregma and 3 mm from dorsal border) and the amygdala/BNST. For the last region, samples were removed with a 2-mm diameter punch tool according to the brain atlas of Paxinos and Watson (1.0 to −2.5 mm from bregma, 1.5 mm from the midline, and 1 mm from the ventral border). For analysis, each sample was composed of punches from two randomly paired animals of the same genotype in order to obtain sufficient amounts of tissue. Thus, for example, one sample from the VH contained four dissected tissues – the left and right VH from two genotype-matched subjects. For all qPCR experiments, data were normalized using the corresponding GAPDH mRNA expression because this mRNA species has been shown previously to be a stably expressed reference gene (Rhinn et al., 2008; Taki, Abdel-Rahman, & Zhang, 2014). All assays had similar optimum PCR efficiencies, and all samples were assayed in duplicate during the same procedure. The results are presented as percent differences normalized to gene expression in the wild-type group using the ΔΔ Ct relative quantification method (Schmittgen & Livak, 2008).
Each sample tube was immediately homogenized in lysis buffer for RNA extraction. Total RNA was isolated using the Direct-zol RNA MiniPrep Plus (Zymo Research, Irvine, CA, catalog R2072), according to manufacturer’s instructions. Fifty ng of total RNA was reverse-transcribed using the iScript Reverse Transcription Supermix (BioRad, Hercules, CA) according to manufacturer’s instructions. Synthesized cDNA corresponding to 500 ng of total RNA was used in each quantitative real-time PCR (qPCR) reaction. qPCR was performed using TaqMan FastStart Essential DNA Probes Master Mix (Roche Diagnostics, Indianapolis, IN) according to manufacturer’s instructions. PrimeTime® Std qPCR Assays designed by IDT (Integrated DNA Technologies, Coralville, IA) were performed using CRH (NM_205769; forward primer 5′-AGA AAG GAG AAG AGG AAG AAA ACC-3′ and reverse primer 5′-CCG CAG CCG CAT GTT AG-3′), CRH-R1 (NM_007762; forward primer 5′-TGC CTT TCC CCA TCA TTG TG-3′ and reverse primer 5′-GCC CTG GTA GAT GTA GTC AGT A-3′), and the reference gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH; NM_0080845′AAT GGT GAA GGT CGG TGT G-3′ and reverse primer 5′-GTG GAG TCA TAC TGG AAC ATG TAG-3′) in duplicate on a LightCycler 96 (Roche Diagnostics, Indianapolis, IN).
ELISAs
Corticosterone levels were measured using the corticosterone ELISA kit (Enzo Life Sciences, Farmingdale, NY, catalog ADI-900-097), according to manufacturer’s instructions. For the corticosterone assay, plasma was diluted 1:40 with assay buffer. The absorbance was read at 405 nm using an iMark microplate reader (BioRad, Hercules, CA). Sample concentrations for corticosterone were calculated from a standard curve using GraphPad Prism software (GraphPad, La Jolla, CA). The sensitivity of the assay was 27 pg/mL with a range of detection up to 20,000 pg/mL. All samples were assayed in duplicate and all samples from the experiment were assayed during a single procedure.
ACTH levels were measured using the ACTH ELISA kit (Enzo Life Sciences, Farmingdale, NY, catalog ENZ-KIT138) according to manufacturer’s instructions. For the ACTH assay, plasma was diluted 1:1. The absorbance was read at 450 nm using an iMark microplate reader (BioRad, Hercules, CA). Sample concentrations for ACTH were calculated from a standard curve using GraphPad Prism software (GraphPad, La Jolla, CA). The sensitivity of the assay was 0.46 pg/mL with a range of detection up to 165 pg/mL. All samples from the experiment were assayed in duplicate during a single procedure.
Adrenalectomy
Shortly after the time of sacrifice, left and right adrenal glands were harvested from each mouse and weighed.
Statistical analyses
Behavioral data, hormone levels, and BECs were analyzed by ANOVA using SPSS (23.0) to evaluate differences across the experimental conditions (repeated measure including all 10 days and then by unlocked and locked averages). We employed one-sample t tests to further address the hypothesis that the locked-wheel condition would result in increased consumption compared to drinking on unlocked days by asking within each genotype, whether the difference in average drinking in the two experimental conditions was significantly different than the null hypothesis of zero. One-way ANOVA was used to measure genetic variation in total ethanol consumption and preference, hormone levels, adrenal gland weights, and (independently for each brain region and each gene of interest, with Bonferroni corrections) Crh and Crh-R1 expression. Specific between-group comparisons were performed using Tukey (HSD) test where appropriate. Pearson correlation was used to evaluate relationships with BEC, CORT, and Crh expression. In all cases the α level was set at 0.05.
Results
One wild-type female became ill and abruptly stopped eating, drinking, and running in the last few days of the experiment; therefore, she was euthanized and her data from the brief period of aberrant behavior were omitted from analyses.
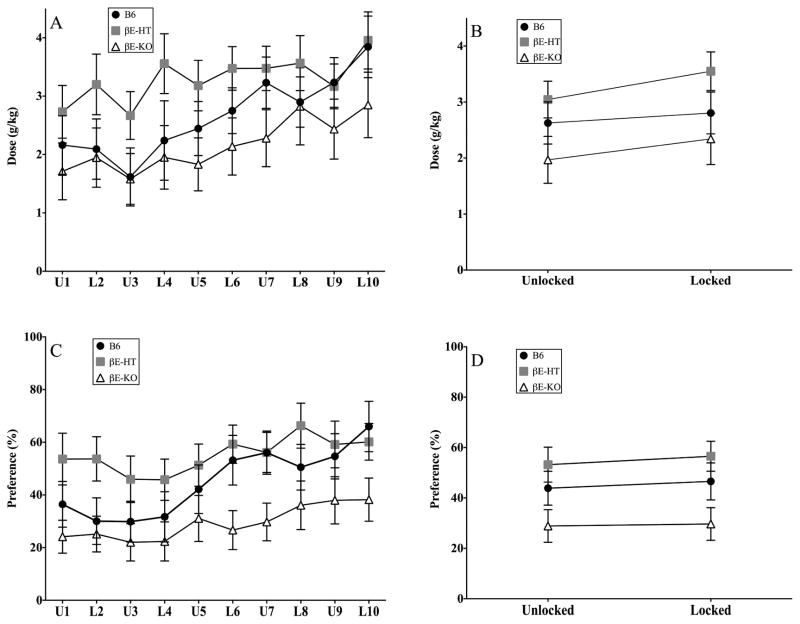
Drinking across the 10 experimental days was variable, reflecting an overall tendency to increase consumption over the test period. Daily group averages are presented in Fig. 1A and 1C (g/kg and preference), and these data are collapsed across experimental conditions in Fig. 1B and 1D (g/kg and preference averaged for the 5 days of unlocked and locked activity wheels). Repeated-measure analyses showed that while genotype did not influence the amount of ethanol consumed across the entire testing period (F[2,32] = 2.298, p > 0.05) or the two experimental conditions (F[1,33] = 2.170, p > 0.05) there was an increase in drinking over days (F[9,288] = 6.588, p < 0.001) and across conditions (F[2,66] = 14.741, p < 0.01). However, changes in consumption did not depend upon genotype across either the 10 experimental days (F[18,288] = 0.712, p > 0.05) or across the average drinking in the two test conditions (F[2,33] = 1.18, p > 0.05).
Fig. 1.
A & C show group means ± SEM for daily consumption and preference and B & D show averaged consumption and preference across the experimental conditions of the study for each group.
In the repeated-measure ANOVA evaluating ethanol preference there was a main effect of genotype across the entire 10-day experiment (F[2,31] = 4.125, p < 0.05), as well as when preference was averaged within the two experimental conditions (F[2,33] = 3.686, p < 0.05). There were also main effects of time on ethanol preference (F[9,279] = 6.193, p < 0.001) across the experimental period, but not when comparing averages of locked and unlocked conditions (F[1,33] = 1.146, p > 0.05), nor was there a significant interaction between condition and genotype as preference changes were small and uniform across groups (F[18,279] = 1.011, p > 0.05 and F[2,33] = .119, p > 0.05). Tukey’s post hoc analysis demonstrated that βE-HT mice preferred the ethanol solution more than the βE-KO subjects in both the daily, and averaged, repeated-measure ANOVAs.
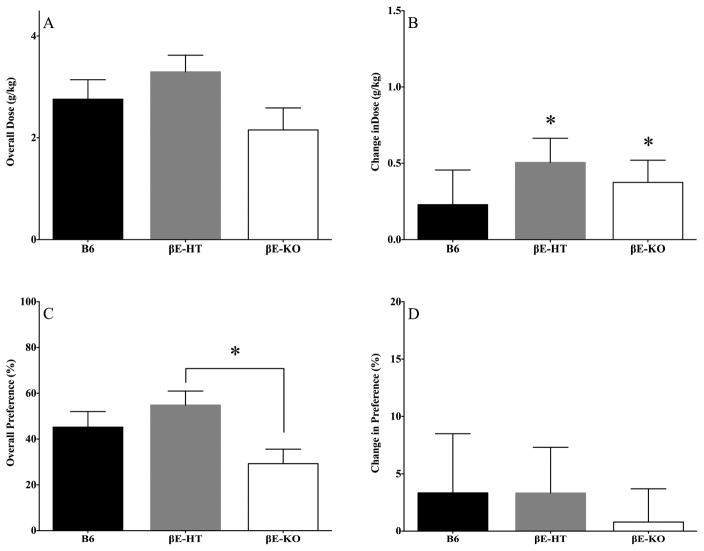
In studies with appreciably more statistical power, we had previously found that female βE-HT mice self-administered higher doses of ethanol than B6 and βE-KO mice (Grisel et al., 1999; Williams et al., 2007). Here, because there were neither main effects of genotype nor interactions involving genotype, we could not do post hoc analyses on consumption. Therefore, we collapsed across all days of the experimental period, to probe for main effects of genotype in one-way ANOVAs for consumption and preference. These data are shown in Fig. 2A and 2C, respectively. Though there were no overall effects of genotype on the g/kg of ethanol self-administered (F[2,35] = 2.17, p > 0.05), genotype did influence overall preference for the drug (F[2,35] = 3.675, p < 0.05). Post hoc Tukey HSD revealed that a significant difference existed between the βE-HT and βE-KO mice; p < 0.05. Finally, in a direct test of our hypothesis that low endorphin levels would increase susceptibility to stress-induced drinking when the wheels were locked, we evaluated the difference in average consumption and preference between locked and unlocked days for each genotype separately (Fig. 2B & 2D). βE-HT and βE-KO mice increased alcohol consumption in response to the stressor, but B6 mice did not, as evidenced by one-sample t tests comparing the difference between locked and unlocked consumption to the null hypothesis, zero (βE-HT mice: t[12] = 3.193, p < 0.01; βE-KO mice: t[9] = 2.577, p < 0.05; and B6 mice: t[8] = 1.011, p > 0.05). One-sample t tests revealed no change in preference for any of the genotypes in response to the stressor (Fig. 2D; B6 t[8] = 0.647, p > 0.05; βE-HT t[12] = 0.836, p > 0.05; βE-KO t[9] = 0.274, p > 0.05). As can be seen in Fig. 2B, only β-endorphin-deficient subjects consumed more alcohol on locked than unlocked days.
Fig. 2.
A & C show group means ± SEM for overall alcohol consumption and preference, collapsing across the entire 10-day experimental period and B &D show the difference, in consumption and preference, between averages of locked and unlocked days by genotype. *denotes significance at p < 0.05.
Surprisingly, genotypes did not differ in their pattern of wheel activity. Both overall rotations and total time spent running were the same across groups (Table 1: F[2,35] = 0.539 and F[2,35] = 0.725, respectively).
Table 1.
Group means ± SEM for 24-h wheel rotations and duration of running
| B6 | βE-HT | βE-KO | |
|---|---|---|---|
| Rotations | 31956 ± 2527 | 34158 ± 1282 | 35288 ± 3004 |
| Duration (minutes) | 359.71 ± 13.72 | 379.22 ± 12.36 | 379.21 ± 14.42 |
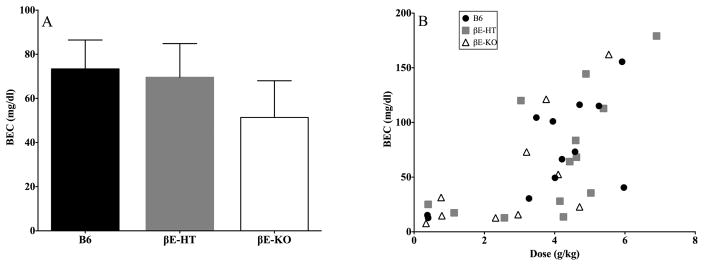
One-way ANOVA comparing BECs between groups revealed no differences (Fig. 3A; F[2,32] = 0.563, p > 0.05), though we did observe the expected relationship between ethanol consumed on the final day of the experiment and BEC (Fig. 3B; Pearson correlation r = 0.667, 2-tailed p < 0.001), and this did not depend upon genotype (p > 0.05).
Fig. 3.
A shows the average (± SEM) blood ethanol content (BEC) in each genotype and B shows the correlation between alcohol consumed on the final day and resultant BEC (r = .667, p < 0.05).
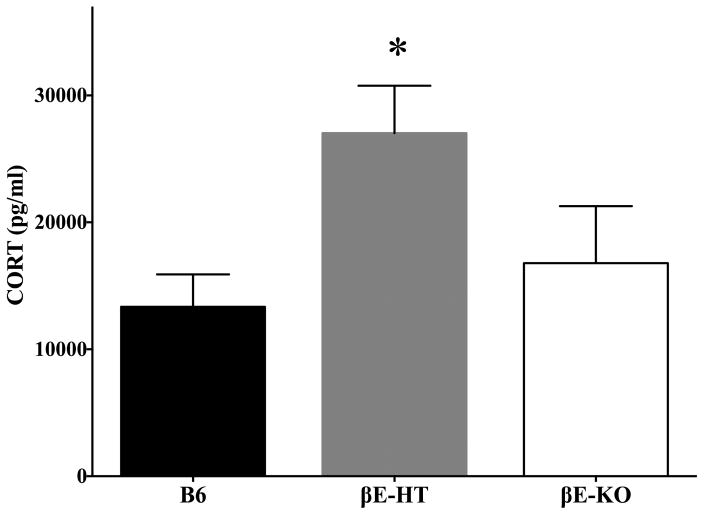
Fig. 4 depicts corticosterone (CORT) levels, also immediately following the final drinking session. CORT was elevated in βE-HT mice compared to either B6 or βE-KO mice, and these did not differ from each other (F[2,30] = 6.603, p < 0.01, Tukey HSD: B6/βE-HT p < 0.01, βE-KO/βE-HT p < 0.05). Two high statistical outliers were removed from the analysis of CORT levels (1 βE-KO, 1 βE-HT). One-way ANOVA investigating genotypic differences in ACTH levels revealed no significant group differences (F[2,32] = 0.947, p > 0.05; data not shown), but this assay may have been insufficiently sensitive as measures of ACTH detected in our ELISA ranged from 2–65 pg/μL, with an average of 31 ± 2.82 pg/μL.
Fig. 4.
Group means ± SEM for corticosterone levels (*denotes significant increase compared to the other two groups, at p < 0.05).
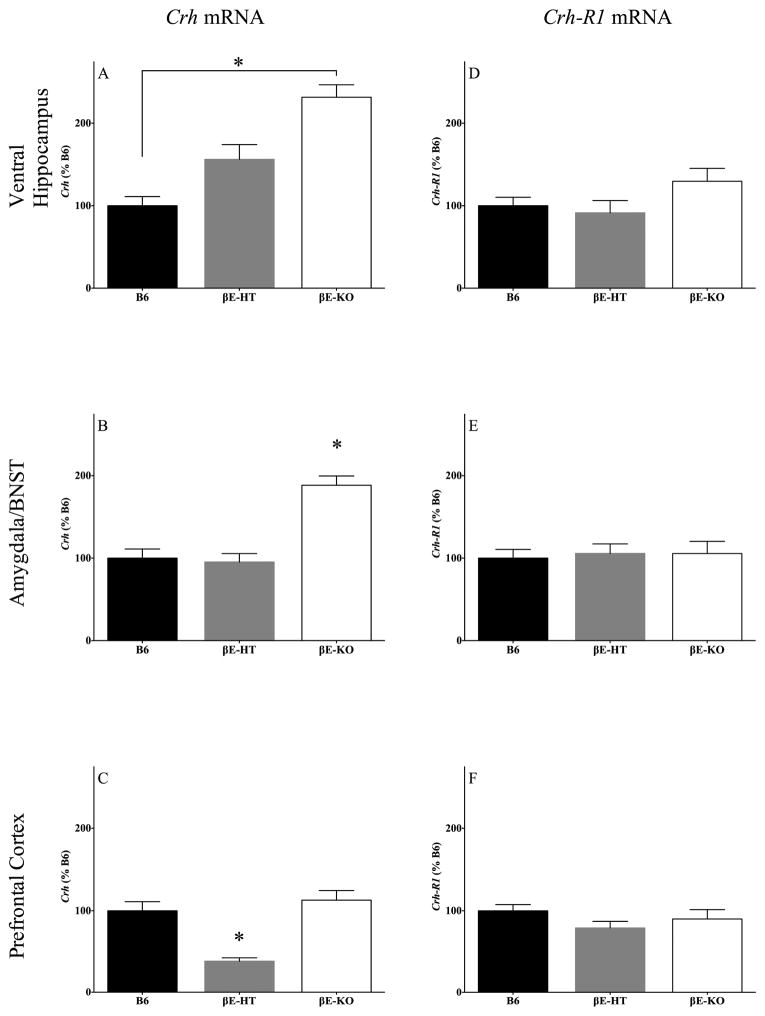
Genotype influenced Crh expression in all brain regions examined (Fig. 5A–5C). In the ventral hippocampus, Crh expression varied significantly (F[2,14] = 18.67, p < 0.05) and was lower in the B6 mice compared with the βE-KO mice (Fig. 5A), but the Crh expression in βE-HT mice was not significantly different from the other two genotypes. In the amygdala/BNST, Crh expression also differed overall (F[2,14] = 22.0, p < 0.05) but was similar in the B6 and βE-HT mice (Fig. 5B), which exhibited significantly less Crh mRNA compared with the βE-KO mice (p < 0.05). In the dorsomedial prefrontal cortex, Crh expression differed (F[2,14] = 18.31, p < 0.05; Fig. 5C) in that the βE-KO and B6 mice were similar and βE-HT mice showed lower expression than either of these genotypes (p < 0.05). In contrast, there were no genotypic differences in the expression of Crh-R1 in any of the brain regions examined (Fig. 5D–5F; VH: F[2,14] = 2.049, p > 0.05; amygdala/BNST: F[2,14] = 0.076, p > 0.05; PFC: F[2,14] = 1.472, p > 0.05).
Fig. 5.
Group means ± SEM for Crh mRNA levels in the ventral hippocampus (A), amygdala/BNST (B), and dorsomedial prefrontal cortex (C). Also shown are group means ± SEM for Crh-R1 mRNA levels in the ventral hippocampus (D), amygdala/BNST (E), and dorsomedial prefrontal cortex (F). *significance at p < 0.05.
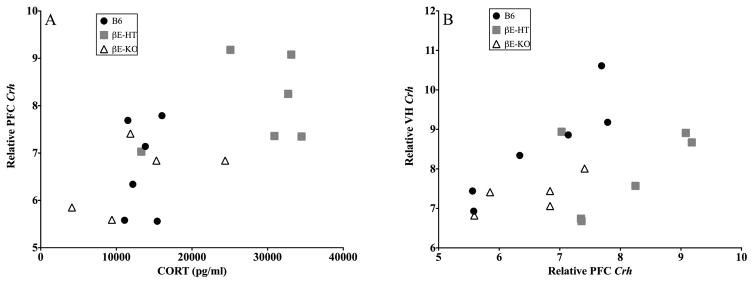
There appeared to be an overall relationship between dmPFC Crh mRNA and plasma CORT levels as shown in Fig. 6A and demonstrated by a Pearson correlation; r = −0.649, p < 0.05. There was also a relationship between dmPFC Crh mRNA and VH Crh mRNA (Fig. 6B; Pearson correlation r = 0.487, p < 0.05). Neither of these relationships depended on genotype in our small sample. (Note that brain samples were pooled for analysis; see Methods.)
Fig. 6.
A depicts the correlation between dorsomedial prefrontal cortex Crh mRNA levels and plasma corticosterone levels (r = 0.649, p < 0.05) and B shows the correlation between Crh mRNA in the dorsomedial prefrontal cortex and the ventral hippocampus (r = 0.487, p < 0.05).
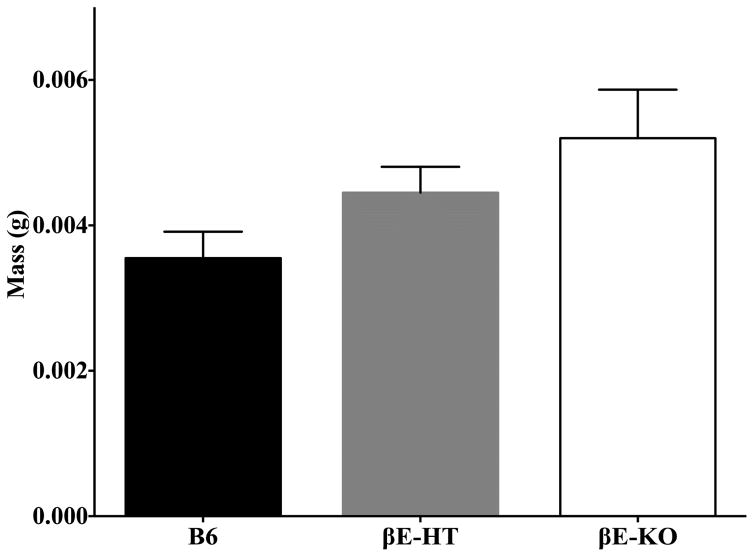
Though there were no significant differences between the adrenal gland weights across genotypes, there was a tendency, especially in the left adrenal glands, for an inverse relationship between β-E levels and mass. ANOVA of combined left and right adrenals by genotype was F[2,20] = 2.421, p = 0.114; for the left adrenals alone F[2,20] = 3.167, p = 0.064; left adrenal data shown in Fig. 7.
Fig. 7.
Group means ± SEM for left adrenal gland weights.
Discussion
The primary goal of this experiment was to examine β-endorphin’s role in the tendency for female mice to voluntarily consume alcohol in response to stress. We found that β-endorphin deficiency increased the propensity to self-administer ethanol in response to frustration stress induced by preventing rotations of a home-cage activity wheel. Oral self-administration of ethanol has been studied in these mice previously (Grisel et al., 1999; Racz et al., 2008; Williams et al., 2007) and current findings corroborate previous studies suggesting that, relative to wild-type subjects (C57BL/6J; B6), heterozygous mice that have reduced levels of β-endorphin tend to consume more ethanol, while those lacking the peptide entirely consume the least. Our aim in the current study was not to evaluate drinking levels between genotypes so much as to evaluate how each genotype changes drinking patterns in response to stress. Because previous research indicated that β-endorphin dampens behavioral responses to stress exposure (Barfield et al., 2010, 2012; Grisel et al., 2008), we were not surprised to find here that transgenic mice with low or absent β-endorphin were prone to stress-induced drinking.
In addition to measuring alcohol consumption patterns in B6, βE-HT, and βE-KO during periods of locked and unlocked home-cage activity wheels, we also assessed several factors associated with the neuroendocrine stress response. On the last day of the experiment, following 14 days of limited 2-h access to 20% ethanol, combined with manipulation of running wheel availability, plasma CORT was elevated in βE-HT mice relative to either B6 or βE-KO lines. There was an inverse relationship between levels of βE and Crh mRNA in the ventral hippocampus, with decreasing endorphin associated with increasing levels of mRNA. However, Crh mRNA was differentially regulated in βE-HT and βE-KO mice in the extended amygdala/BNST and PFC. Message in the amygdala/BNST for Crh was about twice as high in βE-KO mice as in either of the other lines, but in the PFC, βE-KO had about the same levels as B6, while Crh mRNA in the high-drinking βE-HTs was much reduced. These data, adding to a body of both clinical and basic evidence (cf. del Arbol et al., 1995; Thiagarajan, Mefford, & Eskay, 1989), support the idea that β-endorphin modulates endocrine and behavioral components of the stress response and may contribute to an increased susceptibility for heavy drinking. Moreover, the use of low-endorphin heterozygous mice (βE-HT) in our study enables a more nuanced analysis for the role of this peptide beyond what has been shown in earlier studies only comparing knockouts and wild-types (Mogil et al., 2000; Racz et al., 2008) and may better model the human condition.
β-endorphin produces its effects by acting on μ, δ, and κ opioid receptors, binding preferentially to the μ receptor (Hallberg & Nyberg, 2003). There are no differences in opioid binding or receptor expression levels between wild-types (B6) and βE-KO (Mogil et al., 2000). Thus the behavioral differences in stress responses and drinking may result from consequences of the opioid manipulation acting indirectly though non-opioid circuitry. Neither we, nor others, have compared plasma CORT, or mRNA for Crh and its receptor, between experimentally naïve βE-HT and βE-KO and B6 controls. It is therefore not possible to conclude from this study whether the alterations in mRNA are mediated directly by β-endorphin or reflect genetic differences dependent upon the context of 2 weeks of limited access to free-choice ethanol, running on an activity wheel, or both. However, overall differences in consumption were modest, and there were neither genotype differences in blood ethanol concentration on the last day of access, nor in voluntary locomotor activity on the running wheel, so we can assume that neither ethanol exposure nor wheel running account directly for the changes in mRNA or CORT.
Despite enhanced behavioral responses to stress in this and previous studies, we were surprised that in our running and drinking animals, βE-KO and B6 mice do not differ in Crh mRNA in the prefrontal cortex or in plasma corticosterone levels. However, the present data suggest dysregulation of neuroendocrine stress response in the amygdala/BNST and hippocampus particularly, where βE-KO mice express approximately twice as much Crh transcript as wild-types. The inverse relationship between Crh mRNA expression levels and β-endorphin in the VH mirrors differences previously seen in anxiety-like behavior, with mice completely lacking β-endorphin having the most anxious phenotype and highest levels of Crh mRNA (Barfield et al., 2010; Grisel et al., 2008). Such data suggest alterations of the HPA axis associated with β-endorphin deficiency, although Rubinstein and colleagues (1996) found no differences in either basal or restraint stress-induced plasma CORT levels or Crh mRNA in the paraventricular nucleus of the hypothalamus. We had previously (Grisel et al., 2008) found enlarged adrenal glands as a result of β-endorphin deficiency, though the earlier study by Rubinstein did not, and although there were not significant effects of genotype on adrenal weight in this study, a strong tendency was evident in this cohort of subjects. We did not observe genotypic differences in ACTH levels, perhaps because our assay was not sufficiently sensitive. Constitutive loss of gene function can result in adaptive changes in brain and behavior (cf. Gerlai, 1996), and it may be that the altered behavioral phenotype and gene expression in this model result from downstream effects of other, related neural systems. In part, the utility of these and other knockout models may be realized in their capacity to elucidate such related circuitry, but in terms of face validity at least, the heterozygote mice may be a more relevant model of the human condition because low β-endorphin (rather than absent) has been linked in humans to both stress responses and alcohol consumption.
Stress and the ability to cope with stressful situations have been implicated as causal factors in the development of alcoholism (Bolton, Cox, Clara, & Sareen, 2006; Brown, Vik, Patterson, Grant, & Schuckit, 1995; Gianoulakis et al., 1989). In this study, female βE-HT and βE-KO mice, but not B6 mice, increased alcohol consumption in response to the stress of having an appetitive activity wheel blocked, suggesting susceptibility to stress-induced drinking is linked to low β-endorphin levels. Clinical research shows that stress increases β-endorphin, which in turn negatively modulates the stress response (Schedlowski et al., 1995). Furthermore, β-endorphin levels have been correlated with excessive drinking in humans (Froehlich et al., 1990; Gianoulakis, Dai, & Brown, 2003; Wand et al., 1998).
The effects of β-endorphin on free-choice drinking in the home cage with intermittent activity wheel access were concomitant with alterations in mRNA for the CRH peptide, but not its receptor, in the three brain regions we examined, including the ventral hippocampus, extended amygdala, and dorsal medial prefrontal cortex. The VH, among other functions, helps contextualize environmental stimuli and alerts other brain areas to stressful situations and sends glutamatergic projections to the basolateral amygdala, linking the excitation of these two regions (Stamatakis et al., 2014). The amygdala and BNST also play a major role in assessing stressors and helping to coordinate anxiety-related behaviors. The amygdala is activated by stress and has glutamatergic projections that lead to both the medial prefrontal cortex and the BNST. The BNST, located between the amygdala and the nucleus accumbens, connects stress and reward centers of the brain (Sharko, Kaigler, Fadel, & Wilson, 2013). Our samples combined the BNST and amygdala, so we are not able to parse specific contributions of these areas, but nonetheless found that βE-KO mice, the genotype that least tended to prefer and consume ethanol, showed about twice the Crh mRNA expression in this limbic area. Despite our hypothesis that βE-KO mice would be less likely to use ethanol to self-medicate due to the inability for alcohol consumption to alleviate stressed state (Grisel et al., 1999), we found that mice entirely deficient in the peptide did increase intake on locked days relative to days when the wheels were unlocked. Hyperexcitability of the BNST has been seen in chronic drug use and is associated with increased stress susceptibility (Silberman & Winder, 2013; Stamatakis et al., 2014). It may be that CRH in the BNST and amygdala remains overactive and unregulated in βE-KO mice, but can be regulated in the βE-HT mice, possibly mediated by a drinking-induced synthesis of β-endorphin. The BNST sends CRH to the paraventricular nucleus of the hypothalamus, which is an excitatory signal increasing release of ACTH and CORT (Myers et al., 2015). However, Rubinstein and colleagues (1996) reported no change in CRH message in this brain region.
The dmPFC also contributes to processing of emotionally salient information. Glutamatergic projections extend between the mPFC and both the amygdala and the BNST. We propose that significantly lower levels of Crh expression in the dmPFC for the βE-HT mice may be linked to effective self-medication of high stress sensitivity by consumption of ethanol. Because CORT acts in the dmPFC to inhibit the HPA axis via GABAergic projections to the posterior nucleus of the hypothalamus (Myers et al., 2015), the high levels of plasma CORT may explain the low Crh message in βE-HT mice. This idea is supported by the overall negative correlation between plasma CORT and dmPFC Crh mRNA, but it remains possible that differences in drinking (though not statistically evident across the experimental study, and modestly reported elsewhere; Grisel et al., 1999; Williams et al., 2007) produce these effects on changes in mRNA and CORT.
It might have been assumed that mice lacking β-endorphin would be less active on the running wheels, an expectation related to the proposed role of β-endorphin in producing the “runner’s high” (Dinas, Koutedakis, & Flouris, 2011). However, we did not observe differences in running as a function of genotype. Recently, endocannabinoids were implicated in the euphoric effects of running (Fuss et al., 2015), though locomotor activity is no doubt mediated by a constellation of genetic, chemical, and anatomical influences. Because there were no differences in wheel running related to genotype, however, we are able to attribute our results to the efficacy of the stressor and the subsequent response to that stress, rather than a difference in the salience of the activity wheel as a function of β-endorphin levels.
Our previous investigation of the effect of restricted wheel access on drinking (Piza-Palma et al., 2014) showed that female but not male B6 mice increased alcohol consumption in response to the stressor, and therefore we did not include males in the present study. However, it remains possible that low β-endorphin would also shift the reward sensitivity curve in male mice in this experimental context. Therefore, ongoing studies are aimed at better understanding sex differences in the response to restricted wheel access both generally and as a function of β-endorphin level, as well as other stress-related neuroendocrine factors. A limitation of the present study is that we did not assess endocrine- and CRH-related measures in alcohol-naïve animals, and so we are unable to determine whether endocrine and Crh expression differences between groups are directly attributable to differences in β-endorphin or a result of alcohol exposure interacting with constitutive differences in the opioid. Nonetheless, because we employed a between-subject (genotype) design, and there were no differences in voluntary activity or the dose of ethanol self-administered throughout the experiment, our data support the overarching hypothesis that low β-endorphin increases susceptibility to the negatively reinforcing effects of ethanol. It should be noted however, that because both alcohol and stress impact the nervous system and behavior broadly, there are undoubtedly myriad genetic influences on their complex relationship.
Women are a disproportionately growing subset of the overall population of people with alcohol-use disorders (Greenfield et al., 2010) and also suffer from stress-related psychiatric disorders at about double the rate of men (Kessler, McGonagle, Swartz, Blazer, & Nelson, 1993; Marcus et al., 2005). Here we have shown that the increase in voluntary consumption of ethanol in female mice precipitated by blocking access to a positive reinforcer is dependent upon β-endorphin, an opioid peptide whose heritable levels are known to correlate with the clinical liability toward alcohol-use disorders. Therefore, understanding the link between stress susceptibility and the causes of alcoholism is likely to be beneficial for the development of interventions and treatments in this increasingly at-risk group.
Highlights.
β-endorphin-deficient mice are prone to increase voluntary alcohol consumption in response to frustration stress.
Limited access to ethanol, intermittent access to an activity wheel, and β-endorphin deficiency are associated with neuroendocrine regulation in brain regions associated with stress.
Low β-endorphin appears to increase susceptibility to the negatively reinforcing effects of alcohol.
Acknowledgments
The authors thank Carson Mafrice, Ceilia Severini, Zachary Kozick, and Ian Vogel for their assistance with data collection. This work was supported by NIH grant #R15 AA022506 (J.E.G), the Douglas K. Candland Undergraduate Research Fund (C.E.M), and Bucknell University’s Presidential Fellows program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amir S. Involvement of endogenous opioids with forced swimming-induced immobility in mice. Physiology & Behavior. 1982;28:249–251. doi: 10.1016/0031-9384(82)90070-1. [DOI] [PubMed] [Google Scholar]
- Barfield ET, Barry SM, Hodgin HB, Thompson BM, Allen SS, Grisel JE. Beta-endorphin mediates behavioral despair and the effect of ethanol on the tail suspension test in mice. Alcoholism: Clinical and Experimental Research. 2010;34:1066– 1072. doi: 10.1111/j.1530-0277.2010.01182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barfield ET, Moser VA, Hand A, Grisel JE. β-endorphin modulates the effect of stress on novelty-suppressed feeding. Frontiers in Behavioral Neuroscience. 2013;7:19. doi: 10.3389/fnbeh.2013.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC, Lopez MF, Doremus-Fitzwater TL. Effects of stress on alcohol drinking: a review of animal studies. Psychopharmacology. 2011;218:131–156. doi: 10.1007/s00213-011-2443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, et al. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146:1650–1673. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- Beery AK, Zucker I. Sex bias in neuroscience and biomedical research. Neuroscience and Biobehavioral Reviews. 2011;35:565–572. doi: 10.1016/j.neubiorev.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton J, Cox B, Clara I, Sareen J. Use of alcohol and drugs to self-medicate anxiety disorders in a national representative sample. The Journal of Nervous and Mental Disease. 2006;194:818–825. doi: 10.1097/01.nmd.0000244481.63148.98. [DOI] [PubMed] [Google Scholar]
- Brown SA, Vik PW, Patterson TL, Grant I, Schuckit MA. Stress, vulnerability and adult alcohol relapse. Journal of Studies on Alcohol. 1995;56:538–545. doi: 10.15288/jsa.1995.56.538. [DOI] [PubMed] [Google Scholar]
- Buckingham JC. Stimulation and inhibition of corticotrophin releasing factor secretion by beta endorphin. Neuroendocrinology. 1986;42:148–152. doi: 10.1159/000124266. [DOI] [PubMed] [Google Scholar]
- Burk LR, Armstrong JM, Goldsmith HH, Klein MH, Strauman TJ, Costanzo P, et al. Sex, temperament, and family context: how the interaction of early factors differentially predict adolescent alcohol use and are mediated by proximal adolescent factors. Psychology of Addictive Behaviors. 2011;25:1–15. doi: 10.1037/a0022349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annual Review of Physiology. 2005;67:259–284. doi: 10.1146/annurev.physiol.67.040403.120816. [DOI] [PubMed] [Google Scholar]
- Crabbe JC. Use of animal models of alcohol-related behavior. Handbook of Clinical Neurology. 2014;125:71–86. doi: 10.1016/B978-0-444-62619-6.00005-7. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Belknap JK. The complexity of alcohol drinking: studies in rodent genetic models. Behavior Genetics. 2010;40:737–750. doi: 10.1007/s10519-010-9371-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crusio WE. Gene targeting studies: new methods, old problems. Trends in neurosciences. 1996;19:186–187. doi: 10.1016/s0166-2236(96)20023-2. [DOI] [PubMed] [Google Scholar]
- del Arbol JL, Aquirre JC, Raya J, Rico J, Ruiz-Requena MA, Miranda MT. Plasma concentrations of beta-endorphin, adrenocorticotropic hormone, and cortisol in drinking and abstinent chronic alcoholics. Alcohol. 1995;12:525–529. doi: 10.1016/0741-8329(95)00038-0. [DOI] [PubMed] [Google Scholar]
- Dinas PC, Koutedakis Y, Flouris AD. Effects of exercise and physical activity on depression. Irish Journal of Medical Science. 2011;180:319–325. doi: 10.1007/s11845-010-0633-9. [DOI] [PubMed] [Google Scholar]
- Ehringer MA, Hoft NR, Zunhammer M. Reduced alcohol consumption in mice with access to a running wheel. Alcohol. 2009;43:443–452. doi: 10.1016/j.alcohol.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Froehlich JC, Harts J, Lumeng L, Li TK. Naloxone attenuates voluntary ethanol intake in rats selectively bred for high ethanol preference. Pharmacology, Biochemistry, and Behavior. 1990;35:385–390. doi: 10.1016/0091-3057(90)90174-g. [DOI] [PubMed] [Google Scholar]
- Fuss J, Steinle J, Bindila L, Auer MK, Kirchherr H, Lutz B, et al. A runner’s high depends on cannabinoid receptors in mice. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:13105–13108. doi: 10.1073/pnas.1514996112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlai R. Gene-targeting studies of mammalian behavior: is it the mutation or the background genotype? Trends in Neurosciences. 1996;19:177–181. doi: 10.1016/s0166-2236(96)20020-7. Erratum in Trends in Neurosciences, (1996), 19, 271. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C. Endogenous opioids and addiction to alcohol and other drugs of abuse. Current Topics in Medicinal Chemistry. 2009;9:999–1015. doi: 10.2174/156802609789630956. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C, Béliveau D, Angelogianni P, Meaney M, Thavundayil J, Tawar V, et al. Different pituitary beta-endorphin and adrenal cortisol response to ethanol in individuals with high and low risk for future development of alcoholism. Life Sciences. 1989;45:1097–1109. doi: 10.1016/0024-3205(89)90167-7. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C, Dai X, Brown T. Effect of chronic alcohol consumption on the activity of the hypothalamic-pituitary-adrenal axis and pituitary beta-endorphin as a function of alcohol intake, age, and gender. Alcoholism: Clinical and Experimental Research. 2003;27:410–423. doi: 10.1097/01.ALC.0000056614.96137.B8. [DOI] [PubMed] [Google Scholar]
- Greenfield SF, Back SE, Lawson K, Brady KT. Substance abuse in women. The Psychiatric Clinics of North America. 2010;33:339–355. doi: 10.1016/j.psc.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisel JE, Bartels JL, Allen SA, Turgeon VL. Influence of beta-Endorphin on anxious behavior in mice: interaction with EtOH. Psychopharmacology. 2008;200:105– 115. doi: 10.1007/s00213-008-1161-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisel JE, Mogil JS, Grahame NJ, Rubinstein M, Belknap JK, Crabbe JC, et al. Ethanol oral self-administration is increased in mutant mice with decreased β-endorphin expression. Brain Research. 1999;835:62–67. doi: 10.1016/s0006-8993(99)01384-0. [DOI] [PubMed] [Google Scholar]
- Hallberg M, Nyberg F. Neuropeptide conversion to bioactive fragments -- an important pathway in neuromodulation. Current Protein & Peptide Science. 2003;4:31–44. doi: 10.2174/1389203033380313. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. Journal of Affective Disorders. 1993;29:85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- Keyes KM, Grant BF, Hasin DS. Evidence for a closing gender gap in alcohol use, abuse, and dependence in the United States population. Drug and Alcohol Dependence. 2008;93:21–29. doi: 10.1016/j.drugalcdep.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low MJ, Hayward MD, Appleyard SM, Rubinstein M. State-dependent modulation of feeding behavior by proopiomelanocortin-derived beta-endorphin. Annals of the New York Academy of Sciences. 2003;994:192–201. doi: 10.1111/j.1749-6632.2003.tb03180.x. [DOI] [PubMed] [Google Scholar]
- Marcus SM, Young EA, Kerber KB, Kornstein S, Farabaugh AH, Mitchell J, et al. Gender differences in depression: findings from the STAR*D study. Journal of Affective Disorders. 2005;87:141–150. doi: 10.1016/j.jad.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Grisel JE, Hayward MD, Bales JR, Rubinstein M, Belknap JK, et al. Disparate spinal and supraspinal opioid antinociceptive responses in beta-endorphin-deficient mutant mice. Neuroscience. 2000;101:709–717. doi: 10.1016/s0306-4522(00)00422-x. [DOI] [PubMed] [Google Scholar]
- Myers B, Carvalho-Netto E, Wick-Carlson D, Wu C, Naser S, Solomon MB, et al. GABAergic Signaling within a Limbic-Hypothalamic Circuit Integrates Social and Anxiety-Like Behavior with Stress Reactivity. Neuropsychopharmacology. 2015;41:1530–1539. doi: 10.1038/npp.2015.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechnick RN. Effects of opioids on the hypothalamo-pituitary-adrenal axis. Annual Review of Pharmacology and Toxicology. 1993;32:353–382. doi: 10.1146/annurev.pa.33.040193.002033. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Reed C, Pastor R. Preclinical evidence implicating corticotropin-releasing factor signaling in ethanol consumption and neuroadaptation. Genes, Brain, and Behavior. 2015;14:98–135. doi: 10.1111/gbb.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piza-Palma C, Barfield ET, Brown JA, Hubka JC, Lusk C, Schonhar CA, et al. Oral self-administration of EtOH: sex-dependent modulation by running wheel access in C57BL/6J mice. Alcoholism: Clinical and Experimental Research. 2014;38:2387–2395. doi: 10.1111/acer.12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotsky PM. Pathways to secretion of adrenocorticotropin: a view from the portal. Journal of Neuroendocrinology. 1991;3:1–9. doi: 10.1111/j.1365-2826.1991.tb00231.x. [DOI] [PubMed] [Google Scholar]
- Racz I, Schürmann B, Karpushova A, Reuter M, Cichon S, Montag C, et al. The opioid peptides enkephalin and beta-endorphin in alcohol dependence. Biological Psychiatry. 2008;64:989–997. doi: 10.1016/j.biopsych.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall CL, Roberts JS, Del Boca FK, Carroll KM, Connors GJ, Mattson ME. Telescoping of landmark events associated with drinking: a gender comparison. Journal of Studies on Alcohol. 1999;60:252–260. doi: 10.15288/jsa.1999.60.252. [DOI] [PubMed] [Google Scholar]
- Rhinn H, Marchand-Leroux C, Croci N, Plotkine M, Scherman D, Escriou V. Housekeeping while brain’s storming Validation of normalizing factors for gene expression studies in a murine model of traumatic brain injury. BMC Molecular Biology. 2008;9:62. doi: 10.1186/1471-2199-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu CH, Brown LL, Finn DA, Garland T, Jr, et al. Mouse inbred strain differences in ethanol drinking to intoxication. Genes, Brain, and Behavior. 2007;6:1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Ribeiro SC, Kennedy SE, Smith YR, Stohler CS, Zubieta JK. Interface of physical and emotional stress regulation through the endogenous opioid system and mu-opioid receptors. Progress in Neuro-psychopharmacology & Biological Psychiatry. 2005;29:1264–1280. doi: 10.1016/j.pnpbp.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Rubinstein M, Mogil JS, Japón M, Chan EC, Allen RG, Low MJ. Absence of opioid stress-induced analgesia in mice lacking beta-endorphin by site-directed mutagenesis. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:3995–4000. doi: 10.1073/pnas.93.9.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar DK, Kuhn P, Marano J, Chen C, Boyadjieva N. Alcohol exposure during the developmental period induces beta-endorphin neuronal death and causes alteration in the opioid control of stress axis function. Endocrinology. 2007;148:2828–2834. doi: 10.1210/en.2006-1606. [DOI] [PubMed] [Google Scholar]
- Schedlowski M, Flüge T, Richter S, Tewes U, Schmidt RE, Wagner TO. Beta-endorphin, but not substance-P, is increased by acute stress in humans. Psychoneuroendocrinology. 1995;20:103–110. doi: 10.1016/0306-4530(94)00048-4. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nature Protocols. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Sharko AC, Kaigler KF, Fadel JR, Wilson MA. Individual differences in voluntary ethanol consumption lead to differential activation of the central amygdala in rats: relationship to the anxiolytic and stimulant effects of low dose ethanol. Alcoholism: Clinical and Experimental Research. 2013;37(Suppl 1):E172–E180. doi: 10.1111/j.1530-0277.2012.01907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberman Y, Winder DG. Emerging role for corticotropin releasing factor signaling in the bed nucleus of the stria terminalis at the intersection of stress and reward. Frontiers in Psychiatry. 2013;4:42. doi: 10.3389/fpsyt.2013.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis AM, Sparta DR, Jennings JH, McElligott ZA, Decot H, Stuber GD. Amygdala and bed nucleus of the stria terminalis circuitry: Implications for addiction-related behaviors. Neuropharmacology. 2014;76(Pt B):320–328. doi: 10.1016/j.neuropharm.2013.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens MA, Wand G. Stress and the HPA axis: role of glucocorticoids in alcohol dependence. Alcohol Research: Current Reviews. 2012;34:468–483. [PMC free article] [PubMed] [Google Scholar]
- Taki FA, Abdel-Rahman AA, Zhang B. A comprehensive approach to identify reliable reference gene candidates to investigate the link between alcoholism and endocrinology in Sprague-Dawley rats. PLoS One. 2014;9:e94311. doi: 10.1371/journal.pone.0094311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiagarajan AB, Mefford IN, Eskay RL. Single-dose ethanol administration activates the hypothalamic-pituitary-adrenal axis: exploration of the mechanism of action. Neuroendocrinology. 1989;50:427–432. doi: 10.1159/000125259. [DOI] [PubMed] [Google Scholar]
- Thoits PA. Stress and health: major findings and policy implications. Journal of Health and Social Behavior. 2010;51(Suppl):S41–S53. doi: 10.1177/0022146510383499. [DOI] [PubMed] [Google Scholar]
- Wand GS, Mangold D, El Deiry S, McCaul ME, Hoover D. Family history of alcoholism and hypothalamic opioidergic activity. Archives of General Psychiatry. 1998;55:1114–1119. doi: 10.1001/archpsyc.55.12.1114. [DOI] [PubMed] [Google Scholar]
- Williams SB, Holloway A, Karwan K, Allen S, Grisel JE. Oral self-administration of ethanol in transgenic mice lacking β-endorphin. Impulse Online Journal 2007 [Google Scholar]
- Yamada K, Nabeshima T. Stress-induced behavioral responses and multiple opioid systems in the brain. Behavioural Brain Research. 1995;67:133–145. doi: 10.1016/0166-4328(94)00150-e. [DOI] [PubMed] [Google Scholar]
- Young-Wolff KC, Kendler KS, Prescott CA. Interactive effects of childhood maltreatment and recent stressful life events on alcohol consumption in adulthood. Journal of Studies on Alcohol and Drugs. 2012;73:559–569. doi: 10.15288/jsad.2012.73.559. [DOI] [PMC free article] [PubMed] [Google Scholar]