Abstract
Purpose of Review
Allograft vasculopathy (AV) is the leading cause of late allograft loss following solid organ transplantation. Ischemia reperfusion injury (IRI) and donor specific antibody (DSA)-induced complement activation confer heightened risk for AV via numerous innate immune mechanisms including MyD88, HMGB1, and complement induced non-canonical NF-kB signaling.
Recent Findings
The role of MyD88, a signal adaptor downstream of the toll-like receptors (TLR), has been defined in an experimental heart transplant model, which demonstrated that recipient MyD88 enhanced AV. Importantly, triggering receptor on myeloid receptor 1(Trem1), a MyD88 amplifying signal, was present in rejecting human cardiac transplant biopsies and enhanced the development of AV in mice. HMGB1, a nuclear protein that activates TLRs, also enhanced the development of AV. Complement activation elicits assembly of membrane attack complexes (MAC) on endothelial cells which activate non-canonical NF-kB signaling, a novel complement effector pathway that induces pro-inflammatory genes and potentiates endothelial cell mediated alloimmune T cell activation, processes which enhance AV.
Summary
Innate immune mediators including HMGB1, MyD88, and non-canonical NFκB signaling via complement activation contribute to AV. These pathways represent potential therapeutic targets to reduce AV after solid organ transplantation.
Keywords: toll-like receptor, MyD88, non-canonical NF-kB, membrane attack complex, allograft vasculopathy
INTRODUCTION
Allograft vasculopathy (AV) is a highly prevalent vaso-occlusive condition that affects up to 51% of patients 10 years post-transplant [1]. In AV, neointimal and less commonly thrombotic lesions form in large- and small-caliber vessels of the transplanted graft, leading to ischemic complications and allograft loss. In the setting of heart transplantation, where AV is most widely studied and is referred to as cardiac allograft vasculopathy or CAV, AV lesions affect the epicardial coronary and intramyocardial microvessels [2] and is recognized as the leading cause of graft loss after one year post-heart transplantation. Ischemia reperfusion injury (IRI) and development of donor specific antibodies (DSA) are both strong, independent risk factors for the development of AV.
AV is a fundamentally distinct entity from coronary artery disease (CAD). Given its unique pathophysiological underpinnings involving alloimmune T cell activation, AV is clinically refractory to many repositioned agents intended for treating CAD [3]. Moreover, contemporary immunosuppressive regimens to halt alloimmune adaptive responses have yielded only incremental or no therapeutic benefit regarding secondary or tertiary prevention [4,5]. Future agents specifically designed for treating AV are required, and for the rational design of such therapies an improved understanding of the mechanisms underlying AV is urgently needed. Here, we review the role of innate immune signaling molecules and pathways, including HMGB, MyD88 and complement induced non-canonical NF-kB, in the development of AV.
MyD88: a central pathway enhancing chronic allograft rejection
MyD88 is a signal adaptor protein that is downstream of all the TLRs, except TLR3 and also IL-1 and IL-18. TLRs are key innate immune receptors that are expressed on stromal and hematopoietic cells and activated by both microbial and non-microbial activators [6,7]. MyD88 has been established to enhance acute allograft rejection and break transplant tolerance [8–10]. In humans, patients with low expression of MyD88 in peripheral blood mononuclear cells exhibit a significant increase in operational transplant tolerance as compared to renal transplant recipients with evidence of chronic rejection [11]. Mechanistically, MyD88 signaling in an experimental model of kidney transplantation abrogates spontaneous transplant tolerance [12]. MyD88 impaired the expansion of regulatory T cells (T regs) and increased anti-donor IL-17 immune responses [12,13]. Interestingly, the authors of this study recently reported that IL-17 enhanced both acute and chronic allograft rejection in a spontaneous model of murine renal allograft acceptance [14]. The MyD88 expressing cells that mediate AV have not been determined. Prior studies have indicated that NK cells and macrophages may promote AV and represent potential possibilities [15, 16].
In a MHC class II mismatched murine cardiac allograft model, deficiency of MyD88 in the recipient enhanced cardiac allograft survival with reduced evidence of AV [17]. This study also found that there were increased numbers of Trem-1+ immune cells within human heart transplant biopsy specimens with evidence of acute graft rejection [17]. Trem1 is an immunoglobulin superfamily member that is expressed on a variety of innate immune cells including dendritic cells (DCs), macrophages and neutrophils acts as an amplifying signal for MyD88 [18]. Importantly, pharmacological inhibition of Trem1 enhanced cardiac allograft survival with reduced AV development in the murine MHC class II mismatched model [17]. Trem1 inhibition also reduced T cell allograft infiltration and reduced anti-donor IFN-γ T cell responses within the spleen of cardiac allograft recipients [17,18]. Thus, this study defines a role for Trem-1 as MyD88 amplification signal that enhances AV.
IRI leads to cellular necrosis and induces sterile inflammation [19]. During cellular necrosis, substances are released that are typically not sensed by the innate immune system [18,19]. High mobility group box 1 (HMGB1) is a nuclear protein that enhances gene transcription [20]. It has been shown to activate TLR 4 and TLR9, TLRs upstream of MyD88 [21–22]. Prior studies have found a role for HMGB1 in acute allograft rejection as well as cardiac, renal and hepatic IRI [23–27]. Recently, in a MHC class II mismatched cardiac allograft model, pharmacological blockade of HMGB1 reduced AV and was associated with reduced numbers of graft infiltrating Th1 and Th17 producing T cells [28]. Importantly, blockade of HMGB1 correlated with reduced numbers of inflammatory monocytes and macrophages within the spleen of cardiac allograft recipients [28]. Although not focused on AV, a recent study found that blockade of HMGB1 reduced vascular rejection and anti-donor B cells in a murine xenograft model (rat grafts into murine recipients) [29]. Overall, these studies indicate that HMGB1 enhances vascular disease and AV in mice.
Lung allografts are also susceptible to chronic allograft rejection. Chronic rejection after lung transplantation is termed bronchiolitis obliterans syndrome (BOS). Prior human studies have implicated TLR4 in the development of BOS [30]. A recent study has found that the level or hyaluronan (HA) a glycosaminoglycan that activates MyD88 [31], is increased after lung transplantation in humans and correlates with the development of BOS [32]. Interestingly, in the same study the investigators found that administration of HA broke lung transplant tolerance in mice via a MyD88-dependent process [30,32]. Other activators of innate immunity, specifically the S100 proteins or calgranulins, which can induce vascular inflammation [33] have also been found in the BAL of lung transplant recipients that exhibited signs of BOS [34]. A recent experimental study found that calgranulins were upregulated within cardiac transplants [35] but whether calgranulins associate with AV after cardiac transplantation is yet to be studied.
Complement activation as a link between IRI and AV
Activation of the complement cascade occurs in response to IRI or DSA binding to HLA molecules on endothelial cells (EC), the sites where AV lesions occur. Interestingly, a recent murine study demonstrated severe renal IRI enhanced humoral immunity [36]. Specifically, bilateral, but not unilateral renal IRI in mice, enhanced the production of antigen specific IgG1 levels [36]. Furthermore, this study demonstrated that complement activation was critical for the amplified antigen specific humoral response induced by renal IRI as mice that are deficient in factor B, a critical component of the alternate complement cascade, exhibited an abrogated humoral response [36]. Although this study employed a native renal IRI model and not an organ transplant model, it implies the IRI after graft implantation enhances the generation of donor specific antibodies (DSA), which could enhance complement activation to promote AV.
DSA and terminal complement induce inflammatory signaling in the graft vasculature, a potential connection to AV
DSA binding to HLA I [37] and II [38] molecules on the graft vasculature potentiates the proliferative and migratory capacities of EC and promotes AV in a manner that involves activation of Akt, a serine/threonine kinase regulating protein translation and metabolism [39]. DSA biding to HLA class I molecules via integrin β4 activates Akt [40], which potentiates EC proliferation and migration. Underscoring its clinical significance, activated Akt phospho-targets, including S6 kinase and S6 ribosomal protein, are significantly upregulated in patients with antibody-mediated rejection [41], a clinical risk factor for development of AV. However, future studies will be required to determine if Akt activation is a target for preventing or retarding the development of AV after organ transplantation.
Complement activation occurs as a result of DSA binding to surface HLA molecules on EC and is a risk factor for cardiac AV [42]. Complement activation induces generation of anaphylatoxins C3a and C5a and assembly of membrane attack complexes (MACs) that intercalate into the surface membranes of target cells within graft tissue. Locally-produced anaphylatoxins as a result of de novo synthesis of alternative complement components by ECs [43] signal via G protein-coupled receptors and promote transplant rejection [44] by affecting T cell activation [45,46] and suppression [47]. Such activation could promote the development of AV.
MAC, previously believed to promote inflammation by inducing necrolysis via osmotic diffusion, similarly transmits pro-inflammatory and pro-thrombotic signals in target cells. ECs express high levels of complement regulatory proteins, in particular CD59, which, via CREB-mediated enhancement of Sp1 transcription factor activity [48], upregulates CD59 surface expression and restricts autologous MAC-induced cell death. In this context, MAC deposition on nucleated cells like EC induces cellular phenotypes that could promote AV including ERK- [49] and JNK- [50] mediated stress kinase activation, NLRP3 inflammasome activation [51, 52], and platelet activation [53,54]. Overall, these studies support the notion that MAC drives pathologic signaling processes in vascular cells that may promote AV.
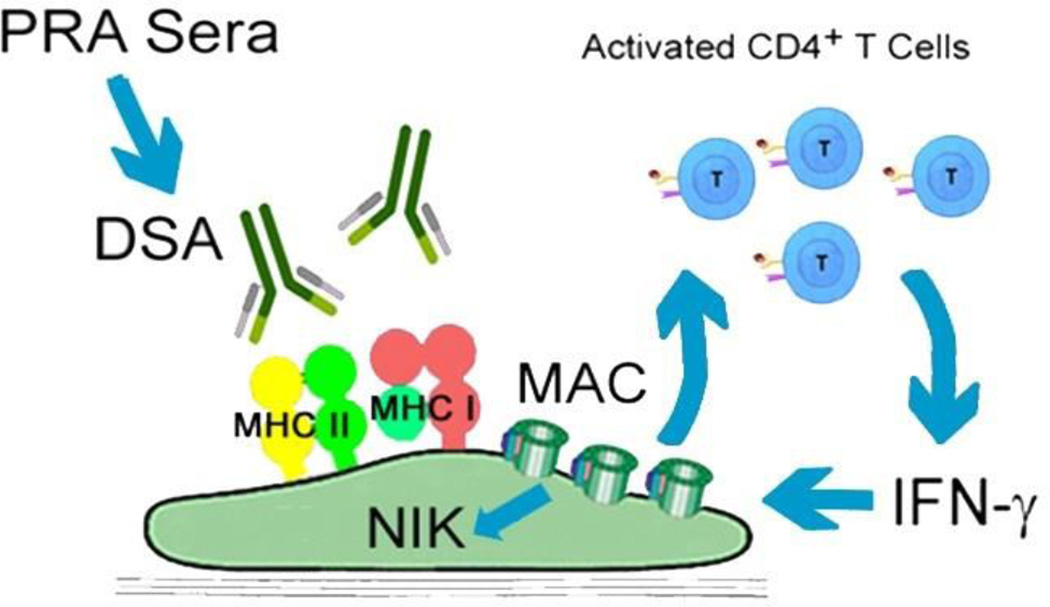
By modeling the complement-activating effects of human DSA with 'high' panel reactive antibody (PRA) sera from allo-sensitized transplant candidates, we identified a novel MAC effector pathway, non-canonical NF-κB, which was required for MAC-treated ECs to upregulate inflammatory genes and potentiate EC-mediated elaboration of IFNγ, a cytokine that promotes AV, from CD4+ alloimmune T cells [55], (Fig. 1). Endothelial non-canonical NF-κB activation was furthermore detected in human coronary arteries in a humanized mouse model and in patient biopsy specimens with chronic antibody mediated rejection (CAMR) [56], a condition strongly associated with AV. Subsequent studies involving genome wide siRNA screening revealed a novel endosome-based mechanism for MAC to induce non-canonical NF-κB activation. In this mechanism, endocytosis of MAC and the formation of MAC+Rab5+Akt+ vesicles were required for non-canonical NF-κB signal activation [57]. Together, our studies show that noncanonical NF-κB is a MAC effector pathway involved in AV requiring an endosome based signaling pathway for its activation.
Figure 1. Mechanisms of MAC Induction of ECs to enhance AV.
PRA sera from allo-sensitized transplant candidates bound DSA on MHC expressed on EC, causing assembly of MAC on EC surfaces. MAC potentiated EC-mediated activation of CD4+IFN-γ+ T cells in a non-canonical NF-kB inducing kinase (NIK) dependent manner. Inflammatory cytokines produced by activated T cells (e.g., IFN-γ) enhance the development of AV.
Conclusions and therapeutic potential
The alarmingly high prevalence and morbidity attributable to AV underscores a generalized inefficacy of current medical therapies. As discussed above, blockade of HMGB1 in mice reduces AV [25]. MyD88 inhibition via gene silencing enhanced cardiac allograft survival along with rapamycin treatment [34] but whether this reduced AV is unknown. Besides the MyD88 signaling pathway, complement inhibition has also emerged as a putative strategy for blocking AV lesion formation. Two recent studies targeted endogenous complement regulators CD59 [58] and Crry [59], a murine complement regulatory ortholog inhibiting C3, using a CR2 fusion protein and single chain antibody, respectively, to relevant sites in vivo in murine hosts to block complement activation in response to IRI. Another recent study used mAb to functionally block proximal activation with anti-C1s [60]. In addition to these antibody based therapies, pharmacologic agents blocking complement effector pathways like non-canonical NF-κB could be explored as putative strategies for inhibiting AV. Given the paucity of new therapies to reduce the development of AV [3], clinical studies to examine if either inhibition of activators of the MyD88 pathway or the complement activation are safe and effective in reducing AV should be considered.
Key Points.
Innate immunity and complement activation enhance AV
MyD88 promotes AV in part via activation of HMGB1 released after graft IRI
Non-canonical NF-κB is a novel complement effector pathway that requires an endosome-based signaling mechanism to elicit EC activation to promote AV
Acknowledgments
None
Financial Support: DJ is supported by NIH grant 1K99 HL125895. DRG is supported by NIH grants AG050096, HL130669 and AG028082.
Abbreviations
- CAD
coronary artery disease
- IRI
ischemia reperfusion injury
- HMGB1
High mobility group box 1
- DSA
donor-specific antibody
- CAMR
Chronic Antibody-Mediated Rejection
- TLR
toll-like receptor
- MAC
membrane attack complex
Footnotes
Conflicts of Interest: The authors report no conflict of interests.
References
** Outstainding interest
* Special Interest
- 1.Taylor DO, Edwards LB, Boucek MM, Trulock EP, Aurora P, Christie J, Dobbels F, Rahmel AO, Keck BM, Hertz MI. Registry of the International Society for Heart and Lung Transplantation: Twenty-Fourth Official Adult Heart Transplant Report-2007. J Heart Lung Transplant. 2007;26:769–781. doi: 10.1016/j.healun.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Lu WH, Palatnik K, Fishebin GA, Lai C, Levi DS, Perens G, Alejos J, Kobashigawa J, Fishbein MC. Diverse Morphologic Manifestations of Cardiac Allograft Vasculopathy: A Pathologic Study of 64 Allograft Hearts. J Heart Lung Transplant. 2011;30:1044–1050. doi: 10.1016/j.healun.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Gupta S. Drugs for the Prevention and Treatment of Cardiac Allograft Vasculopathy. Cardiovasc Pharm. 3:123–130. [Google Scholar]
- 4.Arora S, Ueland T, Wennerblom B, Sigurdadottir V, Eiskjær H, Bøtker HE, Ekmehag B, Jansson K, Mortensen SA, Saunamaki K, Simonsen S, Gude E, Bendz B, Solbu D, Aukrust P, Gullestad L. Effect of everolimus introduction on cardiac allograft vasculopathy--results of a randomized, multicenter trial. Transplantation. 2011;92:235–243. doi: 10.1097/TP.0b013e31822057f1. [DOI] [PubMed] [Google Scholar]
- 5.Eisen HJ, Kobashigawa J, Starling RC, Pauly DF, Kfoury A, Ross H, Wang SS, Cantin B, Van Bakel A, Ewald G, Hirt S, Lehmkuhl H, Keogh A, Rinaldi M, Potena L, Zuckermann A, Dong G, Cornu-Artis C, Lopez P. Everolimus versus mycophenolate mofetil in heart transplantation: a randomized, multicenter trial. Am J Transplant. 2013;13:1203–1216. doi: 10.1111/ajt.12181. [DOI] [PubMed] [Google Scholar]
- 6.Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwasaki A, Medzhitov R. Regulation of Adaptive Immunity by the Innate Immune System. Science. 2010;327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldstein DR, Tesar BM, Akira S, Lakkis FG. Critical role of the Toll-like receptor signal adaptor protein MyD88 in acute allograft rejection. J. Clin. Invest. 2003;111:1571–1578. doi: 10.1172/JCI17573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker WE, Nasr IW, Camirand G, Tesar BM, Booth CJ, Goldstein DR. Absence of Innate MyD88 Signaling Promotes Inducible Allograft Acceptance. J Immunol. 2006;177:5307–5316. doi: 10.4049/jimmunol.177.8.5307. [DOI] [PubMed] [Google Scholar]
- 10.Thornley TB, Brehm MA, Markees TG, Shultz LD, Mordes JP, Welsh RM, Rossini AA, Greiner DL. TLR Agonists Abrogate Costimulation Blockade-Induced Prolongation of Skin Allografts. J Immunol. 2006;176:1561–1570. doi: 10.4049/jimmunol.176.3.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braudeau C, Ashton-Chess J, Giral M, Dugast ELS, Pallier A, Braud C, Moreau A, Renaudin K, Soulillou JP, S B. Contrasted blood and intragraft toll-like receptor 4 mRNA profiles in operational tolerance versus chronic rejection in kidney transplant recipients. Transplantation. 2008;86:130–136. doi: 10.1097/TP.0b013e31817b8dc5. [DOI] [PubMed] [Google Scholar]
- 12.Wu H, Noordmans GA, O’Brien MR, Ma J, Zhao CY, Zhang GY, Kwan TKT, Alexander SI, Chadban SJ. Absence of MyD88 Signaling Induces Donor-Specific Kidney Allograft Tolerance. Journal of the American Society of Nephrology. 2012;23:1701–1716. doi: 10.1681/ASN.2012010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu H, Chadban SJ. Roles of Toll-like receptors in transplantation. Current Opinion in Organ Transplantation. 2014;19:1–7. doi: 10.1097/MOT.0000000000000038. [DOI] [PubMed] [Google Scholar]
- 14.Kwan T, Chadban SJ, Ma J, Bao S, Alexander SI, Wu H. IL-17 Deficiency Attenuates Allograft Injury and Prolongs Survival in a Murine Model of Fully MHCM is matched Renal Allograft Transplantation. American Journal of Transplantation. 2015;15:1555–1567. doi: 10.1111/ajt.13140. [DOI] [PubMed] [Google Scholar]
- 15.Hirohashi T, Chase CM, Della Pelle P, Sebastyian D, Alessandrini A, Madsen JC, Russell PS, Colvin RB. A Novel Pathway of Chronic ALlograft REjection Mediated by NK Cells and Alloantibody. American Journal of Transplantation. 2012;12:313–321. doi: 10.1111/j.1600-6143.2011.03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitchens WH, Chase CM, Uehara S, Cornell LD, Colvein RB, Russell PS, Madsen JC. Macropahge Depletion Suppresses Cardiac Allograft Vasculopathy in MIce. American Journal of Transplantation. 2007;7:2675–2682. doi: 10.1111/j.1600-6143.2007.01997.x. [DOI] [PubMed] [Google Scholar]
- 17. Schiechl G, Brunner SM, Kesselring R, Martin M, Ruemmele P, Mack M, Hirt SW, Schlitt HJ, Geissler EK, Fichtner-Feigl S. Inhibition of Innate Co-Receptor TREM-1 Signaling Reduces CD4+ T Cell Activation and Prolongs Cardiac Allograft Survival. American Journal of Transplantation. 2013;13:1168–1180. doi: 10.1111/ajt.12186. ** This study is the 1st to examine the role of TREM-1 in the development of AV in mice
- 18.Klesney-Tait J, Turnbull IR, Colonna M. The TREM receptor family and signal integration. Nat Immunol. 2006;7:1266–1273. doi: 10.1038/ni1411. [DOI] [PubMed] [Google Scholar]
- 19.Shen H, Kreisel D, Goldstein DR. Processes of Sterile Inflammation. J Immunol. 2013;191:2857–2863. doi: 10.4049/jimmunol.1301539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Helena Erlandsson Harris UA. Mini-review: The nuclear protein HMGB1 as a proinflammatory mediator. European Journal of Immunology. 2004;34:1503–1512. doi: 10.1002/eji.200424916. [DOI] [PubMed] [Google Scholar]
- 21.Kim S, Kim SY, Pribis JP, Lotze M, Mollen KP, Shapiro R, Loughran P, Scott MJ, Billiar TR. Signaling of HIgh Mobility Group Box 1 (HMGB1) through Toll-like REceptor 4 in Macrophages Requires CD14. Molecular Medicine. 2013;19:88–98. doi: 10.2119/molmed.2012.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, Parroche P, Drabic S, Golenbock D, Sirois C, et al. Toll-like receptor 9-dependent activation by DNA containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8:487–496. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 23.Wu H, Ma J, Wang P, Corpuz TM, Panchapakesan U, Wyburn KR, Chadban SJ. HMGB1 Contributes to Kidney Ischemia Reperfusion Injury. J Am Soc Nephrol. 2010;21:1878–1890. doi: 10.1681/ASN.2009101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang Y, Yin H, Han J, Huang B, Xu J, Zheng F, Tan Z, Fang M, Rui L, Chen D, et al. Extracellular Hmgb1 Functions as an Innate Immune-Mediator Implicated in Murine Cardiac Allograft Acute Rejection. Am J Trans. 2007;7:799–808. doi: 10.1111/j.1600-6143.2007.01734.x. [DOI] [PubMed] [Google Scholar]
- 25.Rabadi MM, Ghaly T, Goligorksy MS, Ratliff BB. HMGB1 in renal ischemic injury. American Journal of Physiology - Renal Physiology. 2012;303:F873–F885. doi: 10.1152/ajprenal.00092.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu H, Li J, Wang S, Liu K, Wang L, Huang L. Hmgb1-TLR4-IL-23-IL-17A Axis Promote Ischemia-Reperfusion Injury in a Cardiac Transplantation Model. Transplantation. 2013;5:1448–1454. doi: 10.1097/TP.0b013e318293b7e1. [DOI] [PubMed] [Google Scholar]
- 27.Kamo N, Ke B, Ghaffari AA, Shen X-d, Busuttil RW, Cheng G, Kupiec-Weglinski JW. ASC/caspase-1/IL-1β signaling triggers inflammatory responses by promoting HMGB1 induction in liver ischemia/reperfusion injury. Hepatology. 2013;58:351–362. doi: 10.1002/hep.26320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zou H, Yang Y, Gao M, Zhang B, Ming B, Sun Y, Chen H, Tang X, Chen Z, Xiong P, et al. HMGB1 Is Involved in Chronic Rejection of Cardiac Allograft via Promoting Inflammatory-Like mDCs. American Journal of Transplantation. 2014;14:1765–1777. doi: 10.1111/ajt.12781. ** Interesting study that provides data that HMGB1 blockade reduces AV in mice.
- 29.Li JH, Zhao B, Zhu XH, Wang L, Zou HJ, Chen S, Guo H, Ruan YL, Zheng F, Xiang Y, et al. Blockade of Extracellular HMGB1 Suppresses Xenoreactive B Cell Responses and Delays Acute Vascular Xenogeneic Rejection. American Journal of Transplantation. 2015 doi: 10.1111/ajt.13275. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 30.Palmer SM, Burch LH, Trindade AJ, Davis RD, Herczyk WF, Reinsmoen NL, Schwartz DA. Innate Immunity Influences Long-term Outcomes after Human Lung Transplant. Am. J. Respir. Crit. Care Med. 2005;171:780–785. doi: 10.1164/rccm.200408-1129OC. [DOI] [PubMed] [Google Scholar]
- 31.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11:1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 32. Todd JL, Wang X, Sugimoto S, Kennedy VE, Zhang HL, Pavlisko EN, Kelly FL, Huang H, Kreisel D, Palmer SM, et al. Hyaluronan contributes to bronchiolitis obliterans syndrome and stimulates lung allograft rejection through activation of innate immunity. Am J Respir Crit Care Med. 2014;189:556–566. doi: 10.1164/rccm.201308-1481OC. ** Combined murine mechanistic study and human translational study that indicates that HA enhances BOS.
- 33.Oesterle A, Hofmann Bowman MA. S100A12 and the S100/Calgranulins: Emerging Biomarkers for Atherosclerosis and Possibly Therapeutic Targets. Arterioscler Thromb Vasc Biol. 2015;35:2496–2507. doi: 10.1161/ATVBAHA.115.302072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Saito T, Liu M, Binnie M, Sato M, Hwang D, Azad S, Machuca TN, Zamel R, Waddell TK, Cypel M, et al. Distinct Expression Patterns of Alveolar “Alarmins” in Subtypes of Chronic Lung Allograft Dysfunction. American Journal of Transplantation. 2014;14:1425–1432. doi: 10.1111/ajt.12718. *Important clinical study correlating calgranulins with BOS after lung transplantation.
- 35.Shen H, Heuzey E, Mori D, Wong C, Colangelo CM, Chung L, Bruce C, slizovskiy I, Booth CJ, Kreisel D, et al. Haptoglobin Enhances Cardiac Transplant Rejection. Circulation Research 10.1161/circresaha.116.305406. 2015;116:1670–1679. doi: 10.1161/CIRCRESAHA.116.305406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fuquay R, Renner B, Kulik L, McCullough JW, Amura C, Strassheim D, Pelanda R, Torres R, Thurman JM. Renal Ischemia-Reperfusion Injury Amplifies the Humoral Immune Response. Journal of the American Society of Nephrology. 2013;24:1063–1072. doi: 10.1681/ASN.2012060560. ** Murine study that revealed that renal IRI enhances humoral immunity.
- 37.Naemi FM, Carter V, Kirby JA, Ali S. Anti-donor HLA class I antibodies: pathways to endothelial cell activation and cell-mediated allograft rejection. Transplantation. 2013;16:258–266. doi: 10.1097/TP.0b013e3182985504. [DOI] [PubMed] [Google Scholar]
- 38.Lion J, Taflin C, Cross AR, Robledo-Sarmiento M, Mariotto E, Savenay A, Carmagnat M, Suberbielle C, Charron D, Haziot A, GLotz D, Mooney N. HLA class II antibody activation of endothelial cells promotes Th17 and disrupts regulatory T lymphocyte expansion. American Journal of Transplantation. 2015 doi: 10.1111/ajt.13644. m J Transplant. 2015, In Press. [DOI] [PubMed] [Google Scholar]
- 39.Jin YP1, Valenzuela NM, Ziegler ME, Rozengurt E, Reed EF. Everolimus inhibits anti-HLA I antibody-mediated endothelial cell signaling, migration and proliferation more potently than sirolimus. Am J Transplant. 2014;14:806–819. doi: 10.1111/ajt.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang X, Rozengurt E, Reed EF. HLA class I molecules partner with integrin β4 to stimulate endothelial cell proliferation and migration. Sci Signal. 2010;3:ra85. doi: 10.1126/scisignal.2001158. **Important study defining a novel signaling mechanism involving integrin b4 used by donor specific antibody to activate Akt in ECs.
- 41.Li F, Wei J, Valenzuela NM, Lai C, Zhang Q, Gjertson D, Fishbein MC, Kobashigawa JA, Deng M, Reed EF. Phosphorylated S6 kinase and S6 ribosomal protein are diagnostic markers of antibody-mediated rejection in heart allografts. J Heart Lung Transplant. 2015;34:580–587. doi: 10.1016/j.healun.2014.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frank R, Molina MR, Goldberg LR, Wald JW, Kamoun M, Lal P. Circulating donor-specific anti-human leukocyte antigen antibodies and complement C4d deposition are associated with the development of cardiac allograft vasculopathy. Am J Clin Pathol. 2014;142:809–815. doi: 10.1309/AJCPTLBEU5BQ8SHN. [DOI] [PubMed] [Google Scholar]
- 43. Raedler H1, Yang M, Lalli PN, Medof ME, Heeger PS. Primed CD8(+) T-cell responses to allogeneic endothelial cells are controlled by local complement activation. Am J Transplant. 2009;9:1784–1795. doi: 10.1111/j.1600-6143.2009.02723.x. * Conceptually interesting study showing that stimulation of ECs with TNF-a or IL-1 elicited synthesis of alternative complement components, C3 and factor B, resulting in local complement activation characterized by the generation of C5a and enhanced alloimmune T cell activation
- 44.Pratt JR, Basheer SA, Sacks SH. Local synthesis of complement component C3 regulates acute renal transplant rejection. Nat Med. 2002;8:582–587. doi: 10.1038/nm0602-582. [DOI] [PubMed] [Google Scholar]
- 45.Strainic MG, Liu J, Huang D, An F, Lalli PN, Muqim N, Shapiro VS, Dubyak GR, Heeger PS, Medof ME. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity. 2008;28:425–435. doi: 10.1016/j.immuni.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cravedi P, Leventhal J, Lakhani P, Ward SC, Donovan MJ, Heeger PS. Immune cell-derived C3a and C5a costimulate human T cell alloimmunity. Am J Transplant. 2013;13:2530–2539. doi: 10.1111/ajt.12405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strainic MG, Shevach EM, An F, Lin F, Medof ME. Absence of signaling into CD4+ cells via C3aR and C5aR enables autoinductive TGF-β1 signaling and induction of Foxp3+ regulatory T cells. Nat Immunol. 2013;14:162–1671. doi: 10.1038/ni.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Du Y, Teng X, Wang N, Zhang X, Chen J, Ding P, Qiao Q, Wang Q, Zhang L, Yang C, Yang Z, Chu Y, Du X, Zhou X, Hu W. NF-κB and enhancer-binding CREB protein scaffolded by CREB-binding protein (CBP)/p300 proteins regulate CD59 protein expression to protect cells from complement attack. J Biol Chem. 2014;289:27112724. doi: 10.1074/jbc.M113.525501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pippin JW, Durvasula R, Petermann A, Hiromura K, Couser WG, Shankland SJ. DNA damage is a novel response to sublytic complement C5b-9-induced injury in podocytes. J Clin Invest. 2003;111:877–885. doi: 10.1172/JCI15645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elimam H, Papillon J, Takano T, Cybulsky AV. Complement-mediated activation of calcium-independent phospholipase A2γ: role of protein kinases and phosphorylation. J Biol Chem. 2013;288:3871–3885. doi: 10.1074/jbc.M112.396614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Laudisi F, Spreafico R, Evrard M, Hughes TR, Mandriani B, Kandasamy M, Morgan BP, Sivasankar B, Mortellaro A. Cutting edge: the NLKRP3 inflammasome links complement-mediated inflammation and IL-1β release. J Immunol. 2013;191:1006–1010. doi: 10.4049/jimmunol.1300489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Triantafilou K, Hughes TR, Triantafilou M, Morgan BP. The complement membrane attack complex triggers intracellular Ca2+ fluxes leading to NLRP3 inflammasome activation. J Cell Sci. 2013;126(Pt 13):2903–2913. doi: 10.1242/jcs.124388. **ref 47 and 48. Studies identifying a role for MAC-induced activation of innate immunity via NLRP3 inflammasome activation, resulting in elaboration of IL-1b and IL-18b and implicating a role for MAC-induced subcellular calcium mishandling and mitochondrial dysfunction.
- 53.Martel C, Cointe S, Maurice P, Matar S, Ghitescu M, Théroux P, Bonnefoy A. Requirements for membrane attack complex formation and anaphylatoxins binding to collagen-activated platelets. PLoS One. 2011;6:e18812. doi: 10.1371/journal.pone.0018812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ota H, Fox-Talbot K, Hu W, Qian Z, Sanfilippo F, Hruban RH, Baldwin WM., 3rd Terminal complement components mediate release of von Willebrand factor and adhesion of platelets in arteries of allografts. Transplantation. 2005;79:276–2781. doi: 10.1097/01.tp.0000146195.76904.d3. [DOI] [PubMed] [Google Scholar]
- 55.Zhou J, Qin L, Yi T, Ali R, Li Q, Jiao Y, Li G, Tobiasova Z, Huang Y, Zhang J, Yun JJ, Sadeghi MM, Giordano FJ, Pober JS, Tellides G. Interferon-γ-mediated allograft rejection exacerbates cardiovascular disease of hyperlipidemic murine transplant recipients. Circ Res. 2015;117:943–955. doi: 10.1161/CIRCRESAHA.115.306932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jane-Wit D, Manes TD, Yi T, Qin L, Clark P, Kirkiles-Smith NC, Abrahimi P, Devalliere J, Moeckel G, Kulkarni S, Tellides G, Pober JS. Alloantibody and complement promote T cell-mediated cardiac allograft vasculopathy through noncanonical nuclear factor-κB signaling in endothelial cells. Circulation. 2013;128:2504–2516. doi: 10.1161/CIRCULATIONAHA.113.002972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jane-wit D, Surovtseva YV, Qin L, Li G, Liu R, Clark P, Manes TD, Wang C, Kashgarian M, Kirkiles-Smith NC, Tellides G, Pober JS. Complement membrane attack complexes activate noncanonical NF-κB by forming an Akt+ NIK+ signalosome on Rab5+ endosomes. Proc Natl Acad Sci U S A. 2015;112:9686–9691. doi: 10.1073/pnas.1503535112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Marshall KM, He S, Zhong Z, Atkinson C, Tomlinson S. Dissecting the complement pathway in hepatic injury and regeneration with a novel protective strategy. J Exp Med. 2014;211:1793–1805. doi: 10.1084/jem.20131902. **Conceptually novel strategy using a fusion antibody to target the endogenous anti-MAC activity of CD59 to sites of complement activation in vivo
- 59.Atkinson C, Qiao F, Yang X, Zhu P, Reaves N, Kulik L, Goddard M, Holers VM, Tomlinson S. Targeting pathogenic post ischemic self-recognition by natural IgM to protect against posttransplantation cardiac reperfusion injury. Circulation. 2015;131:1171–80. doi: 10.1161/CIRCULATIONAHA.114.010482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thomas KA, Valenzuela NM, Gjertson D, Mulder A, Fishbein MC, Parry GC, Panicker S, Reed EF. An Anti-C1s Monoclonal, TNT003, Inhibits Complement Activation Induced by Antibodies Against HLA. Am J Transplant. 2015;15:2037–2049. doi: 10.1111/ajt.13273. [DOI] [PMC free article] [PubMed] [Google Scholar]