Abstract
Rationale
The identification of circulating endothelial progenitor cells has led to speculation regarding their origin as well as their contribution to neovascular development. Two distinct types of endothelium make up the blood and lymphatic vessel system. However, it has yet to be determined whether there are distinct lymphatic-specific circulating endothelial progenitor cells.
Objective
This study aims to isolate and characterize the cellular properties and global gene expression of lymphatic-specific endothelial progenitor cells.
Methods and Results
We isolated circulating endothelial colony forming cells (ECFCs) from whole peripheral blood. These cells are endothelial in nature, as defined by their expression of endothelial markers and their ability to undergo capillary morphogenesis in three-dimensional culture. A subset of isolated colonies express markers of lymphatic endothelium, including VEGFR-3 and Prox-1, with low levels of VEGFR-1, a blood endothelial marker, while the bulk of the isolated cells express high VEGFR-1 levels with low VEGFR-3 and Prox-1 expression. The different isolates have differential responses to VEGF-C, a lymphatic endothelial specific cytokine, strongly suggesting that there are lymphatic specific and blood specific ECFCs. Global analysis of gene expression revealed key differences in the regulation of pathways involved in cellular differentiation between blood and lymphatic-specific ECFCs.
Conclusion
These data indicate that there are two distinguishable circulating ECFC types, blood and lymphatic, which are likely to have discrete functions during neovascularization.
Keywords: endothelial progenitor, lymphatic endothelial progenitor, VEGFR-3, Prox-1
Introduction
It has long been thought that new vessels in adults derive from induced growth of existing vasculature, or angiogenesis. In 1997, however, circulating endothelial progenitor cells (EPCs) were found in human peripheral blood, and it was proposed that these cells play a role in seeding angiogenesis and/or post-natal vasculogenesis.1 However, numerous studies aimed at elucidating the function of circulating EPCs have yielded conflicting results. EPCs are mobilized from the bone marrow,2 and can home to sites of ischemia and tumor angiogenesis.1,3 Whether they directly contribute to and incorporate into new vasculature is controversial (reviewed in Yoder and Ingram4). One source of the controversy is the lack of a distinct marker, or set of markers, that definitively identify EPCs. Several methods have been developed to identify the types and developmental stages of endothelial progenitor cell differentiation.5-13 However, the characteristics of isolated endothelial progenitor cells is dependent upon the method of isolation and the source of the cells. Thus, a better understanding of endothelial cell development and progenitor cell functions is necessary for evaluating their role in postnatal vasculogenesis and tumor-associated angiogenesis.
Following development of the blood vascular system, a subset of cells in the cardinal vein begin to express genes, such as sox18 and prox-1, that control differentiation of endothelial cells along a lymphatic lineage.14-16 These cells then bud from the cardinal vein, further differentiate into lymphatic endothelium and organize into lymphatic vasculature. Interestingly, during neovascularization, endothelial progenitor cells have been found to contribute to new lymphatic vasculature.17,18 This suggests that endothelial progenitor cells can somehow differentiate into lymphatic vasculature, although the mechanism for this differentiation is unclear.
Although lymphatic-specific endothelial progenitor cells have been hypothesized, the characterization of lymphatic marker expression in these cells has been limited. One study identified a sub-population of CD34+ cells isolated from fetal liver that express vascular endothelial growth factor receptor-3 (VEGFR-3).19 These cells proliferated in non-adherent culture and expressed the progenitor marker CD133, suggesting the presence of lymphatic progenitor cells. Long-term culture of non-adherent CD34+ cells lead to the accumulation of adherent endothelial-like cells, which lack CD133 and express markers of either/both blood and lymphatic endothelium. Another study found that mononuclear cells isolated from murine bone marrow could produce endothelial-like cells that expressed lymphatic markers when cultured in the presence of VEGF-A and/or VEGF-C.20 These cells, when injected into several mouse angiogenesis models, contributed to lymphatic neovascularization. Similarly, CD34+/VEGFR-3+ cells isolated from human umbilical cord blood can be differentiated toward a lymphatic endothelial cell phenotype when grown in the presence of VEGF-C.21 Interestingly, Nguyen, et. al.22 found that endothelial progenitor cells isolated from umbilical cord blood were genetically similar to human dermal lymphatic endothelial cells, despite the fact that they lack important lymphatic markers podoplanin and prox-1. Taken together, these studies hint at the presence of circulating lymphatic endothelial progenitor cells. However, isolation and characterization of distinct blood and lymphatic endothelial progenitor cells has not been shown.
In this study, we isolated endothelial colony-forming cells from adult whole peripheral blood. These cells have high proliferative potential, and exhibit characteristics consistent with endothelial cells. We found that these cells express markers indicative of either blood-specific or lymphatic-specific endothelium but not both. These cells also demonstrate differential responses to the lymphatic-specific vascular endothelial growth factor C. Global gene expression analysis identified numerous differences between the blood and lymphatic ECFCs including differences in the expression of key molecules involved in cellular differentiation that may play a role in defining the specification of these cells.
Materials and Methods
Cells
Blood samples were obtained from the Puget Sound Blood Bank as either outdated whole blood (approximately 500 ml per unit) or leukocyte reduction filters, which yielded between 4-7×108 leukocytes after elution. Whole blood was mixed 1:2 with phosphate-buffered saline (PBS) containing 10,000 units per liter heparin and 0.02% EDTA. Cells from leukocyte reduction filters were eluted with 200ml PBS containing 10,000 units per liter heparin and 0.02% EDTA. ECFCs were then isolated and cultured as previously described.23 Individual colonies of cells were clonally expanded. All experiments were performed on cells between passage 8 and 15.
Primary mature human dermal microvascular endothelial cells (HMVECs) were obtained from Lonza. Cells were maintained in EGM-2 growth media (Lonza) and experiments were performed on cells between passage 7 and 12.
Antibodies and reagents
Antibodies to CD31 (product number 555444), CD34 (550760), CD45 (555480), CD14 (555396), and CD146 (550314) were obtained from BD Biosciences. VE-cadherin antibody was obtained from Cell Signaling (product number 2500). Antibody to CD133 was obtained from Miltenyi Biotec (130-090-851). Prox-1 antibody was obtained from Abcam (ab11941). VEGF-A was obtained from PeproTech (100-20) and VEGF-C was from R&D Systems (2179-VC).
Flow cytometric analysis
Monolayers of cells were washed once with PBS containing 0.04% EDTA and incubated with cell dissociation solution (Sigma) to remove cells from plates. Cells were washed with PBS and fixed in 4% paraformaldehyde for 10 minutes on ice. Cells were then pelleted and resuspended in blocking buffer (Tris-buffered saline (TBS) with 1% BSA) for 20 min on ice. Cells were pelleted and incubated with primary antibody or IGG control prepared in TBS with 1% BSA for 30 min on ice. Following incubation, cells were washed twice with TBS/1% BSA and then incubated with secondary antibody conjugated to Alexafluor 488 (Invitrogen) for 30 min on ice. The stained cells were washed twice with TBS containing 1% BSA, resuspended in TBS containing 1% BSA, and analyzed by FACScan caliber flow cytometer (Becton-Dickinson, Franklin Lakes, NJ). Data was analyzed using FloJo flow cytometry analysis software (Tree Star, Inc.; Ashland, OR).
RNA isolation and quantitative RT-PCR
Total RNA was isolated from ECFCs using the NucleoSpin RNA kit (Macherey-Nagle). One hundred or 500 ng of total RNA was used in a SuperScript III, Platinum SYBR green, one-step, quantitative reverse transcription PCR (RT-PCR; Invitrogen) according to manufacturer's protocols with the primers for either GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (forward, 5'-AAG GTG AAG GTC GGA GTC AAC G-3'; reverse, 5'-TGG AAG ATG GTG ATG GGA TTT C-3'), prox-1 (forward, 5’-AGT GCA ATG CAG GAA GGA TT-3’; reverse, 5’-CCA CTT GAT GAG CTG AGA GG-3’), or VEGFR-3 (forward, 5’-GAC AGC TAC AAG TAC GAG CAT CTG-3’; reverse 5’-CTG TCT TGC AGT CGA GCA GAA-3’). Relative abundances of prox-1 and VEGFR-3 mRNA were normalized by the delta threshold cycle method to the abundance of GAPDH.
Immunoblot analysis
Cells were harvested and lysed with RIPA buffer (20 mM Tris [pH 7.0], 2 mM EGTA, 5 mM EDTA, phosphatase inhibitors, protease inhibitors, and 1% Triton X-100). Samples were rocked at 4°C for 30 minutes, and then spun at 6,000 × g at 4°C. Cell extracts were fractionated on a sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel, and the proteins were transferred electrophoretically to Immobilon P polyvinylidene difluoride membranes (Millipore) in Tris-glycine buffer (25 mM Tris, 192 mM glycine, 20% methanol). Blots were incubated with the indicated antibody and subsequently with HRP-conjugated secondary antibody (Jackson ImmunoResearch). Immunoreactive proteins were visualized by chemiluminescence using the Amersham ECL Plus Western blotting detection reagents (GE Healthcare).
Immunofluorescence
ECFCs were fixed with 4% (wt/vol) paraformaldehyde in phosphate-buffered saline. Cells were incubated in TBS containing 1% normal goat serum followed by incubation with primary antibodies diluted in TBS containing 1% BSA for 1 hour. Cells were then incubated with fluor-conjugated secondary antibodies for 1 hour. For uptake of acetylated LDL, cells were incubated with fluorescently labeled acetylated LDL in growth medium for 4 hours. Cells were then washed with PBS and fixed as above. For prox-1 staining, cells were fixed with 100% cold methanol and blocked in PBS containing 1% BSA and 5% normal goat serum, followed by incubation with primary antibody in PBS containing 1% BSA for 2 hours. Cells were then incubated with biotin-conjugated anti-rabbit antibody (Vector labs) for 1 hour and Texas Red Streptavidin (Vector Labs) for 1 hour. All slides were mounted in medium containing DAPI (4',6'-diamidino-2-phenylindole; Vectashield, Vector Laboratories) before being viewed under a Nikon microscope.
Three-dimensional culture of endothelial cells
Matrigel (10 mg/ml; BD Biosciences, Bedford, MA) was applied at 0.5 ml/35 mm in a tissue culture dish and incubated at 37°C for at least 30 min to harden. Cells were removed using trypsin-EDTA, washed with growth medium once, and resuspended at 1.5 × 105 cells per ml in growth medium. Cells (1 ml) were gently added to the Matrigel-coated plates, incubated at 37°C, monitored for 24 h, and photographed in digital format using a Nikon microscope.
Proliferation assay
Cells (1 × 105) were plated on 6-well tissue culture dishes in growth medium containing VEGF-C (100ng/ml; R&D Systems), or vehicle control. Plates were incubated for 4 days, during which the cells were fed with fresh medium with or without VEGF-C on the second day. After 4 days, cells were counted with a hemocytometer.
Scratch wound assay
Cells (2 × 105) were plated on 6-well tissue culture dishes and allowed to reach confluence (2–3 days). After aspirating the medium, cell layers were wounded using a 1 ml micropipette tip. Plates were then rinsed with PBS, fed with growth medium with or without VEGF-A or –C (100ng/ml) and 1μM 5-fluorouracil (Sigma), and wounds were observed and photographed at 0 and 24h. The distance migrated was measured using Adobe Photoshop.
RNA-sequencing
Total RNA was isolated from ECFCs and HMVECs using the NucleoSpin RNA kit (Macherey-Nagle, Bethlehem, PA). RNA was further concentrated and purified using the RNA Clean and Concentrator kit (Zymo Research, Irvine, CA). Purified RNA samples were processed at the Fred Hutchison Cancer Research Center Genomic Resources core facility and sequenced using an Illumina HiSeq 2000. Image analysis and base calling were performed using RTA v1.17 software (Illumina). Reads were aligned to the Ensembl's GRCh37 release 70 reference genome using TopHat v2.08b and Bowtie 1.0.0.24,25 Counts for each gene were generated using htseq-count v0.5.3p9. Differentially expressed genes were determined using the R package EdgeR (Bioconductor). Genes were called significant with a |logFC| > 0.585 and a false discovery rate of <0.05. Gene Ontology enrichment was performed using Cytoscape and BINGO.26
Using cytoscape software and the Gene Ontology classification application BINGO, we determined the Gene Ontology terms that were highly enriched among the blood and lymphatic specific expressed genes. The top 20 most highly enriched categories for each cell type are listed in Table S2. Additionally, The data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus27 and are accessible through GEO Series accession number GSE54416 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE54416) for the ECFCs and GSE74322 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE74322) for the HMVECs.
Results
Isolation of ECFCs
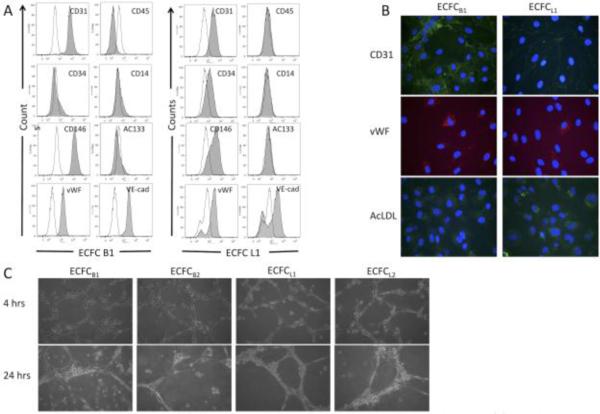
Multiple clones of ECFCs were isolated from whole blood using previously published methods as described in the materials and methods.5 The isolation was performed six separate times, from a total of the equivalent of three liters of blood. Endothelial precursor cells are extremely rare cells, comprising between 0.01% and 0.0001% of mononuclear cells28, however, due to the extended time between collection of the sample and isolation of the cells, our yield was considerably lower. Each isolation yielded between 2-8 colonies, however, of these we were only able to culture and expand 9 clones into cell lines. One of these clones was clearly not endothelial, due to its elongated shape and lack of endothelial marker expression, and is not included in this manuscript. Figure 1 shows the morphology of four separate clones of ECFCs, which exhibit the typical cobblestone morphology that is characteristic of endothelial cells. The remaining 8 cell lines were expanded and measured for their expression of endothelial markers. Figure 2A shows a representative flow cytometry experiment demonstrating expression of endothelial markers on the isolated ECFCs. As shown in Table 1, the four representative ECFC clones express markers of endothelium, including VEGF receptor 2, CD31, CD146, VE-cadherin, and von Willebrand Factor. None of the clones expressed significant levels of the monocyte/macrophage markers CD45 or CD14 (Figure 2A and Table 1). To further confirm that the isolated cells were of endothelial origin, the expression and localization of CD31 and von Willebrand Factor were analyzed by immunofluorescence. All cells expressed CD31 at their sites of cell-cell junctions (Figure 2B) and clones B1 and B2 had consistent expression of von Willebrand Factor in intracellular Weibel-Palades Bodies (Figure 2B). In contrast, von Willebrand Factor was detected in only approximately 50% of cells from clone L1 and L2 (Figure 2B). However, all four cell clones were able to uptake Acetylated LDL, as shown in Figure 2B, and organized into capillary-like networks when plated on Matrigel (Figure 2C). Taken together, these data suggest that the cells isolated are of endothelial origin and were expanded from bona fide endothelial colony forming cells.
Figure 1. Phase images of ECFCB and ECFCL clones.
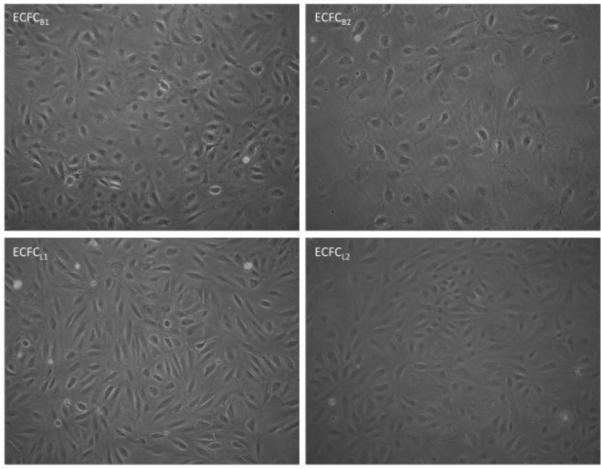
Confluent monolayers of each ECFC clone expanded from single cell isolates from human whole blood are shown. Magnification is 40x.
Figure 2. Isolated clones express endothelial markers, uptake Ac-LDL, and undergo capillary morphogenesis.
(A) ECFC clones B1 and L1 were stained for endothelial or monocyte/macrophage markers and the expression was analyzed by flow cytometry. Shaded areas are primary antibody, unshaded areas are IGG control. (B) ECFC clones B1 and L1 were stained for CD31 (green) or von Willebrand Factor (red) or incubated with fluorescent-labeled AcLDL (green). Nuclei in all samples were stained with DAPI (blue). (C) ECFC clones B1, B2, L1, and L2 were plated on Matrigel. Cells were monitored for capillary morphogenesis and photographs were taken at the indicated times. These experiments were each performed 3 separate times with identical results.
Table 1. Expression of cellular markers on isolated ECFC clones.
Isolated cells were analyzed for marker expression as noted. (+) symbols denote expression was detected in all cells. (+/−) denotes expression was detected on a subset of cells.
| Endothelial | ECFCB1 | ECFCB2 | ECFCL1 | ECFCL2 |
|---|---|---|---|---|
| CD34 (flow cytometry) | - | - | + | + |
| CD31 (flow cytometry) | + | + | + | + |
| CD146 (flow cytometry) | + | + | + | + |
| FLK-1 (R2) (Western blot) | + | + | + | + |
| Flt-1 (R1) (Western blot) | + | + | - | - |
| vWF (flow cytometry) | + | + | +/− | +/− |
| VE-Cadherin (flow cytometry) | + | + | +/− | + |
| uptake AcLDL (immunofluorescence) | + | + | + | + |
| Capillary Morphogenesis | + | + | + | + |
| Stem Cell | ||||
| ac133 (flow cytometry) | - | - | - | - |
| Monocyte/Macrophage | ||||
| CD45 (flow cytometry) | - | - | - | - |
| CD14 (flow cytometry) | - | - | +/− | - |
| Lymphatic | ||||
| Flt-4 (R3) (Western blot) | - | - | + | + |
| Podoplanin (Western blot) | - | - | + | + |
| LYVE-1 (Western blot) | - | - | + | + |
| Prox-1 (immunofluorescence and PCR) | - | - | + | + |
Expression of lymphatic-specific markers
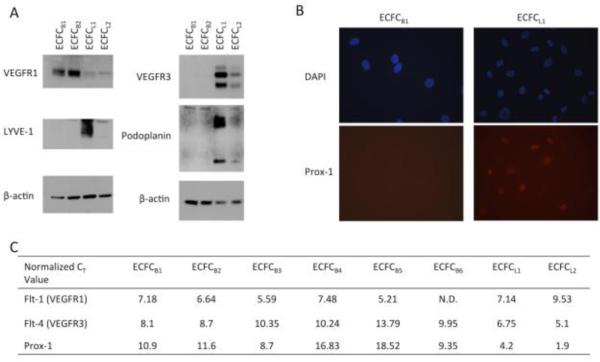
Several markers have been found which distinguish endothelial cells derived from blood vasculature from those derived from the lymphatic vasculature.29 Blood vasculature endothelial cells express high levels of the vascular endothelial growth factor receptor-1 (VEGFR-1). In contrast, the lymphatic endothelium expresses high levels of VEGFR-3, as well as other markers, such as prox-1, podoplanin, and LYVE-1, while largely lacking VEGFR-1. To determine the origin of the ECFC's isolated from whole blood, we examined the expression of these genes on isolated ECFCs. The expression of VEGFR-1 and VEGFR-3 proteins in the clones was determined by Western blot analysis. Interestingly, the expression of lymphatic-specific genes is not uniform amongst ECFCs. The majority of ECFCs isolated from whole blood expressed high levels of VEGFR-1, indicative of blood-specific precursors (Figure 3A). However, two of the clones expressed very low levels of VEGFR-1 but expressed high levels of VEGFR-3. We further analyzed other lymphatic-specific markers and found that both podoplanin and LYVE-1 are expressed on the clones that express VEGFR-3 (Figure 3A, ECFC L1 and L2). In contrast, the clones that lacked VEGFR-3 (ECFC B1 and B2) also lacked podoplanin and LYVE-1 expression.
Figure 3. Expression of lymphatic-specific markers in ECFC clones.
(A) ECFC lysates were separated on a 4-20% SDS-PAGE gel and transferred to PVDF membrane. Blots were probed for VEGFR-1, VEGFR-3, LYVE-1, podoplanin, or β-actin. (B) ECFC B1 and ECFC L1 were stained for Prox-1 protein expression (red) and DAPI (blue). Each experiment was performed 3 times with similar results. (C) RNA was isolated from each ECFC clone. Real-time RT-PCR was performed for VEGFR-1, VEGFR-3, and Prox-1. Values shown are CT values of each gene normalized to the level of GAPDH in each sample.
The transcription factor prox-1 is known to be a major regulator of lymphatic differentiation from venous endothelium. Thus, we analyzed prox-1 expression by immunofluorescence and could detect prox-1 protein expression in ECFC clones L1 and L2, but no expression in ECFC B1 or B2 (Figure 3B). We also compared the expression of VEGFR-1, VEGFR-3, and prox-1 on all the isolated clones by real-time RT-PCR. On average, VEGFR-1 was detected 2 cycles earlier in the blood ECFCs, while VEGFR-3 was detected 4 cycles earlier in the lymphatic ECFCs (Figure 3C). Strikingly, Prox-1 was detected in all lymphatic cells at least 4 cycles sooner than their blood counterparts (Figure 3C). While there is a range of expression levels of lymphatic markers between the two cell lines expressing VEGFR-3, it is clear that these cells express the major lymphatic markers while the all of the cell lines expressing high levels of VEGFR-1 did not express detectable levels of the lymphatic specific markers. Together, these data suggest that there are distinct populations of blood and lymphatic-specific endothelial progenitor cells.
Response to VEGF isoforms
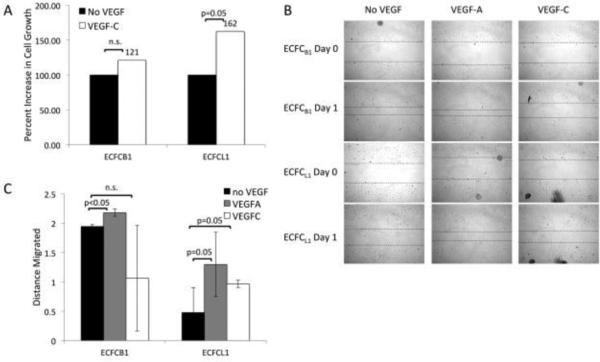
The difference in VEGF receptor expression on the ECFC clones suggests they may respond to different VEGF ligands. On mature blood endothelium, VEGF-A interacts with VEGFR-1 and 2 to promote survival, proliferation, and migration. Lymphatic endothelial cells express VEGFR-3, which binds to VEGF-C and D to induce those functions. To test whether the ECFC clones respond differently to the VEGF isoforms, we treated each cell type with exogenous VEGF isoforms and measured their proliferation and migration. Figure 4A shows the proliferation of cells with and without VEGF. As expected, both cell types proliferate more rapidly in response to VEGF-A, due to the expression of VEGFR-2 on both blood and lymphatic ECFCs (not shown). However, there was only a 20% increase in proliferation of blood ECFCs in response to VEGF-C, which was not statistically significant (Figure 4A, ECFC B1). In contrast, the lymphatic ECFC clones displayed a 60% increase in proliferation in response to VEGFC (p=0.05), a result that was consistent across multiple experiments and cell passage numbers (Figure 4A, ECFC L1). Although it is at the border of statistical significance, this result is consistent with previous results in mature endothelium, where VEGF-C promotes only a moderate proliferative response.29
Figure 4. ECFC response to VEGF-A and VEGF-C.
(A) Cells were plated without VEGF (black bars) or with VEGF-C (white bars) for 4 days and then cell number was quantified with a hemocytometer. (B) Confluent monolayers of ECFC B1 or ECFC L1 were wounded and treated with or without VEGF-A or VEGF-C for 24 hours. Pictures were taken at the time of wounding and 24 hours later. (C) The distance migrated into each wound was quantified and graphed. Data shown is representative of 5 experiments.
To test migration, we performed scratch wound assays on confluent monolayers of ECFCs. We then monitored the migration of each cell type into the wound in the presence or absence of VEGF isoforms. To exclude the contribution of proliferation into the wound, cells were also treated with 5-fluorouracil. There was an increase in migration of ECFC B1 cells in response to VEGF-A but an apparent inhibition of migration in response to VEGF-C. In contrast, ECFC L1 showed increased migration in response to both VEGF-A and VEGF-C (Figure 4B and C). These results indicate that the lymphatic ECFCs respond to VEGF-C and VEGF-A while the blood ECFCs only respond to VEGF-A, consistent with the differential expression of VEGF receptors on these cells and indicating that the lymphatic ECFCs respond to different cues to divide and migrate than the blood ECFCs.
Global gene expression
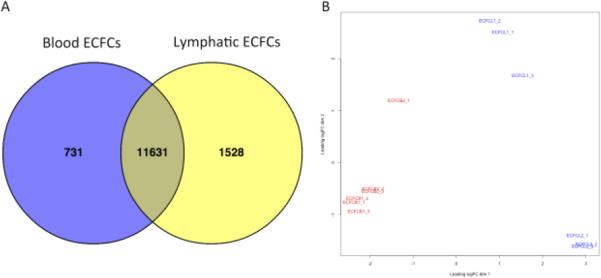
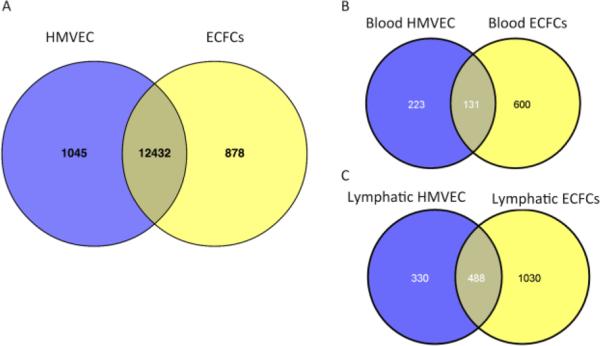
In order to measure the global gene expression differences between blood and lymphatic-specific ECFCs, we performed high-throughput sequencing on four ECFC clones. For each clone, we harvested RNA on three separate days at similar passage number, between 8 and 15. We detected over 16 million reads per sample. As shown in Figure 5A, we found 497 genes to be enriched in the blood-specific ECFCs and 1155 genes to be specific for lymphatic ECFCs. Over 12,000 genes had similar expression patterns between the two cell types. The full list of differentially expressed genes can be found in Supplemental Table I. Using multidimensional scaling, we compared the gene expression of the four cell lines and plotted them in a scatter plot (Figure 5B). This shows that the two blood cell lines (shown in red) cluster together, and are distinct from the two lymphatic cell lines (shown in blue). Importantly, the two lymphatic ECFC lines cluster on the first principal component (X-axis), indicating that they are very similar to each other, while distinctly separable from the two blood ECFC lines. The secondary component analysis (Y-axis) indicates that while very similar, they are distinct cell lines from different patients.
Figure 5. Differential gene expression between blood- and lymphatic-specific ECFCs.
(A) Analysis of gene expression profiles using high-throughput sequencing identified 731 genes enriched in blood-specific ECFCs (blue) and 1528 genes enriched in lymphatic-specific ECFCs (yellow). The majority of identified genes were expressed at similar levels in both cell types (shaded region). (B) Multidimensional scaling scatter plot showing the degree of difference between blood-specific ECFCs (red) and lymphatic-specific ECFCs (blue) for each RNA-sequencing sample.
To determine how the ECFCs were unique we compared gene expression patterns of the four ECFCs described above to primary mature dermal microvascular endothelial cells. We performed similar high-throughput sequencing on primary mature blood and lymphatic HMVEC cell lines as described for the ECFC clones. The genes that were differentially expressed between blood and lymphatic cell lines were then subtracted out, in order to compare those genes that were common to both mature blood and lymphatic HMVECs to those that were common to the precursor blood and lymphatic ECFCs and thereby eliminate the differences in blood versus lymphatic endothelium. Overall, the vast majority of genes are expressed at similar levels in both HMVECs and ECFCs (Figure 6A). However, there were 878 genes that were identified as being enriched in both lymphatic and blood ECFCs as compared to mature endothelium, which represents 6% of the total genes identified in the sequencing run. Interestingly, when we performed Gene Ontology analysis on the lists of genes that are enriched in HMVECs, the most significant GO term identified was cell adhesion, which is consistent with mature endothelial cells adhering to the extracellular matrix rather than being in circulation (Supplementary Table II).
Figure 6. Differential gene expression between mature HMVECs and ECFCs.
(A) Analysis of gene expression profiles using high-throughput sequencing identified 878 genes that are enriched in ECFCs (yellow). The majority of identified genes were expressed at similar levels in both precursor and mature cell types (shaded region). (B) Comparison of blood-enriched genes between blood HMVECs and blood ECFCs. (C) Comparison of lymphatic-enriched genes between lymphatic HMVECs and lymphatic ECFCs.
To determine if the gene expression pattern that differentiates blood ECFCs from lymphatic ECFCs is similar to the pattern that differentiated mature blood endothelial cells from mature lymphatic endothelial cells, we compared blood and lymphatic specific gene expression. For the analysis of blood specific genes we combined the genes that were blood specific in either ECFCs or mature endothelial cells, yielding 954 blood specific genes. Of these genes, 131 are expressed in both mature and ECFC blood-specific cell lines, suggesting their importance for this lineage (Figure 6B). We performed a similar analysis with lymphatic-enriched genes in lymphatic HMVECs and ECFCs. In this case, of the 1848 genes specific to lymphatic lineage cells, we found that 488 genes, over 25%, are expressed in both the mature and ECFC lymphatic cells analyzed (Figure 6C). These genes likely include factors important for lymphatic specification.
To confirm our sequencing data, we performed real-time RT-PCR on select genes (Table 2, denoted with an asterisk). Among the top differentially expressed genes between blood and lymphatic-specific ECFCs are genes involved in regulation of cell differentiation, vasculature development, and endothelial cell differentiation (Table 2; Supplementary Table III). For example, blood-specific ECFCs expressed higher levels of Wnt 9a, and DKK, molecules involved in Wnt signaling. In contrast, lymphatic-specific ECFCs expressed Wnt 5a and 5b. There were also differences in the expression of Notch signaling molecules, with Notch 4 being expressed by blood-specific ECFCs while lymphatic-specific ECFCs expressed Notch 3 and Jagged 1. In addition, the lymphatic ECFCs expressed high levels of cellular differentiation regulators Sonic hedgehog, Inhibitor of DNA-binding 1 and 2 (Id1 and Id2), and prox-1. These differences suggest these molecules may play a role in the differentiation of hemangioblasts toward an endothelial lineage; however further work is necessary to determine the necessity and function of these genes in this process.
Table 2. Differentially expressed genes in Blood and Lymphatic ECFCs.
Gene expression was analyzed by high-throughput RNA sequencing. Differentially regulated genes were subsequently analyzed for Gene Ontology term enrichment. This table shows the expression differences in genes involved in a subset of enriched Gene Ontology terms. A more expansive list of enriched terms can be found in Supplemental Table II.
| Ensembl Code | Fold increase in Blood ECFCs over Lymphatic ECFCs | Fold increase in Lymphatic ECFCs over Blood ECFCs | |
|---|---|---|---|
| Regulation of cell differentiation | |||
| Neuregulin 1 | ENSG00000157168 | 251.6 | |
| Neuroligin 1 | ENSG00000169760 | 211.6 | |
| Transient receptor potential cation channel M2 | ENSG00000142185 | 70.5 | |
| GATA binding protein 6 | ENSG00000141448 | 41.0 | |
| Growth differentiation factor 5 | ENSG00000125965 | 39.0 | |
| Leucine rich repeat containing 17 | ENSG00000128606 | 32.1 | |
| Hepatocyte growth factor | ENSG00000019991 | 25.7 | |
| Interleukin 1 receptor accessory protein-like 1 | ENSG00000169306 | 17.3 | |
| Pre-B-cell leukemia homeobox 1 | ENSG00000185630 | 9.0 | |
| Muscleblind-like splicing regulator 3 | ENSG00000076770 | 8.7 | |
| c-Kit | ENSG00000157404 | 8.0 | |
| Follistatin | ENSG00000134363 | 6.4 | |
| Ectonucleotide pyrophosphatase/phosphodiesterase 1 | ENSG00000197594 | 3.9 | |
| Dickkopf 1 homolog | ENSG00000107984 | 3.7 | |
| Wnt 9A | ENSG00000143816 | 3.7 | |
| Parathyroid hormone-like hormone | ENSG00000087494 | 3.4 | |
| H2.0-like homeobox | ENSG00000136630 | 3.0 | |
| Purinergic receptor P2Y, G-protein coupled, 2 | ENSG00000175591 | 2.6 | |
| Myelin basic protein | ENSG00000197971 | 2.6 | |
| Neuronal cell adhesion molecule | ENSG00000091129 | 2.5 | |
| c-Kit ligand | ENSG00000049130 | 2.5 | |
| Transforming growth factor-beta 3 | ENSG00000119699 | 2.1 | |
| DNA(cytosine-5-)-methyltransferase 3 beta | ENSG00000088305 | 2.1 | |
| Vitamin D receptor | ENSG00000111424 | 2.1 | |
| Homeobox A11 | ENSG00000005073 | 447.9 | |
| Nerve growth factor | ENSG00000134259 | 407.2 | |
| CD36 | ENSG00000135218 | 381.6 | |
| R-Cadherin | ENSG00000179242 | 332.1 | |
| Eyes absent homolog 1 | ENSG00000104313 | 283.3 | |
| GLI family zinc finger 2 | ENSG00000074047 | 147.8 | |
| Wnt 5B | ENSG00000111186 | 88.1 | |
| Deleted in colorectal carcinoma | ENSG00000187323 | 55.6 | |
| Odd-skipped related 1 | ENSG00000143867 | 55.5 | |
| Carbonic anhydrase II | ENSG00000104267 | 44.2 | |
| Wnt 5A | ENSG00000114251 | 34.5 | |
| Lipoprotein lipase | ENSG00000175445 | 34.3 | |
| Chordin | ENSG00000090539 | 30.3 | |
| Integral membrane protein 2C | ENSG00000135916 | 28.7 | |
| Growth hormone receptor | ENSG00000112964 | 27.7 | |
| Interleukin 7 | ENSG00000104432 | 19.5 | |
| GTPase, IMAP family member 5 | ENSG00000196329 | 18.2 | |
| chemokine ligand 5 | ENSG00000161570 | 17.2 | |
| Superoxide dismutase 2 | ENSG00000112096 | 15.7 | |
| Oligodendrocyte myelin glycoprotein | ENSG00000126861 | 15.5 | |
| CD74 | ENSG00000019582 | 14.8 | |
| Kalirin | ENSG00000160145 | 13.7 | |
| Serpin E2 | ENSG00000135919 | 9.3 | |
| SPOCK 2 | ENSG00000107742 | 8.8 | |
| Latent transforming growth factor-beta binding protein 4 | ENSG00000090006 | 8.4 | |
| Interleukin 7 receptor | ENSG00000168685 | 8.0 | |
| MafB | ENSG00000204103 | 7.5 | |
| CD83 | ENSG00000112149 | 7.1 | |
| Teashirt zinc finger homeobox 3 | ENSG00000121297 | 5.5 | |
| ATP-binding cassette G1 | ENSG00000160179 | 5.0 | |
| Cholesteryl ester transfer protein, plasma | ENSG00000087237 | 5.0 | |
| Osteopontin | ENSG00000118785 | 4.9 | |
| Cyclin D2 | ENSG00000118971 | 4.6 | |
| Peroxisome proliferator-activated receptor gamma | ENSG00000132170 | 4.5 | |
| Vanin 1 | ENSG00000112299 | 4.3 | |
| Tribbles homolog 2 | ENSG00000071575 | 3.6 | |
| Receptor-interacting serine-threonine kinase 2 | ENSG00000104312 | 3.5 | |
| Inhibitor of DNA-binding 2 | ENSG00000115738 | 3.4 | |
| Axin 2 | ENSG00000168646 | 3.2 | |
| Voltage-Gated Calcium Channel Subunit Alpha Cav2.1 | ENSG00000141837 | 3.2 | |
| Frizzled 1 | ENSG00000157240 | 3.0 | |
| Toll-like receptor 4 | ENSG00000136869 | 2.8 | |
| PKDCC | ENSG00000162878 | 2.8 | |
| Bambi | ENSG00000095739 | 2.7 | |
| Peroxisome proliferator-activated receptor alpha | ENSG00000186951 | 2.7 | |
| Interleukin 6 receptor | ENSG00000160712 | 2.7 | |
| Homeobox A5 | ENSG00000106004 | 2.3 | |
| Inhibin beta A | ENSG00000122641 | 2.3 | |
| Ephrin A1 | ENSG00000169242 | 2.2 | |
| Peroxisome proliferator-activated receptor delta | ENSG00000112033 | 2.0 | |
| Vasculature development | |||
| Aldehyde dehydrogenase 1A2 | ENSG00000128918 | 444.9 | |
| Notch 4 | ENSG00000206312 | 39.5 | |
| N-cadherin* | ENSG00000170558 | 38.1 | |
| P-selectin | ENSG00000174175 | 17.9 | |
| T-box 3 | ENSG00000135111 | 9.8 | |
| Forkhead box F1 | ENSG00000103241 | 9.0 | |
| Vascular endothelial growth factor C* | ENSG00000150630 | 3.9 | |
| Placental growth factor | ENSG00000119630 | 3.5 | |
| Plasminogen activator, tissue | ENSG00000104368 | 3.4 | |
| Apolipoprotein L domain containing 1 | ENSG00000178878 | 3.3 | |
| Alanyl aminopeptidase | ENSG00000166825 | 2.7 | |
| VEGF receptor 1 (Flt-1)* | ENSG00000102755 | 2.5 | |
| Heme oxygenase 1 | ENSG00000100292 | 2.4 | |
| Podoplanin | ENSG00000162493 | 577.7 | |
| Gap junction protein alpha 4 | ENSG00000187513 | 268.1 | |
| Vav 3 | ENSG00000134215 | 85.8 | |
| Collagen, type XV, alpha 1 | ENSG00000204291 | 80.5 | |
| Chemokine ligand 1 | ENSG00000006210 | 38.6 | |
| BMP binding endothelial regulator | ENSG00000164619 | 37.9 | |
| Sonic hedgehog | ENSG00000164690 | 35.5 | |
| Secretogranin II | ENSG00000171951 | 31.2 | |
| Fibroblast growth factor 1 | ENSG00000113578 | 26.2 | |
| Chondroitin sulfate proteoglycan 4 | ENSG00000173546 | 23.0 | |
| Homeobox A7 | ENSG00000122592 | 14.0 | |
| H-cadherin | ENSG00000140945 | 10.1 | |
| Carcinoembryonic antigen-related cell adhesion molecule 1* | ENSG00000079385 | 9.8 | |
| Angiomotin | ENSG00000126016 | 9.0 | |
| Leptin receptor | ENSG00000116678 | 5.3 | |
| Jagged 1* | ENSG00000101384 | 4.5 | |
| Angiopoietin 1 | ENSG00000154188 | 3.7 | |
| Leukemia inhibitory factor | ENSG00000128342 | 3.6 | |
| Protein tyrosine kinase 2 beta | ENSG00000120899 | 3.5 | |
| Collagen, type VIII, alpha 1 | ENSG00000144810 | 2.5 | |
| Reck | ENSG00000122707 | 2.5 | |
| Collagen, type IV, alpha 1 | ENSG00000187498 | 2.3 | |
| Milk fat globule-EGF factor 8 | ENSG00000140545 | 2.3 | |
| Thymidine Phosphorylase | ENSG00000025708 | 2.2 | |
| Kruppel-like factor 5 | ENSG00000102554 | 2.2 | |
| Fibronectin 1 | ENSG00000115414 | 2.2 | |
| Gastrulation brain homeobox 2 | ENSG00000168505 | 2.1 | |
| Matrix metalloproteinase 19 | ENSG00000123342 | 2.0 | |
| Endothelial cell differentiation | |||
| Prospero homeobox 1* | ENSG00000117707 | 169.4 | |
| Scube 1 | ENSG00000159307 | 58.1 | |
| Inhibitor of DNA-binding 1 | ENSG00000125968 | 3.6 | |
| Notch 3 | ENSG00000074181 | 3.6 | |
Gene expression was confirmed by real-time RT-PCR.
Discussion
The exact identity and function of circulating endothelial precursors has been the subject of much debate. However, their potential for use in angiogenic therapies and diagnostics is still being studied. Careful isolation and characterization of the types of precursor endothelial cells is necessary for understanding their role in postnatal vasculogenesis. Here, we have shown that clonal isolation of endothelial colony-forming cells (ECFCs) from adult human peripheral blood yields cell lines that express either blood vascular or lymphatic-specific markers. Both cell types respond to the cytokine VEGF-A, a major cytokine involved in angiogenesis, while only the lymphatic ECFCs respond to VEGF-C, the major cytokine involved in lymphangiogenesis. While we cannot formally rule out the role of endogenous expression of VEGF-A and VEGF-C in these experiments, the expression levels of both are very low as indicated by the high throughput RNA sequencing data which would indicate that as with mature endothelial cells, ECFCs rely upon exogenous VEGFs. Since all of the ECFC cells were isolated under the same growth conditions, the blood or lymphatic specification was apparently determined before culturing. In addition, both types of cells could be obtained from the blood of a single-donor, ruling out donor-specific influences on ECFC differentiation (not shown). These findings are consistent with previous findings of VEGFR-3 expression on a subset of non-adherent CD34+ cells.19,21 The present study extends that finding to show a definitive characterization of both blood- and lymphatic-specific ECFCs.
Vascular endothelial growth factor signaling plays a key role in vascular development and angiogenesis. The VEGF family of growth factors promotes survival, proliferation and migration of endothelial cells. VEGF-A interacts with VEGFR-1 and VEGFR-2 to induce downstream signaling in blood vascular endothelial cells. In contrast, VEGF-C and VEGF-D are able to bind to VEGFR-3, which is expressed on the lymphatic vasculature. The expression of VEGFR-1 or VEGFR-3 has been shown to delineate between blood and lymphatic endothelial cells, respectively.29 Here we have shown that blood-specific ECFCs express VEGFR-1, while the lymphatic-specific ECFCs express high levels of VEGFR-3 and very little VEGFR-1. However, the expression of VEGFR-3 alone is insufficient to identify lymphatic endothelial cells. Early during embryonic development, VEGFR-3 is expressed on all endothelium, but this expression declines as the lymphatic vasculature develops and VEGFR-3 expression becomes isolated to the lymphatic endothelium.30 It has also been shown that, during sprouting angiogenesis, endothelial tip cells express both VEGFR-1 and VEGFR-3.31 Thus, to determine whether the cells we identified as VEGFR-3+ are truly lymphatic, we analyzed the expression of several other markers that are indicative of lymphatic endothelial cells.15,32,33 We found that the VEGFR-3+ cells also co-expressed the lymphatic markers prox-1, podoplanin, and LYVE-1, suggesting that these cells are fully lymphatic specified.
Previously, microarray analysis was used to identify gene expression differences between blood and lymphatic endothelial cells, where ~300 genes were found to be significantly altered between blood and lymphatic mature endothelial cells.29 In this study we used high-throughput sequencing to identify additional differences, including sets of genes specific to cells of either blood or lymphatic lineage. Of the genes specific to mature blood endothelial cells, 37% are also specific to the blood ECFC clones, though there are also many genes unique to blood ECFCs (Figure 6B). Nearly 60% of the mature lymphatic endothelial cell specific genes are also enriched in both lymphatic ECFC clones analyzed. Again, there are also a large number of genes that are unique only to the lymphatic ECFCs. The genes that are common to both the blood ECFC and mature blood endothelial cells are likely to be important for blood endothelial cell specificity as are the genes common to both the mature lymphatic endothelial cells and the lymphatic ECFCs.
Several potential pathways that may play a role in regulating the differentiation of ECFCs were also identified. For example, we found that blood and lymphatic ECFCs expressed different molecules belonging to the Wnt signaling family. Wnt proteins are secreted molecules that regulate the differentiation of many cell types. Wnt molecules interact with specific cell surface markers to lead to intracellular signaling pathways and gene expression (reviewed in Dejana34). Our sequencing data shows that Wnt 9a and the Wnt pathway inhibitor dickkopf are expressed at higher levels in blood-specific ECFCs, although little is known about how these genes function in endothelial cells. In contrast, Wnt 5a and Wnt 5b are expressed more highly in lymphatic ECFCs. It has previously been shown that Wnt 5a is important for regulation of the differentiation of embryonic stem cells towards endothelial cells (reviewed in Le Bras35). Wnt 5a also promotes endothelial cell proliferation and survival. Interestingly, these Wnt signaling factors are also differentially regulated between mature blood and lymphatic endothelial cells, suggesting they may be important in regulating endothelial cell specificity and should be a focus of future studies into endothelial cell differentiation. We also found that blood and lymphatic ECFCs express different Wnt receptors, frizzled 8 and frizzled 1 and 5, respectively (Supplemental Table I). Thus, Wnt signaling may play an important role in regulating ECFC differentiation and function.
The high-throughput sequencing analysis also identified the Notch signaling pathway as a potential regulator of early endothelial cell differentiation. Notch receptors, and their ligands, have been shown to play important roles in the specification of arterial and venous endothelium.35 During vasculogenesis, arterial endothelial cells express Notch 1 and 4, as well as the ligands Jagged 1, Jagged 2, and DLL4. In contrast, the venous endothelium, which also gives rise to lymphatic endothelium, lacks these markers. Intriguingly, we found that blood-specific ECFCs express the receptor Notch 4, while lymphatic ECFCs express both Notch 3 and Jagged 1. These findings suggest that Notch signaling during development of endothelial precursors could be playing a role in their specification. Due to the rarity of this cell type in the peripheral blood we were unable to analyze the cell surface markers and gene expression prior to plating on a solid matrix in endothelial cell media. Therefore the possibility remains that some of the changes identified occur following plating. However, each cell line has been passaged for months and maintains the specific genotypes indicating that they do not change dramatically during longer passage. Further work is necessary to determine whether and how these pathways are involved in endothelial lineage specification.
Together, the results presented here demonstrate the presence of lymphatic precursor endothelial cells in adult peripheral blood. Additionally, we have screened for differentially expressed genes between these two cell types and have found a large number of genes and pathways that may play a role in endothelial cell differentiation. These cells should prove useful in furthering our understanding of the mechanisms underlying differentiation to blood or lymphatic endothelium, as well as the contribution of circulating endothelial cells to neovascularization.
Supplementary Material
Novelty and Significance.
The identification of circulating endothelial progenitor cells has led to speculation regarding their origin as well as their contribution to neovascular development. Two distinct types of endothelium make up the blood and lymphatic vessel system. However, it has yet to be determined whether there are distinct lymphatic-specific circulating endothelial progenitor cells. Here, we have shown that there are two distinct subsets of ECFCs, blood and lymphatic, which may play distinct roles in post-natal vascularization. There are 1652 differentially expressed genes between blood and lymphatic ECFCS, of which 497 are blood-specific, and 1115 are lymphatic. The identification and characterization of these cells will aid in the understanding of endothelial cell developmental biology as well as neoangiogenesis.
Bullet points.
Circulating lymphatic endothelial colony forming units exist in human blood
Blood and lymphatic ECFCs have differential surface marker and gene expression
Blood and lymphatic ECFCs respond differentially to VEGF-C
ECFCs have distinct gene expression patterns from mature blood and lymphatic ECs
Lymphatic ECFCs may play a role in lymphatic biology and disease
Acknowledgements
None.
Sources of Funding: This research was supported by grant to ML from the NIDCR and the NCI under award numbers PO1DE02195 and R01CA097934 respectively. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- EPC
endothelial progenitor cell
- ECFC
endothelial colony-forming cell
- VEGF
vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors declare no conflict of interest.
References
- 1.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi T, Kalka C, Masuda H, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5(4):434–8. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 3.Le Ricousse-Roussanne S, Barateau V, Contreres JO, Boval B, Kraus-Berthier L, Tobelem G. Ex vivo differentiated endothelial and smooth muscle cells from human cord blood progenitors home to the angiogenic tumor vasculature. Cardiovasc Res. 2004;62(1):176–84. doi: 10.1016/j.cardiores.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 4.Yoder MC, Ingram DA. Endothelial progenitor cell: ongoing controversy for defining these cells and their role in neoangiogenesis in the murine system. Curr Opin Hematol. 2009;16(4):269–73. doi: 10.1097/MOH.0b013e32832bbcab. [DOI] [PubMed] [Google Scholar]
- 5.Mead LE, Prater D, Yoder MC, Ingram DA. Isolation and characterization of endothelial progenitor cells from human blood. Curr Protoc Stem Cell Biol. 2008 doi: 10.1002/9780470151808.sc02c01s6. Chapter 2:Unit 2C.1. [DOI] [PubMed] [Google Scholar]
- 6.Yoder MC, Mead LE, Prater D, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109(5):1801–9. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peichev M, Naiyer AJ, Pereira D, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95(3):952–8. [PubMed] [Google Scholar]
- 8.Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105(1):71–7. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ingram DA, Mead LE, Tanaka H, et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104(9):2752–60. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 10.Gulati R, Jevremovic D, Peterson TE, et al. Diverse origin and function of cells with endothelial phenotype obtained from adult human blood. Circ Res. 2003;93(11):1023–5. doi: 10.1161/01.RES.0000105569.77539.21. [DOI] [PubMed] [Google Scholar]
- 11.Hur J, Yoon CH, Kim HS, et al. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24(2):288–93. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- 12.Case J, Mead LE, Bessler WK, et al. Human CD34+AC133+VEGFR-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Exp Hematol. 2007;35(7):1109–18. doi: 10.1016/j.exphem.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Masuda H, Alev C, Akimaru H, et al. Methodological development of a clonogenic assay to determine endothelial progenitor cell potential. Circ Res. 2011;109(1):20–37. doi: 10.1161/CIRCRESAHA.110.231837. [DOI] [PubMed] [Google Scholar]
- 14.Hong YK, Harvey N, Noh YH, et al. Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev Dyn. 2002;225(3):351–7. doi: 10.1002/dvdy.10163. [DOI] [PubMed] [Google Scholar]
- 15.Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98(6):769–78. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- 16.François M, Caprini A, Hosking B, et al. Sox18 induces development of the lymphatic vasculature in mice. Nature. 2008;456(7222):643–7. doi: 10.1038/nature07391. [DOI] [PubMed] [Google Scholar]
- 17.Religa P, Cao R, Bjorndahl M, Zhou Z, Zhu Z, Cao Y. Presence of bone marrow-derived circulating progenitor endothelial cells in the newly formed lymphatic vessels. Blood. 2005;106(13):4184–90. doi: 10.1182/blood-2005-01-0226. [DOI] [PubMed] [Google Scholar]
- 18.Kerjaschki D, Huttary N, Raab I, et al. Lymphatic endothelial progenitor cells contribute to de novo lymphangiogenesis in human renal transplants. Nat Med. 2006;12(2):230–4. doi: 10.1038/nm1340. [DOI] [PubMed] [Google Scholar]
- 19.Salven P, Mustjoki S, Alitalo R, Alitalo K, Rafii S. VEGFR-3 and CD133 identify a population of CD34+ lymphatic/vascular endothelial precursor cells. Blood. 2003;101(1):168–72. doi: 10.1182/blood-2002-03-0755. [DOI] [PubMed] [Google Scholar]
- 20.Lee JY, Park C, Cho YP, et al. Podoplanin-expressing cells derived from bone marrow play a crucial role in postnatal lymphatic neovascularization. Circulation. 2010;122(14):1413–25. doi: 10.1161/CIRCULATIONAHA.110.941468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan YZ1, Wang HJ, Zhang MH, Quan Z, Li T, He QZ. CD34+ VEGFR-3+ progenitor cells have a potential to differentiate towards lymphatic endothelial cells. J Cell Mol Med. 2014 Mar;18(3):422–33. doi: 10.1111/jcmm.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen VA, Fürhapter C, Obexer P, Stössel H, Romani N, Sepp N. Endothelial cells from cord blood CD133+CD34+ progenitors share phenotypic, functional and gene expression profile similarities with lymphatics. J Cell Mol Med. 2009;13(3):522–34. doi: 10.1111/j.1582-4934.2008.00340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mead LE, Prater D, Yoder MC, Ingram DA. Isolation and characterization of endothelial progenitor cells from human blood. Curr Protoc Stem Cell Biol. 2008 doi: 10.1002/9780470151808.sc02c01s6. Chapter 2:Unit 2C.1. [DOI] [PubMed] [Google Scholar]
- 24.Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25(9):1105–11. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10(3):R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saito R, Smoot ME, Ono K, Ruscheinski J, Wang PL, Lotia S, Pico AR, Bader GD, Ideker T. A travel guide to Cytoscape plugins. Nat Methods. 2012;9(11):1069–76. doi: 10.1038/nmeth.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30(1):207–10. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan SS1, Solomon MA, McCoy JP., Jr. Detection of circulating endothelial cells and endothelial progenitor cells by flow cytometry. Cytometry B Clin Cytom. 2005 Mar;64(1):1–8. doi: 10.1002/cyto.b.20040. [DOI] [PubMed] [Google Scholar]
- 29.Hirakawa S, Hong YK, Harvey N, et al. Identification of vascular lineage-specific genes by transcriptional profiling of isolated blood vascular and lymphatic endothelial cells. Am J Pathol. 2003;162(2):575–86. doi: 10.1016/S0002-9440(10)63851-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaipainen A, Korhonen J, Mustonen T, et al. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci. 1995;92(8):3566–70. doi: 10.1073/pnas.92.8.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tammela T, Zarkada G, Wallgard E, et al. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature. 2008;454(7204):656–60. doi: 10.1038/nature07083. [DOI] [PubMed] [Google Scholar]
- 32.Breiteneder-Geleff S, Soleiman A, Kowalski H, et al. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol. 1999;154(2):385–94. doi: 10.1016/S0002-9440(10)65285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banerji S, Ni J, Wang SX, et al. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144(4):789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dejana E. The Role of Wnt Signaling in Physiological and Pathological Angiogenesis. Circ Res. 2010;107(8):943–52. doi: 10.1161/CIRCRESAHA.110.223750. [DOI] [PubMed] [Google Scholar]
- 35.Le Bras A, Vijayaraj P, Oettgen P. Molecular mechanisms of endothelial differentiation. Vasc Med. 2010;15(4):321–31. doi: 10.1177/1358863X10371685. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.