Abstract
Objective
To test the hypothesis that the lectin-like domain of tumor necrosis factor, mimicked by the TIP peptide, can improve lung function after unilateral orthotopic lung isotransplantation. Because of a lack of a specific treatment for ischemia reperfusion-mediated lung injury, accompanied by a disrupted barrier integrity and a dysfunctional alveolar liquid clearance, alternative therapies restoring these parameters after lung transplantation are required.
Design
Prospective, randomized laboratory investigation.
Setting
University-affiliated laboratory.
Subjects
Adult female rats.
Interventions
Tuberoinfundibular peptide, mimicking the lectin-like domain of tumor necrosis factor, mutant TIP peptide, N,N′-diacetylchitobiose/TIP peptide, and amiloride/TIP peptide were instilled intratracheally in the left lung immediately before the isotransplantation was performed. An additional group received an intravenous TIP peptide treatment, 1.5 mins before transplantation. Studies using isolated rat type II alveolar epithelial cell monolayers and ovine pulmonary endothelial cells were also performed.
Measurements and Main Results
Intratracheal pretreatment of the transplantable left lung with the TIP peptide, but not with an inactive mutant TIP peptide, resulted in significantly improved oxygenation 24 hrs after transplantation. This treatment led to a significantly reduced neutrophil content in the lavage fluid. Both the effects on oxygenation and neutrophil infiltration were inhibited by the epithelial sodium channel blocker amiloride. The TIP peptide blunted reactive oxygen species production in pulmonary artery endothelial cells under hypoxia and reoxygenation and reduced reactive oxygen species content in the transplanted rat lungs in vivo. Ussing chamber experiments using monolayers of primary type II rat pneumocytes indicated that the primary site of action of the peptide was on the apical side of these cells.
Conclusions
These data demonstrate that the TIP peptide significantly improves lung function after lung transplantation in the rat, in part, by reducing neutrophil content and reactive oxygen species generation. These studies suggest that the TIP peptide is a potential therapeutic agent against the ischemia reperfusion injury associated with lung transplantation.
Keywords: tumor necrosis factor, lung transplantation, reperfusion injury, pulmonary edema, rat, sheep, therapeutics
Alveolar liquid clearance from the alveolus into the interstitium is mainly based on active vectorial sodium transport, largely through the highly regulated apical, amiloride-sensitive epithelial sodium channel complex (ENaC) (1), with concomitant passive water transport. The basolaterally expressed, ouabain-inhibitable Na+,K+-adenosine triphosphatase then further drives the vectorial transport into the interstitium and, ultimately, into the lymphatic and blood vessels (2). Because these transport processes can be impaired during acute lung injury (ALI), targeted modulation of Na+ and Cl− transport could represent an effective therapy option. Recently, several studies have demonstrated the usefulness of the β2-adrenergic receptor agonists in this regard (3–6). However, these agents can induce tolerance or unfavorable side effects, such as arrhythmia (7, 8). Furthermore, under conditions of neutrophil-dependent oxidant injury to the alveolar epithelium, as is the case after prolonged hemorrhagic shock in rats, the upregulation of catecholamine-mediated alveolar fluid clearance is blunted (9). Therefore, the search for alternative agents able to activate alveolar liquid clearance, in conditions where there is suboptimal therapeutic benefit with the β2-adrenergic receptor agonists, is clinically important.
A relationship between increased lung epithelial and endothelial permeability and reduced alveolar and lung fluid clearance has been observed in several lung injury models (10, 11), as well as in patients with acute respiratory distress syndrome. Clinically, it has been shown that significant increases in permeability-mediated pulmonary edema may occur in combination with a reduced liquid clearance capacity (12, 13). Submaximal alveolar liquid clearance, combined with ischemia reperfusion (IR) injury in lung transplant patients, is associated with a more prolonged stay in the intensive care unit and longer mechanical ventilation (12). Reactive oxygen species (ROS), the generation of which is increased during IR-mediated ALI (14–16), have been shown to be able both to disrupt pulmonary endothelial barrier integrity (17) and to inhibit ENaC activity (18).
Tumor necrosis factor (TNF) has opposing functions in ALI. TNF receptor signaling can contribute to injury by increasing inflammation. This effect can occur by means of increasing the production of ROS (19), by directly augmenting endothelial permeability (20, 21), and by causing apoptosis of lung microvascular endothelial cells (22–24). Furthermore, TNF was shown to reduce ENaC expression, (25) and in renal cells, this occurs through a TNF receptor 1-induced ceramide-dependent mechanism (26). Conversely, in a rat model of Pseudomonas aeruginosa pneumonia, a TNF-dependent and amiloride-sensitive increase in alveolar fluid resorption was observed (27), most likely mediated by a catecholamine-independent increased sodium uptake by type II pneumocytes (28). Likewise, in a model of intestinal IR and a model of rat bronchial asthma, TNF was shown to stimulate alveolar liquid clearance by an amiloride-sensitive mechanism (29, 30). We and others (31–33) have recently shown that the lectin-like domain of TNF, which is spatially distinct from the receptor-binding sites of the cytokine and which can be mimicked by the 17 amino acid tuberoinfundibular peptide (TIP) (33), increases amiloride-sensitive edema resorption in several rodent models of ex vivo, in situ, and in vivo flooded lungs (34 –36).
Despite the generation of TNF in IR-mediated lung injury, the functional consequences of the lectin-like domain of the cytokine have not been studied in vivo (37). Therefore, the main objective of this study was to assess the effect of the lectin-like domain of TNF, mimicked by the TIP, during IR injury in a unilateral rat lung transplantation model of noncardiogenic ALI. We also investigated whether the action of the TIP in primary rat type II alveolar epithelial cells requires apical or basolateral activation.
Materials and Methods
Cell Culture
Rat alveolar epithelial type II cells were isolated as previously described (38, 39). Cells were isolated by elastase digestion followed by negative selection, using four monoclonal antibodies against cell surface molecules expressed on rat macrophages (CD4/CD32/CD45/RMA) purchased from BD Biosciences-Pharmingen (San Diego, CA). These monoclonal antibodies were preincubated with Dynabeads M-450 (magnetic beads with sheep anti-mouse IgG, Dynal ASA, Oslo, Norway) in 0.1% BSA/PBS. After unbound monoclonal antibodies were removed, rat alveolar epithelial type II cells were mixed with the bead suspension and rocked gently for 30 mins at 4°C. Unbound cells were isolated and plated on polycarbonate Transwells (Corning Costar Co., Cambridge, MA) with a 0.4-μm pore size. Primary cultures of pulmonary arterial endothelial cells were isolated by the explant technique as described previously (40).
Reagents
Amiloride was obtained from Sigma Chemical Company (St. Louis, MO). Cystic fibrosis transmembrane conductance regulator inhibitor (CFTR inh-172), used at 10 μM, was a gift from Dr. Alan S. Verkman (41). Amiloride (Sigma Chemical Company) was used at 0.1 mM. Human TNF TIP and mutant TIP, as acetate salt, were purchased from EMS (Tübingen, Germany). The amino acid sequences were as follows:
Human TIP: CGQRETPEGAEAKPWYC
Human mutant TIP: CGQREAPAGAAAKPWYC;
Both peptides are cyclic through cc oxidation.
Measurement of Hypoxia/Reoxygenation-Induced ROS Production
Sheep pulmonary artery endothelial cells were treated with 50 μg/mL TIP or mutant TIP and were kept in the hypoxia chamber (Coy Labs, Grass Lake, MI) cultured in 0.1% oxygen, 5% CO2, and 94.9% N2. Control conditions were achieved by culturing cells in 21% oxygen, 5% CO2, and 74% N2 in a conventional cell culture incubator. After 90 mins, all of the cells were transferred for 30 mins to a normal cell culture incubator (21% oxygen) and 20 μL of spin-trap stock solution consisting of CMH (20 μM 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine · HCl in Dulbecco's phosphate buffered saline + 25 μM desferrioxamine [Sigma-Aldrich, St. Louis, MO] and 5 μM diethyldithiocarbamate) and 2 μL of dimethyl sulfoxide were added to each well for an additional 30 mins. On completion of incubation, the medium was removed and the adherent cells were trypsinized and pelleted at 500 × g. The cell pellet was washed and suspended in a final volume of 35 μL of Dulbecco's phosphate buffered saline + desferrioxamine and diethyldithiocarbamate, loaded into a 50-μL capillary tube, and analyzed (MiniScope MS200 ESR, Magnettech, Berlin, Germany) at 40-mW microwave power, 3000-mG modulation amplitude, and 100-kHz modulation frequency. Electron paramagnetic resonance (EPR) spectra were analyzed and measured for amplitude, using ANALYSIS version 2.02 software (Magnettech).
Superoxide Quantitation in Lung Tissue
Superoxide levels in the lung tissues of the transplanted control and transplanted TIP-pretreated rats were estimated by EPR assay, using spin-trap compound CMH in the presence and absence of polyethylene glycol-superoxide dismutase (42). Approximately 0.1 g of tissue was sectioned from fresh-frozen lung tissue and immediately immersed, while still frozen, in 200 μL of EPR buffer (PBS supplemented with 5 μM diethyldithiocarbamate [Sigma-Aldrich] and 25 μM desferrioxamine [Sigma-Aldrich]). All samples were then incubated for 30 mins on ice and homogenized for 30 secs with a VWR PowerMAX AHS 200 tissue homogenizer. After incubation, samples were analyzed for protein content, using Bradford analysis (Bio-Rad Laboratories, Hercules, CA). Sample volumes were then adjusted with EPR buffer and 25 mg/mL of CMH hydrochloride to achieve equal protein content and a final CMH concentration of 5 mg/mL. Samples were further incubated for 60 mins on ice and then centrifuged at 14,000 g for 15 mins at room temperature. The supernatant (35 μL) from each sample was loaded into a 50-μL capillary tube and analyzed with a MiniScope MS200 ESR (Magnettech, Berlin, Germany) at a microwave power of 40 mW, modulation amplitude of 3000 mG, and modulation frequency of 100 kHz, with a magnetic strength of 333.95 mT to 3339.94 mT. Resulting EPR spectra were analyzed, using ANALYSIS v.2.02 software (Magnettech), whereby the EPR maximum and minimum spectral amplitudes for the CM · superoxide spin-trap product waveform were quantified.
Cytokine, Chemokine, and Protein Measurements
Rat CINC-3 and rat TNF were determined using the commercially available DuoSet ELISA Kits, which were used according to the manufacturer's instructions (R&D Systems, Wiesbaden, Germany). Similarly, rat interleukin (IL)-10 was determined by ELISA (Biosource Europe SA, Nivelles, Belgium). Protein concentrations were determined using the Pierce BCA protein assay reagent (Thermo Fisher Scientific, Rock-ford, IL).
Measurement of Monolayer Bioelectric Properties
Primary rat alveolar epithelial cells were seeded at a concentration of 1.5 × 106 cells/cm2 in DMEM-H21 medium containing 10% low endotoxin fetal bovine serum, 1% penicillin and streptomycin, and kept at 37°C in a humidified 95% air-5% CO2 environment. Cells were then plated on polycarbonate Transwells (Corning Costar Co., Cambridge, MA) with a 0.4-μm pore size. Twenty-four hours later, nonadherent epithelial cells were removed by washing with PBS, and fresh medium was added to the lower compartments of the Transwells, thus maintaining the alveolar epithelial type II cell monolayers with an air-liquid interface on their apical side. After 72–96 hrs, cells that formed confluent monolayers reaching a transepithelial electrical resistance of >1500 ohms · cm2 were used for experiments. Transepithelial resistance (Rt) (kOhms · cm2) and spontaneous potential difference (mV, apical side as reference) were measured, using the Millicell-ERS (Millipore, Bedford, MA) (43). Transepithelial current (Isc) (μA/cm2) was calculated from the relationship, Isc = spontaneous potential difference/Rt (Ohm's law). The effect of the TIP or the effect of the mutant TIP (both at 10 μg/mL for 1 hr) on bioelectric properties of alveolar epithelial type II cell monolayer was evaluated on day 4 in culture. The TIP or its mutant form was added on both sides of the cell monolayer. In some experiments, the CFTR inh-172 (10 μM), or the ENaC inhibitor amiloride (100 μM) was added together with the TIP.
Animal Experiments
Left-Sided Orthotopic Rat Lung Isotransplantation
Weight-matched (200–250 g) female Wistar rats received orthotopic single left lung isografts. All animals received humane care in compliance with the European Convention of Animal Care. The protocol was approved by the local animals study committee at the University of Konstanz, Germany.
Study Groups
Study groups consisted of: seven vehicle control animals receiving only 500 μL of 0.9% NaCl intratracheally immediately before reimplantation into the left lung; eight animals receiving 125 μg of TIP, intratracheally, four animals receiving 125 μg of TIP plus 100 μM of amiloride intratracheally; four animals receiving 125 μg of TIP plus 625 μg of N,N′-diacetylchitobiose; three animals receiving 125 μg of the inactive mutant of the TIP, “mutant TIP,” intratracheally; three animals receiving 125 μg of TIP IV, into the right ventricle 1.5 mins before reperfusion. Further transplantations were performed in 11 rats, five of which receiving 0.9% NaCl intratracheally as vehicle and six of which receiving 125 μg of TIP to measure ROS. All intratracheal dosing was isovolumetric, using 500 μL of 0.9% NaCl as a vehicle. The dose of 125 μg of TIP was selected as the minimal effective dose, upon performing an initial pilot experiment using four-fold increases in dose.
Donor Procedure
Rats were anesthetized by intraperitoneally administered pentobarbital (50 mg/kg) and heparinized (500 IU/kg). A tracheotomy was carried out, and the rats were ventilated through a 14G cannula (Fio2 = 1.0) by a Unno rodent ventilator (Hugo Sachs Harvard Apparatus, March-Hugstetten, Germany) at a tidal volume of 8 mL/kg. After division of the inferior vena cava and resection of the left appendix of the heart, a small silicon tube was inserted into the main pulmonary artery. Both lungs were flushed with 20 mL of low potassium dextrane solution (Perfadex, provided by Xvivo, Göteborg, Sweden) at a pressure of 20 cm H2O. The trachea was tied at end-inspiration, the heart-lung block was removed, and 16G cuffs were placed around the pulmonary artery and vein (44). The vessels were inverted and tied onto the cuff. The lung was stored in low potassium dextrane solution at 1.5°C until implantation.
Recipient Procedure
Transplantation was performed after 20 hrs of cold ischemia at 1.5°C. The recipient was anesthetized by breathing halothane in a glass chamber followed by intubation. Anesthesia was maintained with 2% halothane. A left lateral thoracotomy was performed in the fourth intercostal space. The left hilum was dissected. After clamping the pulmonary artery and vein with removable microvascular clips, the pulmonary vein and artery were opened and flushed with heparinized saline solution. The intrabronchial instillation of peptides and complexing substances was performed on ice with substance in NaCl 0.9% in a total amount of 500 μL immediately before the lung was implanted (about 25–30 mins before reperfusion). Intravascular delivery of the TIP was performed after implantation at 1.5 mins before reperfusion, as indicated by injection in the right ventricle. Then, the respective cuffs of vein and artery were inserted and fixed with 6-0 Silk. The native left lung was removed, and a running over and over suture with 9-0 Monosof (Tyco Healthcare, Wollerau, Switzerland) for the bronchial anastomosis was carried out. The lung was first reventilated and then reperfused. A chest tube was inserted and the thoracotomy was closed. The chest tube was removed after restoration of spontaneous breathing when the animal was extubated. All animals received a regular analgesia with 35 μL of buprenorphine subcutaneously every 12 hrs.
Statistical Analysis
For normally distributed variables, the mean and sd values are provided, and the mean and sem values are indicated in graphs. To examine differences in the Pao2, a rank transformation was applied to the data due to the small number of observations within each group and a one-way analysis of variance (ANOVA) was conducted on the ranked Pao2 values (Fig. 1). To examine differences in the bronchoalveolar lavage fluid (BALF) polymorphonuclear neutrophilic leukocytes (PMN) content, a rank transformation was applied to the data due to the small number of observations within each group and a one-way ANOVA was conducted on the ranked PMN BALF values (Fig. 2). To examine differences in the relative superoxide production fold change, a rank transformation was applied to the data due to the small number of observations within each group and a two-factor ANOVA was conducted on the ranked relative superoxide production fold change values (Fig. 3B). To examine differences in superoxide between treatments and lung, a repeated-measures ANOVA was performed (Fig. 4). To examine differences in transepithelial current percent of control upon apical treatment of H441 cells, a rank transformation was applied to the data due to the small number of observations within each group and a one-way ANOVA was conducted between the seven groups on the ranked transepithelial current percent of control (Fig. 5A). To examine differences in transepithelial current percent of control upon basolateral treatment of H441 cells, a rank transformation was applied to the data due to the small number of observations within each group, and a two-factor ANOVA was conducted on the ranked transepithelial current percent of control (Fig. 5B). The Kruskal-Wallis test was performed to compare the cytokine and chemokine measurements. The SAS 9.1.3 (Systat, Richmond, CA) was used. p < .05 was considered significant.
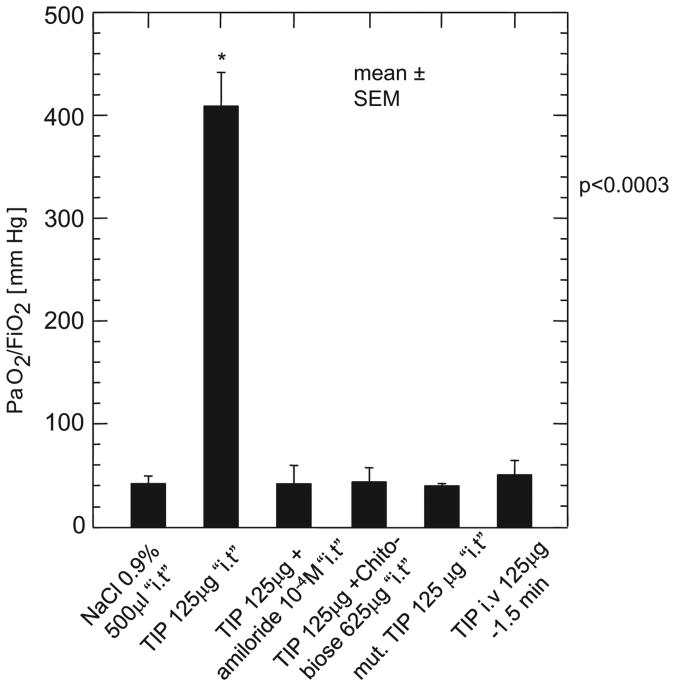
Figure 1.
Oxygenation at 24 hrs after transplantation. At kill, 24 hrs after reperfusion of the left-sided lung transplant, the Pao2/Fio2 ratio was measured after excluding the native right-sided lung by clipping the right-sided stem bronchus and right-sided pulmonary artery. The animals were tracheotomized and ventilated with an Fio2 of 1.0. The TIP peptide significantly increased gas exchange compared with all other study groups. *p < .003 vs. NaCl. Data are mean ± sem. i.t., intratracheally.
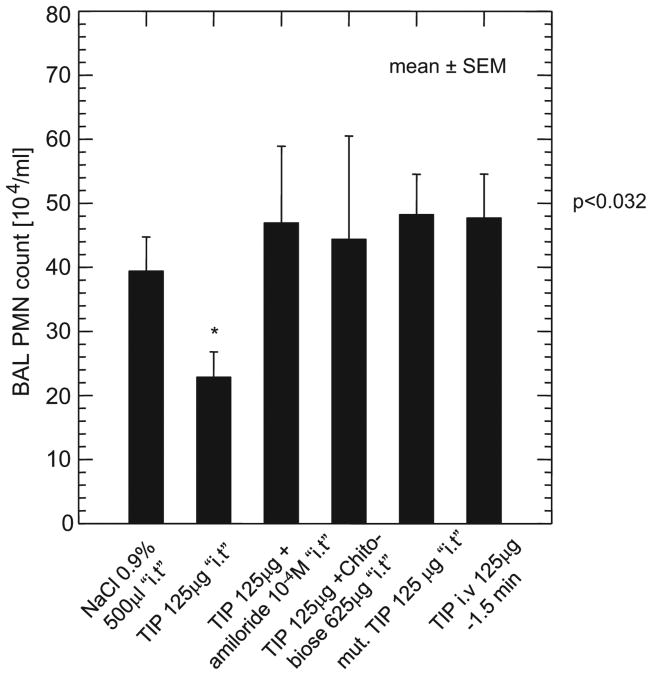
Figure 2.
Bronchoalveolar lavage (BAL) polymorphonuclear neutrophilic leukocytes (PMN) count in the transplanted rats. The TIP peptide intratracheal group had a significantly reduced BAL PMN content compared with all other study groups. *p < .032 vs. NaCl. The absolute PMN count (×104 cells/mL) is given as mean ± sem. i.t., intratracheally.
Figure 3.
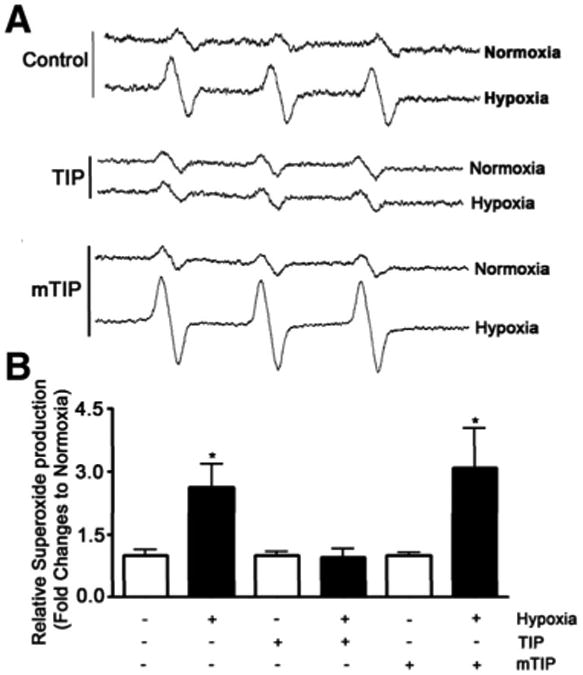
A, Electron paramagnetic resonance waveform spectra of pulmonary artery endothelial cells treated for 120 mins with either saline, TIP peptide (50 μg/mL) or mutant TIP (mTIP) (50 μg/mL) and kept either in normoxia (120 mins) or in hypoxia (90 mins)/reoxygenation (30 mins) conditions; B, intratracheally (i.t.) relative superoxide generation in all groups, as compared with normoxia of control cells. *p < .05 vs. normoxia. Data represent mean ± sem; n = 6.
Figure 4.
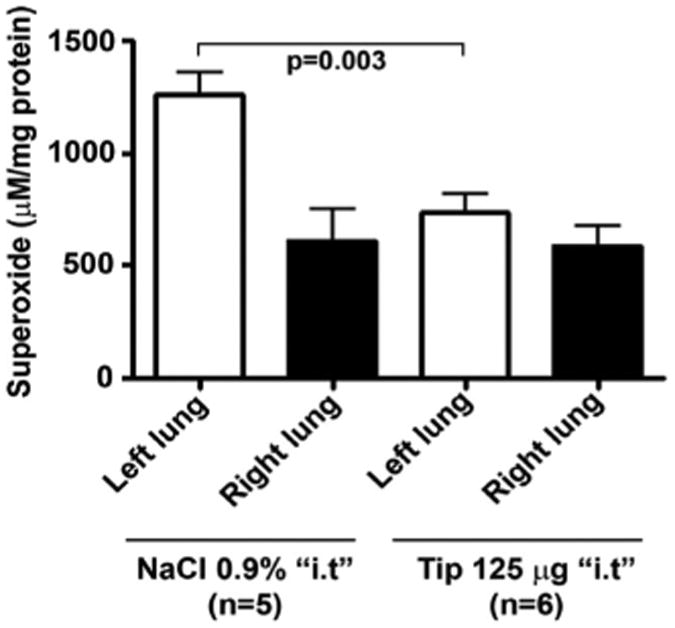
Reactive oxygen species (ROS) generation (assessed by electron paramagnetic resonance measurements in lung homogenates), expressed as μM ROS/mg of protein in transplanted left and corresponding nontransplanted right lungs in control rats treated with 500 μL NaCl i.t. intratracheally (i.t.) (n = 5) vs. TIP peptide-treated rats (n = 6). Lungs were isolated and snap-frozen 24 hrs after transplantation. Data represent mean ± sem.
Figure 5.
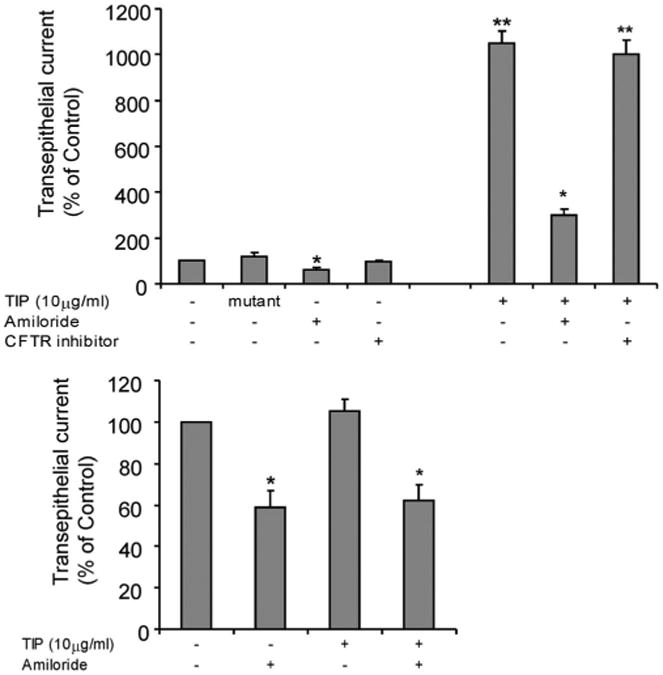
Transepithelial current (Isc) (μA/cm2) in confluent monolayers of primary rat alveolar epithelial type II cells treated for 1 hr with the TIP peptide (10 μg/mL) or the mutant TIP. The TIP or its mutant form was added in either (top) the apical or (bottom) the basolateral side of the cell monolayers. In some experiments, the cystic fibrosis transmembrane conductance regulator (CFTR) inhibitor 172 (10 μM) or the epithelial sodium channel complex inhibitor amiloride (100 μM) was added together with the TIP. *p < .05 vs. control; **p < .005 vs. control. Data are mean ± sem; n = 3.
Results
The TIP Improves Lung Parameters on Orthotopic Left-Sided Rat Isotransplantation
As the main aim of this study, we evaluated the potential role of the TNF-derived TIP in improving lung function in an in vivo setting of IR injury associated with isotransplantation in female Wistar rats (weight, 235 ± 12 g). To assess oxygenation of the transplanted lung, the right bronchus and pulmonary artery were occluded (Fig. 1). As shown in Figure 1, there was a significant difference (p < .0003) in Pao2/Fio2 ratio of the left lung in the group of rats in which 125 μg of TNF TIP was administered, compared with all other study groups. This favorable effect on oxygenation was absent in the rats treated intratracheally with the mutant TIP, in which three amino acids important for the lectin-like activity, i.e., one threonine and two glutamic acids, have been mutated to alanines (33). Interestingly, the beneficial effect of the TIP on oxygenation was completely inhibited by cotreating the animals with 10−4 M amiloride, an inhibitor of the apically expressed ENaC channel, or by pre-exposing the peptide to the oligosaccharide N,N′-diacetylchitobiose (625 μg/lung), which binds to the lectin-like domain of TNF (31–33). Thus, these results indicate that the overall favorable effect of the lectin-like domain of TNF on oxygenation after lung transplantation is mediated by its effect on amiloride-sensitive sodium uptake. Intravascular delivery of the TIP did not provide any improvement in oxygenation (Fig. 1), indicating that the route of application of the peptide is also important to its action.
TIP Reduces PMN Infiltration After Lung Transplantation
In view of the implication of infiltrating PMN in the development of IR-mediated ALI, as well as in the long-term complication bronchiolitis obliterans (45, 46), we have assessed the effect of the TIP on the concentration of these cells in the BALF of the treated rats. The absolute numbers of alveolar macrophages in the BALF did not differ between the study groups (saline group: 30.2 ± 6.7 (n = 7): TIP intratracheal group: 25 ± 13.3 (n = 8); TIP intratracheal + amiloride: 17.8 ± 2.8 (n = 4); TIP intratracheal + N,N′-diacetylchitobiose: 18 ± 8.5 (n = 4); mutant TIP intratracheal: 30.8 ± 5 (n = 3); TIP IV: 30.7 ± 5 (n = 3); all values represent the absolute alveolar macrophage count (×104 cells/mL) as mean ± sd. By contrast, the bronchoalveolar lavage PMN count was significantly decreased in the intratracheally exposed TIP group peptide compared with all other groups (p < .032 vs. control) (Fig. 2). As with the oxygenation, this favorable effect of the TIP was blunted by cotreatment with amiloride (10−4 M) or with the oligosaccharide N,N′-diacetylchitobiose (625 μg/lung) (Fig. 2). There were no significant differences observed among the study groups in BAL content of TNF (median, 4 pg/mL; range, 0–2230 pg/mL), the rat neutrophil chemoattractant and human IL-8 group analogue, CINC-3 (median, 59 pg/mL; range, 5–1433 pg/mL), or the anti-inflammatory cytokine IL-10 (median, 18 pg/mL; range, 0–467 pg/mL) measured at 24 hrs after transplantation. It should be noted, however, that lung tissue concentrations of these cytokines and chemokines may differ from those measured in the BALF and that differences might occur at different time points from the one assessed here.
TIP Reduces Hypoxia-Induced ROS Generation in Ovine Pulmonary Artery Endothelial Cells in Vitro and Blunts IR-Induced ROS Production in Transplanted Rat Lungs in Vivo
As IR-mediated injury of the pulmonary epithelium and endothelium, as well as inhibition of alveolar liquid clearance, is mediated in part by an increased generation of ROS (14–16), we have evaluated, using ovine pulmonary arterial endothelial cells, whether the presence of the TIP is associated with changes in the ROS production in response to hypoxia and reoxygenation. As shown in Figure 3, The TIP, but not the mutant TIP, completely blunted ROS production in sheep pulmonary artery endothelial cells exposed to hypoxia-reoxygenation (0.1% oxygen for 90 mins, then to 21% oxygen for 30 mins). We subsequently investigated whether the TIP at 125 μg/rat intratracheally could also affect ROS generation in transplanted rat lungs. As shown in Figure 4, rat left lung isotransplantation causes a significant increase in ROS generation in the transplanted left, but not in the control right, lung in vehicle-treated lungs, as assessed 24 hrs after transplantation in EPR measurements. Pretreatment of the transplanted lungs with 125 μg of the TIP significantly blunted the increase in ROS generation (Fig. 4). This antioxidant-like effect of the lectin-like domain of TNF therefore might contribute to the improvement in oxygenation observed after lung transplantation.
TIP Initially Activates Apical Sodium Uptake in Rat Type II Pneumocyte Monolayers
Recent data from isolated perfused rabbit lungs suggested that intratracheally instilled TIP primarily activates the basolaterally expressed Na+,K+-adenosine triphosphatase, rather than the apically expressed ENaC (35). We investigated this issue in the rat by comparing the apical vs. basolateral application of the TIP in monolayers of primary rat alveolar type II epithelial cells, assembled in an Ussing Chamber. As shown in Figure 5A, apical application of the TIP, but not of the mutant TIP (both at 10 μg/mL), led to a significantly increased transepithelial current. This effect of the TIP was completely blunted by amiloride (Fig. 5A). Because the apically expressed CFTR regulates ENaC function, we also assessed the effect of the specific CFTR inhibitor, 172 on transepithelial current induced by the TIP. In contrast to amiloride, the inhibition of CFTR did not affect the activity of the TIP (Fig. 5A). In addition, we did not observe any stimulation of transepithelial current on basolateral treatment of the rat type II AEC monolayers (Fig. 5B). Taken together, these results indicate that in the rat, the key ion transporter, or alternatively, the primary receptor affected by the TIP is located at the apical side of the type II alveolar epithelial cells. These data thus suggest an important role for the apically expressed ENaC in the TIP-mediated stimulation of sodium uptake in rat type II alveolar epithelial cells.
Discussion
The primary finding of this study is that the lectin-like domain of TNF, mimicked by the TIP, exerts a favorable effect on organ function in terms of gas exchange and decreased alveolar neutrophil infiltration in the setting of IR injury associated with lung transplantation. Sugita et al. (13) demonstrated that alveolar liquid clearance is decreased in canine lungs at 4 hrs and 8 hrs after transplantation ex vivo and in vivo, and this could not be improved by 10−5 M of the β2-adrenergic receptor agonist terbutaline. Furthermore, in this study, the apically expressed ENaC was found to be decreased at the messenger ribonucleic acid and the protein level in transplanted lungs, suggesting that ENaC, rather than the basolaterally expressed Na+,K+-adenosine triphosphatase, is important in the abnormal alveolar liquid clearance (13). Our data support the role for ENaC as the primary site of action of the TIP in monolayers of primary rat type II alveolar epithelial cells, as the activating effects of the TIP on transepithelial current were limited to the apical side of the cells. These results, thus, suggest that at least in this model, a role for the Na+,K+-adenosine triphosphatase in the action of the TIP, as has been convincingly shown by others in flooded isolated perfused rabbit lungs (36), may be a secondary event to the activation of ENaC and the subsequent increased intracellular sodium concentration. As the study by Sugita and colleagues demonstrated that liquid clearance primarily decreased in the transplanted lung (13), it seems likely that local injury-associated factors may account for this effect.
Our findings also corroborate previous studies that identified a beneficial effect of the lectin-like domain of TNF (31–33) on alveolar edema clearance in flooded lung models in rat, mouse, and rabbit (34–36). In addition, our data in this study as well as our previous published work suggest that the protective effect of the lectin-like domain of TNF is mediated via the activation of Na+ transport. We found that 1) a lectin-deficient mutant of TNF fails to activate both Na+ uptake in the pneumocyte II-like A549 cell line and alveolar liquid clearance in an in situ flooded rat lung model (28); 2) a peptide mimicking this domain, the TIP, efficiently activates alveolar liquid clearance in an ex vivo flooded rat lung and an in situ flooded mouse lung model (34, 35); 3) in an in vivo flooded rat lung model, a complex between TNF and a soluble TNF receptor type 1 construct, stimulated alveolar liquid clearance, a process that was blunted by N,N′-diacetylchitobiose, which binds to the lectin-like domain of TNF (33, 35). Also, in this in vivo flooded rat lung model, the TIP increased alveolar liquid clearance. Taken together, these results indicate that, in flooded lung models, the receptor-binding sites of TNF inhibit, whereas its lectin-like domain activates edema resorption, suggesting a dichotomous role of the cytokine in pulmonary edema resorption (35). These data are limited to the intact lung.
The beneficial effect of the TIP in the lung transplantation model presented here was also accompanied by a decreased PMN concentration in the BALF. These cells are implicated in the important long-term complication of lung transplantation, i.e., bronchiolitis obliterans (46), and furthermore play an important role in complement activation and in the release of mediators, like platelet-activating factor, prostaglandin E2, leukotrienes, ceramide, IL-8 (47, 48), TNF (49), and ROS (14–16). During this process, alveolar epithelial cells and capillary microvascular endothelial cells may undergo apoptotic or necrotic cell death (24). In this context, it is worth noting that an increased generation of the PMN-attractant chemokine, IL-8 in donor lungs, was shown to correlate with graft failure, and that a protective effect toward reperfusion-induced lung injury in rabbits was observed with a monoclonal anti-IL-8 antibody (47, 48). In contrast to the favorable effects of the intratracheal TIP treatment on PMN infiltration, we did not observe a significant effect on alveolar macrophage content or on concentrations of proinflammatory cytokines and chemokines in the BALF 24 hrs after transplantation. It should be noted here that at other time points, differences might be detected between the groups. In any case, the TIP affects specific parameters, such as oxygenation and BALF neutrophil content, during IR-mediated ALI. Whereas the former activity likely implies the activation of sodium uptake by apically expressed ENaC, further studies will be required to unravel the mechanism by which the TIP reduces PMN infiltration. However, because the observed reduction in PMN infiltration in the lungs of TIP-treated animals was abrogated by the intratracheal addition of the ENaC inhibitor amiloride, this suggests that both effects may be linked. In contrast to the intratracheal application, intravascular injection of the TIP 1.5 mins before reperfusion did not improve lung function. Thus, it is conceivable that not only pharmacokinetics or -dynamics, like dosage, binding, or transformation, may influence the peptide's bioactivity, but also the site of action.
It is also worth noting that the effect of the lectin-like domain of TNF likely has physiologic relevance during inflammation and infection. As the soluble TNF receptors are being cleaved by the same enzyme that generates soluble TNF, i.e., the TNF-α converting enzyme (50), complexes between soluble TNF receptors and TNF will form. We have shown that soluble TNF receptors do not inhibit the activity of the lectin-like domain of TNF and that complexes between these receptors and TNF are able to stimulate alveolar liquid clearance in in situ flooded rat lungs (33, 35). At the same time, unfavorable actions of TNF on edema resorption and formation that are mediated by TNF receptor 1 activation are being blocked by the soluble receptors (35). Therefore, the favorable actions of the lectin-like domain of TNF might occur in conditions where both TNF and its soluble receptors are being generated.
Reduced oxidant generation or a respective antioxidant substitution has been shown to restore sodium transport in type II alveolar epithelial cells (18, 51). ROS, apart from influencing ENaC activity and sodium uptake in type II alveolar epithelial cells, can also affect endothelial and epithelial permeability (16, 17). As shown by others (17) and in the data presented herein, exposure of endothelial cells to hypoxia followed by reoxygenation induces the production of ROS, suggesting that endothelium-derived ROS contribute to IR injury. Furthermore, we have shown that the left lung transplantation in rats leads to a significantly increased ROS generation in the transplanted lung 24 hrs after transplantation. It has been shown that ROS can impair expression and organization of the adherens and tight junctional proteins with features similar to those caused by other inflammatory mediators (17).
As the TIP, as demonstrated here for the first time, is able to significantly inhibit ROS generation in endothelial cells on hypoxia-reoxygenation in vitro and on rat lung transplantation in vivo, this may, in part, explain its beneficial effect on oxygenation in the transplanted lungs. Since ROS production is increased during IR injury, this may contribute to both an increased endothelial and epithelial permeability and a reduced lung fluid clearance capacity. By inhibiting ROS generation, the TIP could, on the one hand, preserve ENaC-mediated sodium uptake and, on the other hand, reduce ROS-mediated increases in endothelial permeability. A limitation of our study is that it does not allow discrimination between the effects of the TIP on endothelial and epithelial hyperpermeability, on the one hand, and the effects of the TIP on alveolar liquid clearance, on the other hand. It should be noted, however, that the activation of sodium uptake in alveolar epithelial cells seems to be important for its action in this model, because its protective effects are completely abolished on cotreatment of the lungs with the ENaC inhibitor amiloride.
As therapies that target individual cytokines or other mediators have clinically failed, future therapies aiming at restoring the overall balance of cytokines, oxidants, coagulants, and proteases may ultimately be more successful (52).
We speculate that the TIP may have such therapeutic potential. Interventions like ischemic preconditioning, blocking the pathways leading to ROS generation, and restoring the oxidant/antioxidant balance have been shown to confer protection against liver and lung injuries during IR (16).
Recent results have demonstrated that TNF may be a critical mediator in more inflammatory, immunoregulatory, reparative, or neoplastic processes than previously assumed, which may be of relevance for its therapeutic modification (53, 54). TNF has been shown to increase alveolar fluid resorption in Pseudomonas aeruginosa pneumonia (27) and after intestinal IR injury (29), as well as in an asthma model (30). These studies suggest that the lectin-like domain of TNF may be of physiologic relevance during inflammation or infection. Our data demonstrate that the lectin-like domain of TNF may be a critical mediator in alveolar edema clearance during ALI. In conclusion, we found that the lectin-like domain of TNF, mimicked by the TIP, exerted a protective effect on lung function after transplantation by enhancing gas exchange and decreasing neutrophil content. The magnitude of the effect exerted by the TIP on gas exchange strongly suggests that the lectin-like domain of TNF may be of relevance in case of therapeutic TNF targeting. We therefore suggest that the actions of TNF that are mediated by its lectin-like domain may be beneficial for lung function after lung transplantation.
Acknowledgments
We thank Dr. Michael A. Matthay for critically reading the manuscript.
Supported, in part, by a grant from the Deutsche Forschungsgemeinschaft (FOR 321/2-1; research group “Endogenous tissue injury: Mechanisms of autodestruction”) (JH); the NGFN Network, funded by the German Ministry of Education and Research (HH, TC); and a grant from the Fondation LeDucq (SK, SB).
Footnotes
See also p. 997.
Dr. Lucas holds patents in use of TNF-derived peptides for treating edema. The remaining authors have not disclosed any potential of interest.
References
- 1.Folkesson HG, Matthay MA. Alveolar epithelial ion and fluid transport: Recent progress. Am J Respir Cell Mol Biol. 2006;35:10–19. doi: 10.1165/rcmb.2006-0080SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vadász I, Raviv S, Sznajder JI. Alveolar epithelium and Na, K-ATPase in acute lung injury. Intensive Care Med. 2007;33:1243–1251. doi: 10.1007/s00134-007-0661-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frank JA, Briot R, Lee JW, et al. Physiological and biochemical markers of alveolar epithelial barrier dysfunction in perfused human lungs. Am J Physiol Lung Cell Mol Physiol. 2007;293:L52–L59. doi: 10.1152/ajplung.00256.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiener-Kronish JP, Matthay MA. Beta-2-agonist treatment as a potential therapy for acute inhalational lung injury. Crit Care Med. 2006;34:1841–1842. doi: 10.1097/01.CCM.0000220050.03102.ED. [DOI] [PubMed] [Google Scholar]
- 5.Su X, Robriquet L, Folkesson HG, et al. Protective effect of endogenous beta-adrenergic tone on lung fluid balance in acute bacterial pneumonia in mice. Am J Physiol Lung Cell Mol Physiol. 2006;290:L769–L776. doi: 10.1152/ajplung.00334.2005. [DOI] [PubMed] [Google Scholar]
- 6.Perkins GD, McAuley DF, Thickett DR, et al. The beta-agonist lung injury trial (BALTI): A randomized placebo-controlled clinical trial. Am J Respir Crit Care Med. 2006;173:281–287. doi: 10.1164/rccm.200508-1302OC. [DOI] [PubMed] [Google Scholar]
- 7.Salpeter SR, Ormiston TM, Salpeter EE. Meta-analysis: Respiratory tolerance to regular beta2-agonist use in patients with asthma. Ann Intern Med. 2004;140:802–813. doi: 10.7326/0003-4819-140-10-200405180-00010. [DOI] [PubMed] [Google Scholar]
- 8.Giembycz MA. Phosphodiesterase 4 and tolerance to beta 2-adrenoceptor agonists in asthma. Trends Pharmacol Sci. 1996;17:331–336. [PubMed] [Google Scholar]
- 9.Modelska K, Matthay MA, Brown LA, et al. Inhibition of beta-adrenergic-dependent alveolar epithelial clearance by oxidant mechanisms after hemorrhagic shock. Am J Physiol. 1999;276:L844–L857. doi: 10.1152/ajplung.1999.276.5.L844. [DOI] [PubMed] [Google Scholar]
- 10.Berthiaume Y, Folkesson HG, Matthay MA. Lung edema clearance: 20 years of progress: Invited review: Alveolar edema fluid clearance in the injured lung. J Appl Physiol. 2002;93:2207–2213. doi: 10.1152/japplphysiol.01201.2001. [DOI] [PubMed] [Google Scholar]
- 11.Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163:1376–1383. doi: 10.1164/ajrccm.163.6.2004035. [DOI] [PubMed] [Google Scholar]
- 12.Ware LB, Fang X, Wang Y, et al. Selected contribution: Mechanisms that may stimulate the resolution of alveolar edema in the transplanted human lung. J Appl Physiol. 2002;93:1869–1874. doi: 10.1152/japplphysiol.00252.2002. [DOI] [PubMed] [Google Scholar]
- 13.Sugita M, Ferraro P, Dagenais A, et al. Alveolar liquid clearance and sodium channel expression are decreased in transplanted canine lungs. Am J Respir Crit Care Med. 2003;167:1440–1450. doi: 10.1164/rccm.200204-312OC. [DOI] [PubMed] [Google Scholar]
- 14.Hamvas A, Palazzo R, Kaiser L, et al. Inflammation and oxygen free radical formation during pulmonary ischemia- reperfusion injury. J Appl Physiol. 1992;72:621–628. doi: 10.1152/jappl.1992.72.2.621. [DOI] [PubMed] [Google Scholar]
- 15.Ischiropoulos H, al-Mehdi AB, Fisher AB. Reactive species in ischemic rat lung injury: Contribution of peroxynitrite. Am J Physiol. 1995;269:L158–L164. doi: 10.1152/ajplung.1995.269.2.L158. [DOI] [PubMed] [Google Scholar]
- 16.Peralta C, Bulbena O, Xaus C, et al. Ischemic preconditioning: A defense mechanism against the reactive oxygen species generated after hepatic ischemia reperfusion. Transplantation. 2002;73:1203–1211. doi: 10.1097/00007890-200204270-00004. [DOI] [PubMed] [Google Scholar]
- 17.Lum H, Roebuck KA. Oxidant stress and endothelial cell dysfunction. Am J Physiol Cell Physiol. 2001;280:C719–C741. doi: 10.1152/ajpcell.2001.280.4.C719. [DOI] [PubMed] [Google Scholar]
- 18.Guo Y, DuVall MD, Crow JP, et al. Nitric oxide inhibits Na+ absorption across cultured alveolar type II monolayers. Am J Physiol. 1998;274:L369–L377. doi: 10.1152/ajplung.1998.274.3.L369. [DOI] [PubMed] [Google Scholar]
- 19.Faggioni R, Gatti S, Demitri MT, et al. Role of xanthine oxidase and reactive oxygen intermediates in LPS- and TNF-induced pulmonary edema. J Lab Clin Med. 1994;123:394–399. [PubMed] [Google Scholar]
- 20.Petrache I, Birukova A, Ramirez SI, et al. The role of the microtubules in tumor necrosis factor-alpha-induced endothelial cell permeability. Am J Respir Cell Mol Biol. 2003;28:574–581. doi: 10.1165/rcmb.2002-0075OC. [DOI] [PubMed] [Google Scholar]
- 21.Koss M, Pfeiffer GR, 2nd, Wang Y, et al. Ezrin/radixin/moesin proteins are phosphorylated by TNF-alpha and modulate permeability increases in human pulmonary microvascular endothelial cells. J Immunol. 2006;176:1218–1227. doi: 10.4049/jimmunol.176.2.1218. [DOI] [PubMed] [Google Scholar]
- 22.Lucas R, Garcia I, Donati YR, et al. Both TNF receptors are required for direct TNF-mediated cytotoxicity in microvascular endothelial cells. Eur J Immunol. 1998;28:3577–3586. doi: 10.1002/(SICI)1521-4141(199811)28:11<3577::AID-IMMU3577>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 23.Hamacher J, Lucas R, Lijnen HR, et al. Tumor necrosis factor-alpha and angiostatin are mediators of endothelial cytotoxicity in bronchoalveolar lavages of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2002;166:651–656. doi: 10.1164/rccm.2109004. [DOI] [PubMed] [Google Scholar]
- 24.Stammberger U, Gaspert A, Hillinger S, et al. Apoptosis induced by ischemia and reperfusion in experimental lung transplantation. Ann Thorac Surg. 2000;69:1532–1536. doi: 10.1016/s0003-4975(00)01228-5. [DOI] [PubMed] [Google Scholar]
- 25.Dagenais A, Fréchette R, Yamagata T, et al. Downregulation of ENaC activity and expression by TNF-alpha in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2004;286:L301–L311. doi: 10.1152/ajplung.00326.2002. [DOI] [PubMed] [Google Scholar]
- 26.Bao HF, Zhang ZR, Liang YY, et al. Ceramide mediates inhibition of the renal epithelial sodium channel by tumor necrosis factor-alpha through protein kinase C. Am J Physiol Renal Physiol. 2007;293:F1178–F1186. doi: 10.1152/ajprenal.00153.2007. [DOI] [PubMed] [Google Scholar]
- 27.Rezaiguia S, Garat C, Delclaux C, et al. Acute bacterial pneumonia in rats increases alveolar epithelial fluid clearance by a tumor necrosis factor-alpha-dependent mechanism. J Clin Invest. 1997;99:325–335. doi: 10.1172/JCI119161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fukuda N, Jayr C, Lazrak A, et al. Mechanisms of TNF-alpha stimulation of amiloride-sensitive sodium transport across alveolar epithelium. Am J Physiol Lung Cell Mol Physiol. 2001;280:L1258–L1265. doi: 10.1152/ajplung.2001.280.6.L1258. [DOI] [PubMed] [Google Scholar]
- 29.Börjesson A, Norlin A, Wang X, et al. TNF-alpha stimulates alveolar liquid clearance during intestinal ischemia-reperfusion in rats. Am J Physiol Lung Cell Mol Physiol. 2000;278:L3–L12. doi: 10.1152/ajplung.2000.278.1.L3. [DOI] [PubMed] [Google Scholar]
- 30.Tillie-Leblond I, Guery BP, Janin A, et al. Chronic bronchial allergic inflammation increases alveolar liquid clearance by TNF-alpha-dependent mechanism. Am J Physiol Lung Cell Mol Physiol. 2002;283:L1303–L1309. doi: 10.1152/ajplung.00147.2002. [DOI] [PubMed] [Google Scholar]
- 31.Hession C, Decker JM, Sherblom AP, et al. Uromodulin (Tamm-Horsfall glycoprotein): A renal ligand for lymphokines. Science. 1987;237:1479–1484. doi: 10.1126/science.3498215. [DOI] [PubMed] [Google Scholar]
- 32.Sherblom AP, Decker JM, Muchmore AV. The lectin-like interaction between recombinant tumor necrosis factor and uromodulin. J Biol Chem. 1988;263:5418–5424. [PubMed] [Google Scholar]
- 33.Lucas R, Magez S, De Leys R, et al. Mapping the lectin-like activity of tumor necrosis factor. Science. 1994;263:814–817. doi: 10.1126/science.8303299. [DOI] [PubMed] [Google Scholar]
- 34.Elia N, Taponnier M, Matthay MA, et al. Functional identification of the alveolar edema reabsorption activity of murine tumor necrosis factor. Am J Respir Crit Care Med. 2003;168:1043–1050. doi: 10.1164/rccm.200206-618OC. [DOI] [PubMed] [Google Scholar]
- 35.Braun C, Hamacher J, Morel DR, et al. Dichotomal role of TNF in experimental pulmonary edema reabsorption. J Immunol. 2005;175:3402–3408. doi: 10.4049/jimmunol.175.5.3402. [DOI] [PubMed] [Google Scholar]
- 36.Vadász I, Schermuly RT, Ghofrani HA, et al. The lectin-like domain of tumor necrosis factor-alpha improves alveolar fluid balance in injured isolated rabbit lungs. Crit Care Med. 2008;36:1543–1550. doi: 10.1097/CCM.0b013e31816f485e. [DOI] [PubMed] [Google Scholar]
- 37.Berthiaume Y. Tumor necrosis factor and lung edema clearance: The tip of the iceberg? Am J Respir Crit Care Med. 2003;168:1022–1023. doi: 10.1164/rccm.2308003. [DOI] [PubMed] [Google Scholar]
- 38.Dobbs LG, Gonzalez R, Williams MC. An improved method for isolating type II cells in high yield and purity. Am Rev Respir Dis. 1986;134:141–145. doi: 10.1164/arrd.1986.134.1.141. [DOI] [PubMed] [Google Scholar]
- 39.Dobbs LG. Isolation and culture of alveolar type II cells. Am J Physiol. 1990;258:L134–L147. doi: 10.1152/ajplung.1990.258.4.L134. [DOI] [PubMed] [Google Scholar]
- 40.Wedgwood S, Mitchell CJ, Fineman JR, et al. Developmental differences in the shear stress-induced expression of endothelial NO synthase: Changing role of AP-1. Am J Physiol Lung Cell Mol Physiol. 2003;284:L650–L662. doi: 10.1152/ajplung.00252.2002. [DOI] [PubMed] [Google Scholar]
- 41.Ma T, Thiagarajah JR, Yang H, et al. Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion. J Clin Invest. 2002;110:1651–1658. doi: 10.1172/JCI16112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wiseman DA, Wells SM, Hubbard M, et al. Alterations in zinc homeostasis underlie endothelial cell death induced by oxidative stress from acute exposure to hydrogen peroxide. Am J Physiol Lung Cell Mol Physiol. 2007;292:L165–L177. doi: 10.1152/ajplung.00459.2005. [DOI] [PubMed] [Google Scholar]
- 43.Roux J, Kawakatsu H, Gartland B, et al. Interleukin-1beta decreases expression of the epithelial sodium channel alpha-subunit in alveolar epithelial cells via a p38 MAPK-dependent signaling pathway. J Biol Chem. 2005;280:18579–18589. doi: 10.1074/jbc.M410561200. [DOI] [PubMed] [Google Scholar]
- 44.Stammberger U, Hamacher J, Hillinger S, et al. sCR1sLeX ameliorates ischemia/reperfusion injury in experimental lung transplantation. J Thorac Cardiovasc Surg. 2000;120:1078–1084. doi: 10.1067/mtc.2000.111175. [DOI] [PubMed] [Google Scholar]
- 45.Martinez-Mier G, Toledo-Pereyra LH, McDuffie JE, et al. Neutrophil depletion and chemokine response after liver ischemia and reperfusion. J Invest Surg. 2001;14:99–107. doi: 10.1080/08941930152024228. [DOI] [PubMed] [Google Scholar]
- 46.Elssner A, Vogelmeier C. The role of neutrophils in the pathogenesis of obliterative bronchiolitis after lung transplantation. Transpl Infect Dis. 2001;3:168–176. doi: 10.1034/j.1399-3062.2001.003003168.x. [DOI] [PubMed] [Google Scholar]
- 47.Sekido N, Mukaida N, Harada A, et al. Prevention of lung reperfusion injury in rabbits by a monoclonal antibody against interleukin-8. Nature. 1993;365:654–657. doi: 10.1038/365654a0. [DOI] [PubMed] [Google Scholar]
- 48.Fisher AJ, Donnelly SC, Hirani N, et al. Elevated levels of interleukin-8 in donor lungs is associated with early graft failure after lung transplantation. Am J Respir Crit Care Med. 2001;163:259–265. doi: 10.1164/ajrccm.163.1.2005093. [DOI] [PubMed] [Google Scholar]
- 49.DeMeester SR, Rolfe MW, Kunkel SL, et al. The bimodal expression of tumor necrosis factor-alpha in association with rat lung reimplantation and allograft rejection. J Immunol. 1993;150:2494–2505. [PubMed] [Google Scholar]
- 50.Peschon JJ, Slack JL, Reddy P, et al. An essential role for ectodomain shedding in mammalian development. Science. 1998;282:1281–1284. doi: 10.1126/science.282.5392.1281. [DOI] [PubMed] [Google Scholar]
- 51.Pittet JF, Lu LN, Morris DG, et al. Reactive nitrogen species inhibit alveolar epithelial fluid transport after hemorrhagic shock in rats. J Immunol. 2001;166:6301–6310. doi: 10.4049/jimmunol.166.10.6301. [DOI] [PubMed] [Google Scholar]
- 52.Ware LB. Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Semin Respir Crit Care Med. 2006;27:337–349. doi: 10.1055/s-2006-948288. [DOI] [PubMed] [Google Scholar]
- 53.Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345:1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 54.Lees CW, Ironside J, Wallace WA, et al. Resolution of non-small-cell lung cancer after withdrawal of anti-TNF therapy. N Engl J Med. 2008;359:320–321. doi: 10.1056/NEJMc0800250. [DOI] [PubMed] [Google Scholar]