Abstract
Satellite cells play crucial roles in mediating the growth, maintenance, and repair of postnatal skeletal muscle. Activated satellite cells (myoblasts) can divide symmetrically or asymmetrically to generate progenies that self-renewal, proliferate or differentiate. Pax7 is a defining marker of quiescent and activated satellite cells, but not differentiated myoblast. We demonstrate here that deletion of Lkb1 upregulates Pax7 expression in myoblasts and inhibits asymmetric divisions that generate differentiating progenies. Furthermore, we find that Lkb1 activates the Notch signaling pathway, which subsequently increases Pax7 expression and promotes self-renewal and proliferation while inhibiting differentiation. Mechanistic studies reveal that Lkb1 regulates Notch1 activation through AMPK-mTOR pathway in myoblasts. Together, these results establish a key role of Lkb1 in regulating myoblast division and cell fates choices.
Keywords: AMPK, Liver kinase b1 (Lkb1), Myoblast, mTOR, Notch1, Pax7, Serine/threonine kinase 11 (Stk11)
1. Introduction
Satellite cells, a mixed population of committed myogenic progenitors and non-committed stem cells, play key roles in mediating the growth, maintenance, and regeneration of postnatal skeletal muscle (Collins et al., 2005; Kuang et al., 2007). In adult resting muscles, satellite cells reside beneath the basal lamina and are predominantly in quiescent stage (Brack and Rando, 2012; Liu et al., 2012). Upon muscle degeneration or injury, quiescent satellite cells are activated to repair injured muscles (Kuang and Rudnicki, 2008). Activated satellite cells (myoblasts) divide to generate different progenies that can be distinguished by the expression of two transcription factors Pax7 and MyoD: Pax7+MyoD− (self-renewal), Pax7+MyoD+ (proliferation) and Pax7−MyoD+ (differentiation) (Liu et al., 2012; Zammit et al., 2004). Many signaling pathways and factors, such as Wnt (Brack et al., 2007), Notch1 (Bjornson et al., 2012; Conboy and Rando, 2002; Kuang et al., 2008; Mourikis et al., 2012), interleukin (IL)-6 (Serrano et al., 2008), and insulin-like growth factor 1 (IGF1) (Musaro et al., 2004) have been shown to affect the fate decision of satellite cells.
Liver kinase B1 (Lkb1), also called Serine/Threonine protein kinase 11 (STK11), plays key roles in several cellular processes including cellular polarity (Granot et al., 2009), cell adhesion (Zagorska et al., 2010), cell proliferation (Shan et al., 2014; Waters et al., 2015) and cell energy metabolism (Nakada et al., 2010). In adult β cells, deletion of Lkb1 regulates pancreatic β cell size, polarity, and enhances glucose tolerance in mice (Fu et al., 2009; Granot et al., 2009). Lkb1 can regulate the proliferation of T-cell progenitors and affect functions of the mature T-cell (Zagorska et al., 2010). In hematopoietic stem cells, Lkb1 affects cell survival, regulates quiescence, cell cycle and energy metabolism (Gan et al., 2010; Krock et al., 2011; Nakada et al., 2010; Zagorska et al., 2010). In muscle progenitors, our recent study found that Lkb1 null satellite cells exhibit accelerated proliferation but reduced differentiation (Shan et al., 2014). Moreover, lack of Lkb1 inhibits myogenic differentiation but increased lipid accumulation and enhanced expression of lipogenic genes in myoblasts (Shan et al., 2015). Although these studies indicated that Lkb1 play important roles in muscle stem cells (Shan et al., 2015; Shan et al., 2014), the functions of Lkb1 in division of muscle progenitor cells are unknown. Furthermore, the molecular mechanism through which Lkb1 regulates muscle stem cell fates and Pax7 expression is not clear.
Therefore, in this study, we isolated and cultured primary myoblasts from WT and Lkb1 knockout mice. We found that deletion of Lkb1 promotes self-renewal and proliferation division but inhibits differentiation division in vitro. Moreover, we found that Lkb1 deficiency upregulates Pax7 expression through upregulating Notch1 signaling pathway. Furthermore, we revealed that Lkb1 activates Notch1 pathway, at least partly through AMPK-mTOR pathway. Together, these results demonstrated that Lkb1 plays a critical role in regulating cell fate choice and function of muscle progenitors.
2. Material and methods
2.1. Animals
All procedures involving mice were guided by Purdue University Animal Care and Use Committee. The MyoDCre (stock number 014140) and Lkb1flox/flox (stock number 014143) mouse strains were bought from Jackson Laboratory (Bar Harbor, ME). The Lkb1deletion mice (MyoD-Lkb1) were generated as previous described (Shan et al., 2014). Mice were housed in the animal facility with free access to standard rodent chow and water.
2.2. Myoblast isolation and culture
Primary myoblasts were isolated from WT and Myod-Lkb1 mice by using type I collagenase and dispase B digestion (Shan et al., 2014). Then the cells were seeded on collagen-coated dishes and cultured in growth medium containing F-10 Ham's medium, with 20% fetal bovine serum (FBS), 4 ng/mL basic fibroblast growth factor, and 1% penicillin–streptomycin at 37 °C with 5% CO2. The medium was changed every 2 days.
2.3. Myoblast division and differentiation assay
For myoblast division assay, the WT and Lkb1 deficiency myoblasts were seeded and cultured at very low density for 24 h, and labeled the cells with Pax7 and MyoD. Then the myoblast division was determined as previous described (Liu et al., 2012). For myoblast differentiation, WT and MyoD-Lkb1 myoblasts were seeded onto 6-well plates (1×105 cells per well) and induced with 2% horse serum for 3 days after confluence. Then cultures were collected for RNA, protein and immunostained for myosin heavy chain (MF20) and Pax7 as described previously (Shan et al., 2014). The differentiation indexes were calculated as the ratio of the nuclei within myotubes and MF20 positive mononucleated cells (Shan et al., 2014). In addition, the percentage of undifferentiated Pax7 positive cells was determined as reserve index (Shan et al., 2014).
2.4. Total RNA extraction, cDNA synthesis and real-time PCR
Total RNA was extracted from cells using Trizol Reagent according to the manufacturer's instructions. The purity and concentration of total RNA were measured by Nano drop 3000 (Thermo Fisher) at 260 nm and 280 nm. Then 5 μg of total RNA were reversed transcribed and real-time PCR were performed as described (Shan et al., 2014). The 2-ΔΔCT method was used to analyze the relative changes in gene expression normalized against 18S rRNA as internal control.
2.5. Protein Extraction and Western Blot Analysis
Protein extraction and western blot were conducted as previously described (Shan et al., 2014). Briefly, total protein was isolated from cells using RIPA buffer. Protein concentrations were determined using Pierce BCA Protein Assay Reagent (Pierce Biotechnology). Proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore Corporation) and detect with specific antibodies. Phospho-S6 (p-S6) and S6 antibodies were from Cell signaling, MF20, Pax7 and MyoG (F5D) were from Developmental Studies Hybridoma Bank (DSHB), all other antibodies (MyoD, pAMPK, AMPK, and GAPDH) were from Santa Cruz Biotechnology (Santa Cruz). Secondary antibodies (anti-rabbit IgG or anti-mouse IgG, Jackson ImmunoResearch) were diluted 8,000-fold. Immunodetection was performed using enhanced chemiluminescence (ECL) Western blotting substrate (Pierce Biotechnology) and detected with a Gel Logic 2200 imaging system (Carestream).
2.6. Data Analysis
All experimental data are presented as means ± SEM. Comparisons were made by unpaired two-tailed Student's t-tests or one-way ANOVA, as appropriate. Effects were considered significant at P < 0.05.
3. Results
3.1. Deletion of Lkb1 promotes asymmetric self-renewal division but inhibits asymmetric differentiation division of myoblasts
Our previous studies found that deletion of Lkb1 may affect muscle stem cell fates (Shan et al., 2014). To examine whether Lkb1 deficiency affects the muscle satellite cell division, we isolated and cultured primary myoblasts from WT and MyoD-Lkb1 mice at low density to allow analysis of daughter cells divided from single cells. Consistent with our previous study (Liu et al., 2012), we found that the doublets of the sister cells are predominantly Pax7+ MyoD+/Pax7+ MyoD- (asymmetric self-renewal division), Pax7+ MyoD+/Pax7+ MyoD+ (symmetric proliferation division) or Pax7+ MyoD+/Pax7- MyoD+ (asymmetric differentiation division) (Fig. 1A). Notably, Lkb1 deletion significantly increased the asymmetric self-renewal division (17.4% MyoD-Lkb1 versus 11.5% WT) and symmetric proliferation division (71.8% MyoD-Lkb1 versus 60.4% WT) (Fig. 1B). However, the asymmetric differentiation division was dramatically inhibited (10.8% MyoD-Lkb1 versus 28.1% WT) (Fig. 1B). Consistently, MyoD-Lkb1 myoblasts expressed higher level of the myogenic progenitor marker gene Pax7 and lower level of the myogenic differentiation marker gene Myog (Fig. 2A, B). Moreover, quantitative real time PCR results showed that the mRNA levels of the myoblasts differentiation related genes including myocyte enhancer factor 2C (Mef2c), caveolin 3 (Cav3), cadherin 15 (M-cadherin; Cadh15) and calpain 1 (Capn1) were significantly decreased in the Lkb1-null myoblast (Fig. 2A). Combined with our previous results (Shan et al., 2014), these data indicate that deletion of Lkb1 upregulates Pax7 expression and affects muscle progenitor cell fate decision by inhibiting differentiation division but promotes self-renew and proliferation.
Figure 1. Lkb1 deficiency promotes asymmetric self-renewal division and inhibits asymmetric differentiation division in cultured myoblasts.
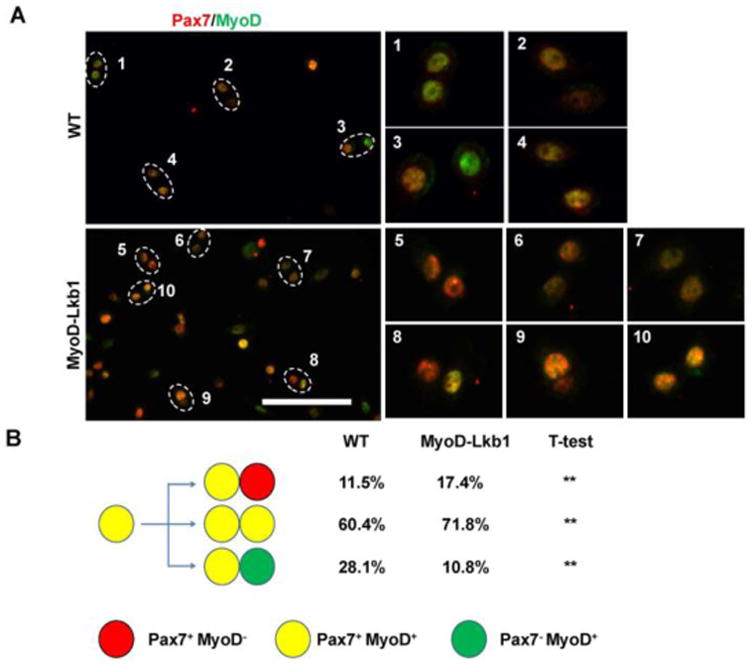
(A) Primary myoblasts cultured at low density and with synchronized cell cycle were stained for Pax7 (red) and MyoD (green). Doublets of Pax7+MyoD+:Pax7+MyoD− sister cells (5, 8, 9) represent asymmetric self-renewal division; Pax7+MyoD+:Pax7+MyoD+(2, 4, 6,7,10) represent symmetric proliferative division; and Pax7+MyoD+:Pax7−MyoD+ (1, 3, 7) represent asymmetric differentiation division. Scale bar: 100 μm. (B) Percentages of asymmetric self-renewal, symmetric proliferative and asymmetric differentiation divisions in the WT and Lkb1 KO myoblasts. n=3 independent experiments, with at least 40 doublets analyzed in each experiment. **P<0.01.
Figure 2. Lkb1 deficiency increases the expression of Pax7 and activities Notch1 signaling pathway in myoblasts.
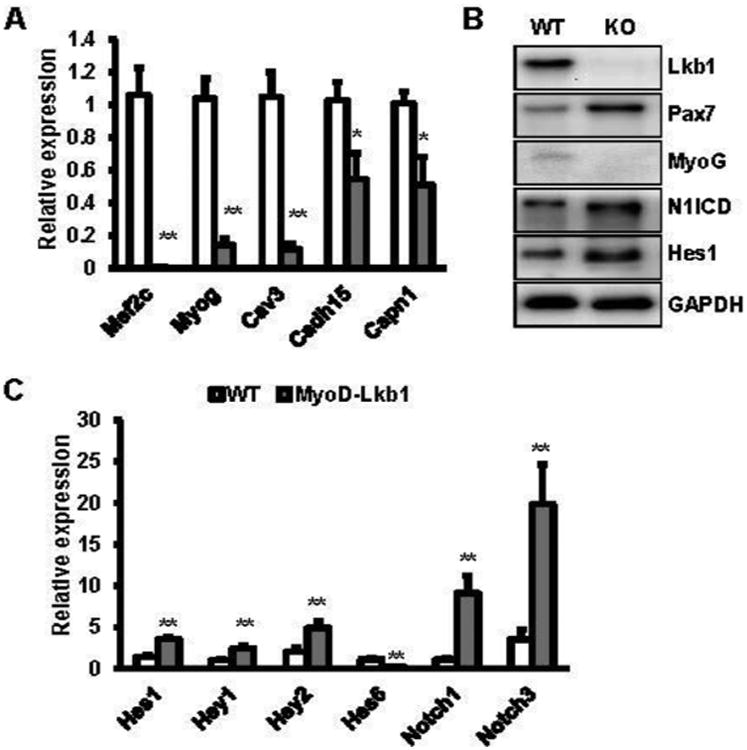
(A) The expression of myogenic differentiation related genes in WT and MyoD-Lkb1 myoblasts. (B) Protein levels of Pax7, MyoG, N1ICD and Hes1 in WT and MyoD-Lkb1 myoblasts. (C) mRNA levels of Notch1 and its target genes in WT and MyoD-Lkb1 myoblasts. Error bars represent SEM, n=6. *P<0.05, **P<0.01
3.2. Lkb1 deficiency activities Notch1 signaling pathway
Previous studies demonstrated that Notch1 signaling pathway regulates Pax7 expression and muscle satellite cell fate (Liu et al., 2012; Wen et al., 2012). Therefore, we examined the effects of Lkb1 deletion on Notch1 signaling pathway. Notably, higher levels of cleaved Notch1 intracellular domain (N1ICD) were found in Lkb1 knockout myoblast compared to the WT myoblasts (Fig. 2B). The protein levels of Notch1 target gene Hes1 was also dramatically increased after deletion of Lkb1 in myoblasts (Fig. 2B). Furthermore, real time PCR results showed that deletion of Lkb1 significantly increased the mRNA levels of Notch1 and its target genes including Hes1, Hey1 and Hey2 (Fig. 2C). In addition, higher level of Notch3 was found in Lkb1 deletion cells (Fig. 2C). Taken together, these results indicate that Lkb1 deficiency activates notch1 signaling pathway in primary myoblasts.
3.3. Lkb1 regulates Pax7 expression and myoblast division through Notch1 signaling pathway
As Lkb1 deficiency upregulates the expression of N1ICD and Notch1 target genes, we examined whether Notch1 signaling pathway are necessary to mediate the effects of Lkb1 on pax7 expression and myoblast division. We first checked the effects of Notch1 on myogenic genes expression in primary myoblasts. We isolated and cultured primary myoblasts from Rosa26-N1ICD mice (Liu et al., 2012), in which N1ICD overexpression can be induced by adenovirus-Cre. Indeed, we found the expression of the Notch1 target genes Hes1 and Hey1 was dramatically increased after adenovirus-Cre infection (Fig. 3A, B). N1ICD overexpression significantly increased Pax7 expression but decreased Myog expression in myoblasts (Fig. 3A, B). By contrast, adenovirus-Cre mediated knockout of Notch1 resulted in reduction of Hes1, Hey1 and Pax7, while upregulation the expression of Myog and embryonic myosin heavy chain (eMHC) (Fig. 3C). Furthermore, treatment with N-[N-(3,5-difluorophenacetyl-L-alanyl)]-S-phenylglycine t-butyl ester (DAPT), an inhibitor of Notch1 signaling pathway, significantly decreased the expression of Hes1, Hey1 and Pax7, but increased Myog expression (Fig. 3 D, E). These results indicate that Notch1 signaling pathway affects Pax7 and Myog expression.
Figure 3. Lkb1 regulates Pax7 expression and myoblast division through Notch signaling pathway.
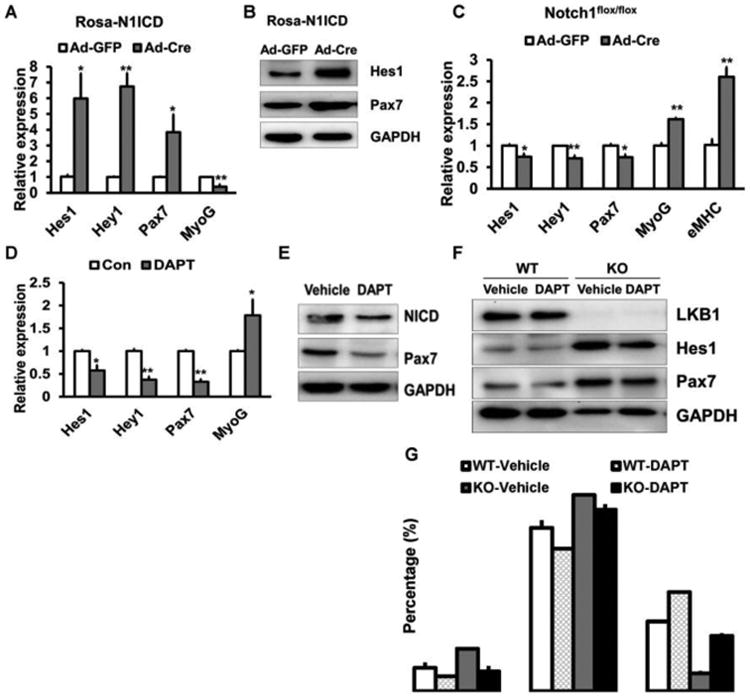
(A, B) The mRNA (A, n=5) and the protein (B) levels of Pax7 in Rosa26-N1ICD myoblasts infected with adenovirus-GFP or adenovirus-Cre.(C) Expression of Pax7 in Notch1flox/flox myoblasts infected with adenovirus-GFP or adenovirus-Cre (n=6). (D, E) Relative mRNA (D, n=6) and protein (E) levels of Pax7 in WT myoblasts treated with Notch inhibitor DAPT. (F) Protein levels of Pax7 in WT and MyoD-Lkb1 myoblasts treated with or without DAPT. (G) Percentages of self-renewal, proliferative and differentiation divisions in the WT and Lkb1 KO myoblasts treated with or without DAPT. n=3 independent experiments, with at least 50 doublets analyzed in each experiment. Error bars represent SEM, *P<0.05, **P<0.01
Next, we investigated whether inhibition of Notch1 signaling pathway can rescue the defects in differentiation division of Lkb1 deficiency myoblast. Western blot results showed that DAPT treatment decreased the protein levels of Hes1 and Pax7 in WT and Lkb1 knockout myoblasts (Fig. 3 F). In addition, the differentiation division of Lkb1 deficiency myoblasts was dramatically increased after DAPT treatment (Fig. 3 G). These results suggest that inhibition of Notch1 signaling pathway can rescue the differentiation division of Lkb1 deficient myoblasts.
To further confirm whether inhibition of Notch1 could rescue the differentiation capacity of Lkb1 deficient myoblasts, we treated and differentiated the WT and Lkb1 knockout myoblasts. Imuunostaining results indicated that inhibition of Notch signaling pathway by DAPT promoted formation of myotubes and decreased the Pax7 positive cells (reserve cells) in WT and Lkb1-KO myoblasts (Fig. 4A). Consistently, quantitative results indicated that DAPT treatment increased the differentiation index while significantly decreased the Pax7+ cells and the reserve index (Fig. 4A, B). Furthermore, western blot and real time PCR results further confirmed that inhibition of Notch pathway dramatically decreased Pax7 expression and rescued the differentiation marker genes expression (Fig. 4C, D). These results suggested that Notch signaling pathway mediates the effects of Lkb1 deletion on Pax7 expression and myoblast division.
Figure 4. Inhibition of Notch signaling pathway affects the myogenic differentiation of WT and MyoD-Lkb1 myoblasts.
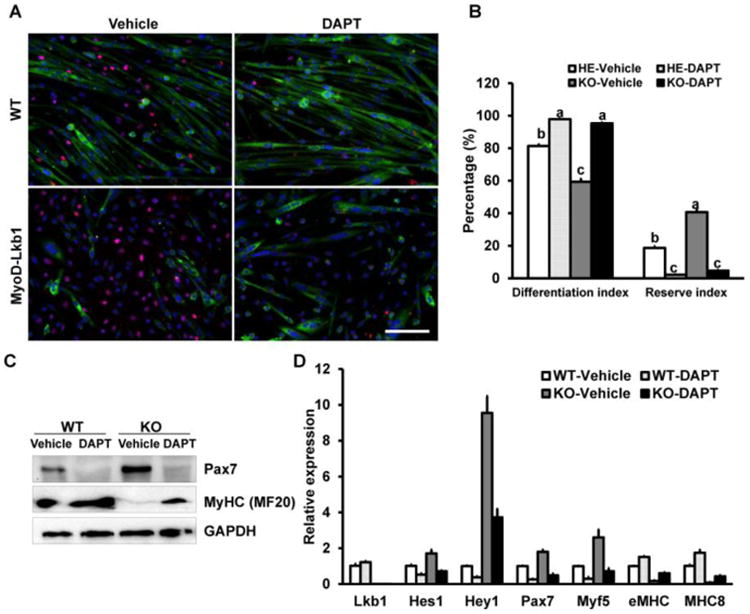
(A) MF20 (Green) and Pax7 (Red) staining showing differentiation efficiency of WT and MyoD-Lkb1 myoblasts treated with vehicle control or DAPT. Scale bars: 100 μm. (B) Inhibition of Notch signaling partially rescued the differentiation efficiency of MyoD-Lkb1 myoblasts. Different letters means P < 0.05. (C, D) The expression of differentiation related genes in WT and Myod-Lkb1 cells treated with or without DAPT. Error bars represent SEM. n=6-8.
3.4. AMPK-mTOR pathway, downstream target of Lkb1, regulates Notch signaling
To investigate how Lkb1 deficiency activates Notch target genes, we examined the AMPK-mTOR pathway, which has been thought as the canonical downstream target of Lkb1 (Lizcano et al., 2004). Indeed, Lkb1 deletion decreased the phosphorylated AMPK (pAMPK, T172) levels but increased the phosphorylated S6 (pS6, S240/244) levels in myoblasts (Fig. 5A). Conversely, AMPK activator AICAR treatment significantly increased the pAMPK levels, decreased the protein levels of phosphorylated S6 (pS6, S240/244) as well as the protein levels of Pax7, N1ICD and Hes1 in wild type myoblasts (Fig.5 B). Real time PCR results further confirmed that activation AMPK inhibits the expression of Pax7 and Notch target genes Hes1 and Hey1 (Fig.5 C). In addition, activation of AMPK decreased the expression of Notch targets (Hes1, Hey1 and HeyL) in C2C12 cells (Fig.5 D). Moreover, mTOR inhibitor rapamycin treatments decreased the protein levels of Hes1 in WT myoblasts (Fig.5 E, F). These data indicated that AMPK-mTOR pathway affects Notch1 signaling pathway in myoblasts.
Figure 5. Lkb1 regulates Notch signaling pathway through AMPK-mTOR pathway.
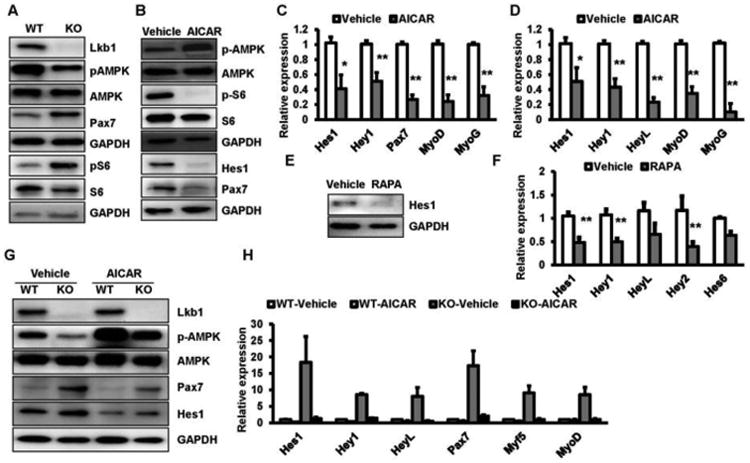
(A) Western blots showing relative levels of phosphorylated AMPK (pAMPK, T172) and pS6 (S240/244) in WT and MyoD-Lkb1 myoblasts. (B) AMPK activator AICAR (1 mM) treatment increased pAMPK level and reduced the levels of pS6, Hes1 and Pax7 proteins. (C, D) AICAR treatment decreased Notch targets in WT myoblasts (C) and C2C12 (D). (E, F) mTOR inhibitor rapamycin (RAPA, 10 nM) treatment decreases the protein (E) and mRNA (F) levels of Notch target genes in WT myoblast. (G, H): AICAR treatment decreases the protein (G) and mRNA (H) levels of Notch target genes in MyoD-Lkb1 myoblasts. Error bars represent SEM, n=7. *P<0.05, **P<0.01.
To further examine whether activation of AMPK can regulate the expression of Notch targets in MyoD-Lkb1 myoblasts, we treated the cells with AICAR. We found that AICAR enhanced the pAMPK levels in WT and Lkb1 KO myoblasts (Fig.5 G). The protein levels of Hes1 and Pax7 were also dramatically decreased after AICAR treatment (Fig.5 G). Furthermore, real time PCR results confirmed that AICAR treatment abolish the effects of Lkb1 deletion on the expression of Notch1 target genes as well as Pax7 in myoblasts (Fig.5 H). Taken together, these results indicate that deletion of Lkb1 affects Notch1 target genes through AMPK-mTOR pathway.
4. Discussion
In this study, we found that deletion of Lkb1 inhibits the differentiation division of myoblasts and upregulates Pax7 expression in myoblasts before or after differentiation. Moreover, we found that Lkb1 deficiency activates Notch signaling pathway, which then upregulates Pax7 expression. Furthermore, we revealed that ablation of Lkb1 activated Notch signaling pathway through AMPK-mTOR pathway. The results indicated that Lkb1 plays a crucial role in skeletal muscle progenitors.
We found that genetic deletion of Lkb1 in myoblasts led to elevated expression of Pax7 but decreased the expression of myogenic differentiation related genes as well as inhibited the differentiation division of myoblasts. It has been shown that the expression and activity of Lkb1 increase during myoblast differentiation (Mian et al., 2012). Overexpression of Lkb1 accelerated myoblast differentiation, whereas knockdown of Lkb1 impaired myoblast differentiation, suggesting Lkb1 positively controls myoblast differentiation (Mian et al., 2012). Our previous study demonstrated that Lkb1 deletion promotes proliferation and inhibits differentiation of muscle progenitors in vivo and in vitro (Shan et al., 2014). Moreover, the expression of Pax7 also affects cell survival, proliferation and differentiation of muscle progenitors (Gunther et al., 2013; Relaix et al., 2006; von Maltzahn et al., 2013). Results from the Pax7 gene conditional knockout mice model indicate that Pax7 is required for the maintenance of quiescent and the normal regenerative capacity of muscle stem cells (Gunther et al., 2013; von Maltzahn et al., 2013). Taken together, the higher levels of Pax7 in Lkb1 deficiency myoblasts may affect the cell fates and cell functions of muscle progenitors.
Notch1 signaling pathway plays critical roles in regulating cell-cell communication during development and postnatal tissue regeneration. It regulates the quiescence/activation, proliferation, and differentiation of myogenic progenitors (Conboy and Rando, 2002; Kuang et al., 2007). Here, we found that deletion of Lkb1 in myoblasts activates Notch signaling pathway, which in turn regulates Pax7 expression and cell fates of muscle progenitors. Consistent to our results, previous studies reported that Lkb1 deficiency modulates Notch signaling in post-mitotic cells (Shorning et al., 2009). Lkb1-null pre-T cells expressed increased levels of Hes-1 and Deltex1 when compared with control cells (Tamas et al., 2010). Inactivation of Lkb1 during pancreatic organogenesis increased expression of the Notch target gene Hes1 and the activated STAT3 gene (Hezel et al., 2008). Notch signaling promotes satellite cell activation and proliferation, but inhibits the differentiation of myogenic progenitors (Conboy et al., 2003; Conboy et al., 2005; Conboy and Rando, 2002; Schuster-Gossler et al., 2007; Vasyutina et al., 2007). Overexpression of N1ICD increased Pax7 positive satellite cells and impaired regeneration of skeletal muscles (Wen et al., 2012). In C2C12 cells, activation of Notch signaling or overexpression of Notch target genes dramatically inhibits myogenic differentiation and the expression of differentiation related genes (Buas et al., 2010; Wen et al., 2012; Wilson-Rawls et al., 1999). During muscle repair process, satellite cell activation and subsequent proliferation is driven by Notch signaling (Brack et al., 2008; Conboy and Rando, 2002). In addition, Notch signaling is necessary for maintaining satellite cells quiescence (Bjornson et al., 2012; Mourikis et al., 2012; Wen et al., 2012), and its deficiency leads to depletion of satellite cells in DMD (Jiang et al., 2014). Moreover, hypoxia treatment activates the Notch signaling pathway and leading to increased levels of Pax7 in myoblasts (Liu et al., 2012), and Notch1 signaling can directly regulate Pax7 expression through RBP-J in myoblast (Wen et al., 2012). Taken together, Lkb1 deficiency in myoblasts activates Notch signaling pathway and subsequently increases Pax7 expression and cell functions.
We demonstrated that Lkb1 regulates Notch1 signaling pathway through AMPK-mTOR pathway. Consistently, a recent study indicates that AMP-activated protein kinase (AMPK) regulates Notch signaling through mTORC1 under the influence of nutrient status (Bi and Kuang, 2015; Li et al., 2014). In mouse and human cells, mTOR is a positive regulator of Notch signaling (Li et al., 2014; Ma et al., 2010; Pollizzi et al., 2009). The expression of Hes1 was dramatically elevated in vitro or in vivo with constitutively active mTOR while reduced by rapamycin treatment (Ma et al., 2010; Pollizzi et al., 2009). Moreover, activation of mTOR leads to upregulation of Notch signaling and the expression of Notch targets both in vitro and in vivo (Ma et al., 2010). It has been reported that mTOR regulates Notch1 signaling pathway through induction of STAT3 (Li et al., 2014; Ma et al., 2010). Our previous study demonstrated that Lkb1 deletion affects myoblast differentiation through phosphorylation of GSK-3β (Shan et al., 2014), which can regulate the expression of Notch signaling pathway and its target Hes1 (Jin et al., 2009). Moreover, interaction network analysis of intracellular active form of NOTCH1 partners revealed that AMPK may also directly interact with Notch1 (Yatim et al., 2012). Therefore, more studies are needed to clarify whether Lkb1/AMPK can directly regulate Notch1 or not in future.
It has been reported that Lkb1 is a master kinase that activates 13 kinases of the AMPK subfamily (Lizcano et al., 2004), including SIK1 (Takemori et al., 2009), MARK (Koh et al., 2006) and MELK (Niesler et al., 2007) that can affect myoblast functions. Here we showed that Lkb1 regulates myoblast function and Notch signaling pathway through AMPK-mTOR pathway. Further studies are needed to elucidate whether other AMPK subfamily members may also be involved in mediating the effect of Lkb1 in myoblast division. In addition, as Lkb1-AMPK pathway plays important roles in nutrient and energy metabolism (Hardie et al., 2012), it is possible that nutrients factors such as serum and amino acids can affect myoblast division through Lkb1-AMPK pathway.
In summary, our present study revealed a crucial role of Lkb1 in regulating Notch signaling and Pax7 expression in muscle progenitor cells. These results enhance our understanding of the function of Lkb1 in muscle stem cells and further suggest that Lkb1 signaling pathway represents a promising target for controlling growth and regeneration of skeletal muscle.
Acknowledgments
The project was partially supported by funding from NIH (R01AR060652) and an incentive grant from Purdue University Office of Vice President for Research (OVPR) to SK, and “Hundred Talents Program” funding from Zhejiang University to TZ. We thank Jun Wu for mouse colony maintenance. The authors declare no conflict of interests.
Abbreviations
- AMPK
AMP-activated protein kinase
- BAT
brown adipose tissue
- DAPT
N-[N-(3,5-difluorophenacetyl-L-alanyl)]-S-phenylglycine t-butyl ester
- FBS
fetal bovine serum
- H&E
hematoxylin and eosin
- Lkb1
Liver kinase B1
- mTOR
mammalian target of rapamycin
- N1ICD
Notch1 intracellular domain
- Pax7
paired-box transcription factor 7
- WAT
white adipose tissue
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bi PP, Kuang SH. Notch signaling as a novel regulator of metabolism. Trends Endocrin Met. 2015;26(5):248–255. doi: 10.1016/j.tem.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornson CRR, Cheung TH, Liu L, Tripathi PV, Steeper KM, Rando TA. Notch Signaling Is Necessary to Maintain Quiescence in Adult Muscle Stem Cells. Stem cells. 2012;30(2):232–242. doi: 10.1002/stem.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brack AS, Conboy IM, Conboy MJ, Shen J, Rando TA. A temporal switch from Notch to Wnt signaling in muscle stem cells is necessary for normal adult myogenesis. Cell stem cell. 2008;2(1):50–59. doi: 10.1016/j.stem.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317(5839):807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- Brack AS, Rando TA. Tissue-specific stem cells: lessons from the skeletal muscle satellite cell. Cell stem cell. 2012;10(5):504–514. doi: 10.1016/j.stem.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buas MF, Kabak S, Kadesch T. The Notch Effector Hey1 Associates with Myogenic Target Genes to Repress Myogenesis. J Biol Chem. 2010;285(2):1249–1258. doi: 10.1074/jbc.M109.046441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, Morgan JE. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122(2):289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Smythe GM, Rando TA. Notch-mediated restoration of regenerative potential to aged muscle. Science. 2003;302(5650):1575–1577. doi: 10.1126/science.1087573. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433(7027):760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Rando TA. The regulation of notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Developmental cell. 2002;3(3):397–409. doi: 10.1016/s1534-5807(02)00254-x. [DOI] [PubMed] [Google Scholar]
- Fu A, Ng ACH, Depatie C, Wijesekara N, He Y, Wang GS, Bardeesy N, Scott FW, Touyz RM, Wheeler MB, Screaton RA. Loss of Lkb1 in Adult beta Cells Increases beta Cell Mass and Enhances Glucose Tolerance in Mice. Cell Metab. 2009;10(4):285–295. doi: 10.1016/j.cmet.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Gan BY, Hu JA, Jiang S, Liu YC, Sahin E, Zhuang L, Fletcher-Sananikone E, Colla S, Wang YA, Chin L, DePinho RA. Lkb1 regulates quiescence and metabolic homeostasis of haematopoietic stem cells. Nature. 2010;468(7324):701–U125. doi: 10.1038/nature09595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granot Z, Swisa A, Magenheim J, Stolovich-Rain M, Fujimoto W, Manduchi E, Miki T, Lennerz JK, Stoeckert CJ, Meyuhas O, Seino S, Permutt MA, Piwnica-Worms H, Bardeesy N, Dor Y. LKB1 Regulates Pancreatic beta Cell Size, Polarity, and Function. Cell Metab. 2009;10(4):296–308. doi: 10.1016/j.cmet.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther S, Kim J, Kostin S, Lepper C, Fan CM, Braun T. Myf5-Positive Satellite Cells Contribute to Pax7-Dependent Long-Term Maintenance of Adult Muscle Stem Cells. Cell stem cell. 2013;13(5):590–601. doi: 10.1016/j.stem.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Bio. 2012;13(4):251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hezel AF, Gurumurthy S, Granot Z, Swisa A, Chu GC, Bailey G, Dor Y, Bardeesy N, DePinho RA. Pancreatic Lkb1 deletion leads to acinar polarity defects and cystic neoplasms. Mol Cell Biol. 2008;28(7):2414–2425. doi: 10.1128/MCB.01621-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang CH, Wen YF, Kuroda K, Hannon K, Rudnicki MA, Kuang SH. Notch signaling deficiency underlies age-dependent depletion of satellite cells in muscular dystrophy. Dis Model Mech. 2014;7(8):997–1004. doi: 10.1242/dmm.015917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin YH, Kim H, Oh M, Ki H, Kim K. Regulation of Notch1/NICD and Hes1 Expressions by GSK-3 alpha/beta. Mol Cells. 2009;27(1):15–19. doi: 10.1007/s10059-009-0001-7. [DOI] [PubMed] [Google Scholar]
- Koh HJ, Arnolds DE, Fujii N, Tran TT, Rogers MJ, Jessen N, Li Y, Liew CW, Ho RC, Hirshman MF, Kulkarni RN, Kahn CR, Goodyear LJ. Skeletal muscle-selective knockout of LKB1 increases insulin sensitivity, improves glucose homeostasis, and decreases TRB3. Mol Cell Biol. 2006;26(22):8217–8227. doi: 10.1128/MCB.00979-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krock B, Skuli N, Simon MC. The Tumor Suppressor LKB1 Emerges as a Critical Factor in Hematopoietic Stem Cell Biology. Cell Metab. 2011;13(1):8–10. doi: 10.1016/j.cmet.2010.12.015. [DOI] [PubMed] [Google Scholar]
- Kuang S, Gillespie MA, Rudnicki MA. Niche regulation of muscle satellite cell self-renewal and differentiation. Cell stem cell. 2008;2(1):22–31. doi: 10.1016/j.stem.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Kuang S, Kuroda K, Le Grand F, Rudnicki MA. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129(5):999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang S, Rudnicki MA. The emerging biology of satellite cells and their therapeutic potential. Trends Mol Med. 2008;14(2):82–91. doi: 10.1016/j.molmed.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Li HL, Lee JY, He CY, Zou MH, Xie ZL. Suppression of the mTORC1/STAT3/Notch1 pathway by activated AMPK prevents hepatic insulin resistance induced by excess amino acids. Am J Physiol-Endoc M. 2014;306(2):E197–E209. doi: 10.1152/ajpendo.00202.2013. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Liu W, Wen Y, Bi P, Lai X, Liu XS, Liu X, Kuang S. Hypoxia promotes satellite cell self-renewal and enhances the efficiency of myoblast transplantation. Development. 2012;139(16):2857–2865. doi: 10.1242/dev.079665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizcano JM, Goransson O, Toth R, Deak M, Morrice NA, Boudeau J, Hawley SA, Udd L, Makela TP, Hardie DG, Alessi DR. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. Embo J. 2004;23(4):833–843. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JH, Meng Y, Kwiatkowski DJ, Chen XX, Feng HY, Sun Q, Zha XJ, Wang F, Wang Y, Jing YL, Zhang S, Chen RR, Wang LM, Wu E, Cai GF, Malinowska-Kolodziej I, Liao Q, Liu YQ, Zhao Y, Sun Q, Xu KF, Dai JW, Han JH, Wu LZ, Zhao RC, Shen HX, Zhang HB. Mammalian target of rapamycin regulates murine and human cell differentiation through STAT3/p63/Jagged/Notch cascade. J Clin Invest. 2010;120(1):103–114. doi: 10.1172/JCI37964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mian I, Pierre-Louis WS, Dole N, Gilberti RM, Dodge-Kafka K, Tirnauer JS. LKB1 Destabilizes Microtubules in Myoblasts and Contributes to Myoblast Differentiation. Plos One. 2012;7(2) doi: 10.1371/journal.pone.0031583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourikis P, Sambasivan R, Castel D, Rocheteau P, Bizzarro V, Tajbakhsh S. A Critical Requirement for Notch Signaling in Maintenance of the Quiescent Skeletal Muscle Stem Cell State. Stem cells. 2012;30(2):243–252. doi: 10.1002/stem.775. [DOI] [PubMed] [Google Scholar]
- Musaro A, Giacinti C, Borsellino G, Dobrowolny G, Pelosi L, Cairns L, Ottolenghi S, Cossu G, Bernardi G, Battistini L, Molinaro M, Rosenthal N. Stem cell-mediated muscle regeneration is enhanced by local isoform of insulin-like growth factor 1. P Natl Acad Sci USA. 2004;101(5):1206–1210. doi: 10.1073/pnas.0303792101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakada D, Saunders TL, Morrison SJ. Lkb1 regulates cell cycle and energy metabolism in haematopoietic stem cells. Nature. 2010;468(7324):653–U669. doi: 10.1038/nature09571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niesler CU, Myburgh KH, Moore F. The changing AMPK expression profile in differentiating mouse skeletal muscle myoblast cells helps confer increasing resistance to apoptosis. Experimental physiology. 2007;92(1):207–217. doi: 10.1113/expphysiol.2006.034736. [DOI] [PubMed] [Google Scholar]
- Pollizzi K, Malinowska-Kolodziej I, Doughty C, Betz C, Ma J, Goto J, Kwiatkowski DJ. A hypomorphic allele of Tsc2 highlights the role of TSC1/TSC2 in signaling to AKT and models mild human TSC2 alleles. Hum Mol Genet. 2009;18(13):2378–2387. doi: 10.1093/hmg/ddp176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relaix F, Montarras D, Zaffran S, Gayraud-Morel B, Rocancourt D, Tajbakhsh S, Mansouri A, Cumano A, Buckingham M. Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells. J Cell Biol. 2006;172(1):91–102. doi: 10.1083/jcb.200508044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster-Gossler K, Cordes R, Gossler A. Premature myogenic differentiation and depletion of progenitor cells cause severe muscle hypotrophy in Delta1 mutants. P Natl Acad Sci USA. 2007;104(2):537–542. doi: 10.1073/pnas.0608281104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano AL, Baeza-Raja B, Perdiguero E, Jardi M, Munoz-Cinoves P. Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab. 2008;7(1):33–44. doi: 10.1016/j.cmet.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Shan TZ, Zhang PP, Bi PP, Kuang SH. Lkb1 Deletion Promotes Ectopic Lipid Accumulation in Muscle Progenitor Cells and Mature Muscles. J Cell Physiol. 2015;230(5):1033–1041. doi: 10.1002/jcp.24831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan TZ, Zhang PP, Liang XR, Bi PP, Yue F, Kuang SH. Lkb1 Is Indispensable for Skeletal Muscle Development, Regeneration, and Satellite Cell Homeostasis. Stem cells. 2014;32(11):2893–2907. doi: 10.1002/stem.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorning BY, Zabkiewicz J, McCarthy A, Pearson HB, Winton DJ, Sansom OJ, Ashworth A, Clarke AR. Lkb1 Deficiency Alters Goblet and Paneth Cell Differentiation in the Small Intestine. Plos One. 2009;4(1) doi: 10.1371/journal.pone.0004264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemori H, Katoh Hashimoto Y, Nakae J, Olson EN, Okamoto M. Inactivation of HDAC5 by SIK1 in AICAR-treated C2C12 myoblasts. Endocrine journal. 2009;56(1):121–130. doi: 10.1507/endocrj.k08e-173. [DOI] [PubMed] [Google Scholar]
- Tamas P, Macintyre A, Finlay D, Clarke R, Feijoo-Carnero C, Ashworth A, Cantrell D. LKB1 is essential for the proliferation of T-cell progenitors and mature peripheral T cells. European journal of immunology. 2010;40(1):242–253. doi: 10.1002/eji.200939677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasyutina E, Lenhard DC, Wende H, Erdmann B, Epstein JA, Birchmeier C. RBP-J (Rbpsuh) is essential to maintain muscle progenitor cells and to generate satellite cells. P Natl Acad Sci USA. 2007;104(11):4443–4448. doi: 10.1073/pnas.0610647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Maltzahn J, Jones AE, Parks RJ, Rudnicki MA. Pax7 is critical for the normal function of satellite cells in adult skeletal muscle. P Natl Acad Sci USA. 2013;110(41):16474–16479. doi: 10.1073/pnas.1307680110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters LR, Walsh NC, Teitell MA. LKB1 regulates germinal center formation and termination. Cell Cycle. 2015;14(14):2183–2184. doi: 10.1080/15384101.2015.1056610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen YF, Bi PP, Liu WY, Asakura A, Keller C, Kuang SH. Constitutive Notch Activation Upregulates Pax7 and Promotes the Self-Renewal of Skeletal Muscle Satellite Cells. Mol Cell Biol. 2012;32(12):2300–2311. doi: 10.1128/MCB.06753-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson-Rawls J, Molkentin JD, Black BL, Olson EN. Activated notch inhibits myogenic activity of the MADS-box transcription factor myocyte enhancer factor 2C. Mol Cell Biol. 1999;19(4):2853–2862. doi: 10.1128/mcb.19.4.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatim A, Benne C, Sobhian B, Laurent-Chabalier S, Deas O, Judde JG, Lelievre JD, Levy Y, Benkirane M. NOTCH1 Nuclear Interactome Reveals Key Regulators of Its Transcriptional Activity and Oncogenic Function. Mol Cell. 2012;48(3):445–458. doi: 10.1016/j.molcel.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorska A, Deak M, Campbell DG, Banerjee S, Hirano M, Aizawa S, Prescott AR, Alessi DR. New Roles for the LKB1-NUAK Pathway in Controlling Myosin Phosphatase Complexes and Cell Adhesion. Sci Signal. 2010;3(115) doi: 10.1126/scisignal.2000616. [DOI] [PubMed] [Google Scholar]
- Zammit PS, Golding JP, Nagata Y, Hudon V, Partridge TA, Beauchamp JR. Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? J Cell Biol. 2004;166(3):347–357. doi: 10.1083/jcb.200312007. [DOI] [PMC free article] [PubMed] [Google Scholar]


