Abstract
During the past one to two decades, substantial progress has been made in our understanding of the immunopathology of type 1 diabetes (T1D) and the potential for immune interventions that can alter the natural history of the disease. This progress has resulted from the use of standardized study designs, endpoints, and, to a certain extent, mechanistic analyses in intervention trials in the setting of new-onset T1D. To date, most of these trials have involved single-agent interventions but, increasingly, future trials will test therapeutic combinations that are based on a compelling scientific rationale and testable mechanistic hypotheses. These increasingly complex trials will benefit from novel trial designs (such as factorial or adaptive designs), enhanced clinical endpoints that more directly assess islet pathology (such as β-cell death assays and islet or pancreatic imaging), improved responder analyses, and sophisticated mechanistic assays that provide deep phenotyping of lymphocyte subsets, gene expression profiling, in vitro T cell functional assessments, and antigen-specific responses. With this developing armamentarium of enhanced trial designs, endpoints, and clinical and mechanistic response analyses, we can expect substantial progress in better understanding the breakdown in immunologic tolerance in T1D and how to restore it to achieve significant and long-lasting preservation of islet function.
Keywords: New-onset T1D, Immune Tolerance Network, β cell, combination therapies, adaptive trials, responder analysis
1. Introduction
Type 1 diabetes (T1D) is characterized by a progressive loss of β-cell function resulting in absolute insulin deficiency. Although the precise etiology remains obscure, the pathogenesis comprises an organ-specific autoimmune process in genetically susceptible individuals involving activated innate immunity and dysregulated humeral and cellular adaptive immune responses [1,2].
The endocrine deficiency in T1D is treated with insulin replacement therapy, which substantially reduces morbidity and mortality. However, despite modern intensive diabetes management – including the use of insulin pumps, continuous glucose monitoring, sensor-augmented insulin pumps, or closed-loop pump-sensor systems (“artificial pancreas”) – normal or near-normal glycemic control (as measured by glycated hemoglobin (HbA1c) < 5.7%) cannot be achieved [3,4]. Even when glycemic control is “good” by current standards (HbA1c < 6.9%), patients with T1D, including children, have a 2-fold greater mortality than their nondiabetic peers [5].
There are currently no disease-modifying interventions for T1D. The restoration of immunologic tolerance is of considerable interest as a means to arrest and possibly reverse the autoimmune destruction of β cells in the pancreas [6]. During the past three decades, substantial efforts have been made to evaluate immunosuppressive and immunomodulatory agents in the clinic [7]. Most T1D trials have been conducted in patients with established or newly diagnosed disease, and this population will be the focus of this review.
2. Targets for immune intervention in T1D
A review of targets for immune intervention and a systematic analysis of the results of intervention trials to date is beyond the scope of this report, and the reader is referred to recent reviews [1,6,7]. Recent decades have witnessed enormous strides in the development of powerful immunomodulatory drugs, most notably fusion proteins and monoclonal antibodies that target specific receptors on B and T cells and a range of cytokines [8]. Many autoimmune disease can now be treated successfully, with evidence of disease modification and induction of remission. However, disease-modifying interventions in T1D have lagged, partly because of inaccessibility of the target organ and partly because the autoimmune process is silent, starting years or decades before diagnosis [9]. Nevertheless, over the past 1-2 decades substantial progress has been made in the design and conduct of intervention trials in T1D [7].
3. Clinical trial designs in T1D
3.1. Standard trial design
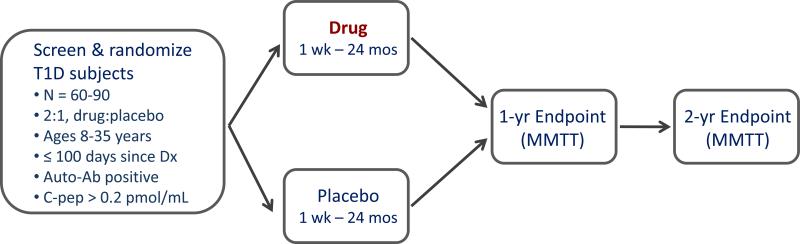
During the past decade, new-onset intervention trials have generally conformed to a common formula, with similar inclusion/exclusion criteria, endpoints, and duration [10,11]. As shown in Fig. 1, these trials are phase 2 proof-of-concept studies with enrolment goals of 60-80 subjects; patients are randomized within 100 days of T1D diagnosis, are autoantibody positive, with a peak C-peptide response of > 0.2 pmol/mL during a mixed meal tolerance test (MMTT), and ages in the range 6-45 years. Eligible patients are randomized 2:1, drug to placebo, in a double-blind, placebo-controlled, 2-arm design. The primary endpoint is the change from baseline in the 2- or 4-hour mean C-peptide area under the curve (AUC) following an MMTT at 12 or 24 months.
Fig. 1.
The standard study design for proof-of-concept trials of novel interventions in new-onset T1D. This is a randomized, placebo-controlled, double-blind phase 2 design, with 2:1 randomization (drug to placebo). Key inclusion criteria are shown. The primary endpoint, generally at 1 year, is the change from baseline in C-peptide area under the curve (AUC) following a mixed-meal tolerance test (MMTT). Secondary endpoints and continued safety follow-up usually extend to 2 years. Reprinted with permission from Ehlers & Nepom [10].
This design has served the community well and has generally provided credible evidence for the presence, or absence, of a signal of efficacy. Nevertheless, this approach has room for improvement: (a) the process is slow, generally 3-5 years from protocol development to completion of the primary analyses; (b) there are a limited number of expert sites and eligible patients, reducing the number of trials that can be conducted; (c) the selection of interventions has often been driven by pragmatic considerations and not by a compelling scientific rationale; and (d) there was often no a priori, testable mechanistic hypothesis and mechanistic insights into success or failure have been limited.
3.2. Alternative trial designs
Current T1D intervention trials are inefficient with respect to speed, the ability to evaluate multiple interventions (including novel combinations), dose optimization, and addressing mechanistic hypotheses. Some of these issues can be addressed by using alternative trial designs.
3.2.1. Factorial designs
Factorial designs are well suited to exploring novel drug combinations while limiting total enrolment [12]. Consider the following example: alefacept can induce partial remission but the effect begins to wane in the 2nd year [13]. One hypothesis is that the induction of tolerance is incomplete but might be augmented by an agent that blocks costimulation (e.g., abatacept [14]) or by an agent that blocks TNFα, an inflammatory cytokine (e.g., etanercept [15]), or the combination of all three. With a 2 × 2 factorial design, there are four treatment groups. One group would receive alefacept alone; one would receive alefacept plus abatacept; one alefacept plus etanercept; and one alefacept plus abatacept plus etanercept. For analyzing the effect of abatacept, the response rate for the two arms which received abatacept are compared to the response rate for the two arms which did not receive abatacept. Analyzing the effect of etanercept is similar. The factorial design has efficiency advantages because each drug is evaluated by comparing outcomes for all patients receiving that drug to outcomes for all patients not receiving that drug [16]. Thus, in the example above, a 2 × 2 factorial design will require only two thirds the number of patients as a 3-arm trial (alefacept vs. alefacept plus abatacept vs. alefacept plus etanercept).
A potential concern with factorial designs is that there could be interactions among the drugs [17]. That is, the effect of abatacept may differ depending on whether or not etanercept is administered. However, that can be a strength because factorial designs are effective at screening for combinations that are synergistic for response [16]. A more important concern is the absence of a placebo group.
There is variability from trial to trial in the rate of C-peptide decline in the placebo group, which is partly a function of age, time since diagnosis, and residual islet function at baseline [18]. This concern is lessened when the core drug (in this case alefacept) is known to have an effect and the primary question in the trial is whether that effect can be enhanced by a second drug. If a placebo group is considered essential, then this can be achieved by evaluating only 2 drugs [17], for example alefacept and abatacept, and the four groups are: alefacept alone, abatacept alone, alefacept plus abatacept, and placebo.
3.2.2. Adaptive designs
Adaptive designs can accelerate the evaluation of novel drug combinations, the sequencing of drug combinations, and dose optimization. A key feature of adaptive designs is that they include prospectively planned opportunities to modify specified aspects of the trial, such as treatment group assignment and overall enrolment [19]. Adaptive designs rely on the use of biomarkers that drive decisions on planned trial modifications and therefore are suitable for interventions with known mechanisms of action.
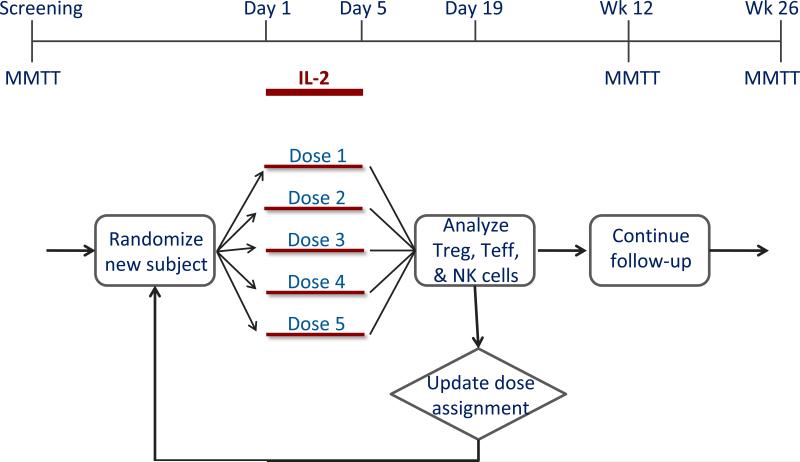
An example of where an adaptive design may be useful is in the development of low-dose IL-2 as a tolerogenic intervention (Fig. 2). While IL-2 promotes both Teff and Treg cells [20], Tregs are exquisitely dependent on IL-2 for growth and stability, and therefore low-dose IL-2 may selectively stimulate and expand Tregs [21]. However, the IL-2/Rapa trial gave mixed results: Tregs were robustly expanded and activated and yet islet function transiently declined, possibly because of unintended expansion of NK cells and eosinophils [22]. The dose used in the IL-2/Rapa trial was probably not low enough. A recent trial explored doses that were even lower [23], but further dose optimization is required to find a dose that selectively targets Tregs with no activation of effector cells.
Fig. 2.
Proposed adaptive trial design for dose optimization of IL-2 in new-onset T1D. The adaptive trial is designed to determine an optimal dose using the continual reassessment method. Subject assignments to one of five IL-2 dose groups is based on ongoing flow cytometric analyses that quantify the relative expansion or activation of Treg, Teff, and NK cells. This design leads to significant efficiencies in terms of total recruitment required to determine an optimal dose. Reprinted with permission from Ehlers & Nepom [10].
The ITN has proposed an adaptive trial design for dose optimization using the continual reassessment method [10]. In this design (Fig. 2), subject assignment to one of 5 dose groups is continually updated based on flow data (Treg, Teff and NK cell frequencies and activation, measured by pSTAT5 levels), such that most subjects are randomized to the effective doses and randomization to ineffective doses is minimized. Based on trial simulations, this design is very efficient, yielding an optimal dose with ≤ 15 subjects [10]. Independently, a group based in Cambridge, UK has reported the designs of two adaptive trials (DILT1D and DILfrequency) that are being conducted to determine the optimal dose and frequency of IL-2 that will expand Tregs without activating Teff populations in T1D [24,25].
Adaptive designs require biomarkers as short-term readouts that can drive trial adaptations, but reliable biomarkers are not generally available for new interventions whose efficacy in T1D is unknown. However, as we better understand the immunologic correlates of response it is conceivable that we will have mechanistic surrogates (e.g., Treg/Teff ratio changes by flow or T cell gene expression changes by RNAseq) that can serve as biomarkers in adaptive trials.
4. Trial endpoints
4.1. Clinical endpoints
The earliest T1D trials focused on clinical endpoints, including glycemic control and reduction or elimination of exogenous insulin therapy (clinical remission). However, it soon became clear that induction of remission is difficult and is complicated by the natural remission that is often observed in the first 6-12 months (“honeymoon period”). Thus, clinical endpoints are blunt tools because (a) it is unknown whether arresting the autoimmune process merely preserves residual islets or leads to restoration of islet cell mass, (b) direct effects on islets versus effects on the metabolic milieu, including insulin sensitivity, cannot be distinguished, and (c) no information is provided on the question of stopping β-cell killing versus reviving β cells that are dysfunctional or dormant. A recent example that illustrates the difficulty of using clinical endpoints was the Protégé study, a phase 3 clinical trial of teplizumab (anti-CD3 mAb) in new-onset T1D. The primary endpoint in Protégé, a composite of glycemic control (HbA1c < 6.5%) and reduced exogenous insulin use (< 0.5 units/kg/day), was not met and hence the trial was declared a failure [26], even though C-peptide secretion was significantly preserved in drug-treated patients at 2 years [27].
Nevertheless, clinical variables remain useful as secondary endpoints and add confidence that a therapeutic intervention can confer a clinically meaningful benefit. Intervention trials generally include intensive diabetes management (using ADA-recommended glycemic targets) for all participants regardless of treatment assignment because it is believed that good glycemic control reduces glucotoxicity and improves the prospects for β-cell recovery following immune intervention. Because of this design feature, assessment of differences in glycemic control between treatment arms is not generally a useful outcome (and, arguably, represents a failure of trial conduct). Nevertheless, between-group differences in HbA1c levels can emerge if the investigational drug results in rapid and significant preservation of β-cell function compared to the control group. This was observed in the rituximab [28], abatacept [14], and AbATE (teplizumab) trials [29], in which C-peptide secretion was significantly preserved by the intervention.
A more useful measure of drug efficacy against a background of standardized diabetes management is a reduction in exogenous insulin use, which requires the use of patient diaries to log daily insulin use. The utility of this approach was shown in the T1DAL (alefacept) trial, in which insulin use was significantly lower in the treatment arm vs. placebo at both 12 and 24 months despite similar levels of glycemic control, consistent with the significant preservation of C-peptide secretion induced by the drug [13,30]. Exogenous insulin use was also significantly decreased at some time points or in aggregate by treatment with rituximab, abatacept, and teplizumab [14,27-29].
Another clinical measure that correlates with islet function is frequency of hypoglycemic events. The Diabetes Control and Complications Trial (DCCT) clearly demonstrated that higher levels of C-peptide secretion resulted in a lower frequency of hypoglycemic events [31]. Until recently it was unclear what effect preservation of C-peptide by an immune intervention would have on hypoglycemia rates: preservation of islet function may decrease hypoglycemia by improving metabolic control and reducing insulin requirements; or, improved islet function may increase rates of hypoglycemia if insulin dose adjustments are not made rapidly enough. The T1DAL trial is the first intervention trial in new-onset T1D that clearly demonstrated a significant reduction in rates of major hypoglycemia in the alefacept group vs. placebo [13,30]. These results provide proof of concept that drug-induced preservation of islet function (vs. differences in natural rates of decline) can reduce hypoglycemia events in the context of intensive diabetes management. This was achieved by providing identical glucometers to all randomized subjects and downloading and recording standardized glucometer data at every clinic visit.
In addition to home glucometers, an approach that should also be evaluated is continuous glucose monitoring (CGM) using subcutaneous sensors that measure interstitial fluid (ISF) glucose. Although changes in ISF glucose levels lag behind changes in blood glucose [32], CGM has the advantage that it can provide a continuous readout over long periods, giving a more complete picture of the number of hypoglycemic events during defined intervals [33]. CGM has been evaluated in patients with established T1D and is approved as an adjunct to blood glucose monitoring. Prospective studies are needed to establish the utility of CGM in the setting of investigational immune interventions in new-onset T1D.
4.2. Measures of islet function
The hallmark of T1D is the progressive loss of endogenous insulin production due to autoimmune inflammation in pancreatic islets called insulitis. Death or functional impairment of β cells can be assessed by measuring endogenous insulin secretion, specifically by measuring C-peptide secretion in response to a stimulus, typically glucagon or a mixed meal (MMTT) [34,35]. The MMTT procedure has been standardized, is safe, and shows acceptable between-test reproducibility [36]. Implementation of standardized MMTTs has facilitated cross-trial comparisons of drug efficacy and has significantly advanced the field.
Meal-stimulated C-peptide responses ≥0.2 pmol/mL result in significant reductions in progression of retinopathy, nephropathy, and rates of hypoglycemia [31]. Additional analyses have revealed that the benefits of residual C-peptide secretion extend below the 0.2 pmol/mL threshold and that there is virtually a linear correlation between C-peptide levels and risk for microvascular complications and hypoglycemia [37,38]. Thus, preservation of stimulated C-peptide secretion is a legitimate primary outcome for intervention therapies [35] and a case can be made that this endpoint should serve as the basis for approval for an intervention therapy in T1D.
However, use of stimulated C-peptide secretion as an endpoint has its limitations. First, the procedures present a burden to patients and clinic staff because of requirements for fasting, lengthy visits, need for IV catheters, occasional intolerance of the liquid mixed meal or glucagon injection, or problems with hypoglycemia. Second, the procedures do not give a true estimate of total insulin secretory capacity. Third, the C-peptide response is dependent on metabolic factors such as insulin sensitivity, prior exercise, or carbohydrate loading. Fourth, the C-peptide response does not distinguish between β-cell killing and functional impairment (β cells are viable but dormant).
4.3. Measures of β-cell death
To overcome the limitations of tests for stimulated C-peptide secretion, there is interest in assays that can directly assess β-cell death. Significant progress has been made with assays that measure plasma levels of demethylated insulin gene DNA, thought to derive from β cells [39-43], or circulating micro-RNAs specific for islets [44,45]. The reader is referred to other articles in this issue for detailed discussion of these approaches.
4.4. Pancreatic imaging
The histopathology of human T1D is not well understood because the affected organ is largely inaccessible to routine biopsies. There has been a limited series (n=29) of laparoscopic pancreatic biopsies in patients with recent-onset T1D [46], in which histopathological findings generally matched those found in post-mortem specimens [47]. However, the laparoscopic biopsies generate small tissue samples, resulting in low yield of affected tissue due to the lobular nature of the disease [46]. Recently, pancreatic tail resections were performed laparoscopically in patients with new-onset T1D, but this study had to be curtailed because of unacceptable complication rates [48]. Therefore, alternatives to tissue biopsies are needed, such as pancreatic imaging.
Pancreatic inflammation is a hallmark of the disease [47,49] and potentially amenable to high-resolution imaging. Various pancreatic and β-cell imaging approaches are under development, with validation in rodent models and in the clinic [50-56]. The next step is to apply these techniques in the setting of intervention trials in new-onset T1D to determine whether pancreatic inflammation is reduced in patients who demonstrate treatment-related islet preservation as determined by stimulated C-peptide secretion; such a trial (the IMAGE-T1D trial) is currently in progress (ClinicalTrials.gov NCT01521520).
4.5. Immunologic surrogates of efficacy
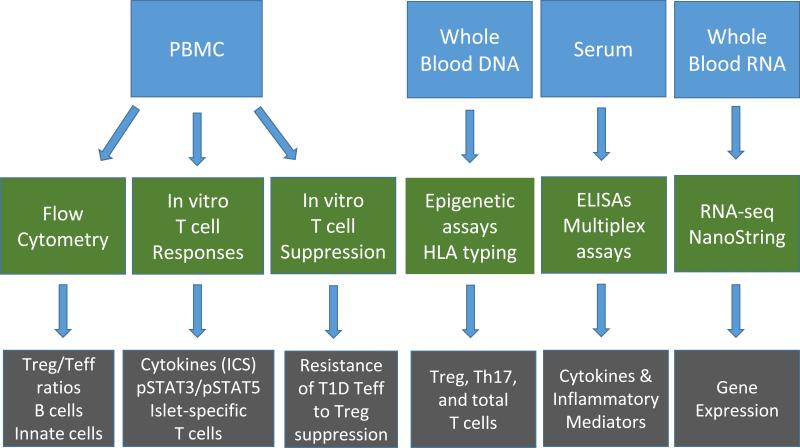
Treatment effects on immune system function are an important outcome of investigational studies, especially immunologic effects that can be correlated with the primary outcome measure (C-peptide). However, progress has been hampered by lack of direct access to the site of pathology, limitations of peripheral blood assays, and non-standardized immunologic assessments. Nevertheless, results from recent trials indicate that improved technologies and use of standardized mechanistic assessments can yield new insights into the pathology, progression, and treatment of T1D [7,57]. Fig. 3 illustrates a standardized sample collection and mechanistic assay scheme for use in new-onset T1D trials.
Fig. 3.
Standardized sample collection and mechanistic assay scheme to identify Immunologic surrogates of efficacy in T1D trials. Trials in new-onset T1D conducted by the Immune Tolerance Network (ITN) employ standardized sample collections (blue boxes: PBMCs, whole blood DNA, serum, and whole blood RNA), which are then analyzed by standardized assay procedures (green boxes: flow cytometry, in vitro T cell assays, epigenetic assays and genotyping, ELISAs and multiplex assays, and RNA-seq or NanoString analyses). Some of the expected outcomes of these assays are shown in the grey boxes.
4.5.1. Flow cytometry
Flow cytometry is the workhorse in intervention trials and benefits from (a) cryopreservation of PBMCs, enabling all trial samples to be analyzed together at the conclusion of the study, and (b) the advent of 12- and 18-color flow, enabling deep phenotyping of all relevant mononuclear cell subsets. Further, standardization of phenotypic markers has facilitated cross-trial comparisons [58]. These advances were highlighted in the T1DAL trial investigating the effects of alefacept, an LFA3-Ig fusion protein that binds to CD2-expressing cells. The T1DAL trial revealed that alefacept preferentially depleted CD4+ and CD8+ effector memory T (Tem) and central memory (Tcm) cells, while sparing naïve (Tn) and regulatory T cells (Tregs); these effects led to a favorable change in the Treg/Tem ratio. These changes were concordant with preservation of β-cell function in alefacept-treated subjects and provided mechanistic support for the hypothesis that targeting memory T cells while sparing Tregs can slow down or halt autoimmunity in T1D and preserve residual β cells [13,30].
Support for a role for memory T cells in T1D was also provided by flow analysis of samples from trials evaluating abatacept and teplizumab. Abatacept significantly slowed down the decline in C-peptide secretion [14] and flow analysis of PBMCs revealed that the drug significantly depleted CD4+ Tcm cells, which correlated with C-peptide preservation at certain time points [59]. In the AbATE and Delay trials, in which teplizumab preserved C-peptide secretion [29,60], flow analyses revealed that there was a decrease in CD4+ and CD8+ Tem cells immediately after therapy followed by an increase in the proportions of CD8+ Tcm cells with a regulatory phenotype at the 2- and 3-month time points in responders [61].
Based on these recent successes and the emergence of common themes, the ITN has developed standardized 12- and 18-color flow panels that will be used in all ongoing and future T1D trials in order to facilitate cross-trial comparisons and search for overarching mechanisms of immune tolerance.
4.5.2. In vitro T cell responses
Use of cryopreserved PBMCs for in vitro responses to cytokines, antigens, etc., opens up new avenues for testing mechanistic hypotheses related to immune interventions [62]. An example of this is the ITN IL-2/Rapa trial, described earlier, in which there was a transient accelerated decline in C-peptide, despite significant increases in Treg frequencies and no changes in Tcm and Tem cells. In vitro studies of thawed PBMCs revealed that stimulation with IL-2 produced enhanced responsiveness in CD25+ T cells as evidenced by increases in pSTAT5 [22]. The functional activation involved Tregs, but likely also involved activated CD25+ conventional T cells which may have contributed to the C-peptide decline [22].
In vitro T cell responses to T1D-specific auto-antigens are an area of significant interest. Assays that reflect antigen-specific activation without the need for HLA restriction or knowledge of the precise epitopes involved would be valuable [63]. This can be achieved by using flow to monitor the induction of cell-surface activation markers, such as CD137 on CD8+ T cells and CD154 on CD4+ T cells, after in vitro incubation with mixtures of antigenic peptides or protein lysates [64,65]. Antigen-specific CD154 activation assays have been used successfully in allergy studies [66] and there are ongoing efforts to optimize these approaches for use in autoimmunity, especially T1D, in which the auto-antigens are known [63].
4.5.3. In vitro T cell suppression
T1D is thought to result, in part, from defects in Treg function or frequency, or from effector T cell (Teff) resistance to Treg-mediated suppression [67-69]. In vitro T cell suppression assays can be performed with previously frozen, CFSE-labeled Teff cells from T1D patients co-cultured with Tregs from healthy controls followed by flow cytometry to quantitate CFSE dye dilution (a marker of cell proliferation) in CD4+ CD25− T cells [68]. This approach can be adapted for use with PBMCs harvested during a clinical trial, and is planned as one of the mechanistic assays for the EXTEND trial (tocilizumab, an IL-6 receptor blocker, in new-onset T1D) which is currently enrolling (ClinicalTrials.gov NCT02293837).
4.5.4. Islet-specific autoreactive T cells
T1D is characterized by the presence of autoreactive Teff cells specific for β-cell antigens. Although autoreactive T cells can also be detected in the peripheral blood of healthy subjects, the hallmark of autoreactive T cells in T1D is their memory phenotype [57]. However, frequencies of autoreactive T cells in peripheral blood are low and available technologies are not sensitive enough to be practical in the clinical trial setting and blood volume constraints. Nevertheless, improvements in technology, notably using MHC-peptide multimers (“tetramers”), are bringing these assays closer to the clinic, particularly for CD8+ autoreactive T cells, which are 10-fold more frequent in peripheral blood than CD4+ autoreactive T cells [63]. An example was the use of CD8+ Qdot technology (a flow-based tetramer assay) in a pilot study of an antigen-specific immunotherapy. In some groups there was preservation of C-peptide and a significant correlation with decreased proinsulin-specific, autoreactive CD8+ T cells [70]. This success may herald the broader use of tetramer technology in T1D intervention trials.
4.5.5. DNA-based assays
T1D susceptibility is strongly influenced by polymorphisms in HLA class II and class I alleles, followed by 40 or more other loci [2]. In most intervention trials, DNA is collected to enable profiling of HLA risk alleles. Although to date an association between specific HLA alleles and responses to immune intervention therapies has not been shown, this remains a possibility, but will likely require larger datasets than currently available. Finding correlations with non-HLA alleles (including the use of whole genome sequencing) will require even larger datasets and is currently impractical. Nevertheless, future meta-analyses on pooled trial data may be a possibility.
Also of interest are epigenetic and epigenomic changes that may relate to T1D immunopathology and responses to therapy. Epigenetic changes (DNA methylation and histone modifications) affect T cell lineage commitment, progression from naïve to effector and memory compartments, and switching between activated and hypo-functional phenotypes [71,72]. Treg stability and function depends on expression of the FoxP3 gene, which is demethylated in a region known as the TSDR (Treg-specific demethylated region) [73]. An assay for the methylation status of the TSDR is available for use on thawed PBMCs and was used on samples from the START trial, confirming the finding that antithymocyte globulin (ATG) therapy led to substantial depletion of Tregs, likely contributing to the lack of efficacy [74]. The FoxP3-driven transcriptional program in Tregs is dependent on the epigenetic regulator Ezh2 and Ezh2-deficient mice succumb to autoimmunity in a pattern similar to FoxP3-deficient mice [75]. Thus, epigenetic changes may be important drivers in autoimmunity and analyses of whole DNA and chromatin from PBMCs should be considered for samples from intervention trials.
4.5.6. Serum cytokines and inflammatory mediators
Therapies that block inflammatory cytokines are successful in diverse autoimmune conditions [76]. However, assessments of serum cytokines in clinical samples have generally not been fruitful, likely because of low concentrations near the detection limits of most assays. Nevertheless, there have been some successes. In the START trial, ATG therapy induced cytokine-release syndrome in all treated subjects, leading to massive increases in serum IL-6 levels and the acute-phase reactants SAA and CRP, which may have contributed to unintended immune activation [70]. Similarly, serum levels of soluble IL-2Rα were significantly elevated in the IL-2/rapamycin trial and correlated with changes in Treg, NK, and eosinophil frequencies, suggesting a prominent role for IL-2-driven immune activation [22].
4.5.7. Gene expression analysis
Collection of blood in RNA-stabilizing Tempus or PAXgene tubes and advances in DNA sequencing have made gene expression profiling in trial samples a reality. Two technologies in particular have emerged: RNA-seq and NanoString [62]. RNA-seq (high-throughput parallel sequencing of cDNA fragments generated from sheared RNA) is an efficient method for transcriptome analyses, with clear advantages over gene expression arrays [77-79]. The NanoString nCounter system captures and counts individual mRNA transcripts, with minimal bias and digital readout, and is more sensitive than microarrays [80]. NanoString was used to analyze differences in gene expression between responders and non-responders to teplizumab in patients with recent-onset T1D, which showed a decrease in genes associated with T cell activation and an increase in genes associated with T cell regulation in responders [61].
5. Responder analyses
C-peptide outcomes in intervention trials demonstrate considerable heterogeneity in treatment responses. This was clearly shown in the AbATE trial, in which a responder analysis revealed that treated subjects who met the criteria for response had robust preservation of C-peptide at 2 years while non-responders were virtually indistinguishable from untreated controls [29]. However, there is currently no consensus on a standard analytical / statistical approach to a responder analysis. Responder analyses are confounded by considerable variability in the rate of C-peptide decline in the control group (which is partly a function of age [81]) such that it is difficult to find categorical cutoffs. This suggests that response / non-response is a continuous rather than binary variable, but a uniform statistical treatment that can be applied to all intervention trials has yet to be developed.
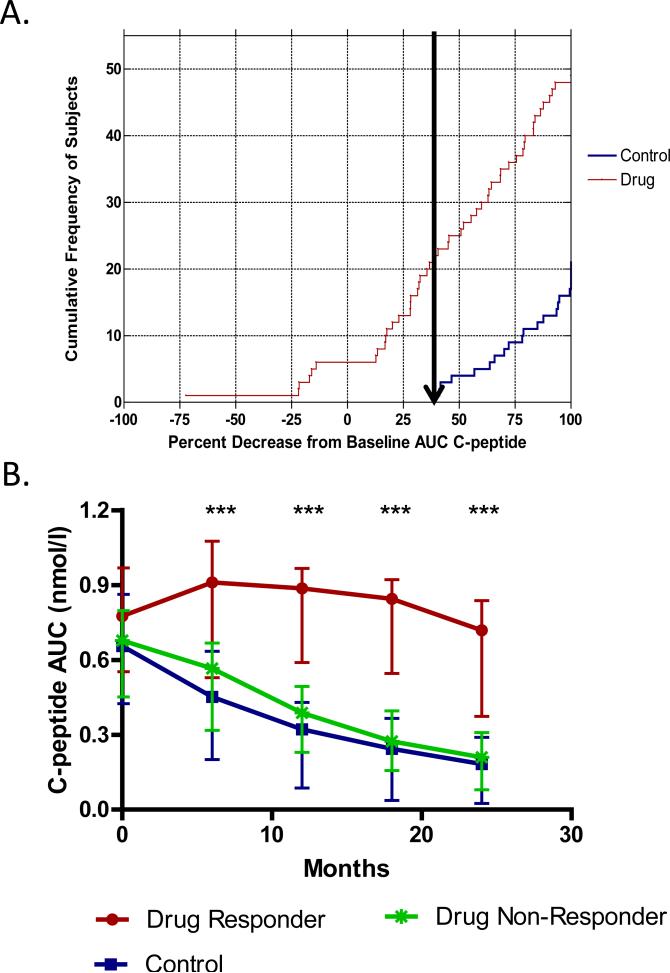
Nevertheless, it is instructive to consider some of the approaches that have been used. In the original teplizumab (anti-CD3) trials, “response” was defined as ≤ 7.5% loss of baseline C-peptide at 12 months, which was based on the interassay CV of the C-peptide assay [82]. This definition was not useful in the AbATE trial and therefore a post hoc analysis was performed to determine a cutoff that identified drug-treated subjects who were clearly different from untreated controls (Fig. 4). This analysis revealed that all of the 25 control subjects lost ≥ 40% of baseline C-peptide at 24 months, and 27 of 49 drug-treated subjects showed a similar loss of ≥ 40%. In contrast, 22 of 49 of the drug-treated subjects lost < 40% of baseline C-peptide, and these subjects were designated “responders” [29]. This analysis proved useful in identifying clinical features that distinguish responders at baseline and is the basis for ongoing mechanistic analyses to better understand the immunologic basis for response to anti-CD3.
Fig. 4.
Responder analysis performed in the AbATE trial to identify clinical responders to teplizumab (anti-CD3 mAb). A. The cumulative frequency of subjects and distribution of percentage decrease from baseline C-peptide AUC at month 24. The arrow shows the smallest percentage loss of C-peptide AUC in the control group. All control subjects (in blue) lost ≥ 40% of their baseline C-peptide secretion; drug-treated subjects (in red) to the left of the arrow lost < 40% of baseline C-peptide and were designated “responders,” while drug-treated subject to the right of the arrow were designated “non-responders.” B. The C-peptide AUC at each time point (means ± 25th and 75th percentiles) is shown for the responders (red line) and non-responders (green line) in the drug-treated group and for the control subjects (blue line). ***P <0.001 between responders and non-responders at each time point based on ANCOVAs. Reprinted with permission from Herold et al. [29].
In the T1DAL trial, 2 thresholds for response were set: complete preservation of baseline C-peptide AUC values at 2 years (complete responders) and preservation of ≥50% of baseline C-peptide AUC values at 2 years (partial responders). In the alefacept group, 87% were partial responders and 30% were complete responders versus 33% and 8%, respectively, in the placebo group [13]. This responder analysis is the basis for ongoing studies to determine the mechanistic basis for response. The complete response criterion for alefacept [13] is similar to the recent recommendation by the Type 1 Diabetes TrialNet Study Group to define response as complete preservation of baseline C-peptide at 6 months [83]. However, complete preservation at 6 months is substantially different from complete preservation at 12 or 24 months, and further work is required to determine how best to apply this criterion.
An alternative approach that treats response as a continuous variable rather than using specific cutoffs was recently reported for the abatacept trial [59]. Flow revealed a significant decrease in CD4+ Tcm cells in the abatacept vs. placebo groups. Based on mixed linear modeling for longitudinal data, including a “lagging” analysis, the changes in CD4+ Tcm cells during the preceding visit were significantly associated with C-peptide change at the current visit, indicating that decreases in CD4+ Tcm correlated with and predicted C-peptide preservation [59]. This type of analysis revealed that “response” does not have to be treated as a categorical variable but can be viewed on a continuum and explored for correlations with plausible immunologic changes. Further analyses will be required to determine whether this approach can be extended to drugs with different mechanisms of action.
6. Conclusions
During the past 1-2 decades, the introduction of standardized trial designs, uniform clinical endpoints, and the increasing implementation of advanced mechanistic analyses have substantially advanced the field. We are now entering the next phase of immune intervention trials in T1D where the focus will be rational combinations of therapeutics based on a strong mechanistic rationale for induction of immunologic tolerance. Successful implementation of novel combination therapy trials will require the exploration of alternative designs that can improve efficiencies and are better suited to evaluating new combinations and associated uncertainties around dosing and timing. Such trials will also require further advances and improvements in β-cell death assays, pancreatic imaging, and mechanistic assessments, especially antigen-specific assays, to facilitate a deeper understanding of drug response and enable the use of biomarkers as surrogate endpoints. The stage is set for the next phase in tackling this hitherto intractable autoimmune disease, and we now have additional tools to make significant inroads in the search for disease-modifying interventions.
Highlights.
There are currently no disease-modifying interventions for the treatment of T1D.
Standardized trial designs have aided evaluation of immune interventions for T1D.
Future trials will evaluate therapeutic combinations with testable mechanistic hypotheses.
We need novel trial designs and endpoints that directly assess β-cell pathology.
Analyses include lymphocyte phenotyping, gene expression, and antigen-specific responses.
Acknowledgments
This publication was supported in part by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under Award Number UM1AI109565. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health. The author thanks Kevan C. Herold, Mark R. Rigby, Carla J. Greenbaum, Steven E. Gitelman, Ashley Pinckney, Lynette Keyes-Elstein, S. Alice Long, Kristina M. Harris, Carol Soppe, and the members of the AbATE, T1DAL, IL-2/Rapa, and START study teams for their numerous contributions to the design, conduct and analysis of the ITN trials referred to in this review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464:1293–1300. doi: 10.1038/nature08933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Todd JA. Etiology of type 1 diabetes. Immunity. 2010;32:457–467. doi: 10.1016/j.immuni.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Klein BEK, Klein R. Further insight on the limits of success of glycemic control in type 1 diabetes. Diabetes. 2015;64:341–343. doi: 10.2337/db14-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farrington C. The artificail pancreas: challenges and opportunities. Lancet Diabetes Endocrinol. 2015;3:937. doi: 10.1016/S2213-8587(15)00430-1. [DOI] [PubMed] [Google Scholar]
- 5.Lind M, Svensson AM, Kosiborod M, Gudbjörnsdottir S, Pivodic A, Wedel H, et al. Glycemic control and excess mortality in type 1 diabetes. N. Engl. J. Med. 2014;371:1972–1982. doi: 10.1056/NEJMoa1408214. [DOI] [PubMed] [Google Scholar]
- 6.Herold KC, Vignali DAA, Cooke A, Bluestone JA. Type 1 diabetes: translating mechanistic observations into effective clinical outcomes. Nat. Rev. Immunol. 2013;13:243–256. doi: 10.1038/nri3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ehlers MR. Immune interventions to preserve β cell function in type 1 diabetes. J. Investig. Med. 2016;64:7–13. doi: 10.1097/JIM.0000000000000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smilek DE, Ehlers MR, Nepom GT. Restoring the balance: immunotherapeutic combinations for autoimmune disease. Dis. Mod. Mech. 2014;7:503–513. doi: 10.1242/dmm.015099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Insel RA, Dunne JL, Atkinson MA, Chiang JL, Dabelea D, Gottlieb PA, et al. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care. 2015;38:1964–1974. doi: 10.2337/dc15-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ehlers MR, Nepom GT. Immune-directed therapy for type 1 diabetes at the clinical level: the Immune Tolerance Network (ITN) experience. Rev. Diabet. Studies. 2012;9:359–371. doi: 10.1900/RDS.2012.9.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenbaum CJ, Harrison LC. Immunology of Diabetes Society, Guidelines for intervention trials in subjects with newly diagnosed type 1 diabetes. Diabetes. 2003;52:1059–1065. doi: 10.2337/diabetes.52.5.1059. [DOI] [PubMed] [Google Scholar]
- 12.Simon R, Freedman LS. Bayesian design and analysis of two × two factorial clinical trials. Biometrics. 1997;53:456–464. [PubMed] [Google Scholar]
- 13.Rigby MR, Harris KM, Pinckney A, DiMeglio LA, Rendell MS, Felner EI, et al. Alefacept provides sustained clinical and immunological effects in new-onset type 1 diabetes patients. J. Clin. Invest. 2015;125:3285–3296. doi: 10.1172/JCI81722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orban T, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, et al. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;378:412–419. doi: 10.1016/S0140-6736(11)60886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mastrandrea L, Yu J, Behrens T, Buchlis J, Albini C, Fourtner S, et al. Etanercept treatment in children with new-onset type 1 diabetes: pilot randomized, placebo-controlled, double-blind study. Diabetes Care. 2009;32:1244–1249. doi: 10.2337/dc09-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simon RM, Steinberg SM, Hamilton M, Hildesheim A, Khleif S, Kwak LW, et al. Clinical trial designs for the early clinical development of therapeutic cancer vaccines. J. Clin. Oncol. 2001;19:1848–1854. doi: 10.1200/JCO.2001.19.6.1848. [DOI] [PubMed] [Google Scholar]
- 17.McAlister FA, Straus SE, Sackett DL, Altman DG. Analysis and reporting of factorial trials. A systematic review. JAMA. 2003;289:2545–2553. doi: 10.1001/jama.289.19.2545. [DOI] [PubMed] [Google Scholar]
- 18.Greenbaum CJ, Beam CA, Boulware D, Gitelman SE, Gottlieb PA, Herold KC, et al. Fall in C-peptide during first 2 years from diagnosis. Evidence of at least two distinct phases from composite Type 1 Diabetes TrialNet data. Diabetes. 2012;61:2066–2073. doi: 10.2337/db11-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones D. Adaptive trials receive boost. Nat. Rev. Drug Disc. 2010;9:345–348. doi: 10.1038/nrd3174. [DOI] [PubMed] [Google Scholar]
- 20.Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. nat. Rev. Immunol. 2012;12:180–190. doi: 10.1038/nri3156. [DOI] [PubMed] [Google Scholar]
- 21.Klatzmann D, Abbas AK. The promise of low-dose interleukin⍰ 2 therapy for autoimmune and inflammatory diseases. Nat. Rev. Immunol. 2015;15:283–294. doi: 10.1038/nri3823. [DOI] [PubMed] [Google Scholar]
- 22.Long SA, Rieck M, Sanda S, Bollyky JB, Samuels PL, Goland R, et al. Rapamycin/IL-2 combination therapy in patients with type 1 diabetes augments Tregs yet transiently impairs beta-cell function. Diabetes. 2012;61:2340–2348. doi: 10.2337/db12-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hartemann A, Bensimon G, Payan CA, Jacqueminet S, Bourron O, Nicolas N, et al. Low-dose interleukin 2 in patients with type 1 diabetes: a phase 1/2 randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2013;1:295–305. doi: 10.1016/S2213-8587(13)70113-X. [DOI] [PubMed] [Google Scholar]
- 24.Waldron-Lynch F, Kareclas P, Irons K, Walker NM, Mander A, Wicker LS, et al. Rationale and study design of the adaptive study of IL-2 dose on regulatory T cells in type 1 diabetes (DILT1D): a non-randomised, open label, adaptive dose finding trial. BMJ Open. 2014;4:e005559. doi: 10.1136/bmjopen-2014-005559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Truman LA, Pekalski ML, Kareclas P, Evangelou M, Walker NM, Howlett J, et al. Protocol of the adaptive study of IL-2 dose frequency on regulatory T cells in type 1 diabetes (DILfrequency): a mechanistic, non-randomised, repeat dose, open-label, response-adaptive study. BMJ Open. 2015;5:e009799. doi: 10.1136/bmjopen-2015-009799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sherry N, Hagopian W, Ludvigsson J, Jain SM, Wahlen J, Ferry RJ, Jr, et al. Teplizumab for treatment of type 1 diabetes (Protégé study): 1-year results from a randomised, placebo-controlled trial. Lancet. 2011;378:487–497. doi: 10.1016/S0140-6736(11)60931-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hagopian W, Ferry RJ, Jr., Sherry N, Carlin D, Bonvini E, Johnson S, et al. Teplizumab preserves C-peptide in recent-onset type 1 diabetes. Two-year results from the randomized, placebo-controlled Protégé trial. Diabetes. 2013;62:3901–3908. doi: 10.2337/db13-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, Becker DJ, Gitelman SE, Goland R, et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N. Engl. J. Med. 2009;361:2143–2152. doi: 10.1056/NEJMoa0904452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herold KC, Gitelman SE, Ehlers MR, Gottlieb PA, Greenbaum CJ, Hagopian W, et al. Teplizumab (anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: metabolic and immunologic features at baseline identify a subgroup of responders. Diabetes. 2013;62:3766–3774. doi: 10.2337/db13-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rigby MR, DiMeglio LA, Rendell MS, Felner EI, Dostou JM, Gitelman SE, et al. Targeting of memory T cells with alefacept in new-onset type 1 diabetes (T1DAL study): 12 month results of a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Diabetes Endocrinol. 2013;1:284–294. doi: 10.1016/S2213-8587(13)70111-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diabetes Control and Complications Trial Research Group Effect of intensive therapy on residual β-cell function in patients with type 1 diabetes in the Diabetes Control and Complications Trial. A randomized, controlled trial. Ann. Intern. Med. 1998;128:517–523. doi: 10.7326/0003-4819-128-7-199804010-00001. [DOI] [PubMed] [Google Scholar]
- 32.Freckmann G, Hagenlocher S, Baumstark A, Jendrike N, Gillen RC, Rössner K, et al. Continuous glucose profiles in healthy subjects under everyday life conditions and after different meals. J. Diabetes Sci. Technol. 2007;1:695–703. doi: 10.1177/193229680700100513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xing D, Kollman C, Beck RW, Tamborlane WV, Laffel L, Buckingham BA, et al. Optimal sampling intervals to assess long-term glycemic control using continuous glucose monitoring Dibetes Technol. Ther. 2011;13:351–358. doi: 10.1089/dia.2010.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DCCT Research Group Effects of age, duration and treatment of insulin-dependent diabetes mellitus on residual β-cell function: observations during eligibility testing for the Diabetes Control and Complications Trial (DCCT) J. Clin. Endocrinol. Metab. 1987;65:30–36. doi: 10.1210/jcem-65-1-30. [DOI] [PubMed] [Google Scholar]
- 35.Palmer JP, Fleming GA, Greenbaum CJ, Herold KC, Jansa LD, Kolb H, et al. C-Peptide Is the appropriate outcome measure for type 1 diabetes clinical trials to preserve β-cell function. Report of an ADA workshop, 21–22 October 2001. Diabetes. 2004;53:250–264. doi: 10.2337/diabetes.53.1.250. [DOI] [PubMed] [Google Scholar]
- 36.Greenbaum CJ, Mandrup-Poulsen T, Friedenberg McGee P, Battelino T, Haastert B, Ludvigsson J, et al. Mixed-meal tolerance test versus glucagon stimulation test for the assessment of β-cell function in therapeutic trials in type 1 diabetes. Diabetes Care. 2008;31:1966–1971. doi: 10.2337/dc07-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steffes MW, Sibley S, Jackson M, Thomas W. Beta-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care. 2003;26:832–836. doi: 10.2337/diacare.26.3.832. [DOI] [PubMed] [Google Scholar]
- 38.Lachin JM, McGee P, Palmer JP. DCCT/EDIC Research Group, Impact of C-Peptide preservation on metabolic and clinical outcomes in the Diabetes Control and Complications Trial. Diabetes. 2014;63:739–748. doi: 10.2337/db13-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akirava EM, Lebastchia J, Galvana EM, Henegariua O, Akiravb M, Ablamunitsa V, et al. Detection of β cell death in diabetes using differentially methylated circulating DNA. Proc. Natl. Acad. Sci. USA. 2011;108:19018–19023. doi: 10.1073/pnas.1111008108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lebastchi J, et al. Immune therapy and β-cell death in type 1 diabetes. Diabetes. 2013;62:1676–1680. doi: 10.2337/db12-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Husseiny MI, Kaye A, Zebadua E, Kandeel F, Ferreri K. Tissue-specific methylation of human insulin gene and PCR assay for monitoring beta cell death. PLoS ONE. 2014;9:e94591. doi: 10.1371/journal.pone.0094591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herold KC, Usmani-Brown S, Ghazi T, Lebastchi J, Beam CA, Bellin MD, et al. β cell death and dysfunction during type 1 diabetes development in at-risk individuals. J. Clin. Invest. 2015;125:1163–1173. doi: 10.1172/JCI78142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fisher MM, Watkins RA, Blum J, Evans-Molina C, Chalasani N, DiMeglio LA, et al. Elevations in circulating methylated and unmethylated preproinsulin DNA in new-onset type 1 diabetes. Diabetes. 2015;64:3867–3872. doi: 10.2337/db15-0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Özcan S. Minireview: microRNA function in pancreatic β cells. Mol. Endocrinol. 2014;28:1922–1933. doi: 10.1210/me.2014-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Erener S, Mojibian M, Fox JK, Denroche HC, Kieffer TJ. Circulating miR-375 as a biomarker of β-cell death and diabetes in mice. Endocrinology. 2013;154:603–608. doi: 10.1210/en.2012-1744. [DOI] [PubMed] [Google Scholar]
- 46.Imagawa A, Hanafusa T, Tamura S, Moriwaki M, Itoh N, Yamamoto K, et al. Pancreatic biopsy as a procedure for detecting in situ autoimmune phenomena in type 1 diabetes. Close correlation between serological markers and histological evidence of cellular autoimmunity. Diabetes. 2001;50:1269–1273. doi: 10.2337/diabetes.50.6.1269. [DOI] [PubMed] [Google Scholar]
- 47.Richardson SJ, Morgan NG, Foulis AK. Pancreatic pathology in type 1 diabetes mellitus. Endocr. Pathol. 2014;25:80–92. doi: 10.1007/s12022-014-9297-8. [DOI] [PubMed] [Google Scholar]
- 48.Krogvold L, Edwin B, Buanes T, Ludvigsson J, Korsgren O, Hyöty H, et al. Pancreatic biopsy by minimal tail resection in live adult patients at the onset of type 1 diabetes: experiences from the DiViD study. Diabetologia. 2014;57:841–843. doi: 10.1007/s00125-013-3155-y. [DOI] [PubMed] [Google Scholar]
- 49.Hanafusa T, Imagawa A. Insulitis in human type 1 diabetes. Ann. N.Y. Acad. Sci. 2008;1150:297–299. doi: 10.1196/annals.1447.052. [DOI] [PubMed] [Google Scholar]
- 50.Laurent D, Vinet L, Lamprianou S, Daval M, Filhoulaud G, Ktorza A, et al. Pancreatic β-cell imaging in humans: fiction or option? Diabetes Obes. Metab. 2016;18:6–15. doi: 10.1111/dom.12544. [DOI] [PubMed] [Google Scholar]
- 51.Normandin MD, Petersen KF, Ding Y-S, Lin S-F, Naik S, Fowles K, et al. In vivo imaging of endogenous pancreatic β-cell mass in healthy and type 1 diabetic subjects using 18F-fluoropropyl-dihydrotetrabenazine and PET. J. Nucl. Med. 2012;53:908–916. doi: 10.2967/jnumed.111.100545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brom M, Woliner-van der Weg W, Joosten L, Frielink C, Bouckenooghe T, Rijken P, et al. Non-invasive quantification of the beta cell mass by SPECT with 111In-labelled exendin. Diabetologia. 2014;57:950–959. doi: 10.1007/s00125-014-3166-3. [DOI] [PubMed] [Google Scholar]
- 53.Denis MC, Mahmood U, Benoist C, Mathis D, Weissleder R. Imaging inflammation of the pancreatic islets in type 1 diabetes. Proc. Natl. Acad. Sci USA. 2004;101:12634–12639. doi: 10.1073/pnas.0404307101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Turvey SE, Swart E, Denis MC, Mahmood U, Benoist C, Weissleder R, et al. Noninvasive imaging of pancreatic inflammation and its reversal in type 1 diabetes. J. Clin. Invest. 2005;115:2454–2461. doi: 10.1172/JCI25048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gaglia JL, Guimaraes AR, Harisinghani M, Turvey SE, Jackson R, Benoist C, et al. Noninvasive imaging of pancreatic islet inflammation in type 1A diabetes patients. J. Clin. Invest. 2011;121:442–445. doi: 10.1172/JCI44339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gaglia JL, Harisinghani M, Aganj I, Wojtkiewicz GR, Hedgire S, Benoist C, et al. Noninvasive mapping of pancreatic inflammation in recent-onset type-1 diabetes patients. Proc. Natl. Acad. Sci. USA. 2015;112:2139–2144. doi: 10.1073/pnas.1424993112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ehlers MR, Rigby MR. Targeting memory T cells in type 1 diabetes. Curr. Diab. Rep. 2015;15:84. doi: 10.1007/s11892-015-0659-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maecker HT, McCoy JP, Nussenblatt R. Standardizing immunophenotyping for the Human Immunology Project. Nat. Rev. Immunol. 2012;12:191–200. doi: 10.1038/nri3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Orban T, Beam CA, Xu P, Moore K, Jiang Q, Deng J, et al. Reduction in CD4 central memory T-cell subset in costimulation modulator abatacept-treated patients with recent-onset type 1 diabetes is associated with slower C-peptide decline. Diabetes. 2014;63:3449–3457. doi: 10.2337/db14-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Herold KC, Gitelman SE, Willi SM, Gottlieb PA, Waldron-Lynch F, Devine L, et al. Teplizumab treatment may improve C-peptide responses in participants with type 1 diabetes after the new-onset period: a randomised controlled trial. Diabetologia. 2013;56:391–400. doi: 10.1007/s00125-012-2753-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.E Tooley J, Vudattu N, Choi J, Cotsapas C, Devine L, Raddassi K, et al. Changes in T-cell subsets identify responders to FcR-nonbinding anti-CD3 mAb (teplizumab) in patients with type 1 diabetes. Eur. J. Immunol. 2016;46:230–241. doi: 10.1002/eji.201545708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ermann J, Rao DA, Teslovich NC, Brenner MB, Raychaudhuri S. Immune cell profiling to guide therapeutic decisions in rheumatic diseases. Nat. Rev. Rheum. 2015;11:541–551. doi: 10.1038/nrrheum.2015.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Odegard JM, Nepom GT, Wambre E. Biomarkers for antigen immunotherapy in allergy and type 1 diabetes. Clin. Immunol. 2015;161:44–50. doi: 10.1016/j.clim.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wolfl M, Kuball J, Ho WY, Nguyen H, Manley TJ, Bleakley M, et al. Activation-induced expression of CD137 permits detection, isolation, and expansion of the full repertoire of CD8+ T cells responding to antigen without requiring knowledge of epitope specificities. Blood. 2007;110:201–210. doi: 10.1182/blood-2006-11-056168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bacher P, Schink C, Teutschbein J, Kniemeyer O, Assenmacher M, Brakhage AA, et al. Antigen-reactive T cell enrichment for direct, high-resolution analysis of the human naive and memory Th cell repertoire. J. Immunol. 2013;190:3967–3976. doi: 10.4049/jimmunol.1202221. [DOI] [PubMed] [Google Scholar]
- 66.Wambre E, James EA, Kwok WW. Characterization of CD4+ T cell subsets in allergy. Curr. Opin. Immunol. 2012;24:700–706. doi: 10.1016/j.coi.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Buckner JH. Mechanisms of impaired regulation by CD4+CD25+FOXP3+ regulatory T cells in human autoimmune diseases. Nat. Rev. Immunol. 2010;10:849–859. doi: 10.1038/nri2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schneider A, Rieck M, Sanda S, Pihoker C, Greenbaum C, Buckner JH. The effector T cells of diabetic subjects are resistant to regulation via CD4+FOXP3+ regulatory T cells. J. Immunol. 2008;181:7350–7355. doi: 10.4049/jimmunol.181.10.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lawson JM, Tremble J, Dayan C, Beyan H, Leslie RDG, Peakman M, et al. Increased resistance to CD4+CD25hi regulatory T cell-mediated suppression in patients with type 1 diabetes. Clin. Exp. Immunol. 2008;154:353–359. doi: 10.1111/j.1365-2249.2008.03810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roep BO, Solvason N, Gottlieb PA, Abreu JRF, Harrison LC, Eisenbarth GS, et al. Plasmid-encoded proinsulin preserves C-peptide while specifically reducing proinsulijn-specific CD8+ T cells in type 1 diabetes. Sci. Transl. Med. 2013;5:191ra82. doi: 10.1126/scitranslmed.3006103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Youngblood B, Hale JS, Ahmed R. T-cell memory differentiation: insights from transcriptional signatures and epigenetics. Immunology. 2013;139:277–284. doi: 10.1111/imm.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vahedi G, Kanno Y, Sartorelli V, O'Shea JJ. Transcription factors and CD4 T cells seeking identity: masters, minions, setters and spikers. Immunology. 2013;139:294–298. doi: 10.1111/imm.12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Spreafico R, Rossetti M, van den Broek T, Jansen NJG, Zhang H, Moshref M, et al. A sensitive protocol for FOXP3 epigenetic analysis in scarce human samples. Eur. J. Immunol. 2014;44:3141–3143. doi: 10.1002/eji.201444627. [DOI] [PubMed] [Google Scholar]
- 74.Gitelman SE, Gottlieb PA, Rigby MR, Felner EI, Willi SM, Fisher LK, et al. Antithymocyte globulin treatment for patients with recent-onset type 1 diabetes: 12-month results of a randomised, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol. 2013;1:306–316. doi: 10.1016/S2213-8587(13)70065-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.DuPage M, Chopra G, Quiros J, Rosenthal WL, Morar MM, Holohan D, et al. The chromatin-modifying enzyme Ezh2 is critical for the maintenance of regulatory T cell identity after activation. Immunity. 2015;42:227–238. doi: 10.1016/j.immuni.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nepom GT, Ehlers M, Mandrup-Poulsen T. Anti-cytokine therapies in T1D: concepts and strategies. Clin. Immunol. 2013;149:279–285. doi: 10.1016/j.clim.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marioni JC, Mason CE, Mane SM, Stephens M, Gilad Y. RNA-seq: an assessment of technical reproducibility and comparison with gene expression arrays. Genome Res. 2008;18:1509–1517. doi: 10.1101/gr.079558.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods. 2008;5:621–628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- 79.Xu X, Zhang Y, Williams J, Antoniou E, McCombie WR, Wu S. Parallel comparison of Illumina RNA-Seq and Affymetrix microarray platforms on transcriptomic profiles generated from 5-aza deoxy-cytidine treated HT-29 colon cancer cells and simulated datasets. BMC Bioinformatics. 2013;14:S1. doi: 10.1186/1471-2105-14-S9-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Geiss GK, Bumgarner RE, Birditt B, Dahl T, Dowidar N, Dunaway DL. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat. Biotech. 2008;26:317–325. doi: 10.1038/nbt1385. [DOI] [PubMed] [Google Scholar]
- 81.Wherrett DK, Chiang JL, Delamater AM, DiMeglio LA, Gitelman SE, Gottlieb PA, et al. Defining pathways for development of disease-modifying therapies in children with type 1 diabetes: a consensus report. Diabetes Care. 2015;38:1975–1985. doi: 10.2337/dc15-1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Herold KC, Gitelman SE, Masharani U, Hagopian W, Bisikirska B, Donaldson D. A single course of anti-CD3 monoclonal antibody hOKT3γ1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes. 2005;54:1763–1769. doi: 10.2337/diabetes.54.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Beam CA, Gitelman SE, Palmer JP. Type 1 Diabetes TrialNet Study Group, Recommendations for the definition of clinical responder in insulin preservation studies. Diabetes. 2014;63:3120–3127. doi: 10.2337/db14-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]