Abstract
Background
It is our hypothesis that high intensity spinal cord stimulation (SCS) to restore an effective cough mechanism using wire leads, will result in significant activation of target neurons without tissue injury or electrode corrosion.
Methods
Adult mini-pigs underwent chronic spinal cord compression, followed by implantation of parallel wire leads on the dorsal epidural surface of the spinal cord, with stimulation contacts at the T9 and T12, and control electrode contacts at the T2 and T5 levels. After 3 months of daily SCS, airway pressure generation (P), tissue in the area of the stimulating and control electrodes and electrode leads were examined. P was also assessed in acute animals, which served as controls.
Results
Mean P at FRC were 54±5cmH2O and 109±11cmH2O in the control and chronically stimulated animals, respectively (p<0.05). There was minimal tissue reaction in the area of the stimulating and control electrodes. All sets of leads revealed no evidence of electrode corrosion.
Comparison with existing methods
Previous porcine models of chronic spinal cord injury (SCI) were developed to study neurological and regenerative outcomes. Our method of chronic SCI porcine model was developed to evaluate the safety of electrical SCS to restore expiratory muscle function.
Conclusion
Chronic SCS with wire lead electrodes results in significant increases in P without evidence of significant adverse tissue reaction, nor evidence of electrode corrosion. This method may be a safe and useful technique to restore a functional cough in spinal cord injured subjects.
Keywords: Expiratory Muscles, Electrical Stimulation, Safety, Rehabilitation
1. Introduction
Optimal electrical stimulation applied to implanted neural prostheses requires stimulus parameters sufficient to activate target neurons or peripheral nerves with the minimum amount of injected current. The success of the implantation also depends on the degree of tissue reaction to the electrical stimulation and any interactions between the tissue and implants. Excessive electrical stimulation can result in neural damage and/or corrosion of metallic implants.
With the goal of activating the expiratory muscles to restore cough in spinal cord injured patients, we have demonstrated in acute animal studies that this can be accomplished with the use of parallel wire leads positioned on the dorsal epidural surface of the spinal cord (Kowalski and DiMarco, 2011). The application of bipolar, high intensity electrical stimulation (40V, 50Hz, 0.2ms pulse width) via electrode contacts (platinum/iridium) at the T9 and T11 levels, results in large airway pressures characteristic of a normal cough.
Based upon the required stimulus parameters and a measured lead impedance of 580Ω, the calculated charge density was ~86 μC/cm2. This value is within the generally accepted safe limits of 100μC/cm2 for tissue damage and 300μC/cm2 for electrode corrosion (Brummer et al., 1977; Brummer and Turner, 1977; Cogan, 2008; Merrill et al., 2005; Yuen et al., 1981). Rose and Robblee (1990), however, suggested lower limits of 50-150μC/cm2 with 0.2ms pulses for electrode corrosion. Moreover, only electrodes with the hemispheric geometries exhibit uniform distribution of charge density (Oldham, 2004). Many electrode designs, including wire electrodes, exhibit non-uniformity of charge density, which may result in substantially higher charge densities at the edges (Cogan, 2008; Kim et al., 1990; Wei and Grill, 2005). In addition, multiple electrodes located in close proximity were stimulated simultaneously, further increasing the potential charge density. Taken together, it is conceivable that the chronic application of these stimulus parameters is potentially harmful.
It is important to note however that most prior safety studies typically employed continuous electrical stimulation for various time periods ranging from a few hours to several weeks and with electrodes in direct contact with neural tissue. In contrast, the proposed application requires only brief intermittent stimulation (0.6s ON-time), 120 times/day, and an extradural electrode location. Each of these factors would be expected to significantly mitigate the potential for neural injury.
The purpose of the present study therefore was to evaluate the effects of chronic electrical stimulation applied to the epidural surface of the spinal cord (T9 and T12 level) to activate expiratory muscles, in an animal model, in the same manner as that which would be employed in our planned clinical trial. Studies were performed in a chronic pig model of spinal cord injury. Outcome measures included measurements of expiratory pressure development to assess cough efficacy, tissue analysis for potential injury and electrode analysis for evidence of potential corrosion. It is our hypothesis that epidural spinal cord stimulation with wire leads, to activate the expiratory muscles, can be applied safely without the development of neural injury and/or electrode corrosion.
2. Methods
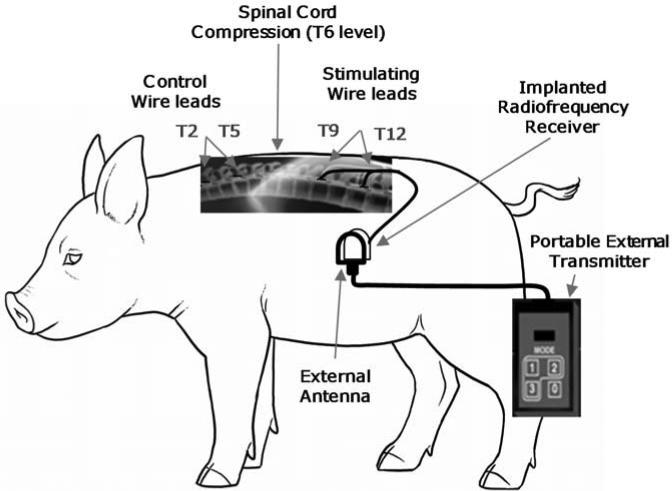
Experiments were performed on 10 mini-pigs (weight: 22.3±0.8 kg) with the approval of the Institutional Animal Care and Use Committee of Case Western Reserve University. All surgical procedures and measurements were the same for each animal. Comparisons were made between six chronic animals that were maintained for three months with daily electrical stimulation (Fig.1) and four animals studied acutely (control animals). Chronic animal procedures, are based on previously described techniques, and therefore presented only briefly (Zurita et al., 2012). Chronic animals were initially pre-medicated with ketamine 20 mg/kg IM, intubated, ventilated and anesthetized with isofluorane (0.5-2%). Intravenous access was obtained via an ear vein to provide IV fluids. Prophylactic antibiotics (Cefazolin 30mg/kg IV) were administered before surgery and every 12h for a total of 4 doses. A catheter inserted in a central ear artery was connected to a calibrated blood pressure transducer for direct arterial measurement of blood pressure. Self-adhering patches were applied to the skin for electrocardiogram and heart rate recording. The level of anesthesia was monitored by response to noxious stimuli. The ventilator was adjusted to maintain an end-tidal CO2 value of 35-45 mmHg. To achieve this value, the tidal volume was initially set at 10ml/kg body weight with a respiratory rate of 15 breaths per minute. Arterial blood pressure, ECG, SpO2, and end-tidal CO2 were monitored throughout the procedures (Waveline Pro Multi-Function Monitor, DRE Inc, Louisville, KY). Body temperature was maintained with a heating blanket (Harvard Apparatus, Cambridge, MA) at 38 ± 0.5°C. Under aseptic conditions, a laminectomy was performed at the T6 level to induce spinal cord injury and allow placement of the stimulating and control wire leads (1.4mm diameter) with two circumferential electrical platinum/10% iridium alloy (Pt/10%Ir) contacts (6mm contact with 50mm center to center spacing) (Ardiem Medical, Inc., Indiana, PA) (Fig.2). Spinal cord injury was induced by the epidural application of two surgical Heifetz's clips for 30 min. via the laminectomy incision, two wire leads (stimulating) were inserted in parallel on the dorsal epidural surface of the spinal cord with one contact positioned at the T9 level (anode) and the other at the T12 (cathode) spinal level under fluoroscopic guidance (Fig.3). Through the same incision, two separate leads (non-stimulating) were positioned rostral to this area over the T2 and T4 spinal level and served as controls. The wires were tunneled under the skin and connected to a radiofrequency receiver (Finetech Medical LTD, Welwyn Garden City, UK), which was implanted under the skin over the lateral portion of the abdominal wall. Stimulation (40V, 50Hz) was applied intra-operatively to verify expiratory muscle contraction. The wounds were covered with hydrofiber dressing, which was then secured with an adhesive bandage.
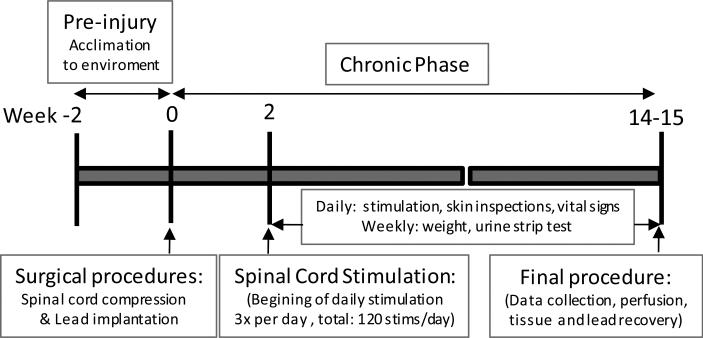
Fig. 1.
Illustration of the chronic experimental procedure.
Fig. 2.
Experimental setup.
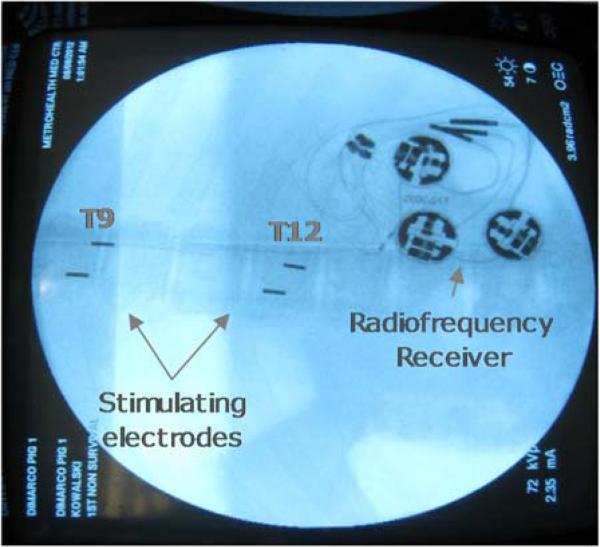
Fig. 3.
Radiograph demonstrating the position of the spinal cord stimulating electrodes and radiofrequency receiver in a chronic animal.
Following surgery, each of the chronic animals was maintained in separate cages. All animals received a fentanyl patch from the time period from 2 days prior to the procedure (to build up adequate systemic drug levels prior to the procedure) through 2 days after the procedure for management of post-operative pain. They also received ketorolac (15 mg IM Q12h) for 2 days after the procedure for management of inflammation and pain. The bladder was expressed and stool eliminated three times per day by manual compression for the first 48 to 72h. Animals were fed with a low energy, high fiber diet to control growth. The condition of the skin was monitored daily. Each animal was weighed weekly.
2.1 Protocol
In the chronic animals, bipolar stimulation of the parallel wires was applied (T9, anode; T12, cathode) to activate the expiratory muscles, 10-14 days post-operatively (Fig.1). This was accomplished by placing the external stimulator transmitter over the implanted receiver and activating the control unit (Fig.2). Electrical stimulation was applied with the maximum stimulus paradigm to be used in a future clinical trial. The stimulus configuration employed in this study was a charge-balanced asymmetric, biphasic waveform with no delay, cathodic-first, with stimulus amplitude 40V, pulse width of 200μs and frequency of 50Hz. Stimulus bursts lasting 0.6s (ON-time) was delivered once every 10s (OFF-time) for a total of 40 stimulations, three times daily, achieving 120 stimulations/day. Based upon the results of our previous clinical trial, this number of stimulations exceeds the maximum expected daily use by 20%, to provide an added margin of safety. The stimulation was well tolerated, as the animals did not manifest any indication of discomfort or pain. The animals were stimulated for ~3 months (mean: 87±2 days), following which the animals underwent a final procedure. During this final procedure, the animals were pre-medicated with ketamine 20 mg/kg IM and anesthetized with pentobarbital (1-2mg/kg/hr), intubated and placed on mechanical ventilation.
2.2 Assessment of airway pressure generation
SCS was applied over a range of stimulus parameters (10-40V, 10-50Hz, 0.2ms). Pressure generation was always assessed during SCS under conditions of tracheal occlusion and following hyperventilation-induced apnea at functional residual capacity (FRC) and over a wide range of lung volumes (between 0.3 liter below and 1.0 liter above FRC). A 2-liter calibration volume syringe was used to apply lung deflations and inflations, the magnitude of which was assessed by the corresponding changes in airway pressure (pre-contractile airway pressures). The relationship between stimulus amplitude (10-40V @ 50Hz and 0.2ms pulse duration) and airway pressure generation was evaluated. A comprehensive evaluation of pressure generation capacity during SCS was performed during the final procedure in the chronic animals and also in the acute animal studies.
2.3 Tissue evaluation
The spinal cord tissue was processed and evaluated microscopically by an independent board-certified veterinary pathologist who specializes in biological safety evaluation (NAMSA, Northwood, OH, USA). Histological evaluations were conducted on all animals to assess the effects of stimulated and non-stimulated electrodes on surrounding spinal tissue. During the final surgical procedure, animals were deeply anesthetized with sodium pentobarbital, exsanguinated and underwent whole body perfusion of 0.9% saline and 10% neutral buffered formalin. A dorsal laminectomy of the spinal cord was performed; the spinal cord adjacent to the electrodes was labeled and the electrodes removed. No electrode migration was observed in any animals. The spinal cord with dura mater was then fixed in 10% neutral buffered formalin to complete the fixation process. Seven sections of spinal cord with dura mater were obtained from each animal at the following sites: at the level of the control electrodes (T2 and T5); between T5 and T6; at T6; between T6 and T9 and, at the level of the stimulating electrodes (T9 and T12) (Fig.3). Tissues were processed using standard histological techniques. Tissues were embedded in paraffin followed by sectioning (5μm thick) and stained with hematoxylin and eosin. The control sites and the stimulated sites were evaluated for irritation including inflammation, tissue necrosis, neovascularization, fibrosis and fatty infiltration.
2.4. Electrode evaluation
At Case Western Reserve University's School of Engineering (Swagelok Center for Surface Analysis of Materials), the platinum/10% iridium alloy (Pt/10%Ir) electrodes (6mm length) were investigated for signs of corrosion including pitting, fatigue fracture (cracking), intergranular corrosion, mechanical damage and surface deposits using the Helio NanoLab 650 Scanning Electron Microscope (accelerating voltage of 5kV with a 1.6nA current). Each lead was examined with 4 different horizontal field widths (HFW) (2.89mm, 256μm, 102μm, 51.2μm) at a 20 degree tilt with 0 and 180 degree rotations.
2.5. Data Analyses
Airway pressures resulting from SCS at specific pre-contractile airway pressures were obtained by interpolation of plots from individual animals. This data was used to determine mean relationships between passive pre-contractile, and generated airway pressures during SCS. Data from the acute and chronic animal groups were compared statistically by oneway ANOVA and Student's t-tests. Data are reported as means ± SE. Statistical significance was determined by P values of <0.05.
The reaction at the implant sites was graded based on the scoring scheme in Annex E of ISO 10993-6, which offers general guidelines for evaluating local tissue responses to implants. Briefly, the intensity of these histological items was ranked as 0 = no response, 1 = minimal, 2 = mild, 3 = moderate and 4 = severe, and the average score was used to determine irritant ranking for stimulated and control sites. The difference in mean irritant score was calculated as follows:
Difference score = mean test score for stimulated site - mean test score for control site
According to ISO guidelines, the implants can be considered nonirritant if the Difference Score ranges between 0.0-2.9 and considered slight, moderate and severe irritants for scores between 3.0 and 8.9, 9.0-15.0, and >15.1, respectively.
3. Results
All animal tolerated chronic intermittent stimulation without evidence of discomfort. SCS resulted in some mild contraction of the hindlimb muscles. No other side effects were observed. Importantly, there were no instances of bowel or bladder leakage.
3.1. Effects of chronic SCS on expiratory pressure generating capacity
Following the first post-surgical week, there was no change in body weight (mean 99.8±2.5% of pre-surgical weight). Subsequently, body weight increased from 18.3±0.6 (pre-surgical weight) to 21.4±0.9kg over the course of the 3 month study period (mean percent increase 17.2±1.7%) (p<0.05).
Body weight of the acute (control) (23.5±1.3kg) and chronic animals (21.4±0.9kg) during the final evaluation were not statistically significantly different (NS).
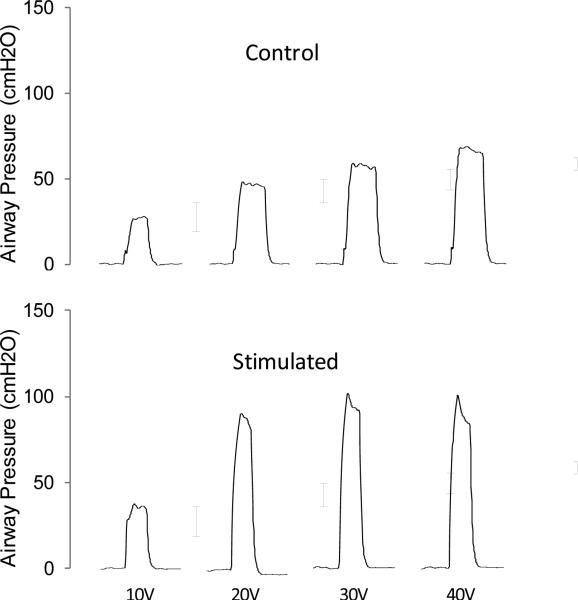
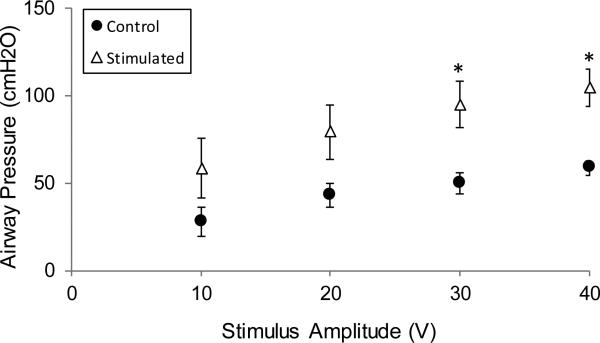
The effect of varying stimulus amplitudes on airway pressure generation during SCS for representative animals and mean data are shown in Figs. 4 and Fig. 5, respectively. In the control and chronic stimulated groups, increases in stimulus amplitude (10-40V) at FRC resulted in progressive increases in airway pressure generation (statistically significant at 30 and 40V; p<0.05 for each).
Fig. 4.
Relationship between stimulus amplitude and airway pressure generation in a representative control (acute) and chronically stimulated animal.
Fig. 5.
Relationship between stimulus amplitude and airway pressure generation in the control (acute animals) (●) and chronically stimulated groups (Δ). The magnitude of airway pressure generation was higher in the chronically stimulated group at each stimulus amplitude, (statistically significant at 30V and 40V) (* p<0.05).
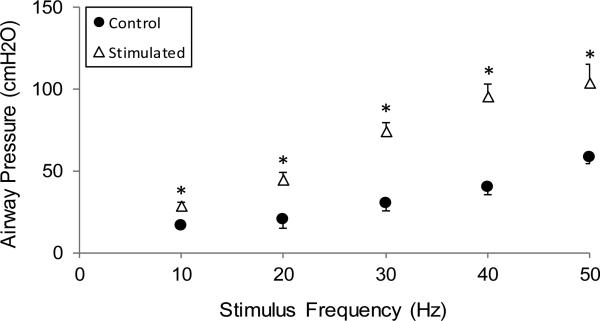
The effects of varying stimulus frequencies (10 - 50Hz) on airway pressure generation during SCS are shown in Fig. 6. In both the control and stimulated groups, increases in stimulus frequencies also resulted in progressive increases in pressure generation (p<0.05, for each).
Fig. 6.
Relationship between stimulus frequency (10-50Hz) and airway pressure generation in the control (acute animals) (●) and the chronically stimulated groups (Δ). Values are group mean values ±SE. In the chronically stimulated group, the magnitude of airway pressure generation during SCS was significantly higher compared to the control group at all stimulus frequences (* p<0.05).
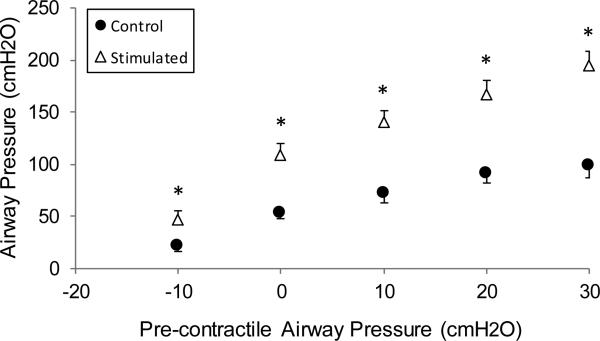
The mean effects of SCS on expiratory airway pressure generation, over a wide range of pre-contractile airway pressure are shown in Fig. 7. As expected, airway pressure generation increased progressively with increasing pre-contractile airway pressure. Following chronic SCS however, airway pressure generation was higher compared with control group values, at all lung volumes (p<0.05, for each if compared to control), ranging between 184 and 201% of control values. For example, mean control airway pressures were 22±3, 54±5 and 99±12 cmH2O for −10 cmH2O, FRC, +30 cmH2O respectively, and 46±10, 109±11 and 194±14 cmH2O respectively, in the chronically stimulated animals (p<0.05, for each).
Fig. 7.
Effect of lower SCS on airway pressure generation over a wide range of lung volumes (expressed as pre-contractile airway pressure) in the control (acute animals) (●) and in the chronically stimulated groups (Δ). At all lungs inflation volumes, the magnitude of airway pressure generation was significantly higher in the stimulated group (* p<0.05).
3.2. Tissue Responses to Chronic SCS
Spinal cord sections were obtained at the same levels in all 6 animals as described in section 2.3.
Marked tissue degeneration/necrosis of both gray and white matter with tissue dropout was observed at the point of induced injury (T6), and at the sites immediately cranial (T5) and caudal (T7) to the site of injury. Spinal cord sections cranial to the site of injury generally showed moderate to marked axonal degeneration, characterized by dilated myelin sheaths, axonal spheroids and macrophages. These changes in the cranial levels (T2 and T5) were often most severe in the medial portion of the dorsal columns and the peripheral tracts of the lateral and ventral funiculi. At spinal cord levels caudal to the site of injury, the axonal degeneration in the lateral and ventral funiculi was more diffuse in the form of extensive lesions in white matter tracts, but the dorsal columns were often somewhat spared. This pattern of changes is consistent with spinal cord trauma. No stimulation-related microscopic changes in neuronal tissue of the spinal cord (T9 and T12 levels) were identified.
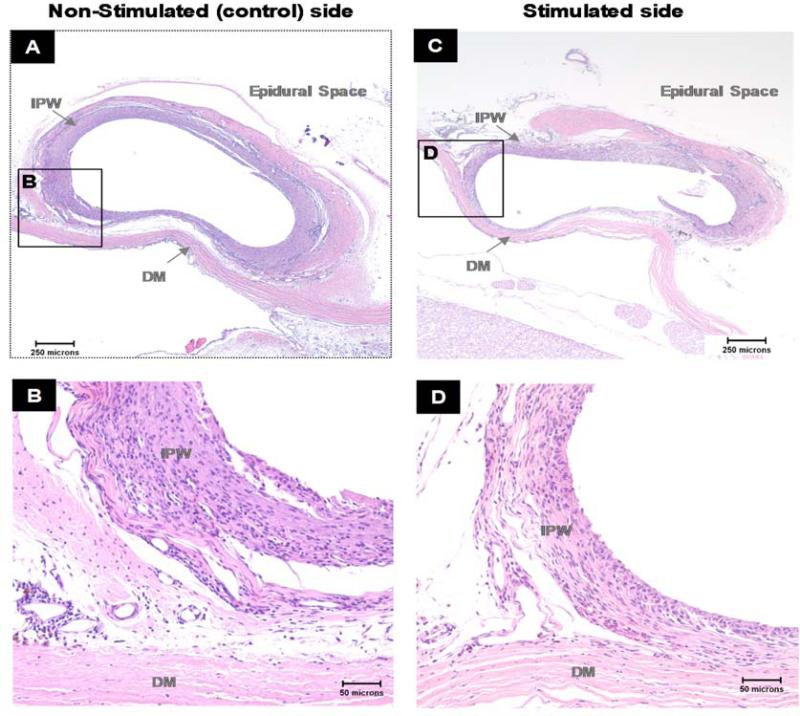
There was minimal reaction to the stimulated and control electrodes in all animals (Fig.8). The electrodes were surrounded by a thin rim of fibrous tissue and minimal to mild inflammatory cells, predominantly lymphocytes and macrophages, with occasional polymorphonuclear cells and giant cells. There were limited microscopic cellular and tissue responses to both the stimulated and control electrodes, and no adverse soft tissue reactions were noted. The average microscopic subcutaneous implant site irritant index and irritant scores for the stimulated and non-stimulated electrode (control) implant were 7.4±0.7 and 9.3±1.1, respectively (NS). Since the group mean irritant scores for the stimulated and non-stimulated electrodes were similar to each other, the stimulated electrodes were considered a non-irritant compared to the non-stimulated electrodes. Therefore, no adverse tissue responses in the spinal cord were attributable to the electrode implants.
Fig. 8.
Photomicrographs of cross-sections of the implanted electrode pockets taken from the non-stimulated side at the T5 spinal level (A,B) and stimulated side at T9 spinal level (C,D) in a representative animal. H&E stain.
B and D - higher magnification of section A and C respectively.
Non-stimulated (control) and stimulated electrode implants were surrounded by a rim of fibrous tissue with minimal to mild infiltration of mixed inflammatory cells (macrophages, polymorphonuclear cells and lymphocytes). The stimulated and control implant pockets could not be visually distinguished and scored similarly on the irritation assessment. IPW – implant pocket wall, DM - dura mater
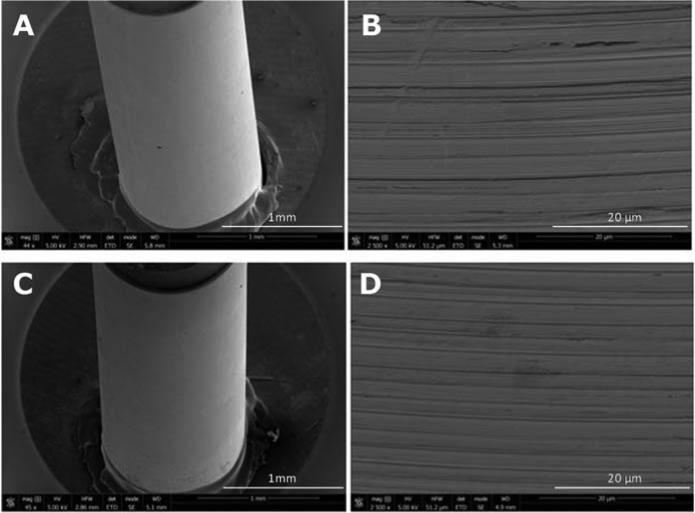
3.3. Effects of Chronic SCS on Implanted Electrodes
An example of the surface condition of the stimulated and control electrode leads is shown in Fig. 9. The stimulating electrodes did not demonstrate any evidence of corrosion including pitting and/or fatigue fracture. However, expected manufacture marks were apparent in all of the evaluated leads. Small scratches presumably due to using metallic forceps during surgery and/or mounting electrodes for evaluation in some samples were observed. The stimulated and non-stimulated electrodes could not be visibly distinguished.
Fig. 9.
Scanning Electron Microscopy (SEM) of a control (A, B) and chronically stimulated electrode (B,D) used in this study. The horizontal lines represent manufacture marks (B,D) which were apparent in all of the evaluated electrodes. All sets of electrodes revealed no evidence of corrosion. More specifically there was no pitting or evidence of fatigue fracture. The stimulated and control electrodes could not be visibly distinguished.
4. Discussion
The results of the present study provide convincing evidence that the stimulus paradigm necessary to fully activate the expiratory muscles, applied with platinum/iridium wire electrodes, can be utilized safely for prolonged periods. Following a 3-month stimulation period, both careful tissue and electrode analyses did not demonstrate any significant tissue reaction at stimulation sites, nor signs of electrode corrosion. Further defining safety, is the fact that the output parameters in terms of the pressure generating capacity of the expiration muscles gradually increased and exceeded control values, as expected, due to training effects.
4.1. Study Limitations
While the spinal cord of the mini-pig is smaller than humans, the mini-pig is the largest animal species known to tolerate paraplegia. Moreover, the mini-pig spinal cord (~0.5 the size of the human cord) was large enough for placement of the same parallel wire leads, expected for use in clinical trials. It is likely therefore that the results obtained in this species are valid.
The stimulus paradigm utilized in the present study exceeded the maximum expected daily use by ~20%. Moreover, due to the smaller spinal cord in mini-pigs, the charge density was applied over a smaller area, compared to that proposed in humans, further enhancing the evidence for safety.
A comprehensive analysis of the response to SCS was not performed during the initial surgery in the chronic animals to minimize operative time and minimize the potential for complications. However, since electrode placement was virtually identical in the acute, control animals, the degree of expiratory muscle activation should have been the same in both groups of animals, allowing for a valid control group.
The results of this study are limited to the specific lead configuration and dimensions, and stimulus paradigm employed in our protocol. It is conceivable, even likely, that use of alternate lead designs or magnitude of charge delivery would have resulted in different results. Therefore, it is not possible to extrapolate these results to other electrodes or use of other stimulus paradigms.
4.2. Comparison to Previous Studies
There are no specific studies evaluating the potential for neural injury or electrode corrosion with wire electrodes employing the proposed stimulus paradigm. However, there has been extensive work assessing the thresholds for neural injury utilizing other electrode systems. This data has been reviewed in several recent articles and therefore is discussed here only briefly (Cogan, 2008; Harnack et al., 2004; Merrill et al., 2005; Merrill, 2010; Van Kuyck et al., 2007).
First, it is important to mention that electrical stimulation was applied with a charge-balanced waveform in this study (i.e. cathodal and anodal phases of equal charge/phase), as charge imbalance has been known for many years to result in a much higher incidence of tissue injury and electrode damage (Merrill et al., 2005).
Yuen et al. (1981) observed neural damage and increased permeability of the blood-brain barrier to Evans Blue dye after 6 hours of continuous stimulation at 50 Hz, at a charge density of 100 μC/cm2 per phase or greater at a charge per phase of 1 μC per phase or greater. Minimal damage occurred in animals stimulated for 20 hours or more at a charge density of 40 μC/cm2. They concluded that this latter charge density was the threshold for neural injury. McCreery and co-investigators determined that charge density and charge per phase interact synergistically to determine the threshold for histologically detectable neural damage (McCreery et al., 1990). In their model, platinum electrodes of various sizes were implanted subdurally over the parietal cortex of adult cats and pulsed continuously for 7 hours at 50Hz. They developed a plot of various combinations of charge density and charge per phase for which localized neural damage occurred. Based upon this plot, the highest calculated charge density of 86 μC/cm2 and charge/phase of 21 μC utilized in the present study, exceed the proposed safety limits. Moreover, a known limitation of charge density and charge per phase estimates with macroelectrodes is the non-uniform current distribution that leads to larger potentials at the edge of disc-shaped electrodes and at the tips of conical electrodes (Cogan, 2008; Kim et al., 1990; Wei and Grill, 2005). For three-dimensional electrodes, there is an additional non-uniformity in potential through the thickness of the electrode coating, which can be quite large (Suesserman et al., 1991). Based upon charge density and charge/phase measurements alone therefore, tissue injury and/or electrode corrosion would have been expected in the present study.
In comparison to these prior studies however, the electrodes were placed in the extradural location, stimulation was provided for very brief periods (~0.5s) and only intermittently in the present study. Each of these factors mitigated against the development of neural injury or electrode corrosion and are the mostly likely explanations of the observed results demonstrating no evidence of tissue injury.
4.3. Clinical Implications
In prior human clinical trials, we determined that electrical stimulation applied to the epidural surface of the spinal cord via platinum/iridium disc electrodes (4mm diameter) was a safe and effective method of restoring an effective cough mechanism in spinal cord injured subjects (DiMarco et al., 2006, 2009a, 2009b, 2014). Use of this technique results in a significant reduction in the incidence of respiratory tract infections and improvement in life quality. Electrode placement however required an invasive procedure involving multiple laminotomy incisions, requiring a ~5-7 hour surgical procedure and 2-3 day hospitalization. In acute animal experiments, we demonstrated that comparable activation of the expiratory muscles could also be achieved with bipolar stimulation utilizing parallel wire electrodes, which can be implanted using minimally invasive techniques. The results of the present study strongly suggest that the intermittent application of high intensity electrical stimulation can be applied safely with wire electrodes in future clinical trials. This method would offer potential patients a safer and less costly method to restore expiratory muscle function and an effective cough mechanism. Ultimately, restoration of an effective cough offers the potential to significantly reduce the morbidity and mortality related to respiratory complications in the spinal cord injured population.
HIGHLIGHTS.
An experimental model of chronic paraplegia was induced in mini-pigs by spinal cord compression.
The effects of long-term, high intensity, intermittent epidural electrical stimulation via wire electrodes on expiratory muscle force generation, potential tissue injury and electrode corrosion, was examined.
The daily application of high intensity electrical stimulation for brief periods to activate the expiratory muscles results in large airway pressures characteristic of a normal cough.
Importantly, electrical stimulation does not result in any evidence of tissue injury or electrode damage.
Acknowledgements
This work was supported by the NIH-NINDS 5U01NS 83696. The authors would like to thank Dana Hromyak and Ujwal Parikh for their assistance with this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brummer SB, McHardy J, Turner MJ. Electrical stimulation with Pt electrodes: Trace analysis for dissolved platinum and other dissolved electrochemical products. Brain Behav Evol. 1977;14:10–22. doi: 10.1159/000124611. [DOI] [PubMed] [Google Scholar]
- Brummer SB, Turner MJ. Electrochemical considerations for safe electrical stimulation of the nervous system with platinum electrodes. IEEE Trans Biomed Eng. 1977;24:59–63. doi: 10.1109/TBME.1977.326218. [DOI] [PubMed] [Google Scholar]
- Cogan SF. Neural stimulation and recording electrodes. Annu Rev Biomed Eng. 2008;10:275–309. doi: 10.1146/annurev.bioeng.10.061807.160518. [DOI] [PubMed] [Google Scholar]
- DiMarco AF, Kowalski KE, Geertman RT, Hromyak DR. Spinal cord stimulation: a new method to produce cough in patients with spinal cord injury. Am J Respir Crit Care Med. 2006;173:1386–9. doi: 10.1164/rccm.200601-097CR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMarco AF, Kowalski KE, Geertman RT, Hromyak DR. Lower thoracic spinal cord stimulation to restore cough in patients with spinal cord injury: results of a National Institutes of Health-sponsored clinical trial. Part I: methodology and effectiveness of expiratory muscle activation. Arch Phys Med Rehabil. 2009;90:717–25. doi: 10.1016/j.apmr.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMarco AF, Kowalski KE, Geertman RT, Hromyak DR, Frost FS, Creasey GH, Nemunaitis GA. Lower thoracic spinal cord stimulation to restore cough in patients with spinal cord injury: results of a National Institutes of Health-sponsored clinical trial. Part II: clinical outcomes. Arch Phys Med Rehabil. 2009;90:726–32. doi: 10.1016/j.apmr.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMarco AF, Kowalski KE, Hromyak DR, Geertman RT. Long-term follow-up of spinal cord stimulation to restore cough in subjects with spinal cord injury. J Spinal Cord Med. 2014;37:380–8. doi: 10.1179/2045772313Y.0000000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnack D, Winter C, Meissner W, Reum T, Kupsch A, Morgenstern R. The effects of electrode material, charge density and stimulation duration on the safety of high-frequency stimulation of the subthalamic nucleus in rats. J Neurosci Methods. 2004;138:207–16. doi: 10.1016/j.jneumeth.2004.04.019. [DOI] [PubMed] [Google Scholar]
- Kim Y, Zieber HG, Wang FE. Uniformity of current density under stimulating electrodes. Crit Rev Biomed Eng. 1990;17:585–619. [PubMed] [Google Scholar]
- Kowalski KE, DiMarco AF. Comparison of wire and disc leads to activate the expiratory muscles in dogs. J Spinal Cord Med. 2011;34:600–8. doi: 10.1179/2045772311Y.0000000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCreery DB, Agnew WF, Yuen TG, Bullara L. Charge density and charge per phase as cofactors in neural injury induced by electrical stimulation. IEEE Trans Biomed Eng. 1990;37:996–1001. doi: 10.1109/10.102812. [DOI] [PubMed] [Google Scholar]
- Merrill DR. The Electrochemistry of Charge Injection at the Electrode/Tissue Interface. Chapter-Implantable Neural Prostheses 2, Part of the series Biological and Medical Physics, Biomedical Engineering. 2010:85–138. [Google Scholar]
- Merrill DR, Bikson M, Jefferts JG. Electrical stimulation of excitable tissue: design of efficacious and safe protocols. J Neurosci Methods. 2005;141:171–98. doi: 10.1016/j.jneumeth.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Oldham K. Electrode “edge effects” analyzed by the Green function method. J Electroanal Chem. 2004;570:163–170. [Google Scholar]
- Rose TL, Robblee LS. Electrical stimulation with Pt electrodes. VIII. Electrochemically safe charge injection limits with 0.2 ms pulses. IEEE Trans Biomed Eng. 1990;37:1118–20. doi: 10.1109/10.61038. [DOI] [PubMed] [Google Scholar]
- Suesserman MF, Spelman FA, Rubinstein JT. In vitro measurement and characterization of current density profiles produced by non-recessed, simple recessed, and radially varying recessed stimulating electrodes. IEEE Trans Biomed Eng. 1991;38:401–8. doi: 10.1109/10.81558. [DOI] [PubMed] [Google Scholar]
- Van Kuyck K, Welkenhuysen M, Arckens L, Raf Sciot R, Nuttin B. Histological Alterations Induced by Electrode Implantation and Electrical Stimulation in the Human Brain: A Review, Neuromodulation: Technology at the Neural Interface. 2007;10:244–61. doi: 10.1111/j.1525-1403.2007.00114.x. [DOI] [PubMed] [Google Scholar]
- Wei XF, 1, Grill WM. Current density distributions, field distributions and impedance analysis of segmented deep brain stimulation electrodes. J Neural Eng. 2005;2:139–47. doi: 10.1088/1741-2560/2/4/010. [DOI] [PubMed] [Google Scholar]
- Yuen TG, Agnew WF, Bullara LA, Jacques P, McCreery DB. Histological evaluation of neural damage from electrical stimulation: considerations for the selection of parameters for clinical application. Neurosurgery. 1981;9:292–99. [PubMed] [Google Scholar]
- Zurita M, Aguayo C, Bonilla C, Otero L, Rico M, Rodriguez A, Vaquero J. The pig model of chronic paraplegia: A challenge for experimental studies in spinal cord injury. Prog Neurobiol. 2012;97:288–303. doi: 10.1016/j.pneurobio.2012.04.005. [DOI] [PubMed] [Google Scholar]