Abstract
The cornea contains a heterogeneous population of antigen-presenting cells with the capacity to contribute to immune responses. Adenovirus keratitis is a severe corneal infection with acute and chronic phases. The role of resident corneal antigen-presenting cells in adenovirus keratitis has not been studied. We utilized transgenic MaFIA mice in which c-fms expressing macrophages and dendritic cells can be induced to undergo apoptosis, in a mouse model of adenovirus keratitis. Clinical keratitis and recruitment of myeloperoxidase and CD45+ cells were diminished in c-fms depleted, adenovirus infected mice, as compared to controls, consistent with a role for myeloid-lineage cells in adenovirus keratitis.
Keywords: epidemic keratoconjunctivitis, adenovirus keratitis, adenovirus, macrophage, dendritic cell, neutrophil, leukocyte, MaFIA mice, host-pathogen interaction
Epidemic keratoconjunctivitis (EKC), a highly contagious eye disease, is one of the most common eye infections worldwide, with outbreaks associated most commonly with health-care transmission during ophthalmologic examinations (Centers for Disease Control 2013). Human adenovirus (HAdV) types 8, 37 and 64 are the principal causes of EKC. Clinical signs of infection include conjunctival lymphoid hyperplasia, hemorrhage, and exudation, preauricular lymphadenopathy, and corneal epithelial erosions, appearing within two weeks after exposure to secretions from another infected person. Following the acute phase, patients develop multifocal, subepithelial, leukocytic infiltrates of the corneal stroma, the hallmark of EKC. Corneal infiltrates can become chronic in up to one-third of cases, and impact corneal transparency which results in reduced vision (Butt and Chodosh 2006). However, no vaccine or specific antiviral treatment is currently available to prevent the corneal manifestations of adenovirus keratitis.
The human cornea is transparent and avascular tissue with epithelial, stromal, and endothelial layers. The stromal layer is a finely organized collagenous matrix populated predominantly by fibroblast-like keratocytes, with a smaller proportion of resident bone marrow-derived cells, including macrophages and dendritic cells (Brissette-Storkus et al. 2002). Constituent cells in the cornea respond to pathogenic or mechanical insults by the induction of chemokines, allowing the initiation of innate and adaptive immune responses (Chinnery et al. 2009; Leal et al. 2010). Specifically, bone marrow derived cells present in the corneal stroma play an important role in the first line of defense against infection (Chinnery et al. 2009).
Using a well-established mouse model of adenovirus keratitis (Chintakuntlawar et al. 2007; Chintakuntlawar and Chodosh 2009; Chintakuntlawar et al. 2010), we previously demonstrated that adenovirus keratitis is a consequence of chemokine expression by infected corneal stromal cells with a lesser contribution from corneal epithelial cells, and subsequent leukocyte recruitment to the subepithelial corneal stroma (Mukherjee et al. 2015). However, a definitive role for corneal dendritic cells and macrophages in the innate immune response to adenovirus keratitis has not been demonstrated. To determine if resident macrophages and dendritic cells contribute to the initial recognition of adenoviruses in the cornea, we utilized Macrophage Fas-Induced Apoptosis (MaFIA) mice, which express eGFP and a membrane bound suicide protein under the control of the myeloid-lineage specific c-fms promoter (Chinnery et al. 2009). In these mice, all macrophages and dendritic cells express eGFP constitutively. When treated with the FK506 dimerizer AP20187, which cross-links to a binding domain in the membrane bound suicide protein, c-fms+ cells in MaFIA mice undergo apoptotic cell death (Burnett et al. 2004; Sun et al. 2010). We show herein that clinical keratitis, myeloperoxidase expression, and infiltration of CD45+ cells were reduced post HAdV-D37 infection of AP20187 treated MaFIA mice, as compared to untreated and control vehicle treated mice. These results suggest a requisite role for resident corneal macrophages and dendritic cells in the development of adenovirus keratitis.
The following methodology was utilized in this study. Human adenovirus type 37 species D (HAdV-D37, GenBank accession number DQ900900.1) was obtained from the American Type Culture Collection (ATCC, Manassas, VA), grown on A549 cells (CCL-185, ATCC), and purified by cesium chloride gradient as previously described (Chintakuntlawar et al. 2007). Purified virus was titered by Tissue Culture Infectious Dose (TCID) assay, and confirmed free of endotoxin and mycoplasma contamination by standard assays. MaFIA (C57BL/6-Tg(Csf1r-EGFP-NGFR/FKBP1A/TNFRSF6)2Bck/J) mice were purchased from Jackson Laboratories (Bar Harbor, ME), and 8 to 12-week old female mice used for experiments. Lyophilized AP20187 (Clontech, Mountain View, CA) was dissolved in 100% ethanol at a concentration of 13.75 mg/ml, and stored protected from light at −20°C. For intraperitoneal (IP) injection, AP20187 stock solution was diluted to 0.55mg/ml in 4% ethanol, 10% PEG-400 and 1.7% Tween-80 in sterile water, and administered by daily IP injection within 30 min after preparation. Injections were continued daily for 5 days, followed by two days rest prior to infection. Vehicle solution was prepared and administered similarly, but without the AP20187. The use of mice in this study was approved by the Massachusetts Eye and Ear Infirmary Animal Care Committee, and animals were treated according to the Association for Research in Vision and Ophthalmology (ARVO) statement for the use of animals in ophthalmic and vision research.
Adenovirus keratitis was induced as previously described (Chintakuntlawar et al. 2007). Briefly, mice were anesthetized by intramuscular injection of ketamine (85 mg/kg) and xylazine (14 mg/kg), and topical anesthetic eyedrops (0.5% proparacaine hydrochloride, Alcon, Fort Worth, TX). Under a surgical microscope (Carl Zeiss Meditec, Inc., Thornwood, NY), 1 μl containing 105 TCID of HAdV-D37 was injected into the central corneal stroma using a heat-pulled, glass micropipette needle and a gas-powered microinjection system (MDI, South Plainfield, NJ) (Chodosh 2006). At 4 days post injection (dpi), corneas were photographed, and mice euthanized using CO2 inhalation, followed by dissection and processing of each cornea for confocal microscopy and flow cytometry.
For confocal microscopy, mouse corneas were harvested at 4 dpi with HAdV-D37, and fixed with 4% paraformaldehyde for 30 minutes at 25°C. To detect neutrophils, cells were fixed and stained with an antibody to myeloperoxidase (Abcam, Cambridge, MA), and with DAPI (Thermo Fisher Scientific, Waltham, MA), a nuclear marker. Corneas were cut radially to flatten them, and coverslipped using mounting medium. Cornea samples were scanned with a Leica laser scanning microscope (TCS SP5, Leica, Heidelberg, Germany). The represented images were confirmed as representative by a masked observer.
For flow cytometry, corneas were harvested at 4 days dpi, cut into 1–2 mm diameter fragments, and digested with 1 mg/ml collagenase type I and 0.5 mg/ml DNase (Sigma Chemical Co., St. Louis, MO). Resulting single-cell suspensions were washed twice in PBS (300 × g, 5 min/wash), incubated on ice for 15 minutes in 100 μl PBS-1% bovine serum albumin (BSA) with 2 μl anti-mouse Fc (BD Pharmingen, San Diego, CA), centrifuged (300 × g, 5 min), and resuspended in 5% normal rat serum (Jackson Immuno Research Inc., West Grove, PA) for 15 minutes on ice. Subsequently, cells were labeled with 4 μl anti-mouse FITC-conjugated anti-CD45 (clone 30-F11, BD Pharmingen), incubated on ice for 30 minutes in the dark, washed 3 times with PBS-1% BSA (300 × g, 5 min/wash), and resuspended in PBS -1% paraformaldehyde. Samples were incubated at 4°C overnight in the dark, pelleted, and resuspended in PBS-1% BSA. Flow cytometry for CD45+ events was performed using a Cytomics FC500 (Beckman Coulter, Brea, CA) flow cytometer.
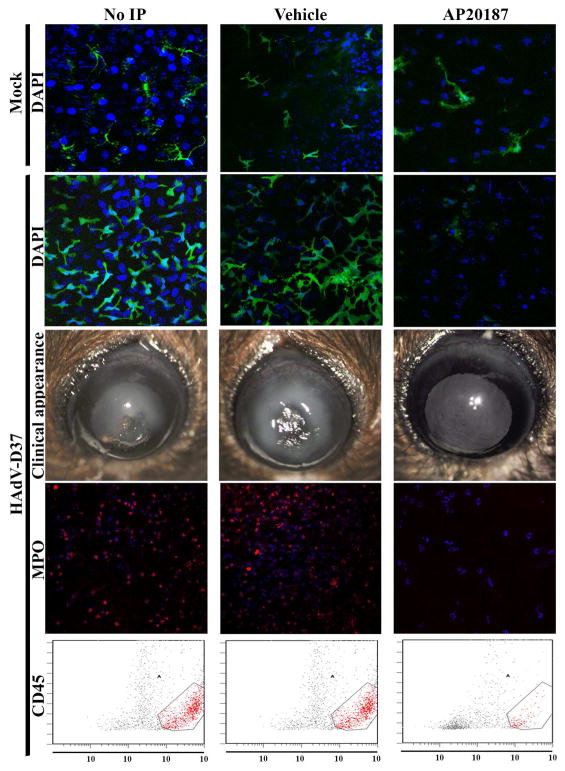
To confirm the presence of resident macrophages and dendritic cells in the healthy mouse cornea, MaFIA mice were injected IP with AP20187 or control vehicle once daily for five days, and euthanized two days later (Chinnery et al. 2009) for confocal microscopy to detect eGFP expressing (c-fms+) cells. As shown, (Fig. 1, top row), eGFP expressing macrophages and dendritic cells were evident in the corneas of untreated and vehicle treated mice, but less common in the AP20187 treated mice, where the remaining eGFP+ cells showed an abnormal morphology.
Fig. 1. The role of myeloid lineage, c-fms+ cells in adenovirus keratitis.
MaFIA mice without pretreatment (left column), pretreated IP daily for 5 days with vehicle (middle column), or with AP20187 (right column), were injected two days later in the right corneal stroma with either buffer control (top row) or 1×105 TCID of HAdV-D37 (rows 2–5) and assessed at 4 days post infection (dpi). Top row. Representative confocal microscopic image from MaFIA mouse cornea two days following the fifth day of IP injections, and 4 days post corneal stromal injection with dialysis buffer as a negative control (Mock), shows a reduction of eGFP expressing cells only in the AP20187 treated animal with an abnormal morphology apparent in the few remaining eGFP+ cells (right panel). Corneal stromal cell nuclei were stained with DAPI (blue). Second row. Confocal microscopy of corneas of HAdV-D37 infected MaFIA mice in the absence of AP20187 pretreatment (untreated or vehicle treated) show corneal infiltration of eGFP+ cells. AP20187 pretreated mice (right panel) did not develop eGFP+ cellular infiltration in the corneal stroma. Third row. Representative clinical photographs of HAdV-D37 infected corneas at 4 dpi. Corneas of untreated and vehicle pretreated mice developed dense corneal opacity (keratitis), while AP20187 pretreated mouse corneas (right panel) remained clear. Fourth row. Confocal microscopic images with anti-myeloperoxidase antibody staining (red) in corneas of HAdV-D37 infected MaFIA mice without AP20187 pretreatment (untreated or vehicle treated) show corneal stromal myeloperoxidase expression. Myeloperoxidase expression in AP20187 pretreated mice was minimal (right panel). Fifth row. Flow cytometry analysis shows greater CD45+ cell infiltration into corneas of HAdV-D37 infected MaFIA mice without AP20187 pretreatment (untreated or vehicle treated), than in AP20187 treated mice. Data are representative of at least two independent experiments, with four mice per time point.
We next examined whether eGFP+ macrophages and dendritic cells were recruited to the corneal stroma in adenovirus keratitis. We pretreated MaFIA mice as described above, followed two days after the last IP injection of AP20187 or vehicle control by injection of 1 μl containing 105 TCID of HAdV-D37, a common agent of EKC, into the central corneal stroma. We euthanized the mice 4 days later, at the peak of HAdV-D37 induced keratitis in this model (Chintakuntlawar et al. 2007). By confocal microscopy, the corneas of untreated and control vehicle treated Mafia mice showed an intense cellular eGFP+ infiltrate, whereas the corneas of the AP20187 Mafia mice contained very few eGFP+ cells (Fig. 1, second row).
To determine whether depletion of macrophages and dendritic cells affects clinically evident adenovirus keratitis, we also examined and photographed the corneas of pretreated and infected mice under a surgical microscope at 4 dpi. Representative photographs are shown (Fig. 1, third row). In both untreated and control vehicle treated MaFIA mice, severe stromal corneal keratitis was evident and typical of adenovirus keratitis in background C57BL/6 mice (Chintakuntlawar et al. 2007), while in AP20187 treated mice, the corneas appeared clinically normal.
Our previous studies demonstrated neutrophil recruitment into the corneas of HAdV-D37 infected mice beginning about 1 dpi with a peak at 4 dpi (Chintakuntlawar et al. 2007; Chintakuntlawar and Chodosh 2009; Chintakuntlawar et al. 2010). To assess the role of corneal macrophage and dendritic cell depletion on subsequent neutrophil infiltration in adenovirus keratitis, we pretreated and infected mice as above. Mice were euthanized and corneas harvested at 4 dpi, and the corneas immunostained with antibody to myeloperoxidase, and examined by confocal microscopy. As shown (Fig. 1, fourth row), untreated and control vehicle treated mouse corneas showed extensive myeloperoxidase expression, completely consistent with what is seen in background C57BL/6 mice (Chintakuntlawar et al. 2007), with dramatically less expression in the corneas of AP20187 pretreated mice. We then performed flow cytometry for CD45+ cells on similarly treated and infected mouse corneas. At 4 dpi, untreated mice and control vehicle treated mice showed significantly more CD45+ events per cornea than AP20187 treated mice (Fig. 1, fifth row).
Using MaFIA mice and an established mouse model of adenovirus keratitis, our data suggests an essential contribution of resident corneal macrophages and dendritic cells towards the development of corneal inflammation in adenovirus infection. In this mouse model, neutrophils are the first infiltrating cells in response to infection, with the maximum number of infiltrating neutrophils typically at 4 dpi (Chintakuntlawar et al. 2007). Infiltration of activated monocytes into the HAdV-D37 infected mouse cornea follows, peaking at 4–8 dpi. These events most closely follow the time course of CXCL1 and CCL2 expression, respectively, by resident corneal stromal cells, possibly explaining the kinetics of cellular infiltration. Others have reported that resident bone-marrow derived cells in the corneal stroma recognize infectious agents and actively assist in the generation of the early innate immune response to infection. For example, myeloid lineage cells in the cornea were shown to play a role in LPS-induced corneal inflammation (Chinnery et al. 2009), and in keratitis induced by Pseudomonas aeruginosa (Sun et al. 2010) and Aspergillus fumigatus (Leal et al. 2010). Our experimental data demonstrates a critical role for resident myeloid cells in neutrophil infiltration into the adenovirus infected cornea. Because AP20187 treatment ablates all myeloid cells in MaFIA mice, not merely those in the corneal stroma, we cannot yet conclude whether infected corneal resident myeloid cells also contribute to infiltration of peripheral myeloid cells to the infected corneal stroma. Such studies could be performed with analysis of CCL2 levels in AP20187 treated, HAdV-D37 infected MaFIA mouse corneas, or by adoptive transfer of wild type peripheral blood myeloid lineage cells into AP20187 treated MaFIA mice after infection.
Although our results indicate a significant contribution of myeloid lineage cells to neutrophil infiltration in adenovirus keratitis, we do not exclude the importance of other resident corneal cells, such as corneal epithelial cells and keratocytes, to the initiation of host cell responses to infection. Prior studies demonstrated unequivocally the capacity of cultured keratocytes to express biologically significant quantities of CXCL8, prior to the expression of other chemokines tested (Natarajan et al. 2003). We also showed in an in vitro 3-dimensional model of the human cornea that in the absence of any other cell type, the presence of keratocytes is sufficient for infiltration of peripheral blood leukocytes upon HAdV-D37 infection (Rajaiya et al. 2015). We also demonstrated previously using CXCL1−/− and CXCR2−/− mice that deficiency of either CXCL1 or its receptor resulted in delayed infiltration of neutrophils, but not reduced numbers of inflammatory monocytes in HAdV-D37 corneal infection (Chintakuntlawar and Chodosh 2009). Thus, the results reported herein and those from prior studies suggest that both keratocytes and myeloid lineage cells in the corneal stroma play critical and perhaps overlapping roles in the early innate immune responses to adenovirus corneal infection.
In summary, we demonstrate that resident myeloid lineage cells in the cornea respond to adenoviral infection by mediating the recruitment of leukocytes to the corneal stroma, manifesting clinically as keratitis. Further investigations will be necessary to elucidate the specific and relative contribution of the various ocular cell types to stromal keratitis after acute epidemic keratoconjunctivitis.
Highlights.
Resident corneal myeloid derived cells recruit leukocytes early in adenovirus keratitis
Adenovirus keratitis is diminished in dendritic and macrophage ablated MaFIA mice
Acknowledgments
This work was supported by National Institutes of Health (NIH) [EY013124, EY021558, and P30 EY014104], a Senior Scientific Investigator Award grant [to JC] from Research to Prevent Blindness, Inc., New York, NY, The Falk Foundation, and the Massachusetts Lions Eye Research Fund.
Footnotes
Potential conflicts of interest.
No author has a conflict of interest related to the subject of this manuscript. James Chodosh has served as a consultant for Novartis, and received travel support from Reliance.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Center for Disease Control and Prevention. Adenovirus-associated epidemic keratoconjunctivitis outbreaks - four states, 2008–2010. Morbidity and Mortality Weekly Report. 2013;62(32):637–641. [PMC free article] [PubMed] [Google Scholar]
- Brissette-Storkus CS, Reynolds SM, Lepisto AJ, Hendricks RL. Identification of a novel macrophage population in the normal mouse corneal stroma. Invest Ophthalmol Vis Sci. 2002;43(7):2264–2271. [PMC free article] [PubMed] [Google Scholar]
- Burnett SH, Kershen EJ, Zhang J, Zeng L, Straley SC, Kaplan AM, Cohen DA. Conditional macrophage ablation in transgenic mice expressing a fas-based suicide gene. J Leukoc Biol. 2004;75(4):612–623. doi: 10.1189/jlb.0903442. [DOI] [PubMed] [Google Scholar]
- Butt AL, Chodosh J. Adenoviral keratoconjunctivitis in a tertiary care eye clinic. Cornea. 2006;25(2):199–202. doi: 10.1097/01.ico.0000170693.13326.fb. [DOI] [PubMed] [Google Scholar]
- Chinnery HR, Carlson EC, Sun Y, Lin M, Burnett SH, Perez VL, McMenamin PG, Pearlman E. Bone marrow chimeras and c-fms conditional ablation (mafia) mice reveal an essential role for resident myeloid cells in lipopolysaccharide/tlr4-induced corneal inflammation. J Immunol. 2009;182(5):2738–2744. doi: 10.4049/jimmunol.0803505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintakuntlawar AV, Astley R, Chodosh J. Adenovirus type 37 keratitis in the c57bl/6j mouse. Invest Ophthalmol Vis Sci. 2007;48(2):781–788. doi: 10.1167/iovs.06-1036. [DOI] [PubMed] [Google Scholar]
- Chintakuntlawar AV, Chodosh J. Chemokine CXCl1/KC and its receptor CXCR2 are responsible for neutrophil chemotaxis in adenoviral keratitis. J Interferon Cytokine Res. 2009;29(10):657–666. doi: 10.1089/jir.2009.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintakuntlawar AV, Zhou X, Rajaiya J, Chodosh J. Viral capsid is a pathogen-associated molecular pattern in adenovirus keratitis. PLoS Pathog. 2010;6(4):e1000841. doi: 10.1371/journal.ppat.1000841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodosh J. Human adenovirus type 37 and the balb/c mouse: Progress toward a restricted adenovirus keratitis model (an American Ophthalmological Society thesis) Trans Am Ophthalmol Soc. 2006;104:346–365. [PMC free article] [PubMed] [Google Scholar]
- Leal SM, Jr, Cowden S, Hsia YC, Ghannoum MA, Momany M, Pearlman E. Distinct roles for dectin-1 and tlr4 in the pathogenesis of aspergillus fumigatus keratitis. PLoS pathogens. 2010;6:e1000976. doi: 10.1371/journal.ppat.1000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Zhou X, Rajaiya J, Chodosh J. Ultrastructure of adenovirus keratitis. Invest Ophthalmol Vis Sci. 2015;56(1):472–477. doi: 10.1167/iovs.14-15635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan K, Rajala MS, Chodosh J. Corneal il-8 expression following adenovirus infection is mediated by c-src activation in human corneal fibroblasts. J Immunol. 2003;170(12):6234–6243. doi: 10.4049/jimmunol.170.12.6234. [DOI] [PubMed] [Google Scholar]
- Rajaiya J, Zhou X, Barequet I, Gilmore MS, Chodosh J. Novel model of innate immunity in corneal infection. In Vitro Cell Dev Biol Anim. 2015;51(8):472–477. doi: 10.1007/s11626-015-9910-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Karmakar M, Roy S, Ramadan RT, Williams SR, Howell S, Shive CL, Han Y, Stopford CM, Rietsch A, et al. Tlr4 and Tlr5 on corneal macrophages regulate pseudomonas aeruginosa keratitis by signaling through Myd88-dependent and -independent pathways. J Immunol. 2010;185(7):4272–4283. doi: 10.4049/jimmunol.1000874. [DOI] [PMC free article] [PubMed] [Google Scholar]