Abstract
Monocytes produce high levels of inflammatory cytokines including IL-6 and TNF-α that are involved in autoimmunity, inflammatory diseases, cardiovascular disease and obesity. Therapies targeting IL-6 and TNF-α have been utilized in treating chronic inflammatory diseases. Oligonol is a lychee fruit-derived low-molecular form of polyphenol mixture, typically catechin-type monomers and oligomers of proanthocyanidins, which are produced by an oligomerization process. Although previous studies reported anti-inflammatory properties of Oligonol, it is unknown whether and how Oligonol suppresses IL-6 and TNF-α production in human monocytes. The results of our study demonstrate that Oligonol (25 μg/ml) decreases the production of IL-6 and TNF-α from human primary monocytes as measured by flow cytometry and ELISA. Such an anti-cytokine effect was likely mediated by the suppression of NF-kB activation without inducing cell death. Our findings raise the possibility of exploring the benefits of Oligonol in controlling inflammatory conditions, especially those associated with monocytes, in humans.
Keywords: Human, cytokines, monocytes, Oligonol, polyphenols
1. Introduction
Monocytes are large circulating leukocytes of the myeloid lineage which represent 5–10% of peripheral leukocytes [1]. Monocytes are armed with pattern recognition receptors such as Toll-like receptors (TLRs) and produce high levels of inflammatory cytokines, including IL-6 and TNF-α, upon activation [1]. The latter cytokines are involved in pathologic conditions including autoimmunity, inflammatory diseases, cardiovascular disease and obesity [2, 3]. In fact, therapeutic approaches neutralizing IL-6 and TNF-α have become a core modality in treating chronic inflammatory diseases such as rheumatoid arthritis (RA), psoriartic arthritis, psoriasis and inflammatory bowel disease [2].
Phytochemicals such as polyphenols that include proanthocyanidins may have immune regulatory properties [4]. The monomeric and oligomeric forms of polyphenols, which are a small portion of the total polyphenols in plants, are the biologically active forms [5]. Oligonol is a lychee fruit-derived low-molecular form of polyphenol mixture, typically catechin-type monomers and oligomers of proanthocyanidins [5]. Oligonol is produced by an oligomerization process that converts high-molecular weight polymeric proanthocyanidins into low-molecular weight oligomeric proanthocyanidins including monomers, dimers and trimers [5]. This process is achieved by mixing proanthocyanidin polymers with tea catechines [6]. Although oligomeric forms of polyphenols (monomers to pentamers) are typically found in less than 10% of the total polyphenols, the process of oligomerization results in the production of Oligonol that contains 15.0 ± 1.1% monomers, 16.3 ± 1.1% dimmers and 4.1 ± 0.7% trimers [7]. Such oligomerization can result in delivering increased levels of oligomeric proanthocyanidins likely by enhancing bioavailability compared to unoligomerized fruit and plant products [6]. Both in vivo and in vitro studies support the immune regulatory and anti-oxidative effects of Oligonol [8–13]. Decreased blood levels of IL-6 were reported in healthy humans after 4 weeks of Oligonol administration [10]. Oligonol attenuated diabetes-induced hepatic damage by reducing oxidative stress and improving lipid metabolism [13]. However, it is largely unknown whether and how Oligonol suppresses the production of the inflammatory cytokines IL-6 and TNF-α in human monocytes that are involved in many pathologic conditions. Here we have addressed this question by demonstrating that Oligonol decreases the production of IL-6 and TNF-α in human monocytes by suppressing NF-κB activation.
2. Materials and Methods
2.1. Human blood cells and culture
This work was approved by the institutional review committee of Yale University. After informed consent, peripheral blood was obtained from healthy adult donors who had no disease affecting the immune system. Peripheral blood mononuclear cells (PBMCs) and monocytes were purified from peripheral blood using Ficoll centrifugation and a negative cell purification kit (Stem Cell Technologies, Vancouver, Canada), respectively [14]. The purity of monocytes was greater than 90%. PBMCs or monocytes were pre-incubated for 60 min with or without Oligonol (Amino Up Chemical, Japan) at indicated doses followed by an additional 1- or 5-hour incubation with lipopolysaccharide (LPS, 500 ng/ml) or PBS (control) in the presence or absence of Oligonol (0–25 μg/ml). Cells and supernatants were harvested for flow cytometry, Western blot analysis and ELISA.
2.2. Flow Cytometry, ELISA and qPCR
For intracellular cytokine analysis, harvested PBMCs were stained with antibodies to CD14, IL-6, TNF-α or isotype antibodies (BD Biosciences, San Jose, CA). Also, the same cells were stained with annexin V and 7-AAD for cell survival analysis. Stained cells were analyzed using an LSRII® flow cytometer (BD Biosciences) and FlowJo software (Tree Star, Ashland, OR). Cell culture supernatants were analyzed for IL-1β, IL-6 and TNF-α by ELISA (eBioscience, San Diego, CA). For qPCR, total RNA was extracted from cells and cDNA was synthesized. The IL6 and TNFA gene levels were analyzed using the appropriate primers (IL6 forward ATGCAATAACCACCCCTGAC, reverse GAGGTGCCCATGCTACATTT; TNFA forward TCCTTCAGACACCCTCAACC, reverse AGGCCCCAGTTTGAATTCTT), with normalization to ACTINB expression.
2.3. Western blot analysis
Monocytes that were pre-incubated for 60 min with or without Oligonol were additionally incubated for 1 hour with or without LPS (500 ng/ml). Cells were harvested and analyzed for phosphorylated NF-κB p65, NF-κB p65 and β-actin using Western blot (antibodies to phosphorylated NF-κB p65 (serine 536) and NF-κB p65 from Cell Signaling Technology, Beverly, MA and to β-actin from Santa Cruz Biotechnology, Dallas, TX).
2.4. Statistical Analysis
Matched t-tests were performed using Microsoft Excel. P values less than 0.05 were considered statistically significant.
3. Results and Discussion
3.1. Oligonol suppresses the frequency of IL-6 and TNF-α expressing monocytes in human PBMCs in response to LPS without inducing cell death
We incubated PBMCs with or without LPS in the presence or absence of low and high doses of Oligonol (10 and 25 μg/ml, respectively). These doses were selected based on a published study analyzing the bioavailability of Oligonol in humans [5]. Monocytes that expressed CD14 and the inflammatory cytokines IL-6 and TNF-α were identified using flow cytometry. Oligonol suppressed the frequency of cells expressing these cytokines in a dose-dependent manner (Figure 1A). The frequency of cells expressing IL-6 was 13.5% ± 3.73 (mean ± standard error of mean (SEM)) in the presence of Oligonol (25 μg/ml) while the frequency of the same cells was 23.4% ± 2.88 in the absence of Oligonol (Figure 1B). Similarly, the frequency of cells expressing TNF-α in monocytes was lower in Oligonol-treated PBMCs than in Oligonol-untreated PBMCs (mean frequency (%) ± SEM, 37.2 ± 6.87 vs. 55.8 ± 7.18) (Figure 1C). We next determined whether such suppression was secondary to cell death. We identified live cells by staining cells with annexin V and 7-AAD followed by flow cytometric analysis. The frequency of live monocytes unstained with annexiv V and 7-AAD was similar between cells incubated with or without Oligonol (Figure 1D–E). In the presence of DMSO (positive control), a large number of cells were stained with annexin V and 7-AAD. These findings suggest that Oligonol suppresses the expression of IL-6 and TNF-α in human monocytes without inducing cell death.
Figure 1. Oligonol suppresses the frequency of IL-6-and TNF-α-expressing monocytes in human peripheral blood mononuclear cells (PBMCs) in response to LPS without inducing cell death.
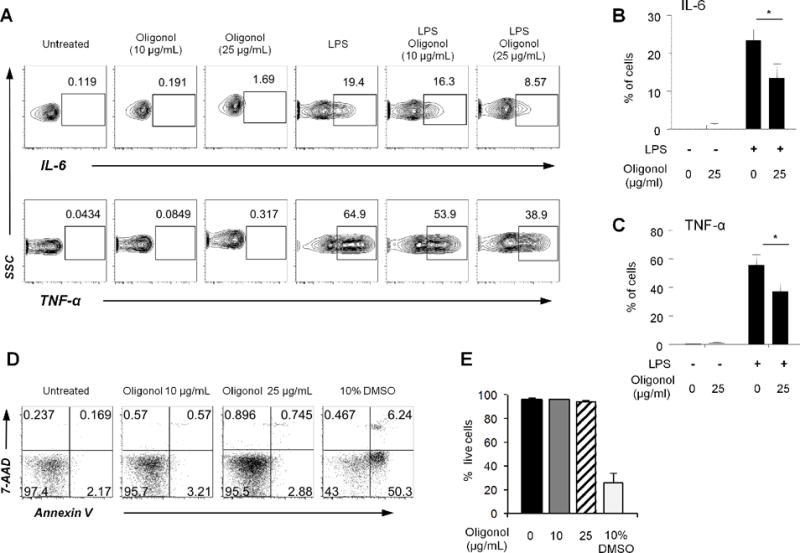
PBMCs were purified from the peripheral blood of healthy human adults. (A–C) PBMCs were pre-incubated for 60 min with or without Oligonol (0–25 μg/ml) followed by an additional 5-hour incubation with or without LPS (500 ng/ml) in the presence of GolgiPlug. Incubated cells were analyzed for monocytes (CD14+) producing IL-6 and TNF-α using flow cytometry. (A) Dot plots show cells expressing cytokines in monocytes. (B–C) The frequency of IL-6- and TNF-α-expressing cells in monocytes is shown. (D–E) PBMCs were incubated for 6 hours in the presence or absence of Oligonol (0–25 μg/ml) or 10% DMSO (positive control for cell death). Cells were stained with annexin V and 7-AAD followed by flow cytometric analysis for live and dead cells. (D) Dot plots show live (annexin V−, 7-AAD−) and dead cells. (D) The frequency of live cells in monocytes. Numbers in dot plots indicate the frequency of cytokine expressing cells or cells stained or unstained with annexinV and/or 7-AAD. (A, D) representative data from 3 independent experiments with 3 donors. (B, C, E) data from 3 donors. Bars and error bars indicate mean and standard error of mean (SEM), respectively. *P < 0.05.
3.2. IL-6 and TNF-α production from purified human monocytes in response to LPS was decreased by Oligonol through suppressing NF-κB activation
To determine the possible mechanism of the suppression of cytokine production by Oligonol, we incubated monocytes with or without LPS in the presence or absence of Oligonol. Similarly to the results of our flow cytometric analysis, Oligonol decreased the levels of IL-6 and TNF-α in culture supernatants as measured by ELISA (Fig 2A). The levels of these cytokines in culture supernatants were lower in cells incubated with Oligonol compared to cells incubated without it (mean (ng/ml) ± SEM, IL-6, 0.79 ± 0.15 vs. 1.76 ± 0.29; TNF-α, 1.18 ± 0.25 vs. 4.01 ± 0.67) (Fig 2B). We also measured the mRNA levels of IL6 and TNFA in monocytes stimulated with LPS in the presence or absence of Oligonol. The mRNA levels of these cytokines in LPS-stimulated monocytes decreased in the presence of Oligonol (relative levels of cytokine mRNA normalized by ACTINB levels in an arbitrary unit in Oligonol-untreated (reference level set as 100) vs. -treated monocytes (mean ± standard error of mean (SEM)), IL6, 100 vs. 28.6 ± 3.8, P = 0.0004; TNFA, 100 vs. 60.9 ± 8.0, P = 0.024). NF-κB is a signaling molecule that is critically involved in the TLR pathway triggered by ligands like LPS as well as in the production of IL-6 and TNF-α [15]. Indeed, Oligonol suppressed the activation of NF-κB (Fig 2C–D). These findings indicate that the suppression of the activation of NF-κB is a mechanism for decreased IL-6 and TNF-α production in human monocytes by Oligonol.
Figure 2. IL-6 and TNF-α production from purified human monocytes in response to LPS was decreased by Oligonol through suppressing NF-κB activation.
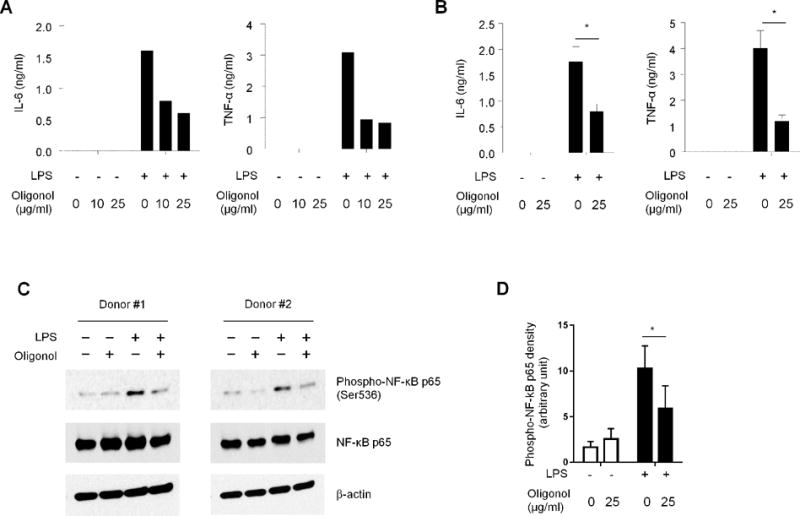
Untouched monocytes (CD14+) were purified from the peripheral blood of healthy adult subjects. (A–B) Purified monocytes were pre-incubated for 60 min with or without Oligonol (10 or 25 μg/ml) followed by an additional 5-hour incubation with or without LPS (500 ng/ml). Culture supernatants were analyzed for IL-6 and TNF-α by ELISA. (A) Cytokine levels in culture supernatant of monocytes incubated with low- and high-dose Oligonol (10 and 25 μg/ml, respectively). (B) Suppression of IL-6 and TNF-α production from monocytes by Oligonol (25 μg/ml). (C–D) Purified monocytes were pre-incubated for 60 min with or without Oligonol (25 μg/ml) followed by an additional 1-hour incubation with or without LPS (500 ng/ml). Cells were harvested and analyzed for the phosphorylated form of NF-κB p65, NF-κB p65 and β-actin using Western blot analysis (n = 5). (A–B) Bars and error bars indicate mean and standard error of mean (SEM), respectively, from (A) 2, (B) 5 (IL-6) and 4 (TNF-α) donors. *P < 0.05.
Monocytes accounting for 5–10% of peripheral blood leukocytes are a potent source of the proinflammatory cytokines IL-6 and TNF-α that are involved in the pathogenesis of inflammatory diseases [1, 2]. Polyphenols found in fruits and leafy vegetables are considered to have functional properties including anti-inflammatory effects [4]. However, the effects of polyphenols on human primary monocytes are less known. Oligonol, a lychee fruit-derived low-molecular form of polyphenol mixture, contains high levels of monomers, dimers and trimers that can result in delivering increased levels of oligomeric forms of polyphenols [5]. Here we have found that Oligonol at a concentration of 25 μg/ml decreases IL-6 and TNF-α production from human primary monocytes by suppressing NF-κB activation without inducing cell death. A previous study reported that this level of polyphenols in serum was reached after taking Oligonol at 100–200 mg/day in humans [5]. We also analyzed IL-1β in the culture supernatants of mononcytes incubated with LPS in the presence or absence of Oligonol (25 μg/ml). Although in our study the production of IL-1β from monocytes in response to LPS was relatively low compared to the production of IL-6 and TNF-α, such IL-1β production was still decreased by Oligonol (IL-1β levels in culture supernatants, mean (pg/ml) ± SEM, 133.7 ± 37.5 vs. 79.4 ± 34.2, P = 0.0462).
The biological significance of our findings is supported by animal studies. Oligonol suppressed NF-κB activation in mouse skin stimulated with a proto-type tumor promoter 12-O-tetradecanoylphorbol-13-acetate (TPA) [10] as well as in the colonic tissue of mice with dextran sulfate sodium-induced colitis [12] and the hepatic tissue of experimental type 2 diabetes model mice [13]. Also, Oligonol decreased NF-κB activation and the expression of IL-6 and TNF-α in the mouse macrophage RAW 264.7 cells [12]. Previous studies reported additional effects of Oligonol on molecules related to inflammation in mice. These include the Oligonol-driven decreased expression of the signal transducer and activator of transcription (STAT) 3, cyclooxygenase-2 (COX-2), and inducible nitric oxide synthase (iNOS) in the tissues of mice with colitis or diabetes [12, 13]. In a human study, decreased blood levels of IL-6 were reported in healthy subjects after 4 weeks of Oligonol administration [11]. Our findings provide a mechanistic explanation for such cytokine suppression by Oligonol. The immune regulatory effect of Oligonol on human monocytes can be relevant in controlling inflammation given the critical role of monocytes in inducing inflammation by directly producing cytokines and affecting other immune cells like T cells [2, 14].
Taken together, we demonstrated that Oligonol, a low-molecular weight phenolic product derived from lychee fruit extract, decreases the production of the inflammatory cytokines IL-6 and TNF-α from human primary monocytes by suppressing the activation of NF-κB. The results of our study raise the possibility of exploring the benefit of Oligonol in controlling inflammation in humans.
Acknowledgments
This work was supported in part by a grant from the National Institutes of Health (AG028069 to IK) and an unrestricted research fund from Amino Up Chemical Co, Sapporo, Japan.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
Insoo Kang received an unrestricted research fund from Amino Up Chemical Co, Sapporo, Japan, the manufacturer of Oligonol that was studied in this work. Takahiro Maeda, Hiroshi Nishioka and Fujii Hajime are employees of Amino Up Chemical Co.
References
- 1.Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 2.Striz I, Brabcova E, Kolesar L, Sekerkova A. Cytokine networking of innate immunity cells: a potential target of therapy. Clin Sci (Lond) 2014;126:593. doi: 10.1042/CS20130497. [DOI] [PubMed] [Google Scholar]
- 3.Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract. 2014;2014:943162. doi: 10.1155/2014/943162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Chu AJ. Antagonism by bioactive polyphenols against inflammation: a systematic view. Inflamm Allergy Drug Targets. 2014;13:34. doi: 10.2174/1871528112666131119211002. [DOI] [PubMed] [Google Scholar]
- 5.Fujii H, Sun B, Nishioka H, Hirose A, Aruoma OI. Evaluation of the safety and toxicity of the oligomerized polyphenol Oligonol. Food Chem Toxicol. 2007;45:378. doi: 10.1016/j.fct.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 6.Miura T, Kitadate K, Fujii H. The function of the next generation polyphenol, “Oligonol”. In: Bagchi D, Lau FC, Ghosh DK, editors. Biotechnology in functional foods and nutraceuticals. Boca Ration: CRC Press; 2010. p. 91. [Google Scholar]
- 7.Tanaka T, Yoshitake N, Zhao P, Matsuo Y, Kouno I, Nonaka G-i. Production of oligomeric proanthocyanidins by fragmentation of polymers. Jpn J Food Chem. 2007;14:134. Japanese. [Google Scholar]
- 8.Yamanishi R, Yoshigai E, Okuyama T, Mori M, Murase H, Machida T, et al. The anti-inflammatory effects of flavanol-rich lychee fruit extract in rat hepatocytes. PLoS One. 2014;9:e93818. doi: 10.1371/journal.pone.0093818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aruoma OI, Sun B, Fujii H, Neergheen VS, Bahorun T, Kang KS, et al. Low molecular proanthocyanidin dietary biofactor Oligonol: Its modulation of oxidative stress, bioefficacy, neuroprotection, food application and chemoprevention potentials. Biofactors. 2006;27:245. doi: 10.1002/biof.5520270121. [DOI] [PubMed] [Google Scholar]
- 10.Kundu JK, Hwang DM, Lee JC, Chang EJ, Shin YK, Fujii H, et al. Inhibitory effects of oligonol on phorbol ester-induced tumor promotion and COX-2 expression in mouse skin: NF-kappaB and C/EBP as potential targets. Cancer Lett. 2009;273:86. doi: 10.1016/j.canlet.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 11.Lee JB, Shin YO, Min YK, Yang HM. The effect of Oligonol intake on cortisol and related cytokines in healthy young men. Nutr Res Pract. 2010;4:203. doi: 10.4162/nrp.2010.4.3.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yum HW, Zhong X, Park J, Na HK, Kim N, Lee HS, et al. Oligonol inhibits dextran sulfate sodium-induced colitis and colonic adenoma formation in mice. Antioxid Redox Signal. 2013;19:102. doi: 10.1089/ars.2012.4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noh JS, Park CH, Yokozawa T. Treatment with oligonol, a low-molecular polyphenol derived from lychee fruit, attenuates diabetes-induced hepatic damage through regulation of oxidative stress and lipid metabolism. Br J Nutr. 2011;106:1013. doi: 10.1017/S0007114511001322. [DOI] [PubMed] [Google Scholar]
- 14.Shin MS, Kang Y, Lee N, Wahl ER, Kim SH, Kang KS, et al. Self Double-Stranded (ds)DNA Induces IL-1beta Production from Human Monocytes by Activating NLRP3 Inflammasome in the Presence of Anti-dsDNA Antibodies. J Immunol. 2013;190:1407. doi: 10.4049/jimmunol.1201195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawai T, Akira S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int Immunol. 2009;21:317. doi: 10.1093/intimm/dxp017. [DOI] [PMC free article] [PubMed] [Google Scholar]


