Abstract
To facilitate drug development for lung delivery, it is highly demanding to establish appropriate airway epithelial cell models as transport barriers to evaluate pharmacokinetic profiles of drug molecules. Besides the cancer-derived cell lines, as the primary cell model, normal human bronchial epithelial (NHBE) cells have been used for drug screenings because of physiological relevance to in vivo. Therefore, to accurately interpret drug transport data in NHBE measured by different laboratories, it is important to know biophysical characteristics of NHBE grown on membranes in different culture conditions. In this study, NHBE was grown on the polyester membrane in different medium and its transport barrier properties as well as cell architectures were fully characterized by functional assays and confocal imaging throughout days of cultures. Moreover, NHBE cells on inserts in different medium were subject to either of AIC (air-interfaced culture) or LCC (liquid-covered culture) condition. Cells in the AIC condition were cultivated on the membrane with medium in the basolateral side only whereas cells with medium in apical and basolateral sides under the LCC condition. Quantitative microscopic imaging with biophysical examination revealed distinct multilayered architectures of differentiated NHBE cells, suggesting NHBE as functional cell barriers for the lung-targeting drug transport.
Keywords: Airway epithelial cell, NHBE, porous membrane, drug transport, differentiation, tight junction
Introduction
There are various models to study absorption, distribution or elimination of inhaled drugs via pulmonary route; in vivo model, isolated perfused lung model, in vitro model [1-4]. Intact organ model should be the most appropriate system to study pharmacokinetics of lung-targeting drug molecules. However, due to its complexity, it is hard to distinguish drug permeation properties of bronchial or alveolar epithelial cells from other tissues in lung. On the other hand, cell culture models are useful to perform mechanistic studies on epithelial cells as drug permeation barriers [5]. As for the lung cell model, there are primary cells and immortalized cell lines available from different locations in the lung [6-8]. Considering that tracheobronchial disposition of drug particulate in the inhaled formulation tends to be significantly greater than alveolar disposition due to the particle size range (5-15 μm) [9], it would be important to characterize bronchial cell models as drug absorption barriers. Bronchial epithelial cell lines such as 16HBE14o- or Calu-3 cells are representative immortalized cell lines which can grow on the porous supports forming tight junctions [6,10,11]. Those cells have been investigated in the context of transport properties of lung epithelial cells (i.e. absorption, metabolism or transporter activity of drug molecules) [6,12-14].
Generally, primary epithelial cells of lung consist of the mixed cell types such as goblet cells, basal cells, or ciliated epithelial cells [12,15]. Thus, primary cell model can be representative in vitro model to capture the intrinsic properties of in vivo lung [16]. Well-established primary cell model would be more useful to investigate functional properties or mechanisms of intact organs under normal or diseased conditions because these cells are likely to be more physiologically relevant to in vivo organs, compared to other cancer-derived cell lines [17]. Shortcomings of primary cell cultures are that those cell types have short life span and require specialized culture skills for expansion in culture flasks [18]. In order to culture primary cells longer, the culture conditions should be optimized in culture media or culture methods. There have been various efforts to optimize culture protocols for lung epithelial cells including airway primary cells [19-22]. Under the optimal culture condition, airway primary cells can be cultured for a longer period of time. Previous reports have shown that these cells can be cultured in serum-free medium supplemented with hormone and growth factors [23,24]. Especially, epithelial cell types are sensitive to the compositions of medium in the absence of serum and their growths are regulated by hormones and various growth factors [25].
The lung epithelial cells can be cultured with different ways such as AIC (air-interfaced culture) or LCC (liquid-covered culture) [5,23]. In AIC, airway epithelial cells can be grown on porous membrane with the medium in only basolateral chamber without medium on the apical side, so-called air-interfaced culture, mimicking oxygen exchange environment in the in vivo airway. On the other hand, in LCC, cells can be cultured with medium in both sides, meaning liquid-covered or submerged culture. When the cells are subjected to drug transport studies in the insert system, tight junction formation is a key factor. Most common techniques to check integrity of cell layers in the Transwell™ insert are TEER (transepithelial electrical resistance) measurement and permeability assessment of a hydrophilic compound mainly transported through a paracellular pathway [5]. TEER measurement serves as an index for the paracellular flow of ions across the cell layers on the membrane. Commonly used paracellular transport markers are Mannitol, Lucifer Yellow, or Fluorescein-Na [13,26]. There have been reports that cell layers on the porous membrane under different culture conditions may show differences in the formation of tight junctions [5,23]. However, especially for the normal primary bronchial epithelial cells, there is no sufficient quantitative descriptions about cell architectures and morphologies which might be important factors governing drug permeation or distribution when the cells are grown on the plain porous supports in serum-free medium under different culture environments (AIC or LCC).
Moreover, even though there were various investigations to find the optimal culture conditions in the medium composition to expand the airway primary cells in a longer period [20,27] and there are a few reports about testing drug permeation properties of the NHBE cell layers in transwell inserts [14,23], the functional properties of NHBE cells were not fully understood in relation to their morphologies on the nucleoporous membrane under different culture conditions. Therefore, we examined morphological properties of NHBE (normal human bronchial epithelial cells) grown on porous supports in serum-free medium with different media composition under either of AIC or LCC condition using biochemical probes and evaluated this primary cell model as a functional barrier for drug permeation.
Materials and Methods
Materials
All the chemicals for Hank's balanced salt solution (HBSS buffer, pH 7.4, 10mM HEPES, 25mM D-Glucose) were obtained from Fisher Scientific, Inc. (Pittsburgh, PA), and Lucifer Yellow CH dipotassium salt (MW: 521.57) was from Sigma-Aldrich. NHBE (normal human bronchial epithelial cells; Clonetics™ passage #1) and BEGM (bronchial epithelial growth medium) bullet kit including BEBM (bronchial epithelial basal medium) and subculture reagents were purchased from Lonza co. (Walkersville, MD). Dulbecco's Modified Eagle Medium: Nutrient Mixture F-12 (DMEM:F12) were from Invitrogen, co (Carlsbad, CA). Hoechst 33342 (abbreviated as “HOE”), MitoTracker® Red CMXRos (“MTR”), LysoTracker® Green DND-26 (“LTG”), and Alexa Fluor® 488 phalloidin were obtained from Molecular Probes, Invitrogen. Nunc® Lab-Tek® I-chamber slide was used for the observation of cells under the microscopy. Transwell™ inserts with polyester membranes (area: 0.33 cm2, pore size: 0.4 μm) were purchased from Corning co (Lowell, MA).
Cell Culture
Normal human bronchial primary epithelial cells (NHBE, Passage #1) were thawed at 37°C water bath according to manufacturer's instruction and cultivated at 75 cm2 flask at 37°C, 5% CO2 incubator to reach 70-80 % confluency. NHBE cells were maintained with the serum-free growth medium (BEGM). BEBM (bronchial epithelial basal medium) was supplemented with human recombinant epidermal growth factor (0.5 ng/ml), insulin (5 μg/ml), transferrin (10 μg/ml), hydrocortisone (0.5 μg/ml), triiodothyronine (6.5 μg/ml), epinephrine (0.5 μg/ml), retinoic acid (50 nM), gentamycin and amphotericin-B (50 μg/ml) and bovine pituitary extract (35 mg/ml) in BEBM bullet kit to make BEGM (bronchial epithelial growth medium). NHBE cells (passage # 2) with density 2.5×105 cells/ml were seeded on porous membrane (area: 0.33 cm2) in the Transwell™ insert in 24-well plate with BEGM medium or differentiation medium. Mixed medium of BEBM and DMEM:F12 supplemented with 8 kinds of growth factors except for BPE (bovine pituitary extract) at 50:50 ratio was used as the “differentiation medium” for NHBE cells on transwell inserts [14,23]. In the case of AIC (air-interfaced culture) condition, the medium in the apical chamber was removed by aspiration after 24 h of cell seeding on transwell inserts and the cells on the membrane was cultured with only medium in the basolateral chamber. The cells grown in inserts with LCC (liquid-covered culture) or submerged culture condition were maintained with the medium in apical and basolateral sides. The medium (growth or differentiation) in the insert was replaced every day while the cells were maintained at 37°C, 5% CO2 incubator.
TEER (Transepithelial Electrical Resistance) Measurement
During the cultures of NHBE cells in inserts in the growth or differentiation medium, the development of tight junctions of the cell layers was followed up by measuring TEER (transepithelial electrical resistance) values using Millipore Millicell® ERS. The apical and basolateral chambers were filled with fresh medium (growth or differentiation medium) before the measurement. The inserts with the cells were equilibrated in the medium at 37°C, humidified CO2 incubator for 30 min. TEER values were measured with two electrodes in Millipore Millicell® ERS submerged into the medium in the insert. TEER values were corrected by subtracting the background TEER values measured in inserts without the cells, only with the media in the both chambers and the area of the membranes (0.33 μm2) was considered in the TEER units (Ω·cm2).
Paracellular Transport Marker Permeability Assay
A hydrophilic compound, Lucifer Yellow (LY), was used to confirm cell layers’ integrity on the porous membrane as a paracellular marker. Apical-to-basolateral (AP→BL) or basolateral-to-apical (BL→AP) transport of LY was studied with NHBE cell layers grown on the polyester membrane in the Transwell™ insert in the growth or differentiation medium under AIC or LCC condition. After the NHBE cells on inserts were equilibrated with HBSS buffer at 37°C for 30 min, 110 μl of 1 mM LY in HBSS buffer (pH 7.4) was added into the apical side of insert where 600 μl of HBSS buffer without LY in the basolateral side for AP→BL transport assay. For BL→AP transport, LY-containing buffer was added into the basolateral compartment while buffer without LY in the apical side. Transport studies were performed for 4 h on a VWR shaking platform at 37°C, 5% CO2 incubator as previously reported [28]. Sample solution was collected from the receiver chamber (basolateral side for AP→BL; apical side for BL→AP) at each time point and from the donor side at the end time point. The signals of LY in the standard and sample solutions were measured at 485 nm (excitation)/540 nm (emission) by the plate reader (BioTEK® Synergy™ BioTEK, co.). Transcellular permeability coefficient, Peff (cm/sec) was calculated by dividing the LY mass transfer rate in the receiver side by the insert area and the initial LY concentration.
Confocal 3D Microscopy and Image Analyses
Inserts with NHBE cells grown in the growth or differentiation medium were washed twice with HBSS buffer. For the actin staining, stock solution of Alexa Fluor® 488 phalloidin (300 U in 1.5 ml methanol) was diluted 40-fold with HBSS buffer to have 5 U/ml for the final use. Cells on the membrane were permeabilized by incubation with 100 μl of 0.1 % Triton-X100 in the apical side and 600 μl of buffer without Triton-X in the basolateral side at the room temperature for 15 min. After washed with buffer twice, the cells in inserts were incubated with 240 μl mixtures of Alexa Fluor® 488 phalloidin and HOE in buffer (100 μl of 5 U/ml Alexa Fluor® 488 phalloidin, 80 μl of 10 μg/ml HOE and 60 μl HBSS) in the apical side and 600 μl buffer in the basolateral side at 37°C, CO2 incubator. After 30 min-incubation, the inserts with the cells and dye solution in the apical side were placed on the Lab-Tek® I-chamber slide mounted with buffer. Zeiss LSM 510-META laser scanning confocal microscope (Carl Zeiss Inc., Thornwood, NJ) with a 60 × water immersion objective was used for scanning the insert through z-axis.
In order to capture morphometric properties of NHBE cells on inserts in the growth or differentiation medium, we used three different biochemical probes for further imaging. Mixed solution (240 μl) of three different dye molecules (10 μg/ml HOE, 80 μl of 1 μM MTR, and 2.5 μM LTG in HBSS) were added into the apical side of inserts with NHBEs in the presence of 600 μl of buffer in the basolateral chamber. After 30 min-incubation at 37°C, 5% CO2 incubator, the inserts with cells were put on chamber slides. Images were acquired across z-axis with 1 μm interval by confocal microscopy at three different fluorescent channels (UV (364 nm), Argon laser (488 nm), Helium neon 1 laser (543 nm)). Z-stack images of NHBE cells on inserts in the growth or differentiation medium under either of AIC or LCC condition were analyzed by MetaMorph software (Molecular Devices, Sunnyvale, CA). After background subtraction, in the thresholded images, region areas around the cells were manually captured by the “Region Measurement” algorithm of software. By assuming a cell as a polyhedron, cell volume was calculated by summation of cell area (polygonal area), sequentially stacked with interval of 1 μm spacing along z-axis according to Simpson's rule for integrations [29].
Statistical Analysis
Plots with the statistical analysis were generated by GraphPad Prism 5 (LaJolla, CA). Data analyses were performed with either an unpaired Student's t-test or a one-way analysis of variance (ANOVA; α = 0.05) as needed. Following ANOVA, a post-hoc Tukey's multiple comparison test was used with p-value < 0.05 as a significance level. Box-and-whiskers plots were displayed with median line and whiskers to 5-95 percentiles.
Results and Discussion
NHBE Cells Showed Differential Development of Tight Junctions Depending on Medium Compositions
To establish suitable airway cell models for drug transport studies, basically it is a very important step to assess intactness of NHBE cells on porous membranes according to the diverse culture environment. In this study, as shown in the diagram in Fig. 1, NHBE cells grown on porous membranes under AIC (air-interfaced culture) or LCC (liquid-covered culture; submerged culture) conditions with different medium compositions were investigated for their functionality as cell barriers for drug permeation studies by both biophysical and morphometric analyses. Intactness of the NHBE cell layers on the membrane in different culture environment was checked by measuring transepithelial resistance (TEER) values from day 1 until day 15 (Fig. 2A and 2B). While medium was maintained in both the apical and basolateral sides for the LCC condition, for AIC the medium was removed on day 1 from the apical side. In the growth medium (BEGM), under either of AIC or LCC condition (Fig. 2A), TEER values kept decreasing during the period and never got higher than 100 Ω·cm2 which is the minimal TEER value in confluent cell monolayers widely used in drug transport studies [30].
Fig. 1.
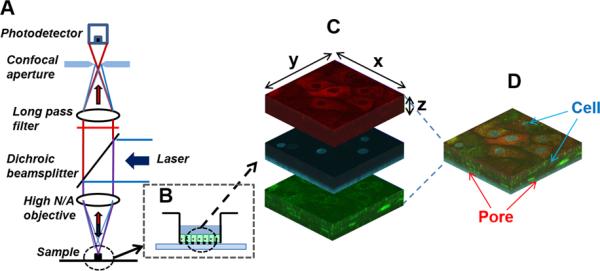
Schematic diagram of experimental settings. (A) Confocal laser fluorescent microscopy geometry with (B) the specimen in Transwell™ insert. With the pinhole at the confocal image plane, this system reduces the out-of-focus noise contribution, while allowing only in-focus signal from the pinhole to be detected. (C) A transwell insert containing the cell layers stained with fluorescent probe molecules (MTR (red), HOE (blue), and LTG (green)) was located on the chamber slide mounted with buffer, followed by confocal microscopic imaging equipped with three different fluorescent channels. (D) With the scanning mode for Z-stacks, the images obtained from the cell layers in porous supports could be reconstructed into three dimensions (3D).
Fig. 2.
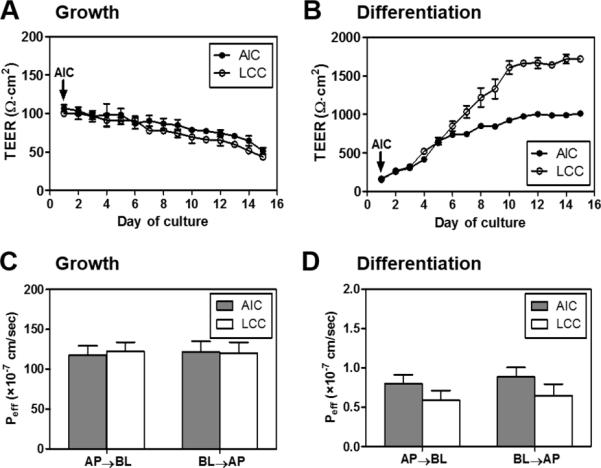
Functional analyses of NHBE cells in inserts grown under different culture conditions. TEER values were measured during the cultures in different conditions; (A) NHBEs in the growth medium (culture conditions were labeled as AIC or LCC) (B) NHBEs in the differentiation medium (AIC or LCC). TEER values of the differentiated NHBEs under AIC and LCC began to show the statistically significant differences from day 6 (unpaired Student's t-test with the 5% significance level. *P<0.05, **P<0.01, ***P<0.001). (C, D) Lucifer Yellow (LY) permeability (Peff) measurements on Day 8 for NHBE in the (C) growth or (D) differentiation medium. Apical-to-basolateral (AP→BL) or basolateral-to-apical (BL→AP) permeability values of NHBE cells on polyester membranes after the LY transport are depicted according to medium compositions and culture conditions (ALC or LCC).
In contrast, in the differentiation medium (BEGM : DMEM with F12 (1:1 mixture) = 1:1), TEER values of NHBE cells in AIC or LCC condition were much higher than those of cells in the growth medium (Fig. 2B). Initially, in the differentiation medium, NHBE cells had average TEER values of 165 Ω·cm2 (± 19.7, standard deviation (S.D.)) and 319 ± 35.7 Ω·cm2 on day 3 for AIC and LCC conditions, respectively. In the AIC condition, TEER values of differentiated NHBE were about 850 ± 18.2 Ω·cm2 on day 8, reaching 924 ± 37.6 Ω·cm2 on day 10. On the other hand, in the LCC condition, TEER values of differentiated NHBE were about 1220 ± 122 Ω·cm2 on day 8, reaching 1607 ± 83.7 Ω·cm2 on day 10. Throughout days of cultures, differentiated NHBE under LCC condition had higher TEER values than those under AIC, which showed similar tendency to the Calu-3 cells showing greater TEER in LCC condition (1185 Ω·cm2 under LCC; 500 Ω·cm2 under AIC) [31]. Previously, it was reported that primary cultured airway epithelial cells of rat, rabbit or human in inserts showed TEER values around 800-1000 Ω·cm2 [32,33]. Based on our TEER measurements in differentiated NHBEs and reported TEER values in primary airway tissue cultures, we could conclude that differentiated NHBE cells in inserts under AIC condition develop confluent airway epithelial cell layers with the appropriate intactness approximately at day 8.
In further, the permeability of Lucifer yellow (LY), a common paracellular transport marker, was also evaluated to confirm the intactness of NHBE cell layer. Plots in Fig. 2C and 2D showed permeability values for LY transport via both apical-to-basolateral and basolateral-to-apical direction (AP→BL; BL→AP) through NHBE cells cultured in either growth or differentiation medium. As shown in Fig. 2C, regardless of AIC or LCC condition, NHBEs cultured in the growth medium showed a high paracellular transport for both directions (avg., 118 × 10−7 cm/sec and 122 × 10−7 cm/sec for AP→BL transport under AIC and LCC condition, respectively). In a sharp contrast, NHBEs cultured in the differentiation medium displayed significantly lower LY permeability (avg., 0.80 × 10−7 cm/sec and 0.59 × 10−7 cm/sec for AP→BL transport under AIC and LCC condition, respectively) than cells cultured in the growth medium; suggesting the formation of intact cell barriers with tight junction formation (Fig. 2D). Together with the TEER values, these LY permeability study results clearly indicated that the morphology and growth profiles of NHBE cells are highly dependent upon the media compositions and moreover, NHBE cell culture in the differentiation medium is optimal for drug transport assays. Regardless of the culture condition, the Peff values of LY transport for both directions were almost identical with no statistical difference (unpaired Student's t-test, p < 0.05). This could be explained by the nature of LY which is a paracellular transport marker transported barely via other pathways that could be affected by the presence of receptors or transporters. Hence, the permeability of LY through the cell layer by either direction has commonly been similar in most of the studies.
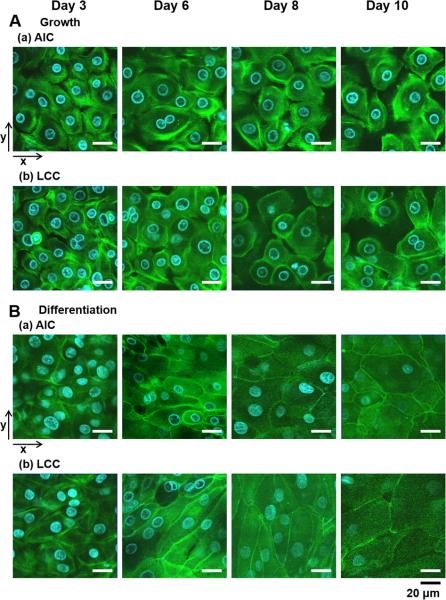
Besides these biophysical measurements for the cell layer intactness, development of tight junction in different culture conditions was examined under a confocal fluorescent microscopy with the actin-staining dye, Alexa Fluor® 488 phalloidin (NHBEs in the growth medium vs. differentiation medium; Fig. 3A and 3B). Regardless of AIC or LCC condition, undifferentiated NHBE in the growth medium formed sparse cell layers without forming tight junctional complexes between cells and could not expand on porous supports during the culture (Fig. 3A), while differentiated NHBE cells formed well-developed tight junctions (Fig. 3B). As NHBE undergoes differentiation with increasing cell population in the defined space of inserts, cell-to-cell interaction facilitates formation of molecular structure of tight junctions between the neighboring cells. Specifically, zonula-occludens protein-1 (ZO-1) plays an important role in organizing components of tight junctions and also linking them to the actin cytoskeleton in the cytoplasm [34].
Fig. 3.
Different development of tight junctions in cell-to-cell contacts in various culture conditions. Actin filaments in NHBE cells grown in different culture conditions were stained with Alexa Fluor® 488 phalloidin (green) and cell nuclei stained with HOE (blue) in HBSS. (A) NHBE in the growth medium under (a) AIC or (b) LCC condition revealed imperfect tight junctions. (B) NHBE in the differentiation medium has developed intact tight junctions under either of (a) AIC or (b) LCC condition. Two-dimensional confocal images were displayed with the arrows for x- and y-axes. The scale bar below indicates 20 μm.
Basically, the growth medium (BEGM) contains low calcium concentration (0.1 mM), generally known as promoting cell division. However as cultures on inserts continues, our results showed that NHBE cells in the growth medium could not expand with proper formation of tight junctions, which suggest additional medium components would be needed for further cell differentiation. The findings from our study informed us that only the differentiated NHBE cells cultured in the appropriate differentiation medium forms an intact cell layer with tight junctions. The differentiation medium contains low concentration of calcium (0.1 mM), identical to the growth medium (BEGM) and other essential growth factors, but without serum. In the serum-supplemented medium, inhibition of cell proliferation and short life span have been reported for airway primary epithelial cells, which might be caused by transforming growth factor (TGF-β) contained in the serum [35]. As key growth factors in the differentiation medium, retinoic acid facilitates the promotion of mucociliary differentiation of airway epithelial cells [20,36,37], while epidermal growth factor (EGF) promotes the formation of multilayered phenotypes [24]. EGF plays a key role to modulate basal expression of mucin genes, with interaction of retinoic acid in a concentration-dependent manner [37]. Other growth factors such as transferrin, hydrocortisone, and triiodothyronine affect cell growth and the cell phenotypes on inserts [35]. The differentiation medium utilized in our experiments contained 50 nM retinoic acid and 0.5 ng/mL EGF, for proper mucociliary differentiation and multilayer formation with appropriate thickness, following previous protocols reported [24,36,37].
Culture Methods (AIC vs. LCC) As Well As Medium Difference Affected Cell Layer Formation on Porous Supports
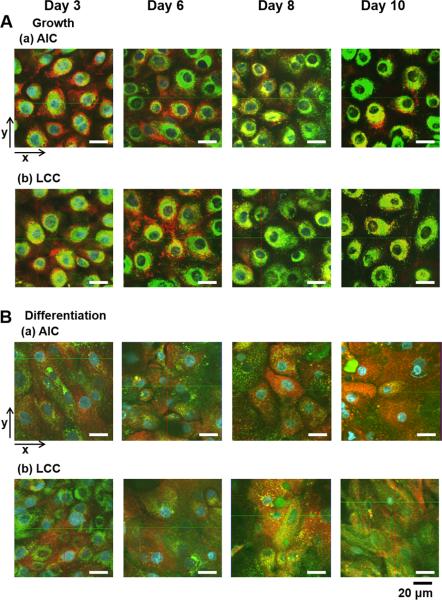
Confocal microscope imaging enabled us to investigate the cell architectures of NHBEs grown on the nucleoporous membrane in different growth conditions with 3D-dimension by scanning Z-stacks. In NHBE cells, cell nuclei, lysosomes, and mitochondria were stained by Hoechst 33342 (HOE; blue color), LysoTracker® Green (LTG; green), and MitoTracker® Red (MTR; red), respectively after 30 min-incubation. Fig. 4 displays two dimensional confocal fluorescent images of NHBE cell layers grown on the membranes under AIC or LCC condition in either of the growth or differentiation medium. As shown in Fig. 4A, LTG and MTR in the cells cultured in the growth medium showed punctuated, homogeneous and dense arrangement around nuclei. In the differentiated NHBEs in surface cell layers, LTG was observed punctuated and dispersed throughout the AIC or LCC period, while MTR was more dispersed specifically after day 8 (Fig. 4B). Since day 8, the fluorescence intensity of MTR in surface cell layers under AIC condition was higher than that under LCC condition (Fig. 4B). This phenomenon could be explained from the difference in the apical microenvironment between AIC and LCC conditions. Airway surface microenvironment in the apical sides could be manipulated by different culture conditions [5,27,35]. Previously, it has been reported that, in submerged culture (LCC) condition, the ciliogenesis and mucociliary movement is inhibited [38], while, under air-interfacing culture (AIC) condition, ultrastructure of airway epithelial cells are well maintained on porous-bottomed inserts with coordinated ciliary movement and mucus secretion from airway surfaces [16,39]
Fig. 4.
Two-dimensional (2D) architectures of NHBE cells on porous supports by confocal fluorescent imaging. Suborganelles (Cell nuclei, mitochondria or lysosomes) in NHBE cells grown in different culture conditions were stained with HOE (blue), MTR (red), and LTG (green) after 30 min-transport experiments with dye mixtures. (A) NHBE in the growth medium under (a) AIC or (b) LCC condition. (B) NHBE in the differentiation medium under (a) AIC or (b) LCC. Two-dimensional confocal images were displayed with the arrow indications (x- or y-axis). The scale bar below indicates 20 μm.
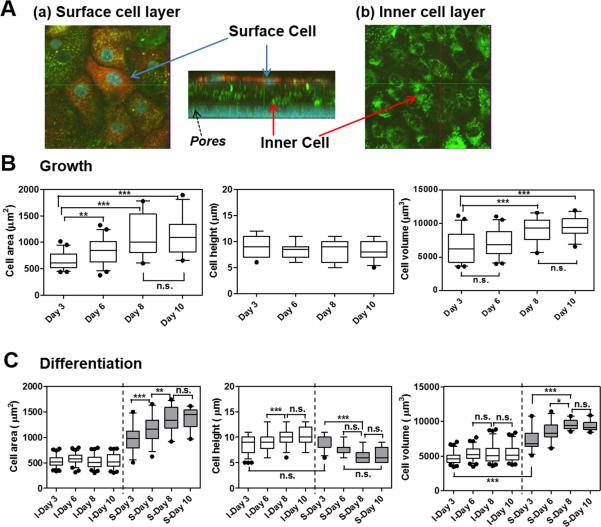
Fig. 5 shows yz planes corresponding to the image sections of xy planes through y-axis in Fig. 4. Regardless of AIC or LCC condition, undifferentiated NHBE cells cultured in the growth medium formed a monolayer and were sparsely distributed (Fig. 4A). Most of these NHBE cells consisted of short and wide squamous cells (Fig. 5A). In a sharp contrast, NHBE cells cultured in the differentiation medium started to form cell multilayers since day 3, and showed mixed cell morphologies of columnar and squamous shapes at the surface cell layers until day 6 (Fig. 4B and 5B). After day 8, differentiated NHBE cells showed more squamous cells in the surface layers. For these NHBE multilayers, surface and inner cell layers were visualized in detail through the z-axis with indications of each cell layer and porous membranes (pore size: 0.4 μm) in Fig. 6A. When the Z-stack images of NHBE cells on day 8 under AIC condition were examined, the inner cell layers were composed of mainly columnar cells, while the surface cell layers of squamous cells (Fig. 6A).
Fig. 5.
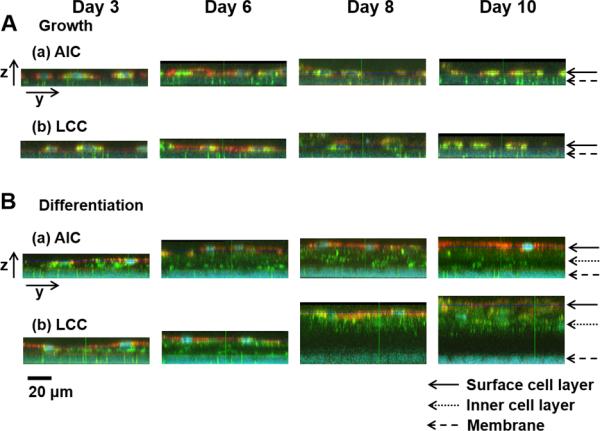
Various layer thicknesses of NHBE cells on porous membranes in inserts during the cultures (NHBEs in the (A) growth and (B) differentiation medium in (a) AIC or (b) LCC condition). In the images of yz planes corresponding to the 2D images of xy planes in Fig. 4, (B) NHBE cells in the differentiation medium formed multilayers with varied thickness of layers while (A) NHBEs in the growth medium showed monolayers with less intact between the cells, decreasing cell densities as a longer period of cultures. The scale bar indicates 20 μm. Cells in the surface or inner layers and porous membranes were indicated by the arrows.
Fig. 6.
Variations in morphological parameters of NHBE cells on different days of AIC cultures. (A) Representative 3D view of the confocal image (z-stack) of NHBE in the differentiation medium on day 8. (a) Surface, (b) inner cell layers and pores of the membrane are indicated with the arrows. From the confocal images, morphometric parameters such as cell area, height, and volume were measured by MetaMorph software for the NHBE cells in the (B) growth medium; (C) differentiation medium on day 3, 6, 8, and 10 under air-interfacing condition. (C) In the case of NHBE cell multilayers in differentiation medium, inner (I) and surface (S) cell layers (separated with the dotted line) showed differences in cell area, height, and volume. Box-and-whisker plots showing median and 5-95 percentiles were depicted with statistical analyses. Statistics was performed with Tukey's multiple comparison test with the 5% significance level. n.s.=not significant, *P<0.05, **P<0.001, ***P<0.0001.
It was noteworthy that, based on the confocal 3D images, we could confirm that NHBEs in the growth medium had low TEER values, because they could not form intact confluent cell layers. In addition, as shown in Fig. 5B, confocal imaging results exhibited that differentiated NHBE cells under LCC condition formed multilayers with intact tight junctions on the porous membranes with a greater thickness than NHBE cells under AIC condition. This difference in the thickness of cell layers appeared to be the critical factor for the significantly higher TEER values in LCC than in AIC conditions. When the total cell layer thicknesses in AIC and LCC conditions were compared, AIC-conditioned NHBE cells in the differentiation medium showed the most physiologically relevant layer thickness [40].
As shown in Fig. 5B (b), from the differentiated NHBE cells after cultured in LCC condition for 8 days, thick dark layers with little staining of LTG or MTR was observed in between membranes and some inner cell layers. This dark layer could be explained by the physicochemical properties of the probes (LTG and MTR) and the thick multilayer features. In the physiological pH environment of medium, MTR with large molecular weight and lower pKa is present as their ionized form and thus possesses slower transport rate, while relatively lipophilic LTG molecules could more efficiently move across the cell layers. Indeed, when compared with NHBEs in the growth medium (Fig. 5A), NHBEs in the differentiation medium (Fig. 5B (a, b)) showed fewer MTR and some amount of LTG transport across inner cell layers from day 6. As differentiated NHBEs cultured in LCC condition formed more inner cell layers than in AIC condition after day 8, longer time is required for both MTR and LTG molecules to go through all the cell layers. Now that differentiated NHBEs were compared with NHBE monolayers in the growth medium after 30-min transport of dye molecules, NHBE multilayers in AIC and LCC condition exhibited dark inner layers with little amount of dye molecules.
Obviously, NHBE cells had different cell morphology and architecture on porous polyester membranes, developing different cell intactness (tight junctions) in the growth vs. differentiation medium. Based on our results of combined functional data (TEER values and LY permeability) and confocal 3D imaging, we found that only differentiated NHBEs cultured in AIC condition could developed physiologically relevant and confluent cell multilayerss on porous membranes throughout the culture period.
Morphometric Analyses Revealed 3D Architectures of Differentiated vs. Undifferentiated NHBE Cells Grown on Membranes
Next, based on the confocal images, quantitative analyses were carried out and morphometric parameters (cell area, height, or volume) were acquired by using MetaMorph software (Fig. 6B and 6C). These morphometric data provided us with important information of the cell phenotypes and characteristics. As shown in Fig. 6B, while the cell height did not change, the cell area and volume of undifferentiated NHBEs cultured in the growth medium gradually increased from day 3 to day 10. In case of NHBEs cultured in differentiation medium, cells in the inner (I) and surface (S) layers displayed highly differential morphometric parameters between each other (Fig. 6C). The cell area and volume of differentiated NHBEs (median area: 580, 511 and 513 μm2; median volume: 5223, 5069 and 5119 μm3 at day 6, 8 and 10, respectively) in the inner layer maintained unchanged along the time course, while those of the NHBEs (median area: 1162, 1331 and 1443 μm2; median volume: 8290, 9367 and 9119 μm3 at day 6, 8 and 10, respectively) in the surface layer increased similar to the undifferentiated NHBEs. Compared with the inner layer differentiated NHBEs, the surface layer differentiated NHBEs possessed significantly larger cell areas and volumes but with smaller cell heights. Based on the morphological characteristics, the undifferentiated NHBEs and surface layer differentiated NHBEs were considered squamous cell phenotypes. On the other hand, differentiated NHBEs in the inner cell layers appeared to be composed of a mixture of cuboidal and columnar cell-types with small cell area with short (5–8 μm) and long (≥12 μm) cell heights, respectively. Generally, cell morphologies are likely to be affected by increasing neighboring cells in the physically defined space during the culture [41].
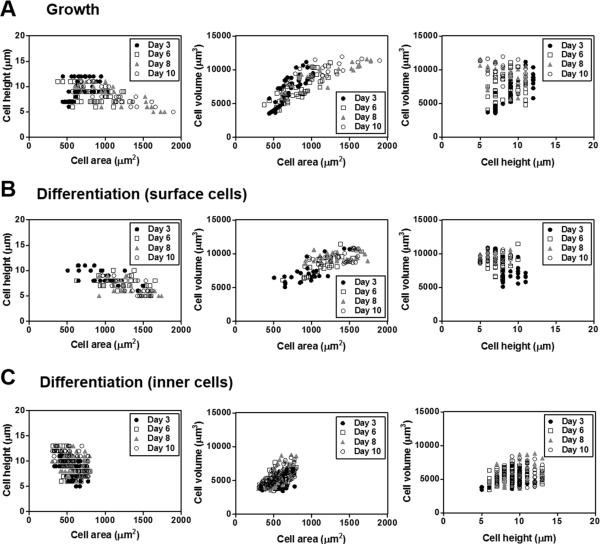
Fig. 7 exhibited the correlation among the morphometric parameters acquired by quantitative analyses of the confocal images of the live cells cultured under AIC condition. The differentiated NHBEs in the inner layers showed no correlation among any morphometric parameter (Fig. 7C). However, in case of both undifferentiated NHBEs and differentiated NHBEs in surface layers, an inverse relationship between the cell area and cell height was observed, while there was a strong correlation between the cell area and the cell volume, indicating that, along the time course, while the cell height got slightly shorter, the cell volume increased by the increase of cell area (Fig. 7A and 7B). Because of the limitation of spatial area on the porous membrane, undifferentiated NHBE cells, forming only cell monolayer, died off after day 10, while differentiated NHBE cells could expand by developing multilayers.
Fig. 7.
Relationship of quantitative morphological parameters in NHBE cells grown in different compositions of media. Cell area, height, and volume were measured by MetaMorph software in the confocal images of the AIC-conditioned NHBE cells in the (A) growth or (B) differentiation medium on day 3, 6, 8, or 10. Scatter plots represents coupled relationships among these parameters, cell area vs. height vs. volume.
Overall, under AIC condition, differentiated NHBE cells have formed intact cell layers without disordered architectures, displaying consistent morphologies to become appropriate cell model for drug transport studies. In our observations, differentiation medium compositions herein were supportive to culture normal primary bronchial cells on the porous polyester membrane under air-interfaced condition to become relevant cell model for the airway epithelium platforms to test absorption and distribution of the inhaled drug molecules. Previous reports suggested that under AIC condition, differentiated human respiratory epithelial cells would be composed of mucus-secreting goblet and basal cells in addition to the ciliated cells [5,8,15]. Given the potential influence of the mucins to the drug transport through the lung epithelium, further studies are underway to characterize the heterogeneous cell population of the differentiated NHBEs and examine the mucus layer thickness on the cell surface. In addition, there have been reports of the influences of extracellular matrix-epithelium interaction on paracrine physiological regulation [19,42]. Thus, the effects of microenvironment at the basolateral side on differentiation of NHBE cells would be also explored by using extracellular matrix fabrication methods [19,43].
Conclusions
In conclusion, differentiated NHBE cells grown on porous polyester membranes under air-interfaced culture (AIC) environment developed cell morphology and architecture relevant to in vivo airway epithelium with intact tight junctions. From our results herein, AIC method appeared to be more proper to culture lung epithelial cells by mimicking the in vivo physiological condition of lung regions interfaced by air. Quantitative microscopic imaging combined with biophysical examination confirmed that differentiated NHBE formed distinct 3-dimensional ultrastructure with confluent multilayers under air interface on porous-bottomed inserts, suggesting NHBE as the appropriate functional cell barrier for the lung-targeting drug transport. Finally, our study design would be useful to investigate biophysical properties of primary airway epithelial cell candidates in order to establish appropriate cell models for studying pharmacokinetic profiles of inhaled drug molecules.
Acknowledgements
This study was financially supported by grants from Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2015R1C1A1A02036781 to M. C. Shin and 2015R1A6A3A01020598 to K. A. Min). Part of this work was funded by NIH grant R01GM078200 to G. R. Rosania.
References
- 1.Sakagami M. In vivo, in vitro and ex vivo models to assess pulmonary absorption and disposition of inhaled therapeutics for systemic delivery. Advanced drug delivery reviews. 2006;58:1030–1060. doi: 10.1016/j.addr.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Tronde A, Norden B, Jeppsson AB, Brunmark P, Nilsson E, Lennernas H, et al. Drug absorption from the isolated perfused rat lung--correlations with drug physicochemical properties and epithelial permeability. Journal of drug targeting. 2003;11:61–74. doi: 10.1080/1061186031000086117. [DOI] [PubMed] [Google Scholar]
- 3.Rothen-Rutishauser BM, Kiama SG, Gehr P. A three-dimensional cellular model of the human respiratory tract to study the interaction with particles. American journal of respiratory cell and molecular biology. 2005;32:281–289. doi: 10.1165/rcmb.2004-0187OC. [DOI] [PubMed] [Google Scholar]
- 4.Nahar K, Gupta N, Gauvin R, Absar S, Patel B, Gupta V, et al. In vitro, in vivo and ex vivo models for studying particle deposition and drug absorption of inhaled pharmaceuticals. European journal of pharmaceutical sciences. 2013;49:805–818. doi: 10.1016/j.ejps.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Forbes B, Ehrhardt C. Human respiratory epithelial cell culture for drug delivery applications. European journal of pharmaceutics and biopharmaceutics. 2005;60:193–205. doi: 10.1016/j.ejpb.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Grainger CI, Greenwell LL, Lockley DJ, Martin GP, Forbes B. Culture of Calu-3 cells at the air interface provides a representative model of the airway epithelial barrier. Pharmaceutical research. 2006;23:1482–1490. doi: 10.1007/s11095-006-0255-0. [DOI] [PubMed] [Google Scholar]
- 7.Elbert KJ, Schafer UF, Schafers HJ, Kim KJ, Lee VH, Lehr CM. Monolayers of human alveolar epithelial cells in primary culture for pulmonary absorption and transport studies. Pharmaceutical research. 1999;16:601–608. doi: 10.1023/a:1018887501927. [DOI] [PubMed] [Google Scholar]
- 8.Steimer A, Haltner E, Lehr CM. Cell culture models of the respiratory tract relevant to pulmonary drug delivery. Journal of aerosol medicine. 2005;18:137–182. doi: 10.1089/jam.2005.18.137. [DOI] [PubMed] [Google Scholar]
- 9.Chan TL, Lippmann M. Experimental measurements and empirical modelling of the regional deposition of inhaled particles in humans. American Industrial Hygiene Association journal. 1980;41:399–409. doi: 10.1080/15298668091424942. [DOI] [PubMed] [Google Scholar]
- 10.Forbes B, Shah A, Martin GP, Lansley AB. The human bronchial epithelial cell line 16HBE14o- as a model system of the airways for studying drug transport. International journal of pharmaceutics. 2003;257:161–167. doi: 10.1016/s0378-5173(03)00129-7. [DOI] [PubMed] [Google Scholar]
- 11.Wan H, Winton HL, Soeller C, Stewart GA, Thompson PJ, Gruenert DC, et al. Tight junction properties of the immortalized human bronchial epithelial cell lines Calu-3 and 16HBE14o. The European respiratory journal. 2000;15:1058–1068. doi: 10.1034/j.1399-3003.2000.01514.x. [DOI] [PubMed] [Google Scholar]
- 12.Forbes II. Human airway epithelial cell lines for in vitro drug transport and metabolism studies. Pharmaceutical science & technology today. 2000;3:18–27. doi: 10.1016/s1461-5347(99)00231-x. [DOI] [PubMed] [Google Scholar]
- 13.Florea BI, Cassara ML, Junginger HE, Borchard G. Drug transport and metabolism characteristics of the human airway epithelial cell line Calu-3. Journal of controlled release. 2003;87:131–138. doi: 10.1016/s0168-3659(02)00356-5. [DOI] [PubMed] [Google Scholar]
- 14.Madlova M, Bosquillon C, Asker D, Dolezal P, Forbes B. In-vitro respiratory drug absorption models possess nominal functional P-glycoprotein activity. The Journal of pharmacy and pharmacology. 2009;61:293–301. doi: 10.1211/jpp/61.03.0003. [DOI] [PubMed] [Google Scholar]
- 15.Ehrhardt C, Forbes B, Kim K-J. Drug Absorption Studies. Springer; NY: 2008. In vitro models of the tracheo-bronchial epithelium. pp. 235–257. [Google Scholar]
- 16.Thornton DJ, Gray T, Nettesheim P, Howard M, Koo JS, Sheehan JK. Characterization of mucins from cultured normal human tracheobronchial epithelial cells. American journal of physiology - Lung cellular and molecular physiology. 2000;278:L1118–1128. doi: 10.1152/ajplung.2000.278.6.L1118. [DOI] [PubMed] [Google Scholar]
- 17.Turner J, Jones CE. Regulation of mucin expression in respiratory diseases. Biochemical Society transactions. 2009;37:877–881. doi: 10.1042/BST0370877. [DOI] [PubMed] [Google Scholar]
- 18.Widdicombe JH, Sachs LA, Morrow JL, Finkbeiner WE. Expansion of cultures of human tracheal epithelium with maintenance of differentiated structure and function. BioTechniques. 2005;39:249–255. doi: 10.2144/05392RR02. [DOI] [PubMed] [Google Scholar]
- 19.Paquette JS, Tremblay P, Bernier V, Auger FA, Laviolette M, Germain L, et al. Production of tissue-engineered three-dimensional human bronchial models. In vitro cellular & developmental biology - Animal. 2003;39:213–220. doi: 10.1290/1543-706X(2003)039<0213:POTTHB>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 20.Guzman K, Gray TE, Yoon JH, Nettesheim P. Quantitation of mucin RNA by PCR reveals induction of both MUC2 and MUC5AC mRNA levels by retinoids. American journal of physiology. 1996;271:L1023–1028. doi: 10.1152/ajplung.1996.271.6.L1023. [DOI] [PubMed] [Google Scholar]
- 21.Choe MM, Tomei AA, Swartz MA. Physiological 3D tissue model of the airway wall and mucosa. Nature protocols. 2006;1:357–362. doi: 10.1038/nprot.2006.54. [DOI] [PubMed] [Google Scholar]
- 22.Yoo JW, Kim YS, Lee SH, Lee MK, Roh HJ, Jhun BH, et al. Serially passaged human nasal epithelial cell monolayer for in vitro drug transport studies. Pharmaceutical research. 2003;20:1690–1696. doi: 10.1023/a:1026112107100. [DOI] [PubMed] [Google Scholar]
- 23.Lin H, Li H, Cho HJ, Bian S, Roh HJ, Lee MK, et al. Air-liquid interface (ALI) culture of human bronchial epithelial cell monolayers as an in vitro model for airway drug transport studies. Journal of pharmaceutical sciences. 2007;96:341–350. doi: 10.1002/jps.20803. [DOI] [PubMed] [Google Scholar]
- 24.Sachs LA, Finkbeiner WE, Widdicombe JH. Effects of media on differentiation of cultured human tracheal epithelium. In vitro cellular & developmental biology - Animal. 2003;39:56–62. doi: 10.1290/1543-706X(2003)039<0056:EOMODO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 25.Moghal N, Neel BG. Integration of growth factor, extracellular matrix, and retinoid signals during bronchial epithelial cell differentiation. Molecular and cellular biology. 1998;18:6666–6678. doi: 10.1128/mcb.18.11.6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lacombe O, Woodley J, Solleux C, Delbos JM, Boursier-Neyret C, Houin G. Localisation of drug permeability along the rat small intestine, using markers of the paracellular, transcellular and some transporter routes. European journal of pharmaceutical sciences. 2004;23:385–391. doi: 10.1016/j.ejps.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Moon SK, Yoo JH, Kim HN, Lim DJ, Chung MH. Effects of retinoic acid, triiodothyronine and hydrocortisone on mucin and lysozyme expression in cultured human middle ear epithelial cells. Acta oto-laryngologica. 2000;120:944–949. doi: 10.1080/00016480050218672. [DOI] [PubMed] [Google Scholar]
- 28.Suresh MV, Wagner MC, Rosania GR, Stringer KA, Min KA, Risler L, et al. Pulmonary administration of a water-soluble curcumin complex reduces severity of acute lung injury. American journal of respiratory cell and molecular biology. 2012;47:280–287. doi: 10.1165/rcmb.2011-0175OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frixione E, Lagunes R, Ruiz L, Urban M, Porter RM. Actin cytoskeleton role in the structural response of epithelial (MDCK) cells to low extracellular Ca2+. Journal of Muscle Research and Cell Motility. 2001;22:229–242. doi: 10.1023/a:1012249629029. [DOI] [PubMed] [Google Scholar]
- 30.Taub ME, Kristensen L, Frokjaer S. Optimized conditions for MDCK permeability and turbidimetric solubility studies using compounds representative of BCS classes I-IV. European journal of pharmaceutical sciences. 2002;15:331–340. doi: 10.1016/s0928-0987(02)00015-5. [DOI] [PubMed] [Google Scholar]
- 31.Ehrhardt C, Fiegel J, Fuchs S, Abu-Dahab R, Schaefer UF, Hanes J, et al. Drug absorption by the respiratory mucosa: cell culture models and particulate drug carriers. Journal of aerosol medicine. 2002;15:131–139. doi: 10.1089/089426802320282257. [DOI] [PubMed] [Google Scholar]
- 32.Mathias NR, Kim KJ, Robison TW, Lee VH. Development and characterization of rabbit tracheal epithelial cell monolayer models for drug transport studies. Pharmaceutical research. 1995;12:1499–1505. doi: 10.1023/a:1016291522345. [DOI] [PubMed] [Google Scholar]
- 33.Galietta LJ, Lantero S, Gazzolo A, Sacco O, Romano L, Rossi GA, et al. An improved method to obtain highly differentiated monolayers of human bronchial epithelial cells. In vitro cellular & developmental biology - Animal. 1998;34:478–481. doi: 10.1007/s11626-998-0081-2. [DOI] [PubMed] [Google Scholar]
- 34.Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. Journal of biological chemistry. 1998;273:29745–29753. doi: 10.1074/jbc.273.45.29745. [DOI] [PubMed] [Google Scholar]
- 35.Wu R, Zhao YH, Chang MM. Growth and differentiation of conducting airway epithelial cells in culture. European respiratory journal. 1997;10:2398–2403. doi: 10.1183/09031936.97.10102398. [DOI] [PubMed] [Google Scholar]
- 36.Gray TE, Guzman K, Davis CW, Abdullah LH, Nettesheim P. Mucociliary differentiation of serially passaged normal human tracheobronchial epithelial cells. American journal of respiratory cell and molecular biology. 1996;14:104–112. doi: 10.1165/ajrcmb.14.1.8534481. [DOI] [PubMed] [Google Scholar]
- 37.Gray T, Koo JS, Nettesheim P. Regulation of mucous differentiation and mucin gene expression in the tracheobronchial epithelium. Toxicology. 2001;160:35–46. doi: 10.1016/s0300-483x(00)00455-8. [DOI] [PubMed] [Google Scholar]
- 38.Neugebauer P, Endepols H, Mickenhagen A, Walger M. Ciliogenesis in submersion and suspension cultures of human nasal epithelial cells. European archives of oto-rhino-laryngology. 2003;260:325–330. doi: 10.1007/s00405-002-0562-y. [DOI] [PubMed] [Google Scholar]
- 39.Matsui H, Randell SH, Peretti SW, Davis CW, Boucher RC. Coordinated clearance of periciliary liquid and mucus from airway surfaces. Journal of clinical investigation. 1998;102:1125–1131. doi: 10.1172/JCI2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choe MM, Sporn PH, Swartz MA. An in vitro airway wall model of remodeling. American journal of physiology - Lung cellular and molecular physiology. 2003;285:L427–433. doi: 10.1152/ajplung.00005.2003. [DOI] [PubMed] [Google Scholar]
- 41.Vunjak-Novakovic G, Freed LE. Culture of organized cell communities. Advanced drug delivery reviews. 1998;33:15–30. doi: 10.1016/s0169-409x(98)00017-9. [DOI] [PubMed] [Google Scholar]
- 42.Nakamura Y, Tate L, Ertl RF, Kawamoto M, Mio T, Adachi Y, et al. Bronchial epithelial cells regulate fibroblast proliferation. American journal of physiology. 1995;269:L377–387. doi: 10.1152/ajplung.1995.269.3.L377. [DOI] [PubMed] [Google Scholar]
- 43.Huang TW, Chan YH, Cheng PW, Young YH, Lou PJ, Young TH. Increased mucociliary differentiation of human respiratory epithelial cells on hyaluronan-derivative membranes. Acta biomaterialia. 2010;6:1191–1199. doi: 10.1016/j.actbio.2009.08.031. [DOI] [PubMed] [Google Scholar]