Abstract
The establishment of protocols to differentiate human pluripotent stem cells (hPSCs) including embryonic (ESC) and induced pluripotent (iPSC) stem cells into functional hepatocyte-like cells (HLCs) creates new opportunities to study liver metabolism, genetic diseases and infection of hepatotropic viruses (hepatitis B and C viruses) in the context of specific genetic background. While supporting efficient differentiation to HLCs, the published protocols are limited in terms of differentiation into fully mature hepatocytes and in a smaller-well format. This limitation handicaps the application of these cells to high-throughput assays. Here we describe a protocol allowing efficient and consistent hepatic differentiation of hPSCs in 384-well plates into functional hepatocyte-like cells, which remain differentiated for more than 3 weeks. This protocol affords the unique opportunity to miniaturize the hPSCs-based differentiation technology and facilitates screening for molecules in modulating liver differentiation, metabolism, genetic network, and response to infection or other external stimuli.
Keywords: Pluripotent stem cells, hepatic differentiation, high-throughput assay, 384-well plates
INTRODUCTION
Hepatic differentiation of patient-derived induced pluripotent stem cells (iPSCs) constitutes a unique in vitro approach to analyze hepatic biology in the context of the genetic background of the patient, and could also hold the key to the large production of hepatocytes for autologous transplantation. Several protocols of hepatic differentiation of human hPSCs have been described recently (Basma et al., 2009; Si Tayeb et al., 2010; Sullivan et al., 2010). They rely on a multi-step process in which the treated cells go through a definitive endoderm stage induced by treatment of colony-type culture of hiPSC with Activin A, followed by hepatic specification to commit the cells toward the hepatic lineage. Finally cells are maturated in vitro to obtain albumin-positive cells, which structurally look like mature hepatocytes. Functional assays demonstrate that these hepatocytes-like cells (HLCs) also secrete albumin, metabolize urea, recapitulate lipid metabolism, and express various isoforms of the cytochrome p450.
These differentiated HLCs retain a strong fetal and poorly mature phenotype, as demonstrated by the persistence of alpha-fetoprotein (AFP) and diminished hepatic functions when compared to primary adult hepatocytes (PHHs). Strategies to improve the differentiation protocol are being explored. For example, treatment of differentiated cells with small-molecule compounds may hold the key to improving hepatic maturation or functionality (Shan et al., 2013). However, in order to screen for small molecules, the differentiation protocol needs to be miniaturized to 384-well plates.
Thus far, the efficient differentiation of hPSCs has relied on a preliminary colony-type culture of hPSCs, directly treated to produce hepatocytes without passage at any stage of the hepatic differentiation (Hannan et al., 2013; Mallanna et al., 2013). Using this approach, efficient differentiation of hPSCs-derived HLCs is usually limited to 6-, 12- or 24-well plates, curtailing the usefulness of this approach in generating cell populations suitable for high-throughput assay (Reviewed in Schwartz et al., 2014). Some published protocols suggested passing definitive endoderm (DE) cells (Hay et al., 2008; Agarwal et al., 2008; Liu et al., 2010; Duan et al., 2010), to allow a more homogeneous and flatter cell population (Inamura et al., 2011), but none of them took advantage of this approach to miniaturize the format of plates. Furthermore the HLCs tend to lose their differentiated phenotype with time without special culturing condition (Khetani and Bhatia, 2008). Here we describe a protocol allowing efficient and consistent differentiation of hESC and hiPSC into functional hepatocyte-like cells in 96- and 384-well plates. We also describe a new HLC culturing condition, allowing maintenance of SC-derived HLCs for more than 3 weeks in these high-throughput formats, with a gradual improvement of their hepatic phenotype and functions.
RESULTS
Limitations of current differentiation protocol
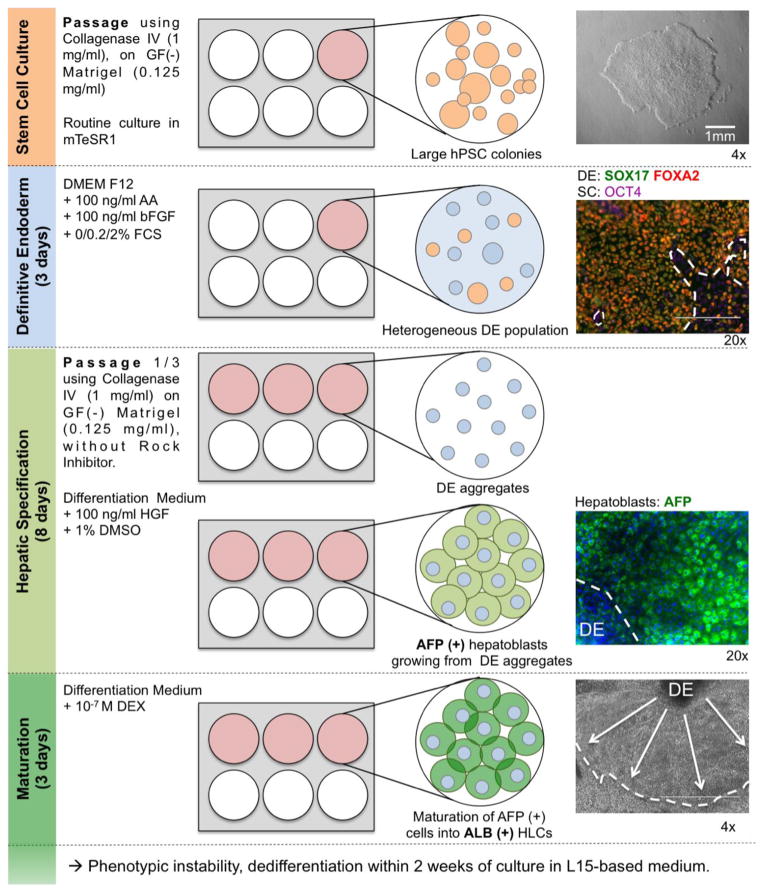
Our previously published protocol (Carpentier et al., 2014), adapted from Basma et al. (Basma et al., 2009) described efficient differentiation of hESCs or hiPSCs into HLCs in 6 or 12-well plates. This protocol allowed us to obtain HLCs with up to 80% of hepatic markers (AFP, ALB and/or AAT)-positive cells, but exhibited several limitations in terms of homogeneity of the HLCs population and miniaturization to smaller-well format (Figure 1). First hPSCs were routinely maintained in a colony-type culture and passaged in aggregates. The definitive endoderm (DE) induction was thus performed on 70–80% confluent large SC colonies treated with 100 ng/ml of Activin A and bFGF in the presence of increasing concentration of FCS (Basma et al., 2009). While allowing up to 60–80% of cells expressing SOX17 and FOXA2 after 3 days of treatment in the best case, this protocol was hampered by a high variability in efficiency of DE induction, because of variability within the lots of Activin A. Moreover, it usually led to a non-homogeneous induction of SOX17/FOXA2-positive DE cells. Importantly, OCT4-positive cells within the DE cell population were still observed at the end of the DE induction stage.
Figure 1. Detailed previous protocol with its main limitations.
Colony-type cultures of hPSCs were differentiated into definitive endoderm population. DE cells were then passed in aggregates, seeded on Matrigel, before undergoing hepatic specification and maturation. One well of a 6-well plate of hPSCs would usually produces 3 wells of a 6-well plate of heterogeneous AFP(+) ALB(+/−) HLCs growing around DE-derived aggregates. Our detailed improved protocol can be found in Figure 7.
Before inducing hepatic specification, we reported that it is preferable to pass the DE cells. Indeed in our hands, direct hepatic specification on confluent DE cells led to overcrowded cell population affecting both the maturation and visualization of the differentiated cells. When passaged, our DE cells exhibited the same limitations as colony-type hPSCs and could not be re-seeded at a single cell level. To allow reproducible and consistent survival of the cells, DE cells had to be passed in a similar way to colony-type hPSCs: re-suspending and re-plating aggregates of approximately 20–50 DE aggregated cells. Treatment with high concentration of HGF and DMSO then induced proliferation of hepatoblast-like cells expanding from those aggregates, leading to a typical non-homogeneous hepatoblasts population. Only those expanded monolayer of AFP-positive cells had the ability to maturate into albumin-positive cells after dexamethasone treatment (Figure 1).
In this context, attempts to pass and differentiate DE cells in 96-well plates were inefficient with heterogeneous cell density and gave rise to inconsistent differentiation from wells to wells. It was also difficult to quantify level of differentiation by IFA of albumin-positive cells because of large cell aggregates.
Next we describe several improvements of our previously published protocol at the different stages from SC culture to hepatic differentiation, allowing for the first time efficient, homogeneous, consistent and reproducible generation of hPSCs-derived HLCs in 384-well plates.
Non-colony-type culture of human pluripotent stem cells
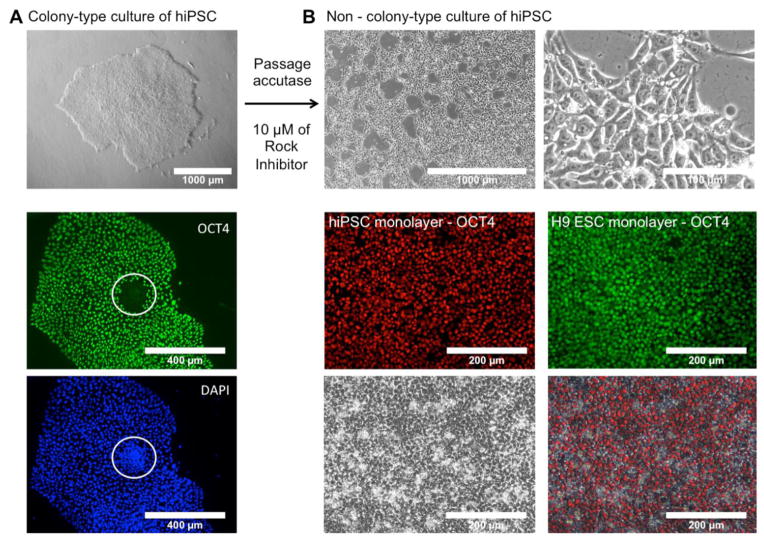
Colony-type culture remains today the standard method to maintain hPSCs in culture. Colony-type culture however presents some disadvantages, such as the heterogeneous state of SCs within colonies (Figure 2A) and common occurrence of chromosomal abnormalities. Recently, Chen et al. described a protocol to adapt human pluripotent SCs to a robust and reliable non-colony type culture (Chen et al., 2012). This approach is based on repeated passage of the hPSCs at single-cell level using gentle dissociation buffer (Accutase), and seeding and maintaining in presence of high concentration of Y-27632 Rock Inhibitor to reduce the high death rate of individual SCs. After a few passages, hPSCs adapted to a single-cell culture are selected and display the same characteristics as hPSCs cultured in colony, in terms of pluripotency and self-renewal.
Figure 2. Adaptation of hPSCs to non-colony type culture.
(A) Colony-type culture of hiPSC showing area of loss of pluripotency marker OCT4 expression.
(B) Colony-type culture of H9ESC and hiPSCs were adapted to a non-colony type culture, by serial passages at a single-cell level, using gentle dissociation buffer and high concentration of Rock Inhibitor (Y-27632). Monolayer adapted hPSCs exhibited SC-like morphology and maintained their expression of pluripotency markers (OCT4). More detailed characterization of expression of pluripotency markers in monolayer-adapted culture of hPSCs is shown in Supplemental Figure 1.
In this context, we used an H9-ESC-derived cell line, named H9ESC-ml (for monolayer), adapted for non-colony type culture by Chen and collaborators (Chen et al., 2012). We also adapted several of our own iPSC cell lines, generated using STEMCCA lentiviruses (Carpentier et al., 2014), to a non-colony type culture following Chen’s protocol. Cell morphology (large nucleus, nucleoli) and expression of various pluripotent markers were assessed routinely in the adapted cells to ensure the maintenance of their pluripotency functions, by IFA, FACS analysis or RTqPCR (Figure 2B and Supplemental Figure 1).
Non colony-type hPSCs were routinely cultured in mTeSR1 on highly concentrated (0.4 mg/ml) growth factor-reduced Matrigel, with daily medium change, and were passed using gentle dissociation buffer (for example, StemPro® Accutase®) in mTeSR1 containing 10 μM Rock Inhibitor Y-27632.
Induction of homogeneous definitive endoderm population
Induction of DE from hPSCs is a critical first step for subsequent efficient hepatic differentiation. Different protocols of induction of DE have been described in the past, all of them relying on treatment with high concentration of Activin A (D’Amour et al., 2005). In order to improve our previously described DE induction protocol (Carpentier et al., 2014), we hypothesized that our non-colony type SC would allow a more homogeneous distribution of the cells and then a more homogeneous effect of AA. Confluent hESCs or hiPSCs were resuspended at a single-cell level using accutase, and 2 million single cells were seeded on low concentration GF (-) Matrigel (0.125 mg/ml) in presence of Y-27632. On the day after, the cells formed a homogeneous monolayer of individual cells, thus potentially maximizing the homogeneity of action of Activin A on the SC population.
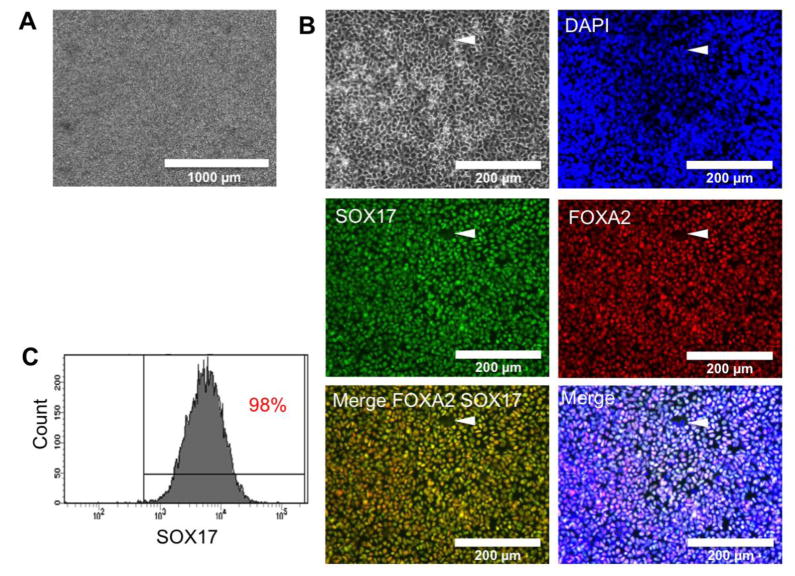
We previously experienced a high variability of effect of Activin A from lot to lot. In order to obtain a more robust and more reproducible DE induction, we investigated the efficiency of the STEMdiff ™ Definitive Endoderm Kit (from StemCell Technologies) on our non-colony type hESCs and hiPSCs, following the manufacturer’s instruction. At the end of the DE induction, the treated cells formed a homogeneous and confluent monolayer of individualized DE cells without aggregates or multicellular structures (Figure 3A). The expression of definitive endoderm markers SOX17 and FOXA2/HNF3B was assessed by immunofluorescence or by FACS analysis. Around 98% of both hiPSCs- and hESCs-derived cells were positive for both markers, assessed by IFA (Figure 3B) and flow cytometry (Figure 3C), and expression of pluripotency (OCT4) was negative (data not shown), confirming a very efficient induction of DE cells on both types of hPSCs investigated here. Each well of a 6-well plate contained between 2 and 2.5 million DE cells. Despite cellular toxicity of Activin A observed during the first few days of DE induction, the cell proliferation during days 3 and 4 allows the maintenance of high number of cells. To be noted, some hiPSCs cell lines exhibited higher cell toxicity or a slow cell growth during the DE induction, leading to less than 2 million cells per well at the end of the induction.
Figure 3. Definitive endoderm induction of non-colony-type hPSCs.
(A) After 4 days of induction, 2 million hESCS or hiPSCs should produce 2–2.5 million confluent DE cells, forming a highly homogeneous monolayer cell population (No aggregates).
(B) Co staining for hiPSCs-derived DE markers FOXA2 and SOX17, confirming the co-expression of these 2 DE markers, while very few cells remain negative for these 2 markers (Arrow).(C) FACS analysis of H9-ESCs-DE cells confirming that 98% are positive for DE marker SOX17. At that time, DE cells are ready for passage before starting hepatic specification. See Supplemental Figure 2 for details about assessing optimal DE seeding density.
Passage of DE cells in various culture formats before hepatic specification
In our previous protocol, hepatic specification could be performed only on passaged aggregates of DE cells, leading to a heterogeneous hepatoblast population (Figure 1). We reasoned that adapting hPSCs to single-cell level and treating single hPSCs for homogeneous DE induction would allow efficient passage of the DE cells at a single cell level.
At the end of the homogeneous DE induction (see Figure 3 for validation), the cell monolayer was resuspended using Accutase, and resuspended in our ‘differentiation medium’ (DMEM-F12, 10 % KOSR, 1% NEAA, 1% glutamine, 1% PS) in the presence of 100 ng/ml of HGF, 1% dimethyl sulfoxide (DMSO) and 10 μM Y-27632. Our experience with differentiation in 6- and 12-w plates showed us that a passage of 1 in 3 usually led to the best level of hepatic differentiation at the end of the differentiation process. To further investigate this optimal concentration of cells, we seeded increasing amounts of H9 ESCs-derived DE cells in wells of 384w plates, and differentiation was carried out as described in this manuscript. At the end of the hepatic maturation (Day 15), hepatic differentiation efficiency was assessed by quantification of the number of AFP- and ALB-positive cells, and quantification of secreted AFP and ALB in the supernatant during the last day of differentiation (Supplemental Figure 2A and 2B). Representative results for the H9-hESC cell line shows that both percentage of positive cells and secretion of ALB (normalized to the number of cells, assessed by automatic quantification of nuclei) show a higher hepatic maturity when about 4000 DE cells were seeded per well (Supplemental Figure 2A and 2C). We then routinely resuspended H9-derived DE cells at a concentration of 125,000 cells per ml, subsequently seeding them at a density of about 79.000 cells/cm2 (see Supplemental Table 1 for volume and cell number per well depending of the plate format).
Importantly, the optimal cell concentration varied from cell line to cell line, and should be evaluated for each cell line using the strategy described here. For example, one of our iPSC cell lines had optimal hepatic differentiation when seeded at 10,000 DE cells per well (data not shown).
Hepatic specification
After seeding of the optimized density of DE cells per cm2 and incubation overnight at 37°C, the hepatic specification was continued for 7 more days by culturing the cells in the differentiation medium with high concentration of hepatocyte growth factor (HGF, 100 ng/ml) in presence of 1% DMSO, with daily medium change, as described previously (Basma et al., 2009; Carpentier et al., 2014). At that point, cells do not undergo apoptosis further and Rock Inhibitor is no longer required. Within days, the cells proliferated and reached confluence, until a point when they became growth arrested.
The exact role of DMSO in this protocol is unclear but is critical. It has been suggested that DMSO promotes hepatic differentiation (Hay et al., 2008; Sullivan et al., 2010), through desacetylation of histones (Baxter et al., 2010) and/or by inducing methylation of the cellular DNA (Basma et al., 2009). DMSO may also stabilize cell membranes (Duan et al., 2010). Importantly, it has been reported (Hay et al., 2008) that subsets of SC remaining within the DE population can lead to other cell type differentiation when cultured in presence of DMSO. This emphasizes the importance of having the purest DE cell population before starting hepatic specification.
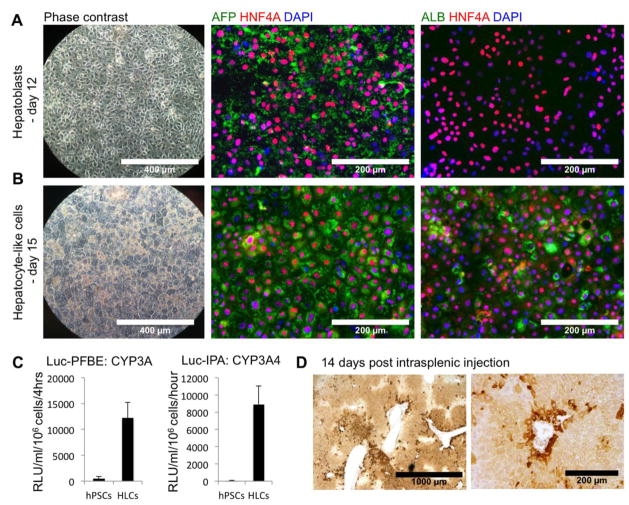
At day 12 of differentiation, the cells formed a confluent monolayer (Figure 4A). They do not display characteristic hepatocyte morphology yet and thus cell differentiation cannot be validated by phase contrast observation only: the nuclei appeared more oval than round, with an intermediate ratio cytoplasm/nucleus. Thus the hepatic specification had to be assessed by IFA for the expression of early hepatic markers, like the hepatocyte nuclear factor 4A (HNF4A), a master regulator of hepatic differentiation, and alpha-fetoprotein (AFP), marker of fetal hepatoblasts. In our protocol, more than 90% of the cells were positive for these 2 markers by IFA (Figure 4A). The cells were also positive for alpha-1-antitrypsin (AAT) (data not shown). Importantly, the cells were negative for albumin or different cytochrome p450 isoforms, markers of more mature hepatocytes. The level of hepatic specification could also be assessed by ELISA. High levels of secreted AFP but no albumin could be detected in the supernatants of culture at day 12 of differentiation (Data not shown).
Figure 4. Hepatic specification and maturation of DE cells passed and differentiated in wells of a 96-well plate.
(A) After 8 days of hepatic specification (day 12), the cells were confluent, but did not display a hepatocyte morphology (phase contrast, magnification: 10x). Cells were positive for HNF4A and AFP, but remained negative for albumin, as assessed by immunofluorescence (Magnification: 20x).
(B) After hepatic maturation (day 15), hepatic-like cells (HLCs) displayed typical polygonal morphology (phase contrast, magnification: 10x) and were positive for AFP, albumin and HNF4A (magnification: 20x).
(C) Activity of the cytochrome P450 3A family (Substrate: Luc-PFBE) and CYP3A4 isoform (Substrate: Luc-IPA) in HLCs compared to hPSCs.
(D) Hep-Par1 immunohistochemistry visualization of engrafted HLCs 14 days after intrasplenic injection of 4 million HLCs in the spleen of MUP-uPA/SCID/Bg mice.
See Supplemental Figure 3 for more functional characterization of HLCs and assessment of intra-hepatic engraftment.
Hepatic Maturation
On day 12, the differentiation medium containing HGF and DMSO was removed and the cells were then cultured for 3 more days in the differentiation medium containing 10−7 M dexamethasone (DEX), a synthetic glucocorticoid, in order to induce hepatic maturation. The medium was replaced daily. As described in our previous protocol, treatment with DEX induced a drastic change of morphology within 2 days, the cells becoming polygonal, displaying tight junctions and small round nuclei (Figure 4B). Lipid droplets were also apparent in the cell cytoplasm. The hepatic maturation of the cells was assessed by immunofluorescence assays. HLCs displayed characteristic nuclear staining for HNF4A and 3B, and strong cytoplasm expression of AFP, ALB (Figure 4B) and AAT (data not shown).
We then assessed hepatic functions of HLCs (Supplemental Figure 1). Secretion of albumin (ALB) is a common indication of hepatic maturation. After 3 days of culture with DEX, the treated cells secrete significant amounts of ALB, confirming hepatic maturation of the cells (Supplemental Figure 1A). We also investigated the effect of Oncostatin M (OSM), a growth factor commonly used instead of (Hay et al., 2008; Si-Tayeb et al., 2010; Sullivan et al., 2010), or in combination with DEX (Agarwal et al., 2008; Liu et al., 2010) to induced hepatic maturation of hepatoblasts into HLCs. Treatment with OSM did not show additional effect on maturation induced by DEX, confirming our previous observations (Supplemental Figure 3A). Hepatic cells are also involved in lipid metabolism: lipid storage in the HLCs was confirmed by Oil Red O staining of lipid droplets (Supplemental Figure 3B), and their capacities to uptake lipoproteins was demonstrated by incubation with Alexa 488-conjugated LDL (Supplemental Figure 3C). The hepatic storage of glycogen was assessed by periodic acid Schiff staining (Supplemental Figure 3D). Finally we observed uptake of Indocyanin Green (ICG), a process mediated by the anion transporter LSAT1 specifically expressed by mature hepatocytes (Supplemental Figure 3E).
More importantly, we assessed the metabolism of PFBE, a drug metabolized by the CYP3A cytochromes, a major family of liver detoxifying enzymes, by our cells. After incubation in presence of Luciferin-PFBE, the release of Luciferin in the supernatant of culture confirmed that our cells have active CYP3A (Figure 4C, left panel). In order to more specifically address the activity of the CYP3A4, a marker of mature hepatocytes, we incubated our HLCs with a CYP3A4-specific substrate Luciferin-IPA. Release of Luciferin in this assay confirms the activity of the CYP3A4 in our cells (Figure 4C, right panel).
At this stage of differentiation, hPSCs-ml-derived HLCs could also be efficiently engrafted in the liver of transgenic MUP-uPA/SCID/Bg mice, as described previously (Carpentier et al., 2014). Within days after intrasplenic injection, increasing levels of human albumin could be detected in the serum of injected mice (data not shown), suggesting repopulation of the mouse liver by the human HLCs. Engrafted HLCs could also be visualized in situ by IHC (Figure 4D, negative control illustrated in Supplemental Figure 3F) and by IFA (Supplemental Figure 3G).
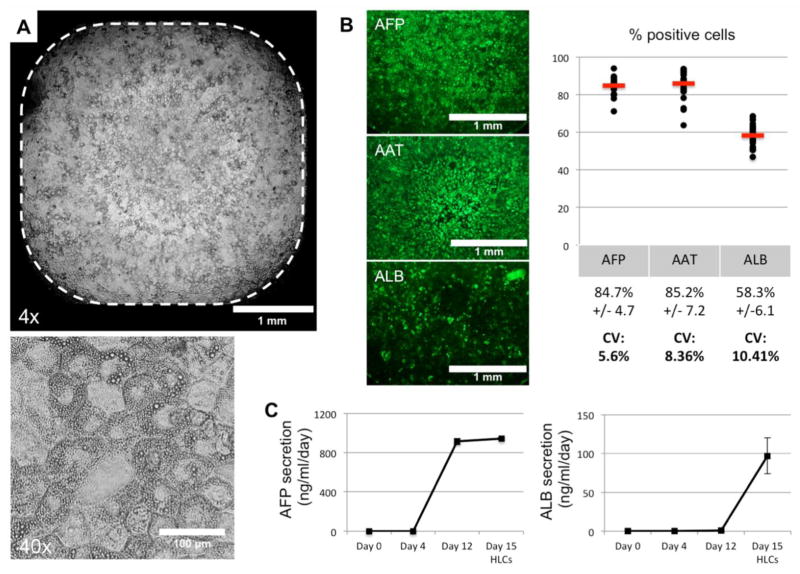
Efficient and reproducible hepatic differentiation in 384-well plates
The advantage of this differentiation protocol is to be able to passage homogeneous DE cells in smaller plate format than the standard plate format necessary to maintained hPSCs culture and treat them with Activin A. In this context, we investigated the efficiency of our protocol for hepatic differentiation in plate format compatible with high throughput assay: 384-well plates. As mentioned earlier, a cell density of 79,000 DE cells per cm2 would correspond to 4400 H9 hESC-derived DE cells per well. This number of resuspended DE cells, previously generated in 6-well plate, was seeded in multiple wells of a 384-well plate previously coated with GF(−) Matrigel, and hepatic specification and maturation were performed as described previously. This approach allowed us to obtain in every well homogeneous monolayer of polygonal cells covering the entire well, without aggregates or empty areas (Figure 5A). After IFA staining for hepatic markers, the percentage of positive cells at day 15 of differentiation was assessed by automated image acquisition, showing a consistent high percentage of AFP(+), AAT(+) and ALB(+) from well to well (Figure 5B). CV% for expression of markers assessed on more than 100 wells by automatic acquisition and analysis show variability below 15%. The IFA results were further confirmed by ELISA. At day 15, AFP secretion is maintained at a similar level as Day 12, confirming that the HLCs did not lose expression of fetal markers. However, different from Day 12 cells, the HLCs now secreted significant level of ALB (Figure 5C), confirming efficient maturation of HLCs in wells of 384-well plates. In order to calculate the CV% for the functional ALB secretion, we used the AlphaLISA technology (Perkin Elmer), with CV% ranging between 5 (raw data) and 15% (quantification using standard curves). These results confirm the homogeneous hepatic differentiation of hPSCs within wells of 384-well plate with phenotypic and functional variability low enough to make it suitable for high-throughput assay.
Figure 5. Assessment of hepatic maturation of H9-ESC-derived HLCs differentiated in 384-well plates.
(A) Phase contrast microscopy showing homogeneous population of polygonal cells within a well of a 384-well plate. Higher magnification illustrates the polygonal morphology, small round nuclei and presence of cytoplasmic lipid droplets.
(B) Automatic quantification of percentage of cells positive for hepatic markers AFP, AAT and ALB within wells of a 384-well plate, with quantification evaluated automatically on more than 100 wells (magnification 4x).
(C) ELISA for AFP and ALB in the supernatants during the differentiation process in 384-well plates.
Long-term maintenance of HLCs
We usually maintained the HLCs in a medium previously used for primary culture of adult human hepatocytes (Podevin et al., 2010) and hPSCs-derived HLCs (Hay et al., 2008; Sullivan et al., 2010; Carpentier et al., 2014), composed of Leibowitz L15 medium, 8.4% FCS, 8.4% Tryptose Phosphate Buffer, 1% NEAA and PS, complemented with 1 μM of insulin (5.8μg/ml) and 10 μM (4.8μg/ml) of hydrocortisone 21-hemisuccinate. However, in this medium, HLCs usually exhibit a characteristic phenotypic instability, with a loss of hepatic morphology within a week, associated with a dedifferentiation of the cells. For example, we showed that HCV-infected HLCs lost the ability to support viral replication during this dedifferentiation process (Carpentier et al., 2014).
In order to improve the stability of the differentiated hepatocytes, we investigated the culture of HLCs in a medium validated for primary culture of adult human hepatocytes (Schulze-Bergkamen et al., 2003) and also used for in vitro differentiation and long-term maintenance of HepaRG cells, a mature hepatic cell model based on in vitro maturation of precursor hepatic-like cells (Lucifora et al., 2014). This medium is based on William’s E Medium, containing 10% FBS, penicillin and streptomycin, complemented with 1 μg/ml of human insulin (0.17 μM), 10 μM of hydrocortisone 21-hemisuccinate (4.8 μg/ml) and 1.8% DMSO. When cultured in this WEM medium, the HLCs displayed a much more stable phenotype, with differentiated HLCs maintained in culture for more than 3 weeks (Figure 6A and B). Interestingly, the typical hepatic morphology of the HLCs continues to improve toward more polygonal cells with smaller nucleus and more binucleated cells (Figure 6B, arrows). What promotes this phenotypic stability is not known, but the DMSO in the WEM culture medium has been described as critical for maintenance of primary hepatocytes phenotype (Schulze-Bergkamen et al., 2003) and also helps stabilize cellular membrane (Duan et al., 2010). Moreover, specific concentration of insulin and hydrocortisone has been shown to maintain hepatic phenotype of primary cultures of human adult hepatocytes for around 15 days (Podevin et al., 2010).
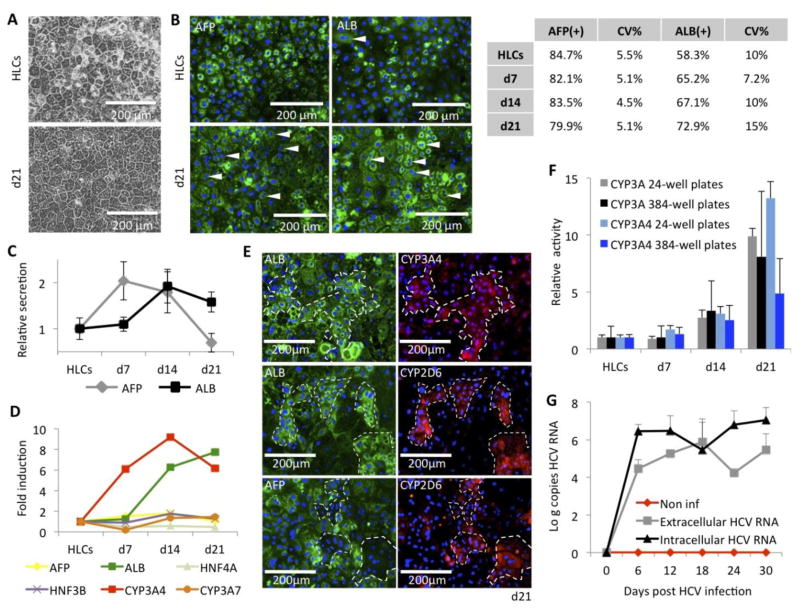
Figure 6. Long-term maintenance of HLCs.
(A) Improved phenotypic stability when HLCs are cultured in the new WEM hepatic maintenance medium, compared to previously described L15 medium.
(B) Immunofluorescence assays for expression of AFP and ALB in HLCs, differentiated in 384-well plates, and maintained for 3 weeks in the WEM medium. (Arrows indicate binucleated cells).Table indicates percentage of positive cells at the different times of culture in WEM medium, assessed by automatic quantification, and their respective coefficient of variation (CV%) from well to well.
(C) Relative secretion of AFP and ALB assessed by ELISA in the supernatants of culture of HLCs, differentiated in 384w plates, and maintained for 3 weeks in the WEM medium.
(D) RTqPCR analysis of hepatic genes in SC-derived HLCs, differentiated in 24-well plates, and maintained for 3 weeks in the WEM medium. See Supplemental Figure 2 for expression relative to control PHHs.
(E) Co-immunostaining for ALB, AFP, CYP3A4 and CYP2D6 in HLCs, differentiated in 24-well plates, after 21 days in WEM medium.
(F) Increased of the CYP3A PFBE and CYP3A4 IPA activities during 3 weeks of culture in WEM medium, on HLCs differentiated and maintained in 24-well plates (grey and light blue bars) and 384-well plates (black and blue bars).
(G) HCV replication in HLCs maintained in WEM medium for up to 1 month, assessed by RTqPCR for intra- and extracellular HCV RNA quantification, expressed respectively as log (copies HCV RNA / mg total cellular RNA) (black line) and log (copies HCV RNA / ml of supernatants) (grey line), compared to non-inoculated HLCs (red line). HLCs were differentiated, inoculated with HCVcc and maintained in 24-well plates.
Characterization of our HLCs, differentiated in 384-well plates, during the 3 weeks of maintenance in the WEM medium by immunofluorescence followed by automatic quantification of the positive cells showed a maintained expression of AFP (>80%) and a progressive increased percentage of cells positive for albumin (From 58% to up to 73%) (Figure 6B). To be noted, not only more cells were positive for albumin, but also the intensity of albumin staining per cell was significantly increased while AFP staining intensity tended to decrease. These results were further confirmed by a consistent increase of albumin concentration in the supernatant (Figure 6C). Secretion of AFP decreased during the 2nd and 3rd week of culture in the hepatic maintenance medium (Figure 6C).
RTqPCR analysis of hepatic genes during culture in the WEM medium shows that ALB mRNA is significantly increased during the 3 weeks of culture (Figure 6D, green line), consistent with results obtained by IFA and ELISA. Importantly, we could also detect an increased expression of CYP3A4 mRNA (Figure 6D, red line), marker of mature hepatocyte. To be noted, the expression of the fetal marker AFP and CYP3A7, the predominant human fetal liver cytochrome, did not increase during that time (Figure 6D, yellow and orange lines). However, when compared to RNA isolated from primary culture of adult hepatocytes, HLCs still exhibited a less mature phenotype (Supplemental Figure 4). Immunofluorescence assays on cells maintained 21 days in the WEM medium show that cells brightly positive for ALB are also positive for CYP3A4 (Figure 6E, upper panels). Interestingly, expression of CYP2D6 was also correlated to ALB bright positive cells (Figure 6E, middle panels), but found mainly in cells negative for AFP (Figure 6E, lower panels)
Assessment of the CYP3A family activity during culture in the WEM medium confirmed an improved activity during the 3 weeks of culture in the WEM medium, both in 24w- and 384wHLCs (Figure 6F). Because CYP3A7 is not overexpressed during that time, we believe that this increase of CYP3A activity is mainly due to CYP3A4. This hypothesis was confirmed by increased activity of the CYP3A4 activity, assessed by incubating the HLCs with Luc-IPA, and observed both in 24w- and 384w-HLCs (Figure 6F). To be noted, attempts to quantify induction of CYP3A4 and 1A2 in HLCs differentiated in 384w plates were not successful (data not shown), suggesting that further maturation is needed to ensure fully mature hepatic functionality of these cells in 384w plates.
Finally, we investigated the maintenance of HCV replication after overnight inoculation of HLCs with JFH1-HCVcc (Wakita et al., 2015), followed by culture in the WEM medium for a month (Figure 6G). While HLCs maintenance in our previous L15 culture medium showed loss of HCV replication (Carpentier et al., 2014), culture in the WEM medium allowed maintenance of HCV replication, demonstrated by quantification of both intracellular and extracellular HCV RNA for more than a month.
Taken together, these results illustrate the functional maintenance of hPSCs-derived HLCs for more than 3 weeks, with an improvement of their hepatic functions (ALB secretion, CYP3A4 activity), and the ability to maintain long-term infection by HCV, a hepatotropic virus notoriously difficult to culture.
DISCUSSION
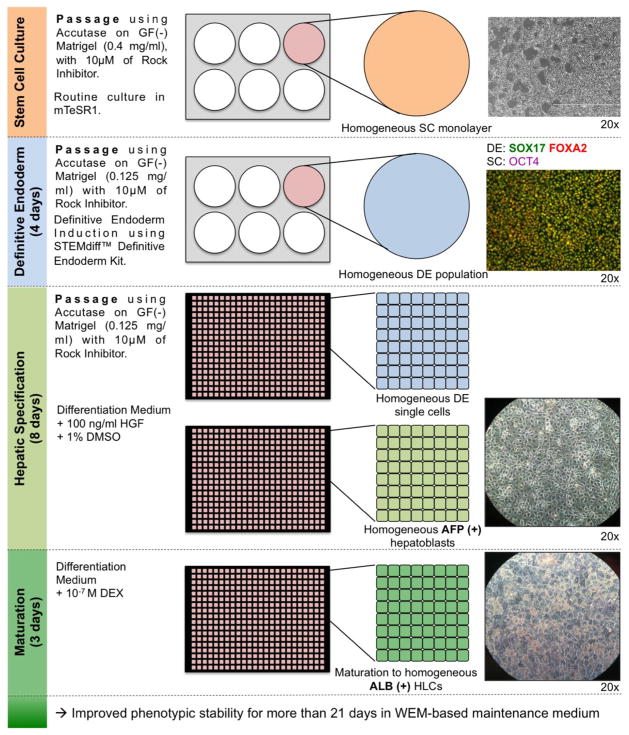
Here we described for the first time the efficient and reproducible differentiation of human pluripotent stem cells into functional hepatocyte-like cells in 384-well plates. We identified here several critical factors that ensure efficiency and reproducibility of hepatic differentiation of hPSCs (Figure 7):
Figure 7. Optimized protocol for differentiation of hPSCs into HLCs within wells of a 384-well plate.
Non-colony-type cultures of hPSCs are differentiated into highly homogeneous definitive endoderm population. DE population homogeneity is assessed for co-expression of SOX17 and FOXA2. DE cells are then passed at a single-cell level on GF(-) Matrigel, before undergoing hepatic specification and maturation in a 384-well plate. At the different stage of hepatic specification and maturation, cells should be assessed for expression of hepatoblast marker AFP and hepatocyte marker ALB. One well of a 6-well plate of hPSCs usually produces over 1 full 384-well plate of highly homogeneous AFP(+) ALB(+) HLCs.
The use of hPSCs maintained in non-colony type culture, allowing a better and more homogeneous passage of the cells at the pluripotent stage.
The use of the STEMdiff Definitive Endoderm kit, which combined with the single-cell culture adapted hPSCs, allows a robust and reproducible induction of highly pure and homogeneous DE cell population.
The passage of monolayer hPSCs-derived DE cells at a single-cell level, allowing subsequent differentiation in smaller-well format (96- and 384-well plates)
A hepatic specification step in presence of DMSO, allowing a more homogeneous hepatic differentiation but also a proliferation of the passaged cells to reach confluence.
While the HLCs generated from this new protocol do not exhibit a significantly more mature hepatic phenotype than what has been described before, we described here for the first time the consistent and reproducible differentiation of HLCs in 384-well plates, which opens the door to their use for high throughput assay. Importantly, we showed here that results for both phenotypic (expression of hepatic markers by IFA) and functional (secretion of ALB assessed by AlphaLISA) characterization of the 384w-HLCs show coefficients of variation below 15%, making them suitable as readout for high-throughput screening. In this context, the screening for compounds that could improve the level of in vitro maturation of HLCs is currently in progress. Recently, a study used micro patterned PHHs (MCPPs) on feeder cells to perform a high-throughput screen in order to identify drugs capable of improving functionality in vitro of PHHs and potentially promoting maturation of SC-derived HLCs (Shan et al., 2013). However this screen was not performed directly on HLCs, because they could not be efficiently differentiated in wells smaller than a 24-well plate. Only candidate drugs selected on adult PHHs were then validated on HLCs, suggesting that drugs specifically acting on early stages of hepatic maturation could have been missed.
In addition, we described a new long-term culture medium allowing maintenance and further maturation of HLCs for more than 3 weeks, in large format and in 384-well plates. During maintenance in WEM medium, HLCs exhibited improved phenotypic stability, increased secretion and higher percentage of cells positive for adult hepatic markers. Importantly, CV% of expression of hepatic markers, like AFP and ALB, was maintained below 15% throughout the maintenance in WEM medium, compatible with use for high-throughput assay.
HLCs maintained in WEM medium also demonstrated an increase of the metabolic activity of CYP3A family and 3A4 cytochromes, consistent with a more mature phenotype. However, CYP activity showed significant variability from well to well, with CV% above 15%. Investigation for compounds improving hepatic maturation should also focus on their effect on homogeneity of maturation, in order to allow high throughput assay related to more mature hepatic functions, like drug metabolism and toxicology assays.
Finally, we demonstrated that HLCs maintained in WEM medium could support productive HCV infection for more than 30 days, suggesting it could be a valuable approach for studying the HCV infection in cells reproducing the genetic background of the patient. To be noted, HLCs maintained in WEM medium also support and maintain productive infection by Hepatitis B Virus (HBV) after in vitro infection (Xia Y et al., manuscript in preparation).
Taken together, these improvements allow for the first time the production of stem cell-derived HLCs in a format suitable for high-throughput assays. PHHs are currently the reference cells for liver metabolism in vitro assay and toxicology studies, but PHHs-based approaches are hampered by very limited cell supply, short-term in vitro culture, and lack of specific donor’s phenotypic and genetic information. The ability to reprogram patient somatic cells into hiPSCs and then to differentiate them into hepatocyte-like cells, and maintain them for more than 3 weeks in 384-w plates opens for the first time the possibility to generate large numbers of patient-specific hepatocytes for high-throughput assay. However, more improvements are still needed to develop a stem cell-derived hepatocyte-like cells model as relevant and mature as adult hepatocytes. In this context, we are currently conducting a high throughput screening of thousands of small compounds for their ability to induce further hepatic maturation of HLCs differentiated in 384-well plates. We hope that this strategy, based on the new protocol described here, will identify new compounds aimed at improving the relevance of the model.
EXPERIMENTAL PROCEDURES
Stem cell culture
Experiments were performed on several iPSC cell lines generated in our laboratory (using STEMCAA-lentivirus reprograming vector) or by other collaborating teams here at NIH, using retro- and lentiviruses. In parallel, protocol of differentiation was validated using the H1, H9 and HSF6 ESC cell lines. All hESCs and hiPSCs cell lines were maintained in a colony-type culture on growth factor-reduced (GF(−)) Matrigel (0.125 mg/ml)(Corning) in mTeSR1 (StemCell Technologies). Colony type H9-ESC and 2 STEMCCA-iPSCs were then adapted to a non-colony type culture following instructions by Chen et al. (Chen et al., 2012). Briefly, hPSCs colonies were dissociated using Accutase (Life Technologies) and single hPSCs were resuspended in mTeSR1 (StemCell technologies) in presence of 10μM of Rock Inhibitor Y-27632 (Millipore) and seeded in wells coated with highly concentrated GF(−) Matrigel (0.4 mg/ml). Monolayer type hPSCs are routinely passed 1 in 8 every 5–6 days. Expression of pluripotency markers was assessed on cells fixed with PFA4% and incubated overnight with antibodies anti-OCT4 (Santa Cruz Biotechnologies, sc-9081, 1:400) or anti-NANOG (BD, N31-355, 1:200) as described previously (Carpentier et al., 2014). FACS analysis were performed on resuspended cells as described previously (Carpentier et al., 2014) using the same set of antibodies. Total RNA was also isolated from monolayer and colony type culture of hPSCS using the GeneJET RNA isolation kit (Thermo Scientific) and pluripotency gene expression was assessed using the Verso 1-step QRT-PCR Low ROX kit (Thermo Scientific).
Definitive endoderm induction
DE was induced using the STEMdiff™ Definitive Endoderm Kit (StemCell Technologies), following manufacturer’s instructions. Briefly, monolayer type culture of hPSCs are resuspended using Accutase (Life Technologies) and 2 million cells were seeded per well of a 6w plates previously coated with GF(−) Matrigel (0.125 mg/mL). The day after, cell monolayer was washed with PBS and then cultured for 4 days in STEMdiff Definitive Endoderm Basal medium with Supplements A and B (for 1 day) and then Supplement B only (for 3 days, with daily medium change). Expression of DE markers was assessed by immunofluorescence assay and flow cytometry on PFA-fixed cells using a combination of antibodies against SOX17 (R&D Systems, AF1924, 1:100) and FOXA2 (Santa Cruz Biotechnology, sc-271103, 1:100) as described previously (Carpentier et al., 2014).
Hepatic specification
Before starting the hepatic specification, DE cells have to be passed. DE cells were resuspended using accutase, and seeded at a concentration of 79000 cells / cm2 (See Supplemental Table 1), in a differentiation medium (High glucose DMEM, F12, 10% KOSR, 1% Glutamine, 1% NEAA, 1% Penicillin/Streptomycin)(Life technologies) containing 100ng/ml of HGF (Peprotech), 1% DMSO (Sigma Aldrich) and 10μM Rock Inhibitor Y-27632. The day after, the medium is changed, and the cells are cultures for 7 more days in Differentiation Medium containing similar concentration of HGF and DMSO, but without Rock Inhibitor Y-27632.
Hepatic maturation
Hepatoblasts are then maturated for 3 days by culturing the cells in differentiation medium containing 10−7M of Dexamethasone (Sigma Aldrich).
HLCs long-term maintenance
Differentiated HLCs could then be maintained for more than 3 weeks in the WEM medium based on William’s E Medium containing 10% FBS, 1% PS, 1 μg/ml of human Insulin (0.17μM) (Sigma Aldrich), 4.8 μg/ml of hydrocortisone 21-hemisuccinate (10 μM) (Sigma Aldrich), and 1.8% DMSO, with medium changed every 2–3 days.
Primary Human Hepatocytes culture
Suspension of freshly isolated or cryopreserved PHHs were seeded on Matrigel and maintained for 2 days in hepatic maintenance medium before their total RNA was isolated and used as control for expression of hepatic genes in HLCs. PHHs were provided by the NIH-funded Liver Tissue Procurement and Cell Distribution System (N01-DK-7-0004/HHSN26700700004C).
Assessment of hepatic differentiation and maturation
Secretion of ALB (Bethyl Laboratories) and AFP (Calbiotech) were assessed by ELISA following manufacturer’s instructions. To be noted, when assessing AFP secretion in WEM medium, the supernatant should be diluted at least 1:100 as the WEM medium affect the sensitivity of the kit. Secretion of ALB in 384-well plates was also assessed by AlphaLISA (Perkin-Elmer), following manufacturer’s instructions. For immunofluorescence assay, cells were fixed using PFA 4%, and stained using a combination of antibodies anti-ALB (Cedarlane, CL2513A, 1:330), anti-AFP (Sigma Aldrich, A8452, 1:330), anti-AAT (Dako, A0012, 1:50), anti-HNF4A (Santa Cruz Biotech, sc-6556, 1:100), anti-CYP3A4 (Abcam, ab135813, 1:50) and anti-CYP2D6 (Sigma Aldrich, AV41675, 1:200). Percentage of positive cells was then assessed using an Image Express Micro (Molecular Devices) and analyzed with Metamorph Cell Scoring software. Total RNA was isolated using the GeneJET RNA isolation kit (Thermo Scientific) and gene expression was assessed using the Verso 1-step QRT-PCR Low ROX kit (Thermo Scientific). Used primers are listed in Supplemental Table 2. Functional assays (ORO staining, Lipoprotein uptake, PAS staining and ICG metabolism) were performed as described previously (Carpentier et al., 2014). CYP450 3A family activity was assessed using the P450-Glo Luc-PFBE CYP3A4 Assay (Promega), and specific CYP3A4 activity was confirmed using the P450-Glo Luc-IPA CYP3A4 Assay (Promega), both following the manufacturer’s instructions with Luc-PFBE and Luc-IPA diluted in differentiation medium instead of WEM medium.
Murine hepatic transplantation of human HLCs was performed in the MUP-uPA/SCID/Bg transgenic mouse model, by intrasplenic injection of 4 million HLCs dissociated using Trypsin-EDTA (Corning) and re-suspended in Saline Buffer, as described previously (Carpentier et al., 2014). In situ detection of engrafted cells was performed on PFA-fixed section of paraffin-embedded liver, using antibodies anti-Hep Par-1 (Dako, M7158, 1:50), anti-ALB (Bethyl Laboratories, A80-229A, 1:500) and anti-AAT (Dako, A0012, 1:50), as described previously (Carpentier et al., 2014).
In vitro HCV infection
HCV infection was performed using JFH1-HCVcc (Genotype 2a), produced in Huh7.5.1 cells as described by Wakita et al. (Wakita et al., 2005). HLCs were incubated overnight with JFH1-HCVcc at a multiplicity of infection of 0.5. The day after, inoculated cells were washed with PBS and maintained in culture in WEM medium. Every 3 days, HCV RNAs were isolated from HLCs using the GeneJET RNA isolation kit (Thermo Scientific) and from supernatants using the GeneJET Viral DNA and RNA Purification Kit (Thermo Scientific). HCV RNA titer was then quantified by RTqPCR as described previously (Carpentier et al., 2014).
Statistics
In vitro data are expressed as average of at least 3 independent experiments, and 2-tailed Student’s t test was performed to assess statistical significance. A P value of less than 0.05 was considered significant.
Results concerning the 384-well plates experiments are expressed as average of at least 100 wells.
The coefficient of variation (CV%) is calculated as:
Animal study approval
HLCs mouse engraftment was performed according to the NIH guidelines for animal care and was approved by the Office of Animal Care and Use, Bethesda, Maryland, USA (protocol NIDDK-K090-LDB-13).
Supplementary Material
Highlights.
Hepatic differentiation of human PSCs in 96- and 384-w plates.
Phenotypic and functional CV% < 15%: suitable for high-throughput assays.
New HLCs culture medium: maintenance and further hepatic maturation for >30 days.
Upon HCV inoculation, detectable intra and extracellular HCV RNA for >30 days.
Acknowledgments
We wish to thank Kevin Chen (NIH Stem Cell Unit) for the monolayer adapted H9-ESC cell line and for his advices concerning adaptation of colony-type culture into monolayer SC culture. We thank also Zhensheng Zhang, Ronda Sapp, Fang Zhang, and Frank Q Li (NIDDK, LDB) for their technical assistance and helpful discussions. This work was supported by the Intramural Research Program of the NIDDK (Z01 DK54504 and DK54505).
Footnotes
Author Contributions
A.C. and T.J.L. designed the experiments. A.C., I.N., V.C., Y.X. and Z.H. performed and analyzed the experiments. A.C., I.N. and T.J.L. wrote and revised the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agarwal S, Holton KL, Lanza R. Efficient differentiation of functional hepatocytes from human embryonic stem cells. Stem Cells. 2008;26:1117–27. doi: 10.1634/stemcells.2007-1102. [DOI] [PubMed] [Google Scholar]
- Basma H, Soto-Gutiérrez A, Yannam GR, Liu L, Ito R, Yamamoto T, Ellis E, Carson SD, Sato S, Chen Y, et al. Differentiation and transplantation of human embryonic stem cell-derived hepatocytes. Gastroenterology. 2009;136:990–9. doi: 10.1053/j.gastro.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter MA, Rowe C, Alder J, Harrison S, Hanley KP, Park BK, Kitteringham NR, Goldring CE, Hanley NA. Generating hepatic cell lineages from pluripotent stem cells for drug toxicity screening. Stem Cell Res. 2010;5:4–22. doi: 10.1016/j.scr.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier A, Tesfaye A, Chu V, Nimgaonkar I, Zhang F, Lee SB, Thorgeirsson SS, Feinstone SM, Liang TJ. Engrafted human stem cell-derived hepatocytes establish an infectious HCV murine model. J Clin Invest. 2014;124:4953–4964. doi: 10.1172/JCI75456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KG, Mallon BS, Hamilton RS, Kozhich OA, Park K, Hoeppner DJ, Robey PG, McKay RDG. Non-colony type monolayer culture of human embryonic stem cells. Stem Cell Res. 2012;9:237–248. doi: 10.1016/j.scr.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotech. 2005;23:1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- Duan Y, Ma X, Zou W, Wang C, Bahbahan IS, Ahuja TP, Tolstikov V, Zern ME. Differentiation and characterization of metabolically functioning hepatocytes from human embryonic stem cells. Stem Cells. 2010;28:674–686. doi: 10.1002/stem.315. [DOI] [PubMed] [Google Scholar]
- Hannan NRF, Segeritz CP, Touboul T, Vallier L. Production of hepatocyte-like cells from human pluripotent stem cells. Nat Protoc. 2013;8:430–437. doi: 10.1038/nprot.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay DC, Zhao D, Fletcher J, Hewitt ZA, McLean D, Urruticoechea-Uriguen A, Black JR, Elcombe C, Ross JA, Wolf R, et al. Efficient differentiation of hepatocytes from human embryonic stem cells exhibiting markers recapitulating liver development in vivo. Stem Cells. 2008;26:894–902. doi: 10.1634/stemcells.2007-0718. [DOI] [PubMed] [Google Scholar]
- Inamura M, Kawabata K, Takayama K, Tashiro K, Sakurai F, Katayama K, Toyoda M, Akutsu H, Miyagawa Y, Okita H, et al. Efficient generation of hepatoblasts from human ES cells and iPS cells by transient overexpression of homeobox gene HEX. Mol Ther. 2011;19:400–407. doi: 10.1038/mt.2010.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khetani SR, Bhatia SN. Microscale culture of human liver cells for drug development. Nat Biotechnol. 2008;26:120–126. doi: 10.1038/nbt1361. [DOI] [PubMed] [Google Scholar]
- Liu H, Ye Z, Kim Y, Sharkis S, Jang YY. Generation of endoderm-derived human induced pluripotent stem cells from primary hepatocytes. Hepatology. 2010;51:1810–1819. doi: 10.1002/hep.23626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucifora J, Xia Y, Reisinger F, Zhang K, Stadler D, Cheng X, Sprinzl MF, Koppensteiner H, Makowska Z, Volz T, et al. Specific and nonhepatotoxic degradation of nuclear hepatitis B virus cccDNA. Science. 2014;343:1221–1228. doi: 10.1126/science.1243462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallanna SK, Duncan SA. Differentiation of hepatocytes from pluripotent stem cells. Curr Proto SC Biology. 2013;26:1G.4.1–1G.4.13. doi: 10.1002/9780470151808.sc01g04s26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podevin P, Carpentier A, Pène V, Aoudjehane L, Carrière M, Zaïdi S, Hernandez C, Calle V, Méritet JF, Scatton O, et al. Production of infectious hepatitis C virus in primary cultures of human adult hepatocytes. Gastroenterology. 2010;139:1355–64. doi: 10.1053/j.gastro.2010.06.058. [DOI] [PubMed] [Google Scholar]
- Schulze-Bergkamen H, Untergasser A, Dax A, Vogel H, Büchler P, Klar E, Lehnert T, Friess H, Büchler MW, Kirschfink M, et al. Primary human hepatocytes – a valuable tool for investigation of apoptosis and hepatitis B virus infection. J Hepatol. 2003;38:736–744. doi: 10.1016/s0168-8278(03)00120-x. [DOI] [PubMed] [Google Scholar]
- Schwartz RE, Fleming HE, Khetani SR, Bhatia SN. Pluripotent stem cellderived hepatocyte-like cells. Biotech Adv. 2014;32:504–513. doi: 10.1016/j.biotechadv.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan J, Schwartz RE, Ross NT, Logan DJ, Thomas D, Duncan SA, North TE, Goessling W, Carpenter AE, Bhatia SN. Identification of small molecules for human hepatocyte expansion and iPS differentiation. Nat Chem Biol. 2013;9:514–521. doi: 10.1038/nchembio.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si Tayeb K, Noto FK, Nagaoka M, Li J, Battle MA, Duris C, North PE, Dalton S, Duncan SA. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology. 2010;51:297–305. doi: 10.1002/hep.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan GJ, Hay DC, Park IH, Fletcher J, Hannoun Z, Payne CM, Dalgetty D, Black JR, Ross JA, Samuel K, et al. Generation of functional human hepatic endoderm from human induced pluripotent stem cells. Hepatology. 2010;51:329–35. doi: 10.1002/hep.23335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakita T, Pietschmann T, Kato T, Date T, Miyamoto M, Zhao Z, Murthy K, Habermann A, Krausslich HG, Mizokami M, et al. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med. 2005;11:791–6. doi: 10.1038/nm1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.