Abstract
This is a brief summary of our studies of NOD autoimmune diabetes examining the events during the initial stage of the process. Our focus has been on antigen presentation events and the antigen presenting cells (APC) inside islets. Islets of non-diabetic mice contain resident macrophages that are developmentally distinct from those in the inter-acinar stroma. The autoimmune process starts with the entrance of CD4+ T cells together with a burst of a subset of dendritic cells (DC) bearing CD103. The CD103+ DC develop under the influence of the Batf3 transcription factor. Batf3 deficient mice do not develop diabetes and their islets are uninfiltrated throughout life. Thus, the CD103+ DC are necessary for the progression of autoimmune diabetes. The major CD4+ T cell response in NOD are the T cells directed to insulin. In particular, the non-conventional 12–20 segment of the insulin B chain is presented by the class II MHC molecule I-Ag7 and elicits pathogenic CD4+ T cells. We discuss that the diabetic process requires the CD103+ DC, the CD4+ T cells to insulin peptides, and NOD specific I-Ag7 MHC-II allele. Finally, our initial studies indicate that beta cells transfer insulin containing vesicles to the local APC in a contact-dependent reaction. Live images of beta cells interactions with the APC and electron micrographs of islet APCs also show the transfer of granules.
1.0 Introduction
1.1
This paper is a brief summary of our recent studies on antigen presentation in the islets of Langerhans of the NOD mouse strain [1–3]. We do not review here the complex process of autoimmune diabetes, but focus entirely on recent experiences from our laboratory examining antigen presentation events in islets. About 80–90% of mice in our NOD colony develop diabetes starting at about the 20th week of age. The first detectable insult in the NOD is an interferon-inducible gene signature evident from 4–6 weeks of age [4]. This is the critical time period in which the autoimmune process is set to move forward. We summarize here the antigen presentation events at this time period focusing on the resident antigen presenting cells (APC) in islets as well as on the profile of the initial T cells.
1.2. The APC of the islets: the macrophages
The presence of APC in islets of non-diabetic animals was described in early histological studies as well as in the analysis of isolated islets. The most recent studies were reviewed by Calderon et al. 2014 which contains references to the initial reports [5]. The APCs in normal islets, i.e. from non-diabetic mice, were initially identified as F4/80 positive cells, a marker of macrophages [5]. When islets were isolated and examined, the myeloid cells were referred to as dendritic cells (DCs) based on their expression of the CD11c marker [6–8]. However, more in-depth examination, including the testing of various cell markers combined with gene expression patterns and developmental studies, identify them as macrophages [1,2].
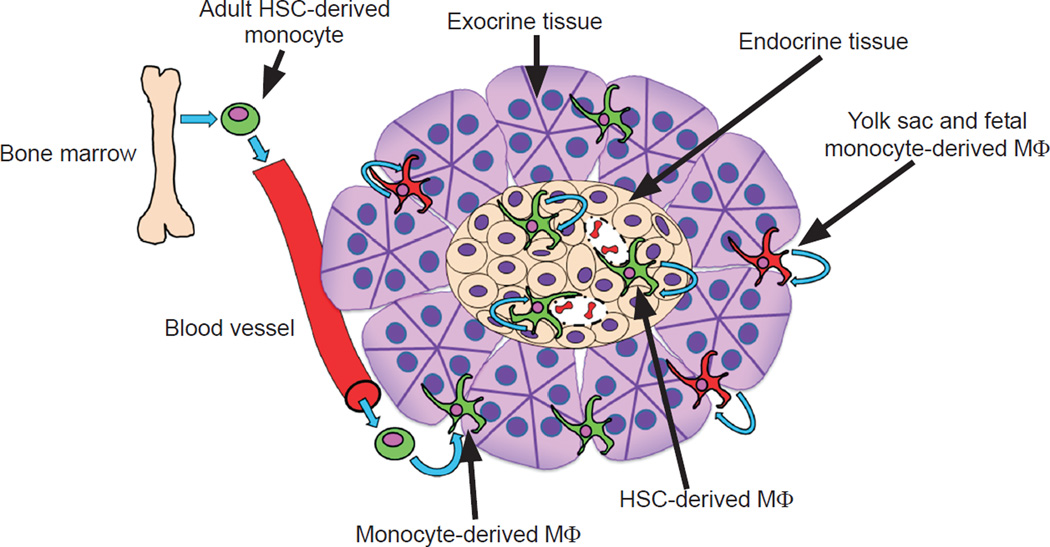
These resident macrophages of the pancreas have distinct features [2] [Figure 1]. The islet macrophages in non-diabetic strains are monolithic, representing only one population based on flow cytometric evaluation [See Table 1]. The pancreatic stromal macrophages parse into two populations based mainly on their expression of CD206 (mannose receptor) and CD301 (CLEC10A). The islet macrophages are present within the islets since birth and derive from definitive hematopoiesis. Under steady state, the islet macrophages do not exchange with monocytes, but proliferate in situ, albeit slowly. In striking contrast to the islet resident macrophages, are those found in the inter-acinar stroma. The CD206/CD301-negative stromal macrophage population is derived from blood monocytes and in constant turnover, and the CD206/CD301+ macrophage is derived from yolk-sac hematopoiesis. The latter tend to concentrate around the pancreatic ducts.
Fig. 1.
The multiple origins of pancreatic macrophages and their maintenance. The illustration depicts the origin of islet-resident macrophages (adult HSC derived, shown in green) and their self-maintenance by in situ proliferation. These show basal M1 features. In contrast, the exocrine pancreatic (stroma) macrophages are composed of two subsets: one in continual replacement by circulating monocytes (adult HSC derived) and not self-maintained and the second derived from yolk sac and fetal liver monocytes (shown in red) and selfmaintained. Half the interacinar macrophages expressed CD206 and CD301 and were preferentially situated among pancreatic ducts. The second set derives from circulating monocytes and did not express CD206 or CD301. All macrophages in the interacinar stroma have M2 features. From Calderon et al. [2] with permission.
Table 1.
Pancreatic macrophage profile under steady state
| Expression marker |
Islet macrophage |
Stroma CD206+ macrophage |
Stroma CD206− macrophage |
|---|---|---|---|
| F4/80 | + | + | + |
| CD11b | + | + | + |
| CD11c | + | + | + |
| MHC-II | + | +/− | + |
| CD64 | + | + | + |
| CD68 | + | + | + |
| LyzM | + | + | + |
| Cx3cr1 | + | +/− | +/− |
| CD206 | − | + | − |
| CD301 | − | + | − |
| Ly6C | − | − | − |
| CD103 | − | − | − |
| Zbtb46 | − | − | − |
| Il1b | + | − | − |
| Tnfa | + | − | − |
| Nos2 | − | − | − |
| Arg1 | − | +/− | +/− |
| Il10 | − | + | − |
| Ym1 | − | − | + |
| Fizz1 | − | + | + |
+, high expression; +/−, low expression; −, negative expression
The turnover of these macrophages was examined by the technique of parabiosis of mice differing in the myeloid marker CD45, i.e. one mouse expressing CD45.1 was parabiosed to another expressing CD45.2. After several weeks of blood exchange, analysis of the islets or the stromal yolk-sac derived cells showed no interchange between the two parabionts. The islet macrophages and stromal yolk-sac derived macrophages, in contrast to monocytes and lymphocytes in the spleen, maintained their initial distribution prior to the parabiosis [2]. Therefore, these resident macrophages are maintained by a low level of replication.
Furthermore, there are differences in gene expression and function between the stromal and islet macrophages. The islet-resident macrophages display a gene expression profile consistent with an M1 activation pattern, while the stromal macrophages exhibit transcripts consistent with an alternative activation pattern, or M2. Additionally, the islet resident and CD206/CD301-negative stromal populations of macrophages express a high-level of MHC-II even under steady state conditions. The macrophages that do not express CD206/CD301 can present exogenous antigen to T cells ex vivo, whereas the CD206/CD301+ macrophages present very weakly. Macrophages in other tissues also differ in origin and properties [reviewed in 9,10].
When mice were lethally irradiated and reconstituted with bone marrow stem cells, the reconstituted macrophages found in the islets and the stroma were still M1 or M2 in their gene expression signatures respectively. From these series of findings, we concluded that the “pancreas anatomy conditions the origin and properties of the resident macrophages” [2]. How each tissue conditions the biology of their resident cells is an important issue for future investigations.
The islet macrophages have a homeostatic or trophic role. This role became apparent in studies from Pollard’s group examining the op/op mouse [11]. This mouse has a natural mutation in the Csf-1 gene and consequently lacks macrophages in many tissues. Their islets are of small size and a number of mice have an abnormal glucose tolerance test [2]. Calderon, et al. showed that most of the op/op mouse islets lack macrophages or contain no more than two per islet. In contrast, islets of normal mice have macrophages ranging in number from 2 to about 20, depending on their size, with a mean of about 10 per islet [6]. The basis of this trophic supportive role of the islet macrophage is not known, but clearly beta cells and the resident macrophages are communicating with each other in a symbiotic relationship.
The islet resident macrophages have a close interaction with blood vessels. Live images of islets isolated from CXCR1-GFP mice show them extending long dendrites amongst the beta cells. Moreover, some of the dendrites penetrate the lumen of the blood vessels [6]. Finally, islet macrophages, as discussed below, have taken up insulin containing granules, on the average of about 10 granules per cell [3].
1.3. The APCs of the islets: the CD103+ DCs
In NOD mice, at 3–6 weeks of age, another APC is found within the islet in small numbers but which increases as diabetes progresses [1]. This minor population is a DC that expresses CD103 (Figure 2). It belongs to the lineage of DC characterized by being efficient in cross presentation, i.e. capable of presenting to CD8+ T cells when taking up proteins or cellular material [12]. At the same time that the CD103+ DC enter the islets, a few CD4+ T cells are also found. It is striking that most of the T cells are found in contact with the islet APCs. There is a correlation in presence of the CD4+ T cells, the CD103+ DC, and the interferon-inducible gene signature of inflammation [1].
Fig. 2.
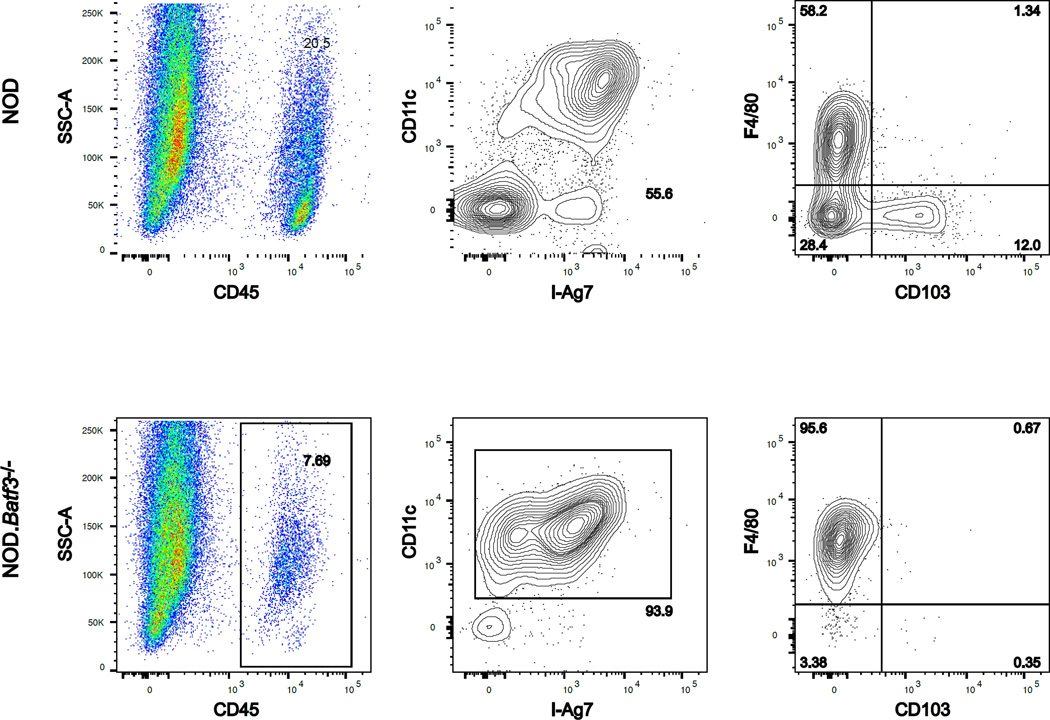
Islet APC composition differs between the NOD and NOD.Batf3−/− mice. Scatter plots represent the flow cytometry of dispersed islets from two NOD (top) or NOD.Batf3−/− (bottom) mice at 12 wks of age. Each gate is predicated upon the panel to the left, e.g. CD11c+ MHCII+ gate displays cells from the CD45+ gate. The NOD shows two APCS, the F4/80+ macrophage and the CD103+ DC, whereas the NOD.Batf3−/− mouse is missing the CD103+ cells.
We have paid attention to T cells found in isolated islets. The islets of non-diabetic mice have a small number of CD45+ cells, ~2–5%, mostly represented by the macrophages described above. However, up to 5% of these CD45+ cells are T cells. We consider this small number as “passenger” T cells, most likely coming from blood in the islet vessels. By direct microscopy, T cells are not found in islets [13]. Finally, also to note, is the importance of isolating the islets free of stromal cells or the perilobular lymphoid aggregates. These represent the bulk of cells in a pancreas and contamination with them can be a serious problem when examining islets [see Suppl Fig 1 of reference 2].
To examine the role that the CD103+ DC is playing in diabetogenesis, we crossed the Batf3 gene knockout mouse onto the NOD background. The Batf3-deficient mice were generated by Dr. Kenneth Murphy. His laboratory found that the Batf3 transcription factor is critical in the differentiation of the common DC precursor to the CD103/CD8α+ alpha lineage [14]. The absence of this DC lineage renders the mouse incapable of cross-presenting antigens to CD8+ T cells. Thus, these mice are susceptible to viral infections and do not reject immunogenic syngeneic tumor cells. We performed microsatellite marker analysis to confirm full backcrossing and SNP analysis on the IDD loci to ensure NOD purity. The Batf3 null mouse (NOD.Batf3−/−), in brief, lacks the CD103/CD8α+ DC lineage of DCs in all lymphoid tissues, confirming the findings made by the Murphy laboratory [14].
The NOD.Batf3−/− mice did not become diabetic when followed for more than a year. Moreover, by histology and flow cytometry, there was no infiltration of hematopoietic cells at any time. As a side note, there is no other NOD model besides genetic depletion of T cells such as the NOD.SCID or NOD.Rag1−/− mice that have as “healthy” islets as those found within the NOD.Batf3−/− mice. Gene expression analysis of the isolated islets did not show the signatures of inflammation that develop in the NOD by the 4th to 8th week of life. In fact, the gene expression analysis of NOD.Rag1−/− and the NOD.Batf3−/− were practically identical [1].
Where is the defect in the NOD.Batf3−/− mice; in the autoreactive T cells or in the APCs? To test these issues, NOD.Batf3−/− mice were lightly irradiated and partially reconstituted with NOD.Rag1−/− bone marrow cells. A few weeks later, the islets and lymphoid tissues now contained the CD103+ DC. The transplant had replaced the defective DC lineage. T cells now entered the islets of these mice resulting in diabetes. As a control, NOD.Batf3−/− reconstituted with bone marrow from a NOD.Rag1−/−xBatf3−/− was ineffective at replacing the DC lineage or causing inflammation within the islets. The key finding here is that the absence of diabetes in the Batf3-deficient mice was caused by the lack of the CD103+ DCs, and that Batf3-deficient T cells are fully competent but unable to initiate diabetogenesis without said DCs. Indeed, in other experiments, T cells isolated from the NOD.Batf3−/− were able to transfer diabetes when injected into NOD.Rag1−/− mice [1].
To further test the requirement of T cell infiltration on the accumulation of the Batf3-dependent DC within the islet, we examined three congenic strains of mice: NOD.B16A, NOD.H4, and NOD.Rag1−/−. NOD.B16A mice, generated by the Eisenbarth laboratory [15,16], are homozygous null for the Ins1 and Ins2 genes. A mutated version of insulin is driven by the insulin promoter. The mutated insulin was engineered with an amino acid change from alanine to tyrosine at position 16 of the B chain, hence B16A. These mice did not become diabetic, and it is generally thought that the change from alanine to tyrosine renders the insulin non-immunogenic to T cells. Indeed, as explained below, most anti-insulin CD4+ T cells did not react with the modified insulin. The NOD.H4 mouse is I-Ak and, therefore, does not have the diabetogenic MHC cass II allele I-Ag7. This strain does not become diabetic. Finally, the NOD.Rag1−/− mouse is deficient in lymphocytes due to their inability to rearrange their antigen receptors during development.
In the islets of NOD mice at 12 weeks of age, there is ~20% CD103+ DCs within the CD11c+ MHCII+ gate. This time point was used as a reference to examine the accumulation of CD103+ DCs in the congenic strains. The NOD.Rag1−/− mice had the basal 5% CD103+ DC. This 5% can be seen at 4–6 weeks of age in wild-type NOD mice. Therefore, T cells are not needed for the initial seeding of the islet by the CD103+ DCs. However, they are necessary for the burst over time. The NOD.H4 was examined to ascertain if non-diabetogenic T cells could initiate a burst of CD103+ DCs within the islet. The percentage of T cells was ~3–4% of the CD45+ population and considered passengers. The CD103+ DCs were at 5% and considered basal. We concluded that non-immunogenic T cells could not drive the expansion of the DCs within the islets.
Finally, to test if insulin reactive T cells, which are generally thought to be the driver or initiating T cells in diabetes, we examined the islets of the NOD.B16A mice. Similar to the NOD.H4 mice, at 12 weeks, the T cell percentage was ~3–4% of the CD45+ cells and the CD103+ DC percentage was 5% of the APCs. Therefore, the congenic mouse studies showed that T cells are unnecessary for the initial seeding of the islets by the Batf3-dependent DCs and that the accumulation of these DCs requires an autoreactive T cell population and, more specifically, an insulin autoreactive T cell population. Taken together, these experiments point to the requirement of CD103+ DCs, insulin reactive T cells, and the expression of the MHC class II IAg7 molecule to initiate the diabetogenic process.
In the absence of the CD103+ DC, the presentation of beta cell epitopes in the pancreatic lymph node was decreased. While presentation of the islet specific glucose-6-phosphotase related protein (IGRP) MHC class I epitope was undetectable, presentation of the BDC2.5 MHC class II epitope was reduced by about half. Importantly the entry of insulin reactive T cells or the BDC2.5 T cells into islets was not detected [1]. Thus, the CD103+ DC controls the entry as well as the priming of T cells. The manner in which CD103+ DC regulate the initiation of diabetes is an issue of continuing investigations, in particular we are concerned on if the macrophages and the CD103+ DC interact or whether they have separate and distinct roles.
1.4. Brief summary of CD4+ T cells to insulin
The first major studies on the reactivity of T cells to insulin identified a number of CD4+ T cells that reacted with a segment of the B chain encompassing the peptide residues 9–23 [17–20, reviewed in 21]. These T cells induced diabetes when transferred into non-diabetic NOD mice. The concept of insulin autoreactivity as the major initiating component of diabetogenesis was championed by many but particularly by George Eisenbarth and his group [22]. Patients with type 1 diabetes show anti-insulin autoantibodies that can be used for prognostic value. Other manipulations supported the role of insulin autoreactivity [21]. Importantly, the findings with the strain generated by Nakayama et al., the “B16A” mouse, discussed previously, displays the critical nature of insulin autoreactivity. This mouse did not develop diabetes, supporting the role of this hot spot in the B chain for diabetes development.
Studies in our laboratory indicated the presence of two major sets of insulin reactive CD4+ T cells directed to closely overlapping segments in the B chain [23–25]. A majority of T cells recognize register 1 encompassing residues 12–20, while a minority recognizes register 2, the 13–21 segment; a one amino acid shift. Importantly, the 12–20 segment is not presented when an APC handles insulin, but rather when presenting denatured insulin or peptide fragments. Processing of insulin by APC results in the presentation of the 13–21. The finding that protein and peptide generate different pMHC-II conformations can be explained by the route each takes inside the APC. Proteins are processed in deep vesicular compartments and subjected to biochemical events that result in editing of the peptides with selection of the strong MHC class II binding epitopes. Peptide or denatured proteins bind by peptide exchange in early vesicles or plasma membrane and are not subject to strong editing, therefore, a broader repertoire of peptides is selected. This selection is important in the context of the autoimmune repertoire. For a more thorough analysis and the relevance to autoimmunity, see our review [26].
The finding that a peptide derived from the B chain is not presented by the processing of insulin may well explain the escape of insulin reactive T cells from thymus control. Insulin is presented by medullary thymic epithelial cells, presumably under the control of Aire transcription factor. The expectation is that the 13–21 segment will be presented and result in elimination of many of the T cells reactive to it. In contrast, the 12–20 segment should not be presented, as there is not a source of large amounts of exogenous denatured or fragmented insulin in the thymus. Immunizations of NOD mice showed no responses to insulin or to the 13–21 peptide, but did elicit a response to 12–20, results that favor this interpretation [23].
The conundrum that developed as soon as these results became apparent is the way in which this epitope is presented, since it did not derive from processing of native insulin. A CD4+ T cell receptor transgenic mouse only recognizing the 12–20 segment provided telling information [25]. The 12–20 reactive CD4+ T cells entered islets without the need of the pancreatic lymph node. From these findings, we speculated that the important role of the pancreatic node is to amplify the immunological process once it has been initiated. Mice lacking lymph nodes have markedly diminished diabetes [27, 28]. Further studies indicated that intra-islet APC presented insulin epitopes. Both the 13–21 peptide derived from insulin processing as well as the 12–20 segment that derived from denatured insulin and insulin catabolic products were detected by T cell assays that discriminated the two epitopes [25]. We have been able to identify within the secretory granules of beta cells a set of vesicles distinct from the insulin bearing ones that contain insulin peptides. We used a monoclonal antibody to the INS-B:9–23 peptide that does not react with insulin or proinsulin [3, 23]. The antibody distinguishes these peptide-containing vesicles. Beta cells from normal non-diabetic mice as well as from NOD contain a number of these reactive vesicles that are compatible with the crinophagic vesicles described in endocrine organs including beta cells [28–30]. The crinophagy pathway has been interpreted as a default pathway to remove excess number of insulin granules and maintain homeostasis in the beta cell [30].
1.5. Insulin-containing vesicles are transferred to resident APCs
Beta cells are in close contact with the resident APCs. In this close contact interaction, secretory granules, as well as the peptide-bearing granule, are transferred to the APC, either to the macrophage or to the CD103+ DC. This transfer was examined by culturing primary beta cells with APC (either DC or macrophages) and then probing the presentation of the insulin peptide-MHC class II complex with T cells to reactive to either of the two epitopes of insulin. Both T cells reacted positively indicating that the APC had acquired immunogenic material processed it and presented it on MHC-II [Fig 3]. The transfer was dependent on both cell contact and viability of the beta cell [3].
Fig. 3.
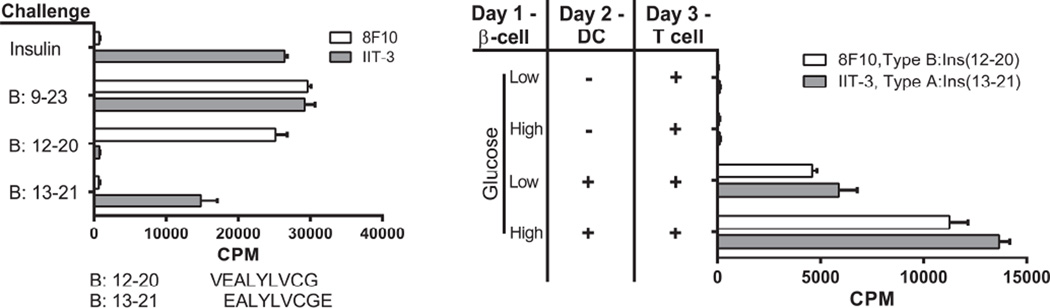
Beta cells transfer of insulin granules to DC. The characterization of the two CD4+ T cells reactive to insulin is shown in the left panel. T cells were incubated with splenic DCs isolated from FMS-like tyrosine kinase 3 ligand (FLT-3L) treated mice. The antigens were either insulin or the B:9–23 peptide, each at 10 µM. 8F10 is a T cell hybridoma that reacts with peptides B:9–23 or B:12–20 (sequence shown below the graph), but not with insulin or B:13–21. The IIT-3 is a T cell hybridoma that reacts with insulin and peptides B:9–23 and B:13–21, but not B:12–20. The panel on the right shows the reactivity of the insulin T cells to beta cell derived antigen. Beta cells are incubated with or without DC, after which CD4+ T cell hybridomas reactive to insulin peptides are added. The bars represent the degree of activation of the T cells as measured by IL-2 production and the proliferation of the IL-2 dependent CTLL-2 cell line as read by counts per minute (CPM). Experiments were performed in the presence of 5mM (Low) or 25mM (High) glucose. From Vomund, et al. with permission from the Proceedings of the National Academy of Science [3].
We examined beta cells from NOD.Rag1−/− mice as well as from the non-diabetic C57BL/6 mouse. There was a very effective transfer of granules, an important finding that indicates that an autoimmune reaction within islets was not required for the passage of the granules. These findings also indicate that cell death is not a prerequisite for the transfer of beta cell material. In fact, streptozotocin-killed cultured beta cells were unable to transfer insulin to APCs. Moreover, we found that human beta cells transferred insulin to APCs derived from NOD mice [3]. (The core human and mouse insulin epitopes have identical sequences).
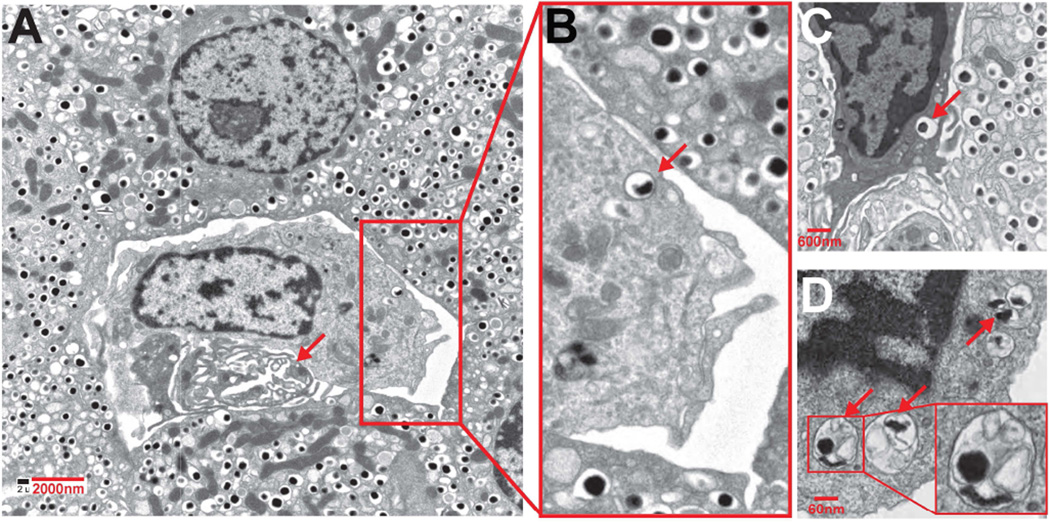
The passage of granules was influenced by glucose concentration. When glucose concentration was increased from the basal 5 mM to 25 mM, about three times more insulin epitope presentation was observed [3]. This passage of secretory vesicles to APCs was documented by electron microscopy of islets of non-diabetic mice and from NOD mice at the early stages of diabetes, i.e. during the first 6 weeks. All intra islet phagocytes contained beta cell granules. Some of the vesicles were the contained an electron dense core surrounded by only one membrane. This was an indication that the whole granule had been taken up. However, a second image showed granules within a double membraned vesicle [3] [Fig 4].
Fig. 4.
Electron micrographs of islets showing phagocytes containing vesicles with the morphology of dense core granules. A represents an islet from an 8-wkold female NOD; B–D are islets taken from NOD.Rag1−/− mice at 14 wk of age. The arrow in A indicates a vessel. In the enlarged area, one of the dense-core granules is indicated by an arrow. In panel C a phagocyte is shown in between beta cells, and an arrow points to a dense-core granule. Panel D shows a portion of a phagocyte with endocytosed material in the form of vesicles containing an electron-dense core, with others containing amorphous content. From Vomund. et al. with permission from the Proceedings of the National Academy of Science [3]
Finally, the passage of granules was documented by live images of insulinoma cells bearing granules expressing a granule membrane protein fused to GFP, ZnT8-GFP, and DC. In these images, as the DC contacted the insulinomas, there was a migration of granules to the contact area followed by a rapid uptake by the DC [3].
The transfer of granules takes place in islets from diabetogenic strains such as the NOD as well as in non-diabetic strains. It is our belief that the transfer of granules from beta cells to APCs has two major consequences. First, it results in skewing the macrophage to an M1 profile of activation. A logical possibility is that this activation profile provides the islet with an early mechanism of defense to unwanted circulating material, including viruses. Second, in the situation of autoimmunity such as in the NOD mouse, the transfer of granules provides the substrate for immunological recognition.
1.6 Brief summary
We have described some of the cellular interactions taking place in the islets at the onset of the diabetic process. These interactions come together in a fine tuned process in the NOD, a strain that contains all the genetic elements that drive the autoimmune process. These interactions involve a close relationship between the beta cells, the two myeloid cell lineages, and the insulin reactive CD4+ T cells. An important substrate is the beta cell granule that gets transferred to the islet APC, a process that appears to be constitutive, but one that has important implications in the NOD mouse. Once these early, islet-intrinsic interactions take place, we believe the pancreatic node enters into action resulting in a rapid amplification of the process. This results in the activation of regulatory cells as well as the CD8+ T cells. At this point, control of the disease becomes difficult. Thus, the autoinflammatory response moves forward leading to the progressive loss of beta cells and eventually diabetes. A better understanding of these early interactions, and their cellular and molecular components, may provide insights applicable to the human disease.
Acknowledgments
We thank all the members of our laboratory who participated in many of the studies discussed in this paper. Jim Mohan, Boris Calderon, and Anthony Vomund led the projects discussed here on insulin reactive T cells, islet macrophages and granule transfer, respectively. Other contributors to these projects included: Xiaoxiao Wan, Jing Hughes, Carolina Valderrama, Katherine Frederick, and Lindsay Moore. Anish Suri, Matteo Levisetti, Michael Gross and Osami Kanagawa made major contributions that preceded the studies described herein. Research reported in this publication was supported by the Juvenile Diabetes Research Foundation 2-SRA-2014-293-Q-R and 17-2013-512, and the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number R01DK058177. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Juvenile Diabetes Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ferris ST, Carrero JA, Mohan JF, Calderon B, Murphy KM, Unanue ER. A minor subset of Batf3-dependent antigen presenting cells in islets of Langerhans is essential for the development of autoimmune diabetes. Immunity. 2014;41:657–669. doi: 10.1016/j.immuni.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calderon B, Carrero JA, Ferris ST, Sojka DK, Moore L, Epelman S, et al. The pancreas anatomy conditions the origin and properties of resident macrophages. J Exp Med. 2015;212:1497–1512. doi: 10.1084/jem.20150496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vomund AN, Zinselmeyer BH, Hughes J, Calderon B, Valderrama C, Ferris ST, et al. CV325 Beta cells transfer vesicles containing insulin to phagocytes for presentation to T cells. Proc Natl Acad Sci USA. 2015;112:5496–5502. doi: 10.1073/pnas.1515954112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carrero JA, Calderon B, Towfic F, Artyomov MN, Unanue ER. Defining the transcriptional and cellular landscape of type 1 diabetes in the NOD mouse. PLoS One. 2013;8:e59701. doi: 10.1371/journal.pone.0059701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calderon B, Carrero JA, Unanue ER. The central role of antigen presentation in islets of Langerhans in autoimmune diabetes. Curr Opin Immunol. 2014;26:32–40. doi: 10.1016/j.coi.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calderon B, Suri A, Miller MJ, Unanue ER. Dendritic cells in islets of Langerhans constitutively present beta cell derived peptides bound to their class II MHC molecules. Proc Natl Acad Sci USA. 2008;105:6121–6126. doi: 10.1073/pnas.0801973105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melli K, Friedman RS, Martin AE, Finger EB, Miao G, Szot GL, et al. Amplification of autoimmune response through induction of dendritic cell maturation in inflamed tissues. J Immunol. 2009;182:2590–2600. doi: 10.4049/jimmunol.0803543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang Q, Adams JY, Tooley AJ, Bi M, Fife BT, Serra P, et al. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banaei-Bouchareb L, Gouon-Evans V, Samara-Boustani D, Castellotti MC, Czernichow P, Pollard JW, et al. Insulin cell mass is altered in Csf1op/Csf1op macrophage-deficient mice. J Leukocyte Biol. 2004;76:359–367. doi: 10.1189/jlb.1103591. [DOI] [PubMed] [Google Scholar]
- 12.Shortman K, Heath WR. The CD8+ dendritic cell subset. Immunol Rev. 2010;234:18–31. doi: 10.1111/j.0105-2896.2009.00870.x. [DOI] [PubMed] [Google Scholar]
- 13.Calderon B, Carrero JA, Miller MJ, Unanue ER. Cellular and molecular events in the localization of diabetogenic T cells to islets of Langerhans. Proc Natl Acad Sci USA. 2011;108:1567–1572. doi: 10.1073/pnas.1018973108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakayama M, Abiru N, Moriyama H, Babaya N, Liu E, Miao D, et al. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature. 2005;435:220–223. doi: 10.1038/nature03523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakayama M, Beilke JN, Jasinski JM, Kobayashi M, Miao D, Li M, et al. Priming and effector dependence on insulin B:9-23 peptide in NOD islet autoimmunity. J Clin Invest. 2007;117:1835–1843. doi: 10.1172/JCI31368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wegmann DR, Norbury-Glaser M, Daniel D. Insulin-specific T cells are a predominant component of islet infiltrates in prediabetic NOD mice. Eur J Immunol. 1994;24:1853–1857. doi: 10.1002/eji.1830240820. [DOI] [PubMed] [Google Scholar]
- 18.Wegmann DR, Gill RG, Norbury-Glaser M, Schloot N, Daniel D. Analysis of the spontaneous T cell response to insulin in NOD mice. J Autoimmun. 1994;7:833–843. doi: 10.1006/jaut.1994.1066. [DOI] [PubMed] [Google Scholar]
- 19.Daniel D, Gill RG, Schloot N, Wegmann D. Epitope specificity, cytokine production profile and diabetogenic activity of insulin-specific T cell clones isolated from NOD mice. Eur J Immunol. 1995;25:1056–1062. doi: 10.1002/eji.1830250430. [DOI] [PubMed] [Google Scholar]
- 20.Halbout P, Briand J-P, Becourt C, Muller S, Boitard C. T cell response to preproinsulin I and II in the nonobese diabetic mouse. J Immunol. 2002;169:2436–2443. doi: 10.4049/jimmunol.169.5.2436. [DOI] [PubMed] [Google Scholar]
- 21.Unanue ER. Antigen presentation in the autoimmune diabetes of the NOD mouse. Annu Rev Immunol. 2014;32:579–608. doi: 10.1146/annurev-immunol-032712-095941. [DOI] [PubMed] [Google Scholar]
- 22.Zhang L, Nakayama M, Eisenbarth GS. Insulin as an autoantigen in NOD/human diabetes. Curr Opin Immunol. 2008;20:111–118. doi: 10.1016/j.coi.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohan JF, Levisetti MG, Calderon B, Herzog JW, Petzold SJ, Unanue ER. Unique autoreactive T cells recognize insulin peptides generated within the islets of Langerhans in autoimmune diabetes. Nat Immunol. 2010;11:350–354. doi: 10.1038/ni.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohan JF, Petzold SJ, Unanue ER. Register shifting of an autoimmune insulin peptide-MHC II complex allows for the escape of diabetogenic T cells from negative selection. J Exp Med. 2011;208:2375–2383. doi: 10.1084/jem.20111502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohan JF, Calderon B, Anderson MS, Unanue ER. Pathogenic CD4+ T cells recognizing an unstable peptide of insulin are directly recruited into islets bypassing local lymph nodes. J Exp Med. 2013;210:2403–2414. doi: 10.1084/jem.20130582. PMC3804950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohan JF, Unanue ER. Unconventional recognition of peptides by T cells and the implications for autoimmunity. Nat Rev Immunol. 2012;12:721–728. doi: 10.1038/nri3294. [DOI] [PubMed] [Google Scholar]
- 27.Gagnerault MC, Luan JJ, Lotton C, Lepault F. Pancreatic lymph nodes are required for priming of β cell reactive T cells in NOD mice. J Exp Med. 2002;196:369–377. doi: 10.1084/jem.20011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levisetti MG, Suri A, Frederick K, Unanue ER. Absence of lymph nodes in NOD mice treated with lymphotoxin-β receptor immunoglobulin protects from diabetes. Diabetes. 2004;53:3115–3119. doi: 10.2337/diabetes.53.12.3115. [DOI] [PubMed] [Google Scholar]
- 29.Halban PA, Wollheim CB. Intracellular degradation of insulin stores by rat pancreatic islets in vitro. An alternative pathway for homeostasis of pancreatic insulin content. J Biol Chem. 1980;255:6003–6006. [PubMed] [Google Scholar]
- 30.Orci L, Ravazzola M, Amherdt M, Yanaihara C, Yanaihara N, Halban P, et al. Insulin, not C-peptide (proinsulin), is present in crinophagic bodies of the pancreatic B-cell. J Cell Biol. 1984;98:222–228. doi: 10.1083/jcb.98.1.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arvan P, Halban PA. Sorting ourselves out: Seeking consensus on trafficking in the beta-cell. Traffic. 2004;5:53–61. doi: 10.1111/j.1600-0854.2004.00152.x. [DOI] [PubMed] [Google Scholar]