Abstract
Significant recent advances in methodologies for the differentiation of pluripotent stem cells to renal progenitors as well as the definition of niche conditions for sustaining those progenitors have dramatically enhanced our understanding of their biology and developmental programing, prerequisites for establishing viable approaches to renal regeneration. In this article, we review the evolution of culture techniques and models for the study of metanephric development, describe the signaling mechanisms likely to be driving progenitor self-renewal, and discuss current efforts to generate de novo functional tissues, providing in depth protocols and niche conditions for the stabilization of the nephronic Six2+ progenitor.
Introduction
Kidney development is mediated by the interactions between two principal lineages of intermediate mesoderm (IM): the ureteric bud (UB) and metanephric mesenchyme (MM). Unlike the development of most epithelial tissues, which are formed from the interactions of existing epithelia and surrounding mesenchymal stroma, the MM contributes to both stroma and the epithelia of the adult kidney. The UB, which forms the collecting duct network, originates in the Wolffian/nephric duct (ND), which itself develops from early rostral intermediate mesoderm. The surrounding MM in the metanephric kidney is comprised of a mixed population of condensed Six2+ self-renewing nephronic progenitors overlying the tips of the ureteric bud (Kobayashi et al., 2008) and Foxd1+ multipotent self-renewing stromal progenitors (Kobayashi et al., 2014), which constrain the Six2+ population (Das et al., 2013). The precursors for the MM arise from the primitive streak but later than those for the ND and instead populate the caudal intermediate mesoderm (Taguchi et al., 2014). The convergence of rostral ND with caudal IM/MM initiates the outgrowth of the UB from the ND as a result of MM signals, i.e., GDNF secretion (Pichel et al., 1996). On the other hand, FGFs from both the UB and MM cause the condensation of MM cells around the bud tip (Barak et al., 2012), producing the so-called cap mesenchyme - the Six2+ self-renewing progenitors. These progenitors undergo mesenchymal-epithelial transition (MET) to form all aspects of the nephronic epithelia, including the podocytes and proximal and distal tubules, beneath the bud tips and merge with the UB to generate a continuous lumen from the glomerulus to the ureter. The remaining MM cells constitute the multipotent Foxd1+ stromal progenitors, which differentiate to other subpopulations of the kidney, including vascular smooth muscle, pericytes, and mesangial cells.
Until recently, efforts to model the process of mammalian metanephric development in culture were limited to explanted primary rudiments – a technique introduced in the 1950s by Clifford Grobstein. In those early studies, Grobstein employed then standard culture conditions, which supported the arborization of the UB along with the induction of nephron formation in cultured intact rudiments (Grobstein, 1953). Moreover, with the separation of MM from the UB, he also demonstrated the responsiveness of MM to inductive signaling (Grobstein, 1956). In these studies he established that embryonic spinal cord could function as a surrogate for the UB in stimulating MET in MM cells to generate tubular epithelia in heterotypic recombinations. Furthermore, differentiation occurred despite the interposition of a porous filter, suggesting that soluble factors were mediating the inductive effects of the spinal cord. Importantly, these results demonstrated that the renal development program and all necessary factors (barring those required for vascular development) are inherent within the metanephros and that minimalistic culture conditions can temporarily stabilize the progenitors, allowing them to respond to inductive cues and to convert to tubular epithelia.
Since the original description of Grobstein’s methods, his model has served admirably with minor modifications for many years as the only available culture system for the study of inductive signaling in metanephric development. Subsequent modifications have facilitated the elimination of spinal cord, which has now been replaced with fragmented UBs or individual growth factors and small molecules that help sustain and/or differentiate the MM progenitors under completely defined serum-free conditions. We previously described culture conditions for the propagation of UB cells long term, and these cells behave in culture like the progenitors from which they originate in that they secrete inductive molecules. Whether they have retained an embryonic character beyond that was not determined. Regardless, those interested in studying UB/collecting duct should refer to those previous publications (Perantoni et al., 1985; Perantoni, 2003). The focus of this article will be on the delineation of niche conditions for the culture of the Six2+ nephronic progenitor.
While development of intact metanephric rudiments cultured in basic media resembles that for tissues in situ, the separated MM does not survive independently without supplementation. The tissue separations not only eliminate paracrine growth factors but also disrupt matrix interactions, which promote progenitor-associated gene expression (Tanigawa et al., 2015). Moreover, secreted autocrine factors are substantially diluted in the considerable volume of culture medium in which the cells are suspended and must be accommodated as well. These various deficiencies and the instability of progenitors subjected to them likely explain why simple reaggregation of fragmented/dissociated metanephroi is insufficient to restore tubulogenesis in the MM. To help overcome these problems, reagents that inhibit apoptosis following tissue disaggregation have been applied. The Rho-dependent kinase inhibitor (ROCKi) Y27632 has been used to stabilize human embryonic stem cells following dissociation (Watanabe et al., 2007) and significantly increased the size of treated intact metanephric rudiments (Meyer et al., 2006). The Davies lab subsequently determined that a 24-hr treatment of such mixed cultures with a ROCKi restores cell viability and permits progression of the MM differentiation program (Davies and Chang, 2014). In fact branching morphogenesis of the UB also occurs under these conditions, yielding reasonably well organized metanephric rudiments. When the same group invoked a two-step process in which expanded UB cysts were formed first in primary aggregates and then isolated and remixed with fresh MM progenitors in small volume cultures, the engineered kidneys developed nephronic structures with reasonably well formed segments that interfaced with the branched UB epithelia (Chang and Davies, 2012). Such observations suggest that if the individual progenitor populations can be properly stabilized, mixed populations may in fact self-organize naturally with limited intervention.
Defining a progenitor’s niche conditions, i.e., the factors that preserve the progenitors in situ, provides clues to the signaling mechanisms employed by the organism to stabilize these cells and insight into how the interplay of various factors regulates the balance between progenitor stability and lineage commitment. Such information can offer alternative treatments or substitute factors capable of sustaining this population. Thus, a small molecule agonist or pathway antagonist may prove equally effective in preserving the progenitor populations. In some cases this approach can significantly impact the cost of propagating a specific population in culture, given the high price of certain signaling ligands. Thus, GSK3 inhibitors are now typically applied to cultured cells in order to activate canonical Wnt signaling rather than treatment with a considerably more expensive Wnt ligand. Furthermore, the successes achieved in stabilizing pluripotent stem cells have now prompted efforts to sustain multipotent tissue-specific progenitors as well. For the MM progenitor, stabilization is a quite recent innovation in the field, although the advance resulted from several earlier observations. Transforming growth factor-α (TGFα) (Rogers et al., 1992) or epidermal growth factor (EGF) (Koseki et al., 1992) inhibit apoptosis in MM populations and partially stabilize the progenitor. Moreover, basic fibroblast growth factor (FGF2) not only promotes MM survival but also stimulates growth and condensation of this lineage (Perantoni et al., 1995). While FGF2 serves as a reasonable substitute in culture, FGF9 and 20 are the principal mediators of FGF-directed MM survival in vivo (Barak et al., 2012). In culture, FGF9 behaves similarly to FGF2 in maintaining MM cells and inducing MM condensation (personal observation); while FGF8, which is also required for MM survival in vivo (Perantoni et al., 2005), is independently incapable of sustaining the MM progenitor (personal observation). Importantly, in the presence of TGFα/FGF2, MM cells temporarily retain their progenitor identity, including a capacity to respond to induction (Tanigawa et al., 2011), a functionality which is lost relatively rapidly over time and with passaging. This is reflected in the substantial decline of Six2+ progenitors in MM populations cultured over one week in these factors (Tanigawa et al., 2015). Regardless, these conditions do provide a useful short-term model for MM cells, since FGF2 stimulates MM proliferation in tissue explants, expanding cell numbers sufficiently for analyses by a variety of molecular and biochemical methodologies. Moreover, cells respond as predicted from murine genetic studies (Das et al., 2013).
To further stabilize niche conditions for the MM progenitor, additional factors have now been identified which not only stimulate proliferation but also sustain and expand the Six2+ population while supporting Pax2 expression (Brown et al., 2015; Tanigawa et al., 2015) , which is apparently also required for MM competency (Brophy et al., 2001). The inclusion of leukemia inhibitory factor (LIF) proved pivotal in our own studies. Prior work demonstrated a role for LIF in MET conversion of uninduced MM progenitors to tubular epithelia (Barasch et al., 1999; Plisov et al., 2001); however, at much reduced LIF concentrations (1 ng/ml vs. 30 ng/ml), the Six2+ progenitor is selectively maintained and expanded when LIF is applied in combination with TGFα/FGF2 and Y27632. Its tissue-specific effects are likely related to the expression of the LIF receptor on the cap mesenchyme cells/Six2+ population but not on Six2-stromal cells (Plisov et al., 2001).
LIF belongs to the IL6 family of cytokines which are predominantly associated with activation of a canonical JAK/STAT signaling mechanism through formation of a heterotypic receptor complex involving gp130. IL6 family members generally induce the simultaneous phosphorylation/activation of STATs 1, 3, and 5 (Nicola and Babon, 2015). This is indeed the case in ES cells (Nemetz and Hocke, 1998), although ectopic expression of a dominant-negative STAT3 construct alone induces differentiation and blocks stem cell self renewal (Niwa et al., 1998). Moreover, constitutive activation of STAT3 in ES cells sustains their stemness (Matsuda et al., 1999). MM progenitors also respond to LIF with rapid phosphorylation of STATs 1, 3, and 5, and STAT3 binds to the Six2 promoter, suggesting a role for STATs in maintenance of MM stemness as well (Tanigawa et al., 2015).
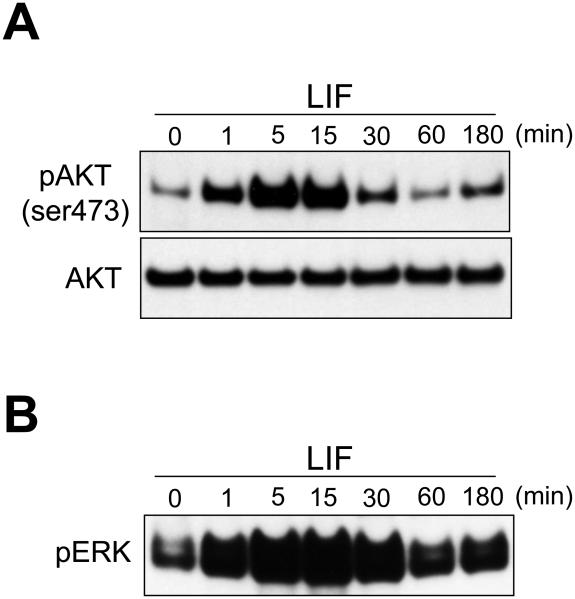
In addition to STATs, LIF also signals through PI(3)-kinase (PI3K) by facilitating an interaction between JAK1 and the p85 subunit of PI3K (Paling et al., 2004). Recent studies demonstrate that signaling through this pathway facilitates MM progenitor self-renewal and that inhibition results in premature differentiation of the progenitor (Lindstrom et al., 2015). Stimulation of PI3K, in turn, activates the serine/threonine kinase AKT, which phosphorylates a variety of targets, including glycogen-synthase kinase (GSK3). Phosphorylation of GSK3 inhibits the axin-GSK3 destruction complex and causes an increase in β-catenin levels, thus activating canonical WNT signaling as well (Fukumoto et al., 2001). There is ample evidence in the metanephros for a required activation of canonical WNT signaling (β-catenin) to sustain competent MM progenitors (Park et al., 2007; Schmidt-Ott et al., 2007). Thusly, LIF could hypothetically activate the canonical WNT pathway through PI3K in the absence of a WNT ligand. Consistent with such an hypothesis, LIF does activate AKT in cultured MM progenitors (Figure 1A). It is possible therefore that in culture LIF may in part replace self-renewal signaling provided in situ by canonical WNT9b activity (Karner et al., 2011).
Figure 1.
LIF rapidly activates A) pAKT and B) pERK in cultured metanephric mesenchyme.
In MM progenitors, the predominant activity of LIF at the lower concentrations involves simultaneous activation of STATs 1, 3, and 5 (Tanigawa et al., 2015), although elevated levels of pJNK (Tanigawa et al., 2015), p38 (not shown), pAKT (Figure 1A), and pERK (Figure 1B) are also demonstrable. Higher LIF concentrations also activate PLCγ (Tanigawa et al., 2015), which stimulates calcium/NFAT signaling (Burn et al., 2011; Tanigawa et al., 2011) to induce MET. The potential role of pJNK in MM progenitor self-renewal was first demonstrated in the context of BMP signaling. In these studies, BMP-induced activation of JNK enhanced MM progenitor self-renewal through a TAK1-dependent mechanism (Blank et al., 2009). Interestingly ROCKi Y27632, which also induces MM proliferation, attenuates JNK signaling (Tanigawa et al., 2015). Given that Tak1 and Jun mutant kidneys are considerably reduced in size, significantly depleted of Six2+ progenitors, and proliferation depressed (Muthukrishnan et al., 2015), the JNK pathway is at least in part responsible for sustaining self-renewal of the MM progenitor. Since LIF robustly activates JNK, its ability to stimulate proliferation might be mediated through JNK activation as well.
The third signaling mechanism initiated by LIF involves activation of the RAS/RAF pathway. LIFR or gp130 recruit and activate the phosphatase SHP2, which in turn induces RAS/RAF signaling (Schiemann et al., 1997). This mechanism is largely attenuated, however, by LIF-induced SOCS3 expression, which competes with SHP2 binding on gp130 and suppresses MAPK signaling (Lehmann et al., 2003). This mechanism likely limits but does not block MAPK signaling in MM progenitors, as ERK activation also occurs following LIF induction (Figure 1B).
Mouse genetic experiments revealed a significant role for Bmp7 in preserving a self-renewing MM progenitor in the cortical nephrogenic zone, and its loss resulted in severe renal dysplasia (Dudley et al., 1995). In culture studies, BMP7 worked synergistically with an FGF to maintain and expand MM progenitors, while inhibiting tubulogenesis (Dudley et al., 1999). This cooperativity has recently been characterized as a coordinated activation of AP-1 transcription (Muthukrishnan et al., 2015). BMP7 primarily activates JUN for AP1-mediated transcription in MM progenitors, while FGF9 preferentially activates FOS. The combination of both factors resulted in significantly elevated levels of AP1 activity, expression of AP1 targets, and proliferation of MM progenitors. Thus BMPs play an important role in preserving and expanding the Six2+ progenitor in vivo and in culture.
Wnt activation of β-catenin-dependent signaling in Six2+ progenitors also preserves this population. Ablation of β-catenin from the Six2-expressing cells results in a substantially depleted cortical nephrogenic zone with very limited nephronic development (Park et al., 2007). On the other hand, kidneys from gain-of-function mutants for β-catenin were increased in size and exhibited enlarged clusters of undifferentiated progenitors surrounding the UB, yet failed to form nephronic structures. Similarly, ectopic expression of β-catenin in cultured MMs enhances survival and induces proliferation but blocks epithelial differentiation. Moreover, inhibition of TCF/LEF activity depletes the nephronic progenitor and attenuates the effects of constitutive β-catenin expression (Schmidt-Ott et al., 2007). Finally, in ChIP-seq studies, considerable overlap in DNA-binding was reported for Six2 and β-catenin (Park et al., 2012), suggesting that they co-regulate several target genes in nephronic progenitors. In vivo the principal activator of β-catenin during development is Wnt9b, which facilitates progenitor proliferation and self-renewal. However, GSK3 inhibitors, such as BIO (Kuure et al., 2007) or CHIR99021 (CHIR) (Araoka et al., 2014), also stabilize β-catenin to activate the canonical Wnt pathway though they suffer from off-target effects, which stimulate NFAT/calcium signaling (Klemm et al., 1997). In lieu of GSK3 inhibition, direct treatment of mESCs with a Wnt ligand has proven more effective at inducing self-renewal than CHIR (ten Berge et al., 2011), presumably again due to off target effects of the small molecule inhibitor. Regardless, the relatively low cost of CHIR has made it an attractive option for both inducing Six2+ cell self-renewal and induction (Brown et al., 2015), and some form of canonical Wnt activation is necessary for progenitor self-renewal.
In addition to factors that promote Six2+ self-renewal, another approach to preserving the Six+ population is through inhibition of differentiation-inducing mechanisms. Accordingly, Bmps activate Smad signaling, which must be suppressed in MM progenitors when high concentrations of Bmps are used to sustain the Six2+ population (Brown et al., 2015). Furthermore, constitutively active Notch2 depletes the Six2+ progenitor population, inhibits Pax2 expression, and causes premature tubule formation in the developing metanephros (Fujimura et al., 2010). Thus, blocking Notch signaling with a γ-secretase inhibitor may also facilitate the preservation of the Six2+ progenitor population (Cheng et al., 2003).
As indicated earlier, some (possibly all) niche factors work synergistically to preserve the Six2+ progenitor. This has been proven for Bmp7 and Fgf2, but other factors may complement their signaling as well. For example, both Bmp7 and LIF activate JNK signaling, which facilitates self-renewal in cultured progenitors. Whether they function synergistically remains to be determined, but certainly one may substitute for the other in activating this pathway. Importantly, it is not unreasonable to speculate that lower concentrations of individual factors in combination may provide more physiological conditions for factors critical to self-renewal, and the reduced concentrations will in turn influence the cost of the medium. We have found, for example that concentrations of Bmp7 above 10 ng/ml are inhibitory to MM propagation when LIF is present at 0.5-1.0 ng/ml (unpublished observation). However, Oxburgh’s lab treats MM cultures with Bmps at 60 ng/ml – a considerably more costly milieu for preserving the Six2+ progenitor. Therefore, when defining niche conditions for propagating progenitors, it is likely as important to establish how these factors synergize relative to one another in culture to optimize their growth.
Methods
Equipment and Supplies: sterile phosphate-buffered saline (PBS) with and without calcium and magnesium; #11 and #15 disposable stainless steel surgical blades with scalpels; dissecting stereomicroscope (minimum 10–25×) with substage and superstage illumination; 1% trypsin solution in PBS lacking calcium and magnesium with 0.5 mg/mL DNase; soybean trypsin inhibitor (2.5 mg/mL) in culture medium; 50:50 Dulbecco’s minimum essential medium (DMEM): Ham’s F12 with L-glutamine (2 mM) and gentamicin (50 μg/mL); stock solutions of medium supplements essential for serum-free culture (Table 1); growth factor stocks (Table 2); regular tissue culture or fibronectin-coated dishes; polycarbonate filters (0.1μm pore size does not obscure tissue).
Table 1.
Serum-free DMEM:F12 basal medium for propagation of metanephric mesenchymal progenitors. All stock solutions are aliquoted to avoid repeated freeze-thaw cycles and stored at −20°C.
| Component | Stock Concentration | Medium Concentration |
|---|---|---|
| glutamine | 200 mM | 2.0 mM |
| gentamicin | 50 mg/ml | 50 μg/ml |
| transferrin, holo, human plasma | 5 mg/ml in PBS | 5 μg/ml |
| insulin, bovine | 5 mg/ml in 0.006 N HCl | 5 μg/ml |
| sodium selenite | 1 × 10−5 M in PBS | 1 × 10−8 M |
| hydrocortisone | 1 × 10−4 M in 95% EtOH | 1 × 10−7 M |
| triiodothyronine | 1 × 10−6 M in DMSO | 1 × 10−9 M |
Table 2.
Recommended ranges for growth factors and small molecule inhibitors added to the basal medium at the time of feeding. Aliquoted growth factors are stored at −80°C. Once thawed, the factors are stored at 4°C until expended.
| Component | Stock Concentration | Medium Concentration |
Source/Catalog # |
|---|---|---|---|
| TGFα (T) | 10 μg/ml in PBS/0.1% BSA | 10 ng/ml | Peprotech/100-16A |
| FGF2 (F) | 100 μg/ml in PBS/0.1% BSA | 50-100 ng/ml | Peprotech/100-18B |
| alternatively, FGF9 | 100 μg/ml in PBS/0.1% BSA | 50-100 ng/ml | Peprotech/100-23 |
| LIF (L) | 10 μg/ml | 1-5 ng/ml | EMD Millipore/LIF2010 |
| Y27632 (Y) | 10 mM in DMSO | 10 μM | Tocris/1254 |
| CHIR 99021 (C) | 10 mM in DMSO | 0.5-1.5 μM | Tocris/4423 |
| Bmp7 (B) | 100 μg/ml in 0.004 N HCl with 0.1% BSA |
5-30 ng/ml | RND Systems/ 5666-BP-010 |
| DAPT (D) | 10 mM in DMSO | 0.5-1.0 μM | EMD Millipore/565770 |
Embryonic age for progenitor isolation
Historically, isolation of MM from the epithelial UB required tissue manipulations at the time the bud invades the MM. For rat and mouse, tissue isolation would occur at E13.5 and E11.5, respectively. At this time, the UB appears as a T-shaped structure and therefore has only undergone primary branching (see Perantoni, 2003, Figure 1, p. 181). Within 24-hrs of this time point, the UB has already initiated tertiary branching, and the MM becomes considerably more difficult to separate with minimal contamination from the UB. It is still possible, however, to achieve at this stage, but beyond this time period, it is extremely challenging to physically separate the tissues. Alternatively, some labs have opted for enzymatic enrichment (Osafune et al., 2006). What with newer lineage-specific fluorescent reporters and FACS sorting, achieving pure populations has become less problematic. Despite the technical improvements though, we do find that the earlier progenitors can be propagated to a greater extent than those isolated from perinatal kidneys. For this reason, we continue to work primarily with progenitors from the early rudiments.
Because metanephric development progresses quite rapidly, timed matings are essential. Breeding is restricted to overnight to ensure a relatively tight window of development. Thus, breeders are paired at the end of the work day and plugged and separated the following morning. For our purposes, when a spermatic plug is detected in a breeding cage in the morning following pairing, that day is recorded as E0.5
Isolation of metanephroi
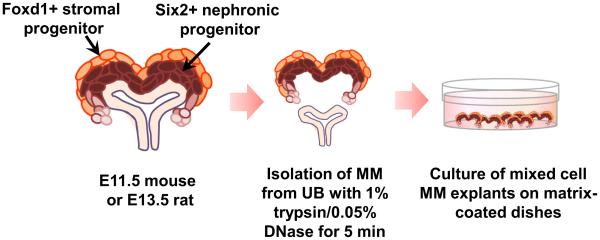
The most challenging aspect of tissue isolation is locating and removing the early primitive rudiments due to their miniscule size. This takes time and practice, and in training new staff, we invariably begin with older embryos and gradually progress to younger ones. To sterilely isolate metanephroi, timed-pregnant females are euthanized according to animal care and use committee requirements, and the ethanol-soaked abdominal area is then opened with scissors and forceps. The intact uterine horns are removed from the females and washed 1–2× with ice-cold PBS to minimize contaminating red blood cells. Embryos are individually removed from the placenta, taking care not to pull them by the umbilical cord, which will distort or tear the urogenital tissues. Intact embryos (3–4) are then placed in 60-mm culture dishes with 1 mL PBS with calcium/magnesium on ice. To avoid tissue degradation, MM isolation proceeds immediately following embryo harvesting. Because of rapid axis extension/body elongation in E11.5 mouse or E13.5 rat embryos, the position of the metanephroi relative to the limbs varies with age. When first forming, the metanephroi are located in the caudal extremity between the hindlimbs and tail. Access to the metanephroi is achieved by cutting through the embryo just below the developing liver and then removing the lower abdominal ectoderm along with the abdominal tissues, i.e., stomach and gut, to open the surgical field. The urogenital tract, including the mesonephros, parallels the embryonic axis on both sides of the somites and can be rolled off of the spinal cord with the embryo positioned on its side using a #15 surgical blade. Upon removal, the surrounding urogenital tissue is separated from the metanephroi with #11 and #15 scalpel blades. Alternatively, some prefer the use of small gauge needles for these dissections, but either way, sharp dissecting instruments are essential. Once the metanephroi have been excised, they are collected in ice-cold PBS. Storage in PBS for more than 1– 2 h is discouraged, as gradual tissue degradation increases the difficulty of cleanly separating and distributing the MMs. UBs are removed from the MMs with a 1% trypsin/ 0.05% DNase solution in PBS without calcium/magnesium (Figure 2). The DNase greatly reduces the “stickiness” of the MMs during and after microdissection, thus preventing tissue loss with pipetting. After a 5–min incubation at room temperature in these enzymes (5-6 metanephroi in 30 µl trypsin/DNase solution), the mesenchyme can be peeled off of the buds using scalpels equipped with #11 surgical blades. Note that mouse MMs are more difficult to isolate cleanly than rat tissues due to a tighter bud adherence, but that should not be a deterrent. Once separated, MMs are transferred into ice-cold culture medium containing soybean trypsin inhibitor (2.5 mg/mL) until explanted to matrix-coated filters or culture dishes for propagation. We generally prefer not to use fetal bovine serum due to its complex and undefined content but it is an option. Moreover, the soybean inhibitor with washing is equally effective at preserving these cells. As described by Cunha and Baskin in this special issue, rudiment trypsinization on ice, while not as rapid as room temperature, offers some advantages. Importantly, the trypsin remains active at the lower temperature, which immobilizes the cell membrane, preventing cellular uptake of the trypsin and minimizing its toxicity.
Figure 2.
Metanephric mesenchyme (MM) is isolated from the ureteric bud (UB) with trypsin/DNase for propagation in culture.
Once the MMs have been isolated, they are most efficiently maintained and propagated on fibronectin-coated tissue culture dishes (Tanigawa et al., 2015). Initially they are cultured intact and passaged at relatively high density (1:3). Otherwise, their ability to proliferate and their competence to respond to induction is degraded. For a 60-mm culture dish, 10 MMs are sufficient to fill the dish over 7-10 days using FTLY medium (Table 2). This occurs even more rapidly with the inclusion of BMP7 and CHIR, which also preferentially increase the Six2+ population. While FTLY medium expands the Six2+ population to comprise nearly two thirds of the total cell numbers over 7 days, we have observed as high as 90% Six2+ rat progenitors when Bmp and CHIR are also included over the same period. However, the addition of CHIR does activate the calcium/NFAT pathway, eventually inducing MET in these cells, which can be largely mitigated by small molecule inhibitors that interfere with this differentiation. After the explants have been expanded for 6-7 days, they are dissociated in clumps with brief trypsinization, diluted in ice-cold PBS and pelleted (1500xg), washed once with PBS, and then seeded 1:3 in culture medium. Cells to be preserved in liquid nitrogen can be directly reconstituted following the PBS wash in Millipore’s ESGRO complete serum-free culture freezing medium for storage. Cultures should be passaged as soon as they near confluence in 3-4 days. As previously described, competent Six2+ progenitors are preserved despite multiple passages over several weeks (Tanigawa et al., 2015).
Isolation of MM progenitors from more developed metanephroi requires some method for sorting the cells from a heterogeneous dissociated population. Expression of a reporter driven by the promoter of a progenitor marker, such as Six2 has proven especially effective, and therefore is generally performed with mouse where the genetic tools are pre-existing. For the isolation of mouse E15.5 Six2-GFP+ cells, metanephroi are incubated with 0.25% Trypsin/EDTA at 37°C for 10 min, and cells are dissociated by pipetting with cold Dulbecco’s modified Eagle’s medium (DMEM) containing trypsin inhibitor or 10% FBS. Dissociated cells are then washed with PBS before resuspension in FACS buffer for sorting. Neonatal kidneys (P0) are dissociated to single cells using a mixture of collagenase XI (Sigma-Aldrich), dispase (Life Technologies), and DNase (Roche) at 37°C for 10 min followed by treatment with 0.25% trypsin-EDTA at 37°C for 5 min. Dissociated cells are washed as described above and then resuspended in FACS buffer. With sorting, both procedures yield Six2+ populations in excess of 90%, and populations of similar purity can be continuously propagated for several passages. In each case where sorting has occurred, the collected cells are first allowed to aggregate (3-10K cells) in round-bottomed low cell-binding 96-well plates (Thermofisher) for 48 hours and then placed on matrix-coated dishes for expansion. This creates conditions more like those of tissue explants and encourages cross-feeding, which promotes cell viability. Subsequent passaging is then performed as for the E11.5 rudiments in clumps following brief trypsinization.
Evaluating the propagated MM progenitors
While nuclear Six2 expression is necessary to sustain the MM nephronic progenitor, as mentioned earlier, it is not sufficient to preserve competency, i.e., an ability to respond to induction. While Pax2 has also been implicated in this response (Brophy et al., 2001; Lusis et al., 2010), there is no evidence to indicate whether Six2 and Pax2 together are sufficient. Therefore, to determine if the MM progenitors are competent, the penultimate assay would be to demonstrate their ability differentiate to form the various segments of the nephron, namely, podocytes and proximal and distal tubules. For this, trysinized cells (10K) are allowed to aggregate as above for 48 hrs and then placed on Whatman Nuclepore filters against embryonic spinal cord and floated on DMEM with 10% FBS. To distinguish MMs from spinal cord, there are a variety of fluorescent reagents available for marking cells prior to co-cultivation. These include Cignal Lenti Reporters (Qiagen) or CellTracker Dyes (Thermofisher), which do not interfere with MET. Co-cultivated tissues are fixed and analysed after 7 days in culture. Antibodies to segment-specific markers (nephrin and Wt1 for podocytes, cadherin-6 for proximal tubules, and cadherin-1 for distal tubules) are applied to tissue sections to confirm the histological appearance of the structures. Interestingly, with the niche conditions we have developed, all three nephronic segments can be visualized in co-cultivation experiments; whereas, the Oxburgh lab reports only proximal and distal tubule formation. Why their conditions do not facilitate podocyte formation is unclear at this time.
While co-cultivation studies provide compelling evidence for the preservation of the MM progenitor, the result is only qualitative. For assessing the MM population quantitatively, we have used a colony assay developed by the Nishinakamura lab (Osafune et al., 2006). Wnt4 is the putative mediator of MET in MM progenitors (Stark et al., 1994; Tanigawa et al., 2011), and MM cells cultured on a feeder layer of Wnt4-expressing cells can be induced to form nephronic tubules (Kispert et al., 1998). By seeding dissociated MM progenitors on a feeder layer of mitomycin C-treated NIH3T3/Wnt4 cells, it is possible to quantify the number of colonies (multicellular epithelial-like structures) developing on the feeder layer after 5-7 days. This assay demonstrated the gradual loss of competent rat MM progenitors in passaged populations and roughly corresponded to the loss of nuclear Six2+ -stained cells (Tanigawa et al., 2015). Importantly, it provides a measure of progenitor stability over time.
Discussion
Despite a long-standing availability of a culture model for the induction of MMs to form nephronic epithelia, the applications of this model were previously somewhat limited due to the miniscule amounts of embryonic tissue derived from each rudiment (5-10K MMs/metanephros). This problem has diminished over time with the development of improved microscale technologies. Still, having access to a reproducible source of MM progenitors would not only allow the evaluation of these cells by a far wider array of methodologies but also expand our options to novel applications of these cells. Notably, rapid advances both in differentiating mouse ESCs/human PSCs as well as redifferentiating iPSCs (both mouse and human) are priming efforts to regenerate de novo a functional kidney. Several labs have now independently generated either UB/collecting duct or MM/nephronic epithelia from hPSCs (Lam et al., 2014; Mae et al., 2013; Taguchi et al., 2014; Xia et al., 2013). Moreover, Little’s lab was able to generate both UB and MM lineages from hPSCs simultaneously (Takasato et al., 2014). More recently, her group demonstrated the remarkable formation of multilineage renal organoids from human iPSCs. Surprisingly, the balance in lineage commitment could be altered by simply prolonging progenitor exposure to CHIR (Takasato et al., 2015). The treatments generated organoids with a collecting duct network, segmented nephrons with podocytes, proximal and distal tubules, interstitium, and vascular elements. Given these remarkable developments in strategies for differentiating PSCs and iPSCs, there is a growing need to understand how to stabilize the progenitors in order to tightly control the differentiation process once the cells have achieved their MM status. While there is clear evidence for UB/MM self organization as described here and in the Introduction, there is still a considerable need for modeling these cells from the time they are generated in vivo to find the optimal balance of factors for treating tissue aggregates that will yield a functional organ. Fortunately, by using current approaches such as ours, modeling the MM progenitor and nephron formation has become readily accessible to most labs and offers new insight into those factors that preserve the progenitor or promote its differentiation. Moreover, they provide a relatively pure system for interrogating the signaling mechanisms that drive either self-renewal or lineage commitment.
In light of recent studies from both the Oxburgh lab and ours, it is apparent that while our approaches show similarities, our defined conditions differ significantly. Oxburgh’s lab employs high levels of Bmps, which are likely responsible for the requirement of a Smad inhibitor – a reagent we have found unnecessary even with the addition of low concentrations of Bmp7. Our medium includes LIF, which may offset the need for more Bmps, since this also activates JNK signalling. With the additions of Bmp7 and CHIR, however, we must include a differentiation inhibitor to block MET in the progenitor population. Presumably, premature commitment is caused in part by activation of the calcium/NFAT pathway, which is stimulated by CHIR. Since Oxburgh also includes CHIR, perhaps robust activation of noncanonical Bmp signalling precludes the need for another pathway inhibitor in his medium. Regardless, given that our conditions fail to stabilize the Six2+ progenitor beyond about one month and five culture passages and that Oxburgh’s conditions fail to support progenitors capable of podocyte differentiation, there is a need for further optimization of these culture conditions. Such studies are likely ongoing in both labs. Finally a significant factor in accessibility to defined niche conditions relates to the cost of the reagents used in the studies. Because most of the factors employed in our media are applied at fairly low (and presumably physiological) levels, our cost of propagating MM progenitors is about an order of magnitude lower for a similar volume of medium than that of others (Brown et al., 2015). Regardless, the efficacy of current models is a significant incentive for embracing this approach to study nephronic progenitors in culture. Whether these cells have been harnessed sufficiently to incorporate into developing or damaged nephrons and become functional will determine precisely how close we are to mimicking nature.
Acknowledgements
This work was supported by a fellowship from the Japanese Society for the Promotion of Science (to S.T.), grant KAKENHI 26860640 from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan (to S.T.), and the Intramural Research Program of the NIH, NCI, CCR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Araoka T, Mae S, Kurose Y, Uesugi M, Ohta A, Yamanaka S, Osafune K. Efficient and rapid induction of human iPSCs/ESCs into nephrogenic intermediate mesoderm using small molecule-based differentiation methods. PLoS One. 2014;9:e84881. doi: 10.1371/journal.pone.0084881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak H, Huh SH, Chen S, Jeanpierre C, Martinovic J, Parisot M, Bole-Feysot C, Nitschke P, Salomon R, Antignac C, Ornitz DM, Kopan R. FGF9 and FGF20 maintain the stemness of nephron progenitors in mice and man. Dev Cell. 2012;22:1191–1207. doi: 10.1016/j.devcel.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barasch J, Yang J, Ware CB, Taga T, Yoshida K, Erdjument-Bromage H, Tempst P, Parravicini E, Malach S, Aranoff T, Oliver JA. Mesenchymal to epithelial conversion in rat metanephros is induced by LIF. Cell. 1999;99:377–386. doi: 10.1016/s0092-8674(00)81524-x. [DOI] [PubMed] [Google Scholar]
- Blank U, Brown A, Adams DC, Karolak MJ, Oxburgh L. BMP7 promotes proliferation of nephron progenitor cells via a JNK-dependent mechanism. Development. 2009;136:3557–3566. doi: 10.1242/dev.036335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brophy PD, Ostrom L, Lang KM, Dressler GR. Regulation of ureteric bud outgrowth by Pax2-dependent activation of the glial derived neurotrophic factor gene. Development. 2001;128:4747–4756. doi: 10.1242/dev.128.23.4747. [DOI] [PubMed] [Google Scholar]
- Brown AC, Muthukrishnan SD, Oxburgh L. A synthetic niche for nephron progenitor cells. Dev Cell. 2015;34:229–241. doi: 10.1016/j.devcel.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burn SF, Webb A, Berry RL, Davies JA, Ferrer-Vaquer A, Hadjantonakis AK, Hastie ND, Hohenstein P. Calcium/NFAT signalling promotes early nephrogenesis. Dev Biol. 2011;352:288–298. doi: 10.1016/j.ydbio.2011.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CH, Davies JA. An improved method of renal tissue engineering, by combining renal dissociation and reaggregation with a low-volume culture technique, results in development of engineered kidneys complete with loops of Henle. Nephron. Experimental nephrology. 2012;121:e79–85. doi: 10.1159/000345514. [DOI] [PubMed] [Google Scholar]
- Cheng HT, Miner JH, Lin M, Tansey MG, Roth K, Kopan R. Gamma-secretase activity is dispensable for mesenchyme-to-epithelium transition but required for podocyte and proximal tubule formation in developing mouse kidney. Development. 2003;130:5031–5042. doi: 10.1242/dev.00697. [DOI] [PubMed] [Google Scholar]
- Das A, Tanigawa S, Karner CM, Xin M, Lum L, Chen C, Olson EN, Perantoni AO, Carroll TJ. Stromal-epithelial crosstalk regulates kidney progenitor cell differentiation. Nat Cell Biol. 2013 doi: 10.1038/ncb2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JA, Chang CH. Engineering kidneys from simple cell suspensions: an exercise in self-organization. Pediatr Nephrol. 2014;29:519–524. doi: 10.1007/s00467-013-2579-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley AT, Godin RE, Robertson EJ. Interaction between FGF and BMP signaling pathways regulates development of metanephric mesenchyme. Genes Dev. 1999;13:1601–1613. doi: 10.1101/gad.13.12.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley AT, Lyons KM, Robertson EJ. A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev. 1995;9:2795–2807. doi: 10.1101/gad.9.22.2795. [DOI] [PubMed] [Google Scholar]
- Fujimura S, Jiang Q, Kobayashi C, Nishinakamura R. Notch2 activation in the embryonic kidney depletes nephron progenitors. J Am Soc Nephrol. 2010;21:803–810. doi: 10.1681/ASN.2009040353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto S, Hsieh CM, Maemura K, Layne MD, Yet SF, Lee KH, Matsui T, Rosenzweig A, Taylor WG, Rubin JS, Perrella MA, Lee ME. Akt participation in the Wnt signaling pathway through Dishevelled. J Biol Chem. 2001;276:17479–17483. doi: 10.1074/jbc.C000880200. [DOI] [PubMed] [Google Scholar]
- Grobstein C. Inductive epitheliomesenchymal interaction in cultured organ rudiments of the mouse. Science. 1953;118:52–55. doi: 10.1126/science.118.3054.52. [DOI] [PubMed] [Google Scholar]
- Grobstein C. Trans-filter induction of tubules in mouse metanephrogenic mesenchyme. Experimental cell research. 1956;10:424–440. doi: 10.1016/0014-4827(56)90016-7. [DOI] [PubMed] [Google Scholar]
- Karner CM, Das A, Ma Z, Self M, Chen C, Lum L, Oliver G, Carroll TJ. Canonical Wnt9b signaling balances progenitor cell expansion and differentiation during kidney development. Development. 2011;138:1247–1257. doi: 10.1242/dev.057646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kispert A, Vainio S, McMahon AP. Wnt-4 is a mesenchymal signal for epithelial transformation of metanephric mesenchyme in the developing kidney. Development. 1998;125:4225–4234. doi: 10.1242/dev.125.21.4225. [DOI] [PubMed] [Google Scholar]
- Klemm JD, Beals CR, Crabtree GR. Rapid targeting of nuclear proteins to the cytoplasm. Current biology : CB. 1997;7:638–644. doi: 10.1016/s0960-9822(06)00290-9. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Mugford JW, Krautzberger AM, Naiman N, Liao J, McMahon AP. Identification of a multipotent self-renewing stromal progenitor population during mammalian kidney organogenesis. Stem cell reports. 2014;3:650–662. doi: 10.1016/j.stemcr.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi A, Valerius MT, Mugford JW, Carroll TJ, Self M, Oliver G, McMahon AP. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell. 2008;3:169–181. doi: 10.1016/j.stem.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koseki C, Herzlinger D, al-Awqati Q. Apoptosis in metanephric development. J Cell Biol. 1992;119:1327–1333. doi: 10.1083/jcb.119.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuure S, Popsueva A, Jakobson M, Sainio K, Sariola H. Glycogen synthase kinase-3 inactivation and stabilization of beta-catenin induce nephron differentiation in isolated mouse and rat kidney mesenchymes. J Am Soc Nephrol. 2007;18:1130–1139. doi: 10.1681/ASN.2006111206. [DOI] [PubMed] [Google Scholar]
- Lam AQ, Freedman BS, Morizane R, Lerou PH, Valerius MT, Bonventre JV. Rapid and efficient differentiation of human pluripotent stem cells into intermediate mesoderm that forms tubules expressing kidney proximal tubular markers. J Am Soc Nephrol. 2014;25:1211–1225. doi: 10.1681/ASN.2013080831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann U, Schmitz J, Weissenbach M, Sobota RM, Hortner M, Friederichs K, Behrmann I, Tsiaris W, Sasaki A, Schneider-Mergener J, Yoshimura A, Neel BG, Heinrich PC, Schaper F. SHP2 and SOCS3 contribute to Tyr-759-dependent attenuation of interleukin-6 signaling through gp130. J Biol Chem. 2003;278:661–671. doi: 10.1074/jbc.M210552200. [DOI] [PubMed] [Google Scholar]
- Lindstrom NO, Carragher NO, Hohenstein P. The PI3K pathway balances self-renewal and differentiation of nephron progenitor cells through beta-catenin signaling. Stem cell reports. 2015;4:551–560. doi: 10.1016/j.stemcr.2015.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusis M, Li J, Ineson J, Christensen ME, Rice A, Little MH. Isolation of clonogenic, long-term self renewing embryonic renal stem cells. Stem Cell Res. 2010;5:23–39. doi: 10.1016/j.scr.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Mae S, Shono A, Shiota F, Yasuno T, Kajiwara M, Gotoda-Nishimura N, Arai S, Sato-Otubo A, Toyoda T, Takahashi K, Nakayama N, Cowan CA, Aoi T, Ogawa S, McMahon AP, Yamanaka S, Osafune K. Monitoring and robust induction of nephrogenic intermediate mesoderm from human pluripotent stem cells. Nature communications. 2013;4:1367. doi: 10.1038/ncomms2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda T, Nakamura T, Nakao K, Arai T, Katsuki M, Heike T, Yokota T. STAT3 activation is sufficient to maintain an undifferentiated state of mouse embryonic stem cells. EMBO J. 1999;18:4261–4269. doi: 10.1093/emboj/18.15.4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer TN, Schwesinger C, Sampogna RV, Vaughn DA, Stuart RO, Steer DL, Bush KT, Nigam SK. Rho kinase acts at separate steps in ureteric bud and metanephric mesenchyme morphogenesis during kidney development. Differentiation; research in biological diversity. 2006;74:638–647. doi: 10.1111/j.1432-0436.2006.00102.x. [DOI] [PubMed] [Google Scholar]
- Muthukrishnan SD, Yang X, Friesel R, Oxburgh L. Concurrent BMP7 and FGF9 signalling governs AP-1 function to promote self-renewal of nephron progenitor cells. Nature communications. 2015;6:10027. doi: 10.1038/ncomms10027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemetz C, Hocke GM. Transcription factor Stat5 is an early marker of differentiation of murine embryonic stem cells. Differentiation; research in biological diversity. 1998;62:213–220. doi: 10.1046/j.1432-0436.1998.6250213.x. [DOI] [PubMed] [Google Scholar]
- Nicola NA, Babon JJ. Leukemia inhibitory factor (LIF) Cytokine & growth factor reviews. 2015;26:533–544. doi: 10.1016/j.cytogfr.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa H, Burdon T, Chambers I, Smith A. Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev. 1998;12:2048–2060. doi: 10.1101/gad.12.13.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osafune K, Takasato M, Kispert A, Asashima M, Nishinakamura R. Identification of multipotent progenitors in the embryonic mouse kidney by a novel colony-forming assay. Development. 2006;133:151–161. doi: 10.1242/dev.02174. [DOI] [PubMed] [Google Scholar]
- Paling NR, Wheadon H, Bone HK, Welham MJ. Regulation of embryonic stem cell self-renewal by phosphoinositide 3-kinase-dependent signaling. J Biol Chem. 2004;279:48063–48070. doi: 10.1074/jbc.M406467200. [DOI] [PubMed] [Google Scholar]
- Park JS, Ma W, O'Brien LL, Chung E, Guo JJ, Cheng JG, Valerius MT, McMahon JA, Wong WH, McMahon AP. Six2 and Wnt regulate self-renewal and commitment of nephron progenitors through shared gene regulatory networks. Dev Cell. 2012;23:637–651. doi: 10.1016/j.devcel.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Valerius MT, McMahon AP. Wnt/beta-catenin signaling regulates nephron induction during mouse kidney development. Development. 2007;134:2533–2539. doi: 10.1242/dev.006155. [DOI] [PubMed] [Google Scholar]
- Perantoni A, Kan FW, Dove LF, Reed CD. Selective growth in culture of fetal rat renal collecting duct anlagen. Morphologic and biochemical characterization. Laboratory investigation; a journal of technical methods and pathology. 1985;53:589–596. [PubMed] [Google Scholar]
- Perantoni AO. The ureteric bud. Tissue-culture approaches to branching morphogenesis and inductive signaling. Methods in molecular medicine. 2003;86:179–192. doi: 10.1385/1-59259-392-5:179. [DOI] [PubMed] [Google Scholar]
- Perantoni AO, Dove LF, Karavanova I. Basic fibroblast growth factor can mediate the early inductive events in renal development. Proc Natl Acad Sci U S A. 1995;92:4696–4700. doi: 10.1073/pnas.92.10.4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perantoni AO, Timofeeva O, Naillat F, Richman C, Pajni-Underwood S, Wilson C, Vainio S, Dove LF, Lewandoski M. Inactivation of FGF8 in early mesoderm reveals an essential role in kidney development. Development. 2005;132:3859–3871. doi: 10.1242/dev.01945. [DOI] [PubMed] [Google Scholar]
- Pichel JG, Shen L, Sheng HZ, Granholm AC, Drago J, Grinberg A, Lee EJ, Huang SP, Saarma M, Hoffer BJ, Sariola H, Westphal H. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature. 1996;382:73–76. doi: 10.1038/382073a0. [DOI] [PubMed] [Google Scholar]
- Plisov SY, Yoshino K, Dove LF, Higinbotham KG, Rubin JS, Perantoni AO. TGF beta 2, LIF and FGF2 cooperate to induce nephrogenesis. Development. 2001;128:1045–1057. doi: 10.1242/dev.128.7.1045. [DOI] [PubMed] [Google Scholar]
- Rogers SA, Ryan G, Hammerman MR. Metanephric transforming growth factor-alpha is required for renal organogenesis in vitro. Am J Physiol. 1992;262:F533–539. doi: 10.1152/ajprenal.1992.262.4.F533. [DOI] [PubMed] [Google Scholar]
- Schiemann WP, Bartoe JL, Nathanson NM. Box 3-independent signaling mechanisms are involved in leukemia inhibitory factor receptor alpha- and gp130-mediated stimulation of mitogen-activated protein kinase. Evidence for participation of multiple signaling pathways which converge at Ras. J Biol Chem. 1997;272:16631–16636. doi: 10.1074/jbc.272.26.16631. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ott KM, Masckauchan TN, Chen X, Hirsh BJ, Sarkar A, Yang J, Paragas N, Wallace VA, Dufort D, Pavlidis P, Jagla B, Kitajewski J, Barasch J. beta-catenin/TCF/Lef controls a differentiation-associated transcriptional program in renal epithelial progenitors. Development. 2007;134:3177–3190. doi: 10.1242/dev.006544. [DOI] [PubMed] [Google Scholar]
- Stark K, Vainio S, Vassileva G, McMahon AP. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature. 1994;372:679–683. doi: 10.1038/372679a0. [DOI] [PubMed] [Google Scholar]
- Taguchi A, Kaku Y, Ohmori T, Sharmin S, Ogawa M, Sasaki H, Nishinakamura R. Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell. 2014;14:53–67. doi: 10.1016/j.stem.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Takasato M, Er PX, Becroft M, Vanslambrouck JM, Stanley EG, Elefanty AG, Little MH. Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nat Cell Biol. 2014;16:118–126. doi: 10.1038/ncb2894. [DOI] [PubMed] [Google Scholar]
- Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, Parton RG, Wolvetang EJ, Roost MS, Chuva de Sousa Lopes SM, Little MH. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature. 2015;526:564–568. doi: 10.1038/nature15695. [DOI] [PubMed] [Google Scholar]
- Tanigawa S, Sharma N, Hall MD, Nishinakamura R, Perantoni AO. Preferential Propagation of Competent SIX2+ Nephronic Progenitors by LIF/ROCKi Treatment of the Metanephric Mesenchyme. Stem cell reports. 2015;5:435–447. doi: 10.1016/j.stemcr.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanigawa S, Wang H, Yang Y, Sharma N, Tarasova N, Ajima R, Yamaguchi TP, Rodriguez LG, Perantoni AO. Wnt4 induces nephronic tubules in metanephric mesenchyme by a non-canonical mechanism. Dev Biol. 2011;352:58–69. doi: 10.1016/j.ydbio.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Berge D, Kurek D, Blauwkamp T, Koole W, Maas A, Eroglu E, Siu RK, Nusse R. Embryonic stem cells require Wnt proteins to prevent differentiation to epiblast stem cells. Nat Cell Biol. 2011;13:1070–1075. doi: 10.1038/ncb2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Nishikawa S, Muguruma K, Sasai Y. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nature biotechnology. 2007;25:681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- Xia Y, Nivet E, Sancho-Martinez I, Gallegos T, Suzuki K, Okamura D, Wu MZ, Dubova I, Esteban CR, Montserrat N, Campistol JM, Izpisua Belmonte JC. Directed differentiation of human pluripotent cells to ureteric bud kidney progenitor-like cells. Nat Cell Biol. 2013;15:1507–1515. doi: 10.1038/ncb2872. [DOI] [PubMed] [Google Scholar]