Abstract
Efforts to restore β-cell number or mass in type 1 diabetes (T1D) must combine an intervention to stimulate proliferation of remaining β-cells and an intervention to mitigate or control the β-cell-directed autoimmunity. This review highlights features of the β-cell, including it being part of a pancreatic islet, a mini-organ that is highly vascularized and highly innervated, and efforts to promote β-cell proliferation. In addition, the β-cell in T1D exists in a microenvironment with interactions and input from other islet cell types, extracellular matrix, vascular endothelial cells, neuronal projections, and immune cells, all of which likely influence the β-cell’s capacity for replication. Physiologic β-cell proliferation occurs in human and rodents in the neonatal period and early in life, after which there is an age-dependent decline in β-cell proliferation, and also as part of the β-cell’s compensatory response to the metabolic challenges of pregnancy and insulin resistance. This review reviews the molecular pathways involved in this β-cell proliferation and highlights recent work in two areas: 1) Investigators, using high-throughput screening to discover small molecules that promote human β-cell proliferation, are now focusing on the dual-specificity tyrosine-regulated kinase-1a and cell cycle-dependent kinase inhibitors CDKN2C/p18 or CDKN1A/p21 as targets of compounds to stimulate adult human β-cell proliferation. 2) Local inflammation, macrophages, and the local β-cell microenvironment promote β-cell proliferation. Future efforts to harness the responsible mechanisms may lead to new approaches to promote β-cell proliferation in T1D.
Keywords: diabetes, autoimmune, cell proliferation, islet
Introduction
In type 1 diabetes (T1D), loss of β-cells leads to inadequate insulin biosynthesis and secretion, leading to hyperglycemia and the need for life-long exogenous insulin replacement. Ideally, an intervention to mitigate or prevent the β-cell-directed autoimmunity coupled with a way to increase β-cell number or mass would reverse the insulin deficiency in T1D. As part of the reviews in this issue of the Journal, this manuscript focuses on recent advances in our understanding of β-cell proliferation, including physiologic and pharmacologic approaches that stimulate β-cell proliferation and the possible timing of interventions to stimulate β-cell proliferation. Throughout we highlight the importance and challenges in translating new information learned from rodent islets to human islets and human T1D by discussing these critical questions:
When does physiologic β-cell proliferation occur?
What pharmacologic approaches stimulate β-cell proliferation?
What are the molecular mechanisms of β-cell proliferation?
Can an autoimmune or inflammatory environment, like that seen in T1D, promote β-cell proliferation?
When in the course of type 1 diabetes would an intervention to stimulate β-cell proliferation be desired?
Background
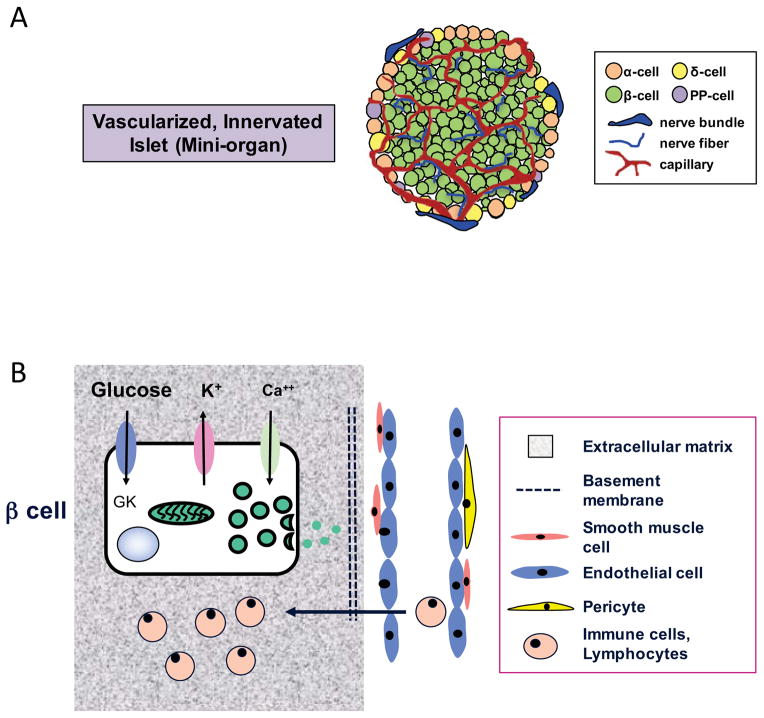
Pancreatic islets are highly vascularized, highly innervated mini-organs that sense nutrients such as glucose and quickly secrete insulin, glucagon, somatostatin, as well as other islet hormones (Figure 1A). While islets are a complex 3-D structure, it is also clear that a β-cell exists within a physiologic microenvironment or “context” which influences β-cell function, survival, and likely proliferation (Figure 1B). For example, the β-cell’s relationship and communication with other islet cell types (by cell-to-cell contact, paracrine signals, and secreted products such as hormone, neurotransmitters, etc.), with the surrounding extracellular matrix, with abutting or near-by endothelial cells and neuronal projections, and in the case of T1D, immune cells, are relevant to efforts to stimulate β-cell proliferation and to ensure that newly arising β-cells function normally and respond appropriately to physiologic cues.
Figure 1. β-cell microenvironment.
(A) Schematic of β cells within a highly vascularized, highly innervated pancreatic islet; (B) Schematic of “context” or interactions of a β cell with other cell types, extracellular matrix, vascular endothelial cells, and immune cells.
Much is known about the rodent islet and its infrastructure and physiology. Fortunately, gaps in our understanding of key infrastructure, events, and processes in human islets are increasingly being investigated. Work over the past decade, especially facilitated by human islets being available for research, has discovered important similarities, yet critical differences, between the rodent islet and the human islet. For example, the adult human islet cell composition is more variable with β-cells accounting for approximately 50–70% of human islet cells compared to rodent islets where there is little variability (75–80% islets are β-cells) [1]. Furthermore, there are differences in islet cell arrangement, islet vascularization, basal insulin secretory rate, and gene expression in rodent and human islets [1].
As part of their sophisticated glucose-sensing and insulin secretion capabilities, β-cells are terminally differentiated cells with a very low baseline proliferation rate (in both mouse and human with human being approximately 10-folder lower). To discover how one could stimulate β-cell proliferation and reverse the β-cell deficiency in T1D, investigators are pursuing two approaches: 1) Harness the mechanisms, growth factors, hormones, signals used under normal physiology to stimulate β-cell proliferation; 2) Search for and identify small molecules or compounds that stimulate β-cell proliferation.
A. When does physiologic β-cell proliferation occur?
Substantial β-cell proliferation occurs in at least three physiologic or compensatory periods: 1) In the early neonatal period; 2) During pregnancy; and 3) In response to insulin resistance. These three time periods have studied longitudinally in mice, but what occurs in humans under these circumstances is unclear.
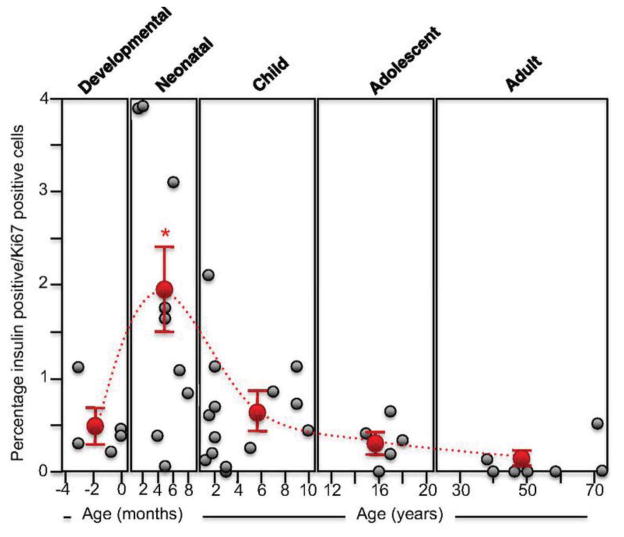
Pancreatic mass and β-cell mass increase early in life in rodents and humans. In humans, an increase in β-cell proliferation has been noted soon after birth and in the first 2–3 years of life [2] with the β-cell proliferation rate declining within and after the first decade of life and thereafter remaining low in the adult period (Figure 2). This age-related decline in the rate of β-cell proliferation occurs in both rodents and humans.
The insulin resistance accompanying pregnancy requires that the mother increase her insulin production and secretion to maintain glucose homeostasis. In rodents, it is clear there is both an increase in insulin biosynthesis/cell and an increase in β-cell β-cell proliferation and number. Some of these islet effects are mediated by lactotrophic hormones such as prolactin and placental lactogen, both of which signal through the prolactin receptor, and other signals such as HGF [3–6]. While in humans there is also a compensatory increase in insulin secretion in vivo, it seems there is little increase in β-cell proliferation or number (based on the small number of human pancreata studied) and neither prolactin nor placental lactogen stimulates human β-cell proliferation as they do in isolated rodents islets. The molecular explanation(s) for why human islets do not respond to a mitogenic stimulus such as prolactin is complex, involving both the prolactin receptor and downstream singling pathways (discussed below).
Insulin resistance, either genetic or the result of obesity, also challenges the β-cells to produce more insulin to maintain glucose homeostasis. In several rodent models of insulin resistance (ob/ob, dbdb, high-fat diet, etc.), there is a dramatic expansion of β-cell mass. In contrast, in obese humans or in human islets transplanted into immunodeficient mice, insulin resistance does not stimulate human β-cell proliferation. Interestingly, using a genetic model insulin resistance (inactivation of hepatic insulin receptor), Kulkarni and colleagues have recently identified the protease inhibitor SerpinB1 as a hepatically derived factor responsible for the marked β-cell proliferation in this model [7]. Culture of human islets with SerpinB1 increased β-cell proliferation.
Figure 2. Human β-cell proliferation as a function of age.
Note the greater rate of proliferation in the first decade of life. Reproduced from [2].
Thus, in early life, both rodent and human β-cells have an increased proliferative capacity, but adult rodent and human islets differ in their proliferative response to the demands of pregnancy and insulin resistance.
B. Are there pharmacologic approaches that stimulate β-cell proliferation?
Using a candidate molecule approach, several classes of drugs, hormones or growth factors such as PPARγ agonists, GLP-1 agonists, DPP-4 inhibitors, GSK3β inhibitors, prolactin, IGF-1, HGF, and PTHRP have been tested for the ability to stimulate β-cell proliferation. While several of these have clear activity in rodent islets, none have proven useful in stimulating human β-cell proliferation. This inability to stimulate human β-cell proliferation has led to intense efforts by several groups to use high-throughput small-molecule screening approaches to identify compounds or small molecules that stimulate β-cell proliferation [8–11]. Interestingly, recent, independent discoveries have identified the dual-specificity tyrosine-regulated kinase-1a (DYRK1A) as the likely target of compounds such as harmine and 5-iodotubercidin, both of which stimulate adult human β-cell proliferation [9,10]. Another group was able to stimulate adult human β-cell proliferation using a RNA interference strategy to silence cell cycle-dependent kinase inhibitors CDKN2C/p18 or CDKN1A/p21 and suggested that p18 and p21 may be attractive targets to promote β-cell proliferation [8]. Efforts to identify additional compounds and to optimize identified compounds are underway. A challenge is that no pathway identified thus far is β-cell specific; thus an approach to only deliver the active compound to β-cells will be required.
C. What are the molecular mechanisms of β-cell proliferation?
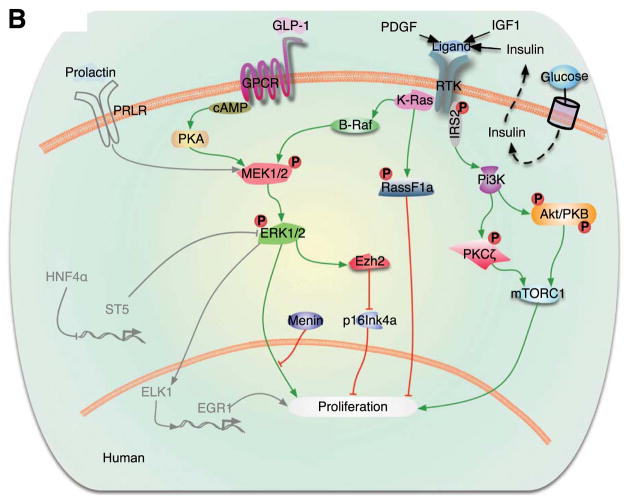
Several intracellular signaling pathways have been implicated in the proliferation of human β-cells, and are extensively reviewed in a three-part series by Stewart and colleagues [12–14]. A few of the key pathways and molecules are depicted in Figure 3. Of note, proliferative mechanisms have been much more extensively studied in rodent β-cells. While expression of most signaling proteins is conserved in humans, a number of pathways that induce proliferation in rodent models do not promote replication in human β-cells. Still other proliferative mechanisms, such as the non-canonical JAK/STAT signaling, and signaling through certain membrane receptors, such as HTR2b, have not yet been extensively investigated in human β-cells [14–16]. Here, we discuss recent information about selected factors and pathways involved in β-cell proliferation.
Figure 3. Signaling pathways, including Ras/Raf/ERK and PI3K/Akt, which regulate human β-cell proliferation.
Pathways depicted in gray represent molecules studied in rodents that have not been investigated in human β-cells. Reproduced from [14].
PI3K/Akt/mTOR axis – see reviews [12,13]. The canonical PI3K pathway is a source of proliferative signals, activating protein kinases PKC and Akt/PKB. Studies of intact and dispersed human islets overexpressing AKT directly have shown increased β-cell proliferation [17], and Yap, another activator of AKT/mTOR signaling, appeared to induce proliferation, though nonspecifically in endocrine cells [18]. PKCζ, which is less often studied as part of the PI3K/mTOR cascade, was recently shown to be necessary for “compensatory” human β-cell replication induced by glucose [19]. Indirect activation of Akt by TGFβ has led to context-dependent effects on β-cell proliferation [20], which suggests that ligands such as TGFβ may activate multiple signaling cascades simultaneously. Leibiger and colleagues recently showed that PI3K-C2α knockdown promoted IR-B/Shc/ERK signaling, inducing proliferation, while still maintaining alternate PKB/Akt signaling necessary for basal β-cell metabolism [21]. It is likely that balancing the activity of multiple pathways will be necessary to promote proliferation while maintaining β-cell identity and function.
Ras/Raf/ERK signaling – see review [14]. There is evidence that pregnancy-induced proliferation in rodents, particularly by prolactin, may act through the ERK pathway [16].
Cell cycle machinery – see reviews [22,23]. In the transition from G1 to S phase, cyclins and cdks are sequentially phosphorylated to drive proliferation. The machinery for cell cycle progression is largely conserved between rodent and human β-cells; however, there are a few key differences. Notably, the β-cells of human islets express high levels of cdk6—absent in rodent β-cells— and low levels of cyclin D2—critical to rodent β-cell proliferation [12]. Recent studies have enumerated additional subtlety. For example, although cdks 4 and 6 and cyclin D3 are readily detectable in both rodent and human β-cells, other cyclins that are abundant in rodent β-cells are not consistently expressed by human β-cells [22,24]. Even amongst the conserved cyclins and cdks, manipulation has produced mixed results in different systems. For example, Cdk5 activation induced proliferation in rodent β-cells, but has previously been implicated in apoptotic pathways of various human cell types [25]. Finally, there is considerable variability between experiments using human β-cells—activation of cyclin isoforms induced β-cell proliferation in human islet grafts [24], but not in the human cell line EndoC-bH1 [26]. A key inter-specific difference is the subcellular localization of cell cycle molecules. In human β-cells the majority of cyclins and cdks are sequestered in the cytoplasm rather than the nucleus, which may contribute to the reluctance of human β-cells to proliferate basally [22,24]. Indeed, there is evidence that cytoplasmic and nuclear trafficking play a regulatory role in human β-cell proliferation [27]. Once in the nucleus, cell cycle molecules can be influenced by additional proteins like menin, which further controls β-cell transcription and replication by modulating methylation activity [28].
-
Hormone and growth factor activity – see reviews [13,14,29]. Recent reviews are an excellent source of information on the role that GPCRs [29], steroid hormones, and pregnancy-related factors [30] play in β-cell proliferation. A selection are summarized here:
Hormones. Some peptide hormones, including parathyroid hormone-related protein (PTHrP) [31,32] and prolactin [33,34], stimulate β-cell replication in vitro and are known mitogens of compensatory β-cell proliferation during rodent pregnancy. Recently it was shown that PRLR-Jak2-Stat5 signaling is not prominent in human β-cells; interestingly, however, murine Stat5a induced proliferation of human β-cells [15] and there is evidence that prolactin may augment traditional T1D therapies [35]. Signaling of steroid hormones progesterone, estrogen, and androgen modulates proliferation, and is disrupted under diabetic conditions [14,34].
Amino acid derivatives. Serotonin, also extensively studied during β-cell adaption during pregnancy [3,36], shows potential for de novo β-cell proliferative activity [37].
Growth factors. Hepatocyte growth factor (HGF) [3,5,6] may exert proliferative effects through the PKC pathway [31]. Altered IGF-1 signaling, via phosphorylation of IRS-2 and GSK3 [38], promoted β-cell proliferation in both isolated and transplanted human islets incubated with protease inhibitor SerpinB1 [7].
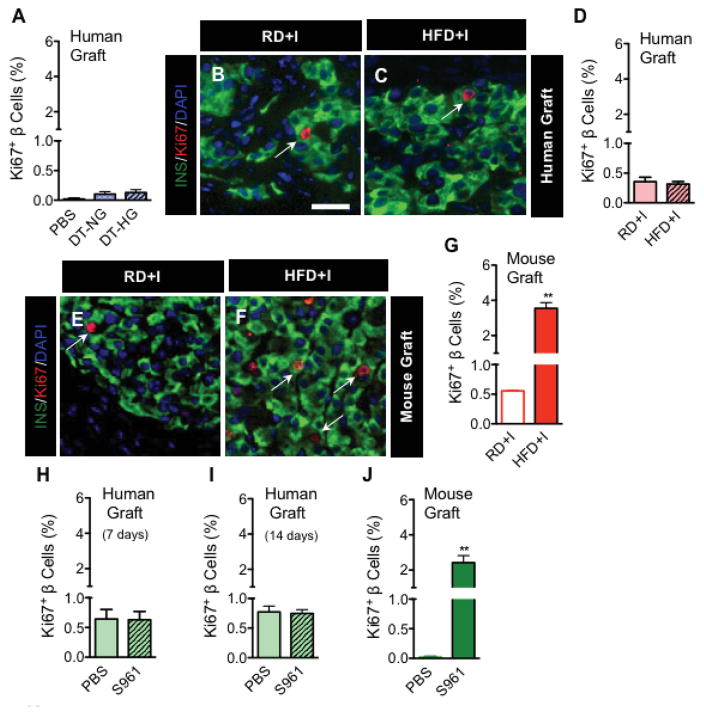
Changes in glucose metabolism. There is robust data indicating that insulin resistance and/or hyperglycemia stimulates β-cell proliferation in rodents [13,19,39]. Porat and colleagues showed that glucose-induced proliferation was regulated by GK metabolism, with increased glycolytic rate causing the β-cell to upregulate proliferative pathways [39]. Carbohydrate response element binding protein (ChREBP) was essential for this downstream proliferative response [13], as was PKCζ-mediated mTOR/cyclin D2 activity [19]. While stimulation at very high glucose concentration increased β-cell proliferation in isolated human islets, lower levels of glucose were not sufficient to produce this effect [19]. Dai and colleagues (Journal of Clinical Investigation, in press, March 2016) recently investigated human β-cell proliferation in vivo, performing human islet transplants in immunodeficient mice and then induction of hyperglycemia +/− insulin resistance. They found that neither chronic nor acute hyperglycemia stimulated β-cell proliferation in human islet grafts, in contrast with grafts of mouse islets (Figure 4). These data again underscore an apparent difference in the proliferative capacity between rodent and human islets.
-
Other potential targets/pharmacological agents. Based on the results of many molecules inducing β-cell proliferation in rodents, several groups have focused on identifying mitogens, receptors, and signaling molecules that have not previously or extensively been studied in β-cells. A small sampling, certainly not exhaustive, is provided here:
Aminopyrazine compounds, which are robustly mitogenic in human β-cells, likely target glycogen synthase kinase-3 beta (GSK3B) and dual specificity tyrosine- phosphorylation- regulated kinase 1A (DYRK1A) [40].
Membrane-bound sodium glucose co-transporter 2 (SGLT2) inhibitor has an insulin-independent effect on β-cells in STZ model, increasing β-cell mass and proliferation rate [41].
Transcription factor Pax4, which is required for β-cell maturation, appears elevated in a subset of mature β-cells that proliferate preferentially during adaptive β-cell expansion [42]. Cultured human islet cells also proliferated upon Pax4 overexpression [43].
Engineered peptides mimicking extracellular matrix (ECM) increased numerous proliferative indexes and β-cell-specific gene expression in rat INS-1 cells; proposed mediators include integrin receptors and subsequent activation of focal adhesion kinase (FAK) and extracellular signal-regulated kinase (ERK) [44].
Circulating fatty acids such as palmitate and oleate, which are elevated considerably during pregnancy, had synergistic effects in the presence of prolactin-induced β-cell proliferation [45]. In certain contexts, lipids have also preserved β-cell proliferative capacity in non-pregnant rodents [46].
Figure 4. Human β-cells do not proliferate in response to hyperglycemia or insulin resistance.
Human islets were transplanted into immunodeficient mice (NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ; abbreviated as NOD-scid IL2rγnull or NSG) that expressed the diphtheria toxin receptor (DTR) on native mouse β-cells (NSG-DTR). Exposure to neither chronic hyperglycemia (induced by DT injection into NSG-DTR mice with human islets); A), nor chronic insulin resistance (induced by placing mice on a high-fat diet; NSG-HFD; B–C), nor acute hyperglycemia and insulin resistance (induced by administration of insulin receptor antagonist, S961; H–I) elicited β-cell proliferation in human islet grafts. In contrast, the same metabolic stressors increased β-cell proliferation rates in mouse islet grafts (E–G; J). Reproduced from Dai et al (JCI, 2016, in press).
D. Does an autoimmune or inflammatory environment, like that seen in T1D, promote β-cell proliferation?
Though macrophages have been studied extensively for their role in autoimmune β-cell destruction during T1D, it has recently become clear that a subset of macrophages also play critical roles in regeneration [47,48], angiogenesis [49,50], and tissue remodeling [51] following injury. These restorative or “M2” macrophages confer anti-inflammatory effects, expressing common signatures of lipoproteins (CD11c, CD206, CD163, CD14) and metabolic genes (Arg1, Fizz1) that are distinct from those of their pro-inflammatory (“M1”) counterparts [52,53]. Furthermore, studies suggest that pancreatic macrophages maintain extreme phenotypic plasticity, including the ability to dynamically interconvert between “M1-like” and “M2-like” populations [49,54–56].
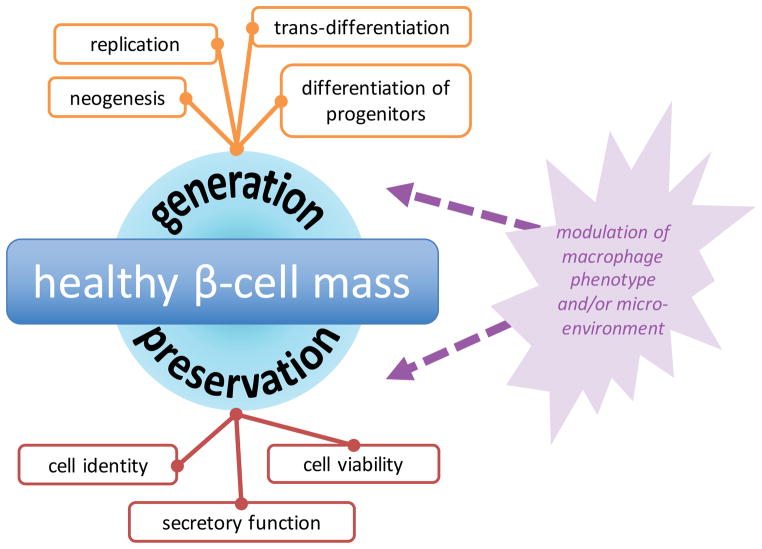
Immune events play a dual role in controlling β-cell proliferation—on one hand, macrophages can promote protective or pro-survival processes, and on the other hand, they can stimulate the production of additional β-cells (Figure 5). In the case of T1D, there is ample evidence that both protection of existing β-cells and generation of new β-cells contribute to alleviation of disease pathogenesis.
Figure 5. Optimizing β-cell mass in T1D.
At any given time, β-cell mass represents the net total of β-cell creation and β-cell loss. Therefore, we can think of expanding a β-cell population as heightening generation (creating more β-cells) or by improving preservation (minimizing β-cell loss). Both generation and preservation of β-cells are facilitated by various mechanisms, which are depicted in the corresponding boxes. Macrophages can affect processes related to both proliferative and protective function.
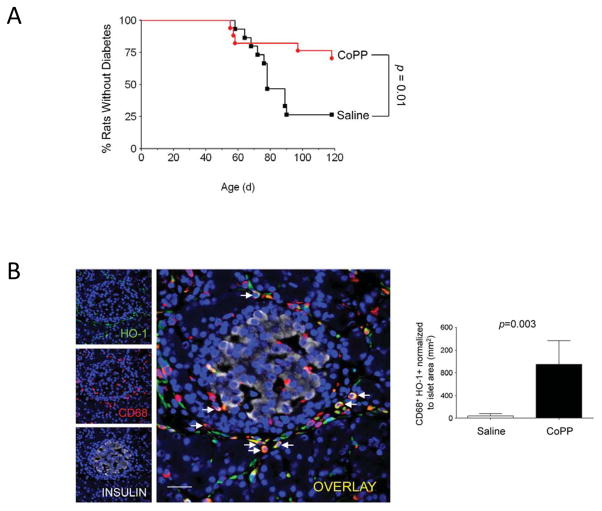
Protection. Recent experiments suggest that M2 macrophages can prevent the onset of inflammatory response in T1D; such protection is likely mediated by secreted peptides with pro-proliferative, anti-inflammatory effects [57]. In particular, several studies focused on the heme oxygenase-1 (HO-1) pathway, whose induction confers protection from oxidative damage [58] and reduces the incidence of T1D in the BioBreeding diabetes-prone (BBdp) rat model [59–61]. Husseini and colleagues showed that this protective effect was achieved through provisional influx of CD68+ HO-1+ macrophages and other cells to peri-islet areas (Figure 6) [61]. Although infiltrating cells were highly heterogenous, most expressed at least one surface marker that is associated with M2 macrophage activation (e.g. CD206, CD163), and whole pancreata showed increased levels of M2-associated genes (Mrc1/CD206, Msr1/CD204, Cx3cr1) and anti-inflammatory cytokines (IL-10, TGFβ1) [61]. Additional signaling pathways that may protect β-cells from oxidative damage and stress include activation of carbon monoxide leasing molecule (CORM)-A1 [62], cell cycle-independent effects of cyclin D2 inhibition [63], and cytokine-activated signaling [64,65].
-
Regeneration. Various chemical methods used to damage to β-cells [56,66,67] enable the study of macrophage-assisted proliferation during the regenerative process. Three major models have emerged:
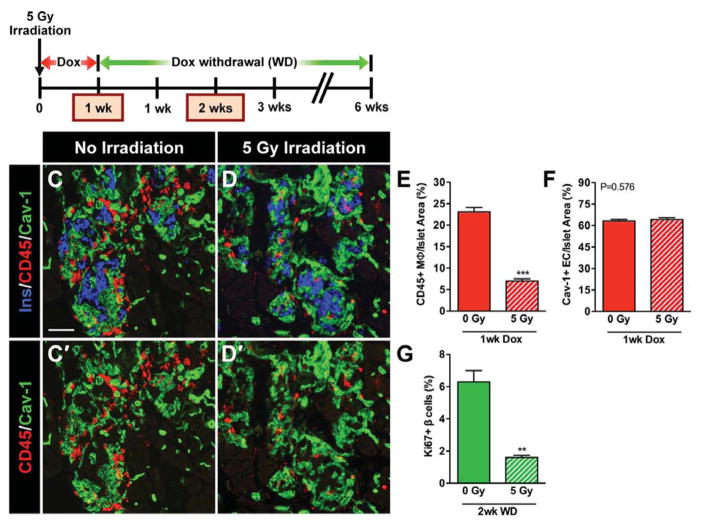
A burst of β-cell proliferative activity following vascular endothelial growth factor A (VEGF-A)-induced β-cell loss, which required the recruitment of macrophages to pancreatic islets (Figure 7) [67]. Islet transplantation experiments indicated that the local islet microenvironment, not a systemic soluble factor, was required for the recovery process, which was likely mediated by a crosstalk between macrophages, ECs, and β-cells [67].
DT-mediated β-cell injury, which initially attracted a large islet-associated M1 population. The population gradually lost this identity and shifted to an M2 phenotype, a change that corresponded to the peak of β-cell regeneration [56]. Ablating either resident macrophages or circulating monocytes strongly impaired this β-cell regenerative response [56].
Pancreatic ductal ligation (PDL), which inflamed exocrine tissue and led to a proliferative β-cell response that was secondary to heavily increased infiltration of M2 macrophages around islets [68]. Macrophage-produced growth factors and cytokines regulated SMAD7 expression in β-cells and subsequently influenced proliferation [53,68,69].
Role of additional cell types in macrophage-mediated regeneration. The dynamic macrophage phenotypes and mechanisms of β-cell expansion exemplify how multiple diverse cell types facilitate tissue regeneration. For example, in addition to infiltrating macrophages stimulated by HO-1 induction, Husseini and colleagues also identified infiltrating CD34+ cells that resembled mesenchymal cells (vimentin+), fibroblasts (α-SMA+), and fibrocytes (collagen V+) [61]. These findings were consistent with previous studies suggesting that ECM components [70,71] and mesenchymal stem cells [72] recruit polarized, proliferative macrophages in response to tissue damage. Complex remodeling of ECM [73] by macrophages in concert with other cell types is an understudied area of β-cell regeneration research and undoubtedly warrants future attention.
Figure 6. HO-1 induction by CoPP prevents T1D and increases CD68+ cells in pancreata of BBdp rats.
CoPP treatment significantly reduced incidence of T1D in BBdp rats (A). Increased number of islet-associated CD68+ (red) and HO-1+ (green) double positive cells (arrows) in 51 days CoPP-injected BBdp rats compared with controls (B). Reproduced from [61].
Figure 7. Recruitment of macrophages into βVEGF-A islets upon VEGF-A induction is necessary for the β-cell proliferative response.
After sublethal irradiation of βVEGF-A mice, Dox-induced VEGF-A expression was maintained for 1 week before islet macrophage infiltration was assessed. Partial bone marrow ablation reduced infiltration of CD45+ macrophages (E), but did not affect intra-islet EC expansion (F). Two weeks following Dox withdrawal, β-cell proliferation was significantly reduced in irradiated mice (G). Reproduced from [67].
E. When in the course of type 1 diabetes would an intervention to stimulate β-cell proliferation be desired?
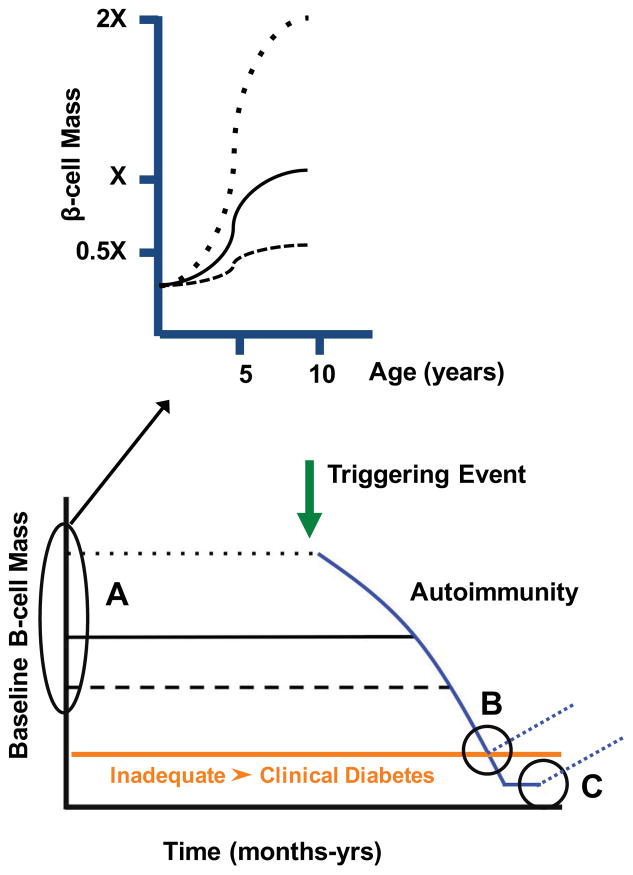
Efforts to stimulate β-cell proliferation to amplify β-cell mass and thus restore insulin secretion could be considered before or after the onset of T1D. For example, β-cell mass is known to vary 3–5 fold across the spectrum of normal individuals, making the “baseline” β-cell mass in individuals a critical determinant of the timing of T1D onset (Figure 8). However, it is not known when an individual’s “baseline” β-cell mass is determined. Potential trajectories include:1) β-cell mass is already different at birth (as the result of genetics or the in utero environment) or 2) individuals have similar β-cell mass at birth, but events in the first decade of life lead to different β-cell mass (Figure 8). In the latter model, it is likely that interventions to increase β-cell mass in the first decade would delay the onset of T1D (“A” scenario in Figure 8). Interventions to stimulate β-cell proliferation to increase β-cell mass could also be applied at the onset of T1D or years after T1D onset with there is only small number of β-cells present (“B” and “C” scenarios in Figure 8). These latter two scenarios would be coupled with an intervention to mitigate or restrain the β-cell-directed autoimmunity.
Figure 8. Timing of interventions to increase β-cell mass in T1D.
Lower panel shows schematic of T1D model with a triggering event initiating an autoimmune process which leads to a slow destruction of β cells and ultimately clinical diabetes when β cell mass is inadequate (orange line). Two horizontal dotted lines highlight the range of baseline β cell mass in non-diabetic individuals and how a difference baseline β cell mass would influence the timing of T1D onset. β cell mass is thought to be largely determined in the first decade of life (upper panel showing the difference trajectories for achieving peak β cell mass. Three time periods (A, B, C) at which interventions might be introduced to influence β cell mass in T1D.
Summary and Future Directions
Thus far, efforts to stimulate human β-cell proliferation to increase β-cell mass have largely been unsuccessful, likely reflecting that human β-cells are terminally differentiated cell with a very low proliferative capacity and are “programmed” not to proliferate. Fortunately, recent efforts have discovered new molecules and pathways that can now be targeted and explored. However, it is likely that more than one pathway will need to be modulated to adequately expand β-cell mass. Furthermore, new information about the islet microenvironment and the ability of cells such as macrophages to promote β-cell proliferation may open new ways to expand β-cell mass. A greater understanding of the human β-cell is needed to successfully translate discoveries and approaches stimulating rodent β-cell proliferation.
Highlights.
New approaches needed to overcome β islet cell’s low proliferative capacity
Physiologic/pharmacologic approaches to stimulate β-cell proliferation discussed
Small molecules promote β-cell proliferation in vitro
Macrophage and islet microenvironment promote β-cell proliferation in vivo
Acknowledgments
This work was supported by grants from the Department of Veterans Affairs (BX000666, BX002223), the National Institute of Diabetes and Digestive and Kidney Diseases (DK68854, DK66636, DK69603, DK63439, DK62641, DK72473, DK89572, DK89538, DK68764, DK92758, DK97829, DK94199, DK104211, DK106755, DK108120), JDRF, and the Vanderbilt Diabetes Research and Training Center (DK20593). Work in the authors’ laboratory has been greatly assisted by the Integrated Islet Distribution Program (https://iidp.coh.org/).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dai C, Brissova M, Hang Y, Thompson C, Poffenberger G, Shostak A, et al. Islet-enriched gene expression and glucose-induced insulin secretion in human and mouse islets. Diabetologia. 2012;55:707–718. doi: 10.1007/s00125-011-2369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gregg BE, Moore PC, Demozay D, Hall BA, Li M, Husain A, et al. Formation of a human β-cell population within pancreatic islets is set early in life. The Journal of Clinical Endocrinology & Metabolism. 2012;97:3197–3206. doi: 10.1210/jc.2012-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ernst S, Demirci C, Valle S, Velazquez-Garcia S, Garcia-Ocaña A. Mechanisms in the adaptation of maternal β-cells during pregnancy, Diabetes Management (London. England) 2011;1:239–248. doi: 10.2217/dmt.10.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C, Snider F, Cross JC. Prolactin Receptor Is Required for Normal Glucose Homeostasis and Modulation of β-Cell Mass during Pregnancy. Endocrinology. 2009;150:1618–1626. doi: 10.1210/en.2008-1003. [DOI] [PubMed] [Google Scholar]
- 5.Demirci C, Ernst S, Alvarez-Perez JC, Rosa T, Valle S, Shridhar V, et al. Loss of HGF/c-Met signaling in pancreatic β-cells leads to incomplete maternal β-cell adaptation and gestational diabetes mellitus. - PubMed - NCBI. Diabetes. 2012;61:1143–1152. doi: 10.2337/db11-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johansson M, Mattsson G, Andersson A, Jansson L, Carlsson PO. Islet endothelial cells and pancreatic beta-cell proliferation: studies in vitro and during pregnancy in adult rats. Endocrinology. 2006;147:2315–2324. doi: 10.1210/en.2005-0997. [DOI] [PubMed] [Google Scholar]
- 7.El Ouaamari A, Dirice E, Gedeon N, Hu J, Zhou J-Y, Shirakawa J, et al. SerpinB1 Promotes Pancreatic β Cell Proliferation. Cell Metabolism. 2016;23:194–205. doi: 10.1016/j.cmet.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robitaille K, Rourke JL, McBane JE, Fu A, Baird S, Du Q, et al. High-Throughput Functional Genomics Identifies Regulators of Primary Human Beta Cell Proliferation. Journal of Biological Chemistry. 2016 doi: 10.1074/jbc.M115.683912. jbc.M115.683912–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang P, Alvarez-Perez JC, Felsenfeld DP, Liu H, Sivendran S, Bender A, et al. A high-throughput chemical screen reveals that harmine-mediated inhibition of DYRK1A increases human pancreatic beta cell replication. Nature Medicine. 2015;21:383–388. doi: 10.1038/nm.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dirice E, Walpita D, Vetere A, Meier BC, Kahraman S, Hu J, et al. Inhibition of DYRK1A stimulates human beta-cell proliferation. Diabetes. 2016:db151127. doi: 10.2337/db15-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walpita D, Hasaka T, Spoonamore J, Vetere A, Takane KK, Fomina-Yadlin D, et al. A human islet cell culture system for high-throughput screening. Journal of Biomolecular Screening. 2012;17:509–518. doi: 10.1177/1087057111430253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kulkarni RN, Mizrachi EB, Ocana AG, Stewart AF. Human β-cell proliferation and intracellular signaling: driving in the dark without a road map. Diabetes. 2012;61:2205–2213. doi: 10.2337/db12-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernal-Mizrachi E, Kulkarni RN, Scott DK, Mauvais-Jarvis F, Stewart AF, Garcia-Ocaña A. Human β-Cell Proliferation and Intracellular Signaling Part 2: Still Driving in the Dark Without a Road Map. Diabetes. 2014;63:819–831. doi: 10.2337/db13-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stewart AF, Hussain MA, Garcia-Ocaña A, Vasavada RC, Bhushan A, Bernal-Mizrachi E, et al. Human β-Cell Proliferation and Intracellular Signaling: Part 3. Diabetes. 2015;64:1872–1885. doi: 10.2337/db14-1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen H, Kleinberger JW, Takane KK, Salim F, Fiaschi-Taesch N, Pappas K, et al. Augmented Stat5 Signaling Bypasses Multiple Impediments to Lactogen-Mediated Proliferation in Human Beta Cells. Diabetes. 2015;64:db150083–3797. doi: 10.2337/db15-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amaral MEC, Cunha DA, Anhê GF, Ueno M, Carneiro EM, Velloso LA, et al. Participation of prolactin receptors and phosphatidylinositol 3-kinase and MAP kinase pathways in the increase in pancreatic islet mass and sensitivity to glucose during pregnancy. J Endocrinol. 2004;183:469–476. doi: 10.1677/joe.1.05547. [DOI] [PubMed] [Google Scholar]
- 17.Dietrich MG, Zuellig RA, Spinas GA, Lehmann R, Tschopp O, Niessen M. Specific and redundant roles of PKBα/AKT1 and PKBβ/AKT2 in human pancreatic islets. Experimental Cell Research. 2015;338:82–88. doi: 10.1016/j.yexcr.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 18.George NM, Boerner BP, Mir SUR, Guinn Z, Sarvetnick NE. Exploiting Expression of Hippo Effector, Yap, for Expansion of Functional Islet Mass. Mol Endocrinol. 2015;29:1594–1607. doi: 10.1210/me.2014-1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lakshmipathi J, Alvarez-Perez JC, Rosselot C, Casinelli GP, Stamateris RE, Rausell-Palamos F, et al. PKC-ζ is essential for pancreatic beta cell replication during insulin resistance by regulating mTOR and cyclin-D2. Diabetes. 2016:db151398. doi: 10.2337/db15-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao X, Fischbach S, Song Z, Gaffar I, Zimmerman R, Wiersch J, et al. Transient suppression of transforming growth factor beta receptor signaling facilitates human islet transplantation. Endocrinology. 2016:en20151986. doi: 10.1210/en.2015-1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leibiger B, Moede T, Paschen M, Yunn NO, Lim JH, Ryu SH, et al. PI3K-C2α Knockdown Results in Rerouting of Insulin Signaling and Pancreatic Beta Cell Proliferation. Cell Reports. 2015;13:15–22. doi: 10.1016/j.celrep.2015.08.058. [DOI] [PubMed] [Google Scholar]
- 22.Fiaschi-Taesch NM, Kleinberger JW, Salim FG, Troxell R, Wills R, Tanwir M, et al. Human pancreatic β-cell G1/S molecule cell cycle atlas. Diabetes. 2013;62:2450–2459. doi: 10.2337/db12-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fiaschi-Taesch N, Bigatel TA, Sicari B, Takane KK, Salim F, Velazquez-Garcia S, et al. Survey of the human pancreatic beta-cell G1/S proteome reveals a potential therapeutic role for cdk-6 and cyclin D1 in enhancing human beta-cell replication and function in vivo. Diabetes. 2009;58:882–893. doi: 10.2337/db08-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fiaschi-Taesch NM, Salim F, Kleinberger J, Troxell R, Cozar-Castellano I, Selk K, et al. Induction of Human β-Cell Proliferation and Engraftment Using a Single G1/S Regulatory Molecule, cdk6. Diabetes. 2010;59:1926–1936. doi: 10.2337/db09-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Draney C, Hobson AE, Grover SG, Jack BO, Tessem JS. Cdk5r1 Overexpression Induces Primary β-Cell Proliferation. J Diabetes Res. 2016;2016:6375804–15. doi: 10.1155/2016/6375804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pal A, Potjer TP, Thomsen SK, Ng HJ, Barrett A, Scharfmann R, et al. Loss-of-Function Mutations in the Cell-Cycle Control Gene CDKN2A Impact on Glucose Homeostasis in Humans. Diabetes. 2016;65:527–533. doi: 10.2337/db15-0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tiwari S, Roel C, Wills R, Casinelli G, Tanwir M, Takane KK, et al. Early and Late G1/S Cyclins and Cdks Act Complementarily to Enhance Authentic Human β-Cell Proliferation and Expansion. Diabetes. 2015;64:3485–3498. doi: 10.2337/db14-1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karnik SK, Hughes CM, Gu X, Rozenblatt-Rosen O, McLean GW, Xiong Y, et al. Menin regulates pancreatic islet growth by promoting histone methylation and expression of genes encoding p27Kip1 and p18INK4c. Pnas. 2005;102:14659–14664. doi: 10.1073/pnas.0503484102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amisten S, Salehi A, Rorsman P, Jones PM, Persaud SJ. An atlas and functional analysis of G-protein coupled receptors in human islets of Langerhans. Pharmacol Ther. 2013;139:359–391. doi: 10.1016/j.pharmthera.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 30.Angueira AR, Ludvik AE, Reddy TE, Wicksteed B, Lowe WL, Layden BT. New insights into gestational glucose metabolism: lessons learned from 21st century approaches. Diabetes. 2015;64:327–334. doi: 10.2337/db14-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vasavada RC, Wang L, Fujinaka Y, Takane KK, Rosa TC, Mellado-Gil JMD, et al. Protein kinase C-zeta activation markedly enhances beta-cell proliferation: an essential role in growth factor mediated beta-cell mitogenesis. Diabetes. 2007;56:2732–2743. doi: 10.2337/db07-0461. [DOI] [PubMed] [Google Scholar]
- 32.Guthalu Kondegowda N, Joshi-Gokhale S, Harb G, Williams K, Zhang XY, Takane KK, et al. Parathyroid hormone-related protein enhances human β-cell proliferation and function with associated induction of cyclin-dependent kinase 2 and cyclin E expression. Diabetes. 2010;59:3131–3138. doi: 10.2337/db09-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arumugam R, Fleenor D, Freemark M. Knockdown of prolactin receptors in a pancreatic beta cell line: effects on DNA synthesis, apoptosis, and gene expression. Endocrine. 2013;46:568–576. doi: 10.1007/s12020-013-0073-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sorenson RL, Brelje TC, Roth C. Effects of steroid and lactogenic hormones on islets of Langerhans: a new hypothesis for the role of pregnancy steroids in the adaptation of islets... - PubMed - NCBI. Endocrinology. 1993;133:2227–2234. doi: 10.1210/endo.133.5.8404674. [DOI] [PubMed] [Google Scholar]
- 35.Hyslop CM, Tsai S, Shrivastava V, Santamaria P, Huang C. Prolactin as an Adjunct for Type 1 Diabetes Immunotherapy. Endocrinology. 2016;157:150–165. doi: 10.1210/en.2015-1549. [DOI] [PubMed] [Google Scholar]
- 36.Kim H, Toyofuku Y, Lynn FC, Chak E, Uchida T, Mizukami HI, et al. Serotonin Regulates Pancreatic β-Cell Mass during Pregnancy. Nature Medicine. 2010;16:804–808. doi: 10.1038/nm.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang G, Rajpurohit SK, Delaspre F, Walker SL. First quantitative high-throughput screen in zebrafish identifies novel pathways for increasing pancreatic β-cell mass. Elife. 2015 doi: 10.7554/eLife.08261.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y, Tanabe K, Baronnier D, Patel S, Woodgett J, Cras-Méneur C, et al. Conditional ablation of Gsk-3β in islet beta cells results in expanded mass and resistance to fat feeding-induced diabetes in mice. Diabetologia. 2010;53:2600–2610. doi: 10.1007/s00125-010-1882-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Porat S, Weinberg-Corem N, Tornovsky-Babaey S, Schyr-Ben-Haroush R, Hija A, Stolovich-Rain M, et al. Control of Pancreatic β-Cell Regeneration by Glucose Metabolism. Cell Metabolism. 2011;13:440–449. doi: 10.1016/j.cmet.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 40.Shen W, Taylor B, Jin Q, Nguyen-Tran V, Meeusen S, Zhang YQ, et al. Inhibition of DYRK1A and GSK3B induces human β-cell proliferation. Nat Commun. 2015;6:8372. doi: 10.1038/ncomms9372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng STW, Chen L, Li SYT, Mayoux E, Leung PS. The Effects of Empagliflozin, an SGLT2 Inhibitor, on Pancreatic β-Cell Mass and Glucose Homeostasis in Type 1 Diabetes. PLoS ONE. 2016;11:e0147391. doi: 10.1371/journal.pone.0147391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lorenzo PI, Fuente-Martín E, Brun T, Cobo-Vuilleumier N, Jimenez-Moreno CM, Herrera Gomez IG, et al. PAX4 Defines an Expandable β-Cell Subpopulation in the Adult Pancreatic Islet. Sci Rep. 2015;5:15672. doi: 10.1038/srep15672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brun T, Franklin I, St-Onge L, Biason-Lauber A, Schoenle EJ, Wollheim CB, et al. The diabetes-linked transcription factor PAX4 promotes {beta}-cell proliferation and survival in rat and human islets. J Cell Biol. 2004;167:1123–1135. doi: 10.1083/jcb.200405148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu J, Liu S, Chen Y, Zhao X, Lu Y, Cheng J. Functionalized self-assembling peptide improves INS-1 β-cell function and proliferation via the integrin/FAK/ERK/cyclin pathway. International Journal of Nanomedicine. 2015;10:3519–3531. doi: 10.2147/IJN.S80502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brelje TC, Bhagroo NV, Stout LE, Sorenson RL. Beneficial effects of lipids and prolactin on insulin secretion and beta-cell proliferation: a role for lipids in the adaptation of islets to pregnancy. J Endocrinol. 2008;197:265–276. doi: 10.1677/JOE-07-0657. [DOI] [PubMed] [Google Scholar]
- 46.Maedler K, Oberholzer J, Bucher P, Spinas GA, Donath MY. Monounsaturated fatty acids prevent the deleterious effects of palmitate and high glucose on human pancreatic beta-cell turnover and function. Diabetes. 2003;52:726–733. doi: 10.2337/diabetes.52.3.726. [DOI] [PubMed] [Google Scholar]
- 47.Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.He H, Xu J, Warren CM, Duan D, Li X, Wu L, et al. Endothelial cells provide an instructive niche for the differentiation and functional polarization of M2-like macrophages. Blood. 2012;120:3152–3162. doi: 10.1182/blood-2012-04-422758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cattin A-L, Burden JJ, Van Emmenis L, Mackenzie FE, Hoving JJA, Garcia Calavia N, et al. Macrophage-Induced Blood Vessels Guide Schwann Cell-Mediated Regeneration of Peripheral Nerves. Cell. 2015;162:1127–1139. doi: 10.1016/j.cell.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sindrilaru A, Peters T, Wieschalka S, Baican C, Baican A, Peter H, et al. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. The Journal of Clinical Investigation. 2011;121:985–997. doi: 10.1172/JCI44490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Calderon B, Carrero JA, Ferris ST, Sojka DK, Moore L, Epelman S, et al. The pancreas anatomy conditions the origin and properties of resident macrophages. J Exp Med. 2015;212:1497–1512. doi: 10.1084/jem.20150496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Gassen N, Van Overmeire E, Leuckx G, Heremans Y, De Groef S, Cai Y, et al. Macrophage dynamics are regulated by local macrophage proliferation and monocyte recruitment in injured pancreas. Eur J Immunol. 2015;45:1482–1493. doi: 10.1002/eji.201445013. [DOI] [PubMed] [Google Scholar]
- 54.Cucak H, Grunnet LG, Rosendahl A. Accumulation of M1-like macrophages in type 2 diabetic islets is followed by a systemic shift in macrophage polarization. J Leukoc Biol. 2014;95:149–160. doi: 10.1189/jlb.0213075. [DOI] [PubMed] [Google Scholar]
- 55.Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, et al. Tissue-Resident Macrophage Enhancer Landscapes Are Shaped by the Local Microenvironment. Cell. 2014;159:1312–1326. doi: 10.1016/j.cell.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Criscimanna A, Coudriet GM, Gittes GK, Piganelli JD, Esni F. Activated macrophages create lineage-specific microenvironments for pancreatic acinar- and β-cell regeneration in mice. Gastroenterology. 2014;147:1106–18.e11. doi: 10.1053/j.gastro.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 57.Pound LD, Patrick C, Eberhard CE, Mottawea W, Wang GS, Abujamel T, et al. Cathelicidin Antimicrobial Peptide: A Novel Regulator of Islet Function, Islet Regeneration, and Selected Gut Bacteria. Diabetes. 2015;64:4135–4147. doi: 10.2337/db15-0788. [DOI] [PubMed] [Google Scholar]
- 58.Lee TS, Chau LY. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nature Medicine. 2002;8:240–246. doi: 10.1038/nm0302-240. [DOI] [PubMed] [Google Scholar]
- 59.Nakamichi I, Habtezion A, Zhong B, Contag CH, Butcher EC, Omary MB. Hemin-activated macrophages home to the pancreas and protect from acute pancreatitis via heme oxygenase-1 induction. J Clin Invest. 2005;115:3007–3014. doi: 10.1172/JCI24912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ndisang JF, Mishra M. The heme oxygenase system selectively suppresses the proinflammatory macrophage m1 phenotype and potentiates insulin signaling in spontaneously hypertensive rats. Am J Hypertens. 2013;26:1123–1131. doi: 10.1093/ajh/hpt082. [DOI] [PubMed] [Google Scholar]
- 61.Husseini M, Wang GS, Patrick C, Crookshank JA, MacFarlane AJ, Noel JA, et al. Heme oxygenase-1 induction prevents autoimmune diabetes in association with pancreatic recruitment of M2-like macrophages, mesenchymal cells, and fibrocytes. Endocrinology. 2015;156:en20151304–3949. doi: 10.1210/en.2015-1304. [DOI] [PubMed] [Google Scholar]
- 62.Nikolic I, Saksida T, Vujicic M, Stojanovic I, Stosic-Grujicic S. Anti-diabetic actions of carbon monoxide-releasing molecule (CORM)-A1: Immunomodulation and regeneration of islet beta cells. Immunology Letters. 2015;165:39–46. doi: 10.1016/j.imlet.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 63.Saavedra-Ávila NA, Sengupta U, Sánchez B, Sala E, Haba L, Stratmann T, et al. Cyclin D3 promotes pancreatic β-cell fitness and viability in a cell cycle-independent manner and is targeted in autoimmune diabetes. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E3405–14. doi: 10.1073/pnas.1323236111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ding Y, Xu Y, Shuai X, Shi X, Chen X, Huang W, et al. Reg3α Overexpression Protects Pancreatic Beta-Cells From Cytokine-Induced Damage and Improves Islet Transplant Outcome. Mol Med. 2014 doi: 10.2119/molmed.2014.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Diaz-Ganete A, Baena-Nieto G, Lomas-Romero IM, Lopez-Acosta JF, Cozar-Castellano I, Medina F, et al. Ghrelin’s Effects on Proinflammatory Cytokine Mediated Apoptosis and Their Impact on β-Cell Functionality. International Journal of Endocrinology. 2015;2015:1–11. doi: 10.1155/2015/235727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Song I, Patel O, Himpe E, Muller CJF, Bouwens L. Beta Cell Mass Restoration in Alloxan-Diabetic Mice Treated with EGF and Gastrin. PLoS ONE. 2015;10:e0140148. doi: 10.1371/journal.pone.0140148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brissova M, Aamodt K, Brahmachary P, Prasad N, Hong JY, Dai C, et al. Islet microenvironment, modulated by vascular endothelial growth factor-A signaling, promotes β cell regeneration. Cell Metabolism. 2014;19:498–511. doi: 10.1016/j.cmet.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xiao X, Gaffar I, Guo P, Wiersch J, Fischbach S, Peirish L, et al. M2 macrophages promote beta-cell proliferation by up-regulation of SMAD7. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E1211–20. doi: 10.1073/pnas.1321347111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ino K, Masuya M, Tawara I, Miyata E, Oda K, Nakamori Y, et al. Monocytes infiltrate the pancreas via the MCP-1/CCR2 pathway and differentiate into stellate cells. PLoS ONE. 2014;9:e84889. doi: 10.1371/journal.pone.0084889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bayan JA, Peng Z, Zeng N, He L, Chen J, Stiles BL. Crosstalk Between Activated Myofibroblasts and β Cells in Injured Mouse Pancreas. Pancreas. 2015;44:1111–1120. doi: 10.1097/MPA.0000000000000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen P, Cescon M, Zuccolotto G, Nobbio L, Colombelli C, Filaferro M, et al. Collagen VI regulates peripheral nerve regeneration by modulating macrophage recruitment and polarization. Acta Neuropathol. 2015;129:97–113. doi: 10.1007/s00401-014-1369-9. [DOI] [PubMed] [Google Scholar]
- 72.Cao X, Han ZB, Zhao H, Liu Q. Transplantation of mesenchymal stem cells recruits trophic macrophages to induce pancreatic beta cell regeneration in diabetic mice. The International Journal of Biochemistry & Cell Biology. 2014;53:372–379. doi: 10.1016/j.biocel.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 73.Liou GY, Döppler H, Necela B, Krishna M, Crawford HC, Raimondo M, et al. Macrophage-secreted cytokines drive pancreatic acinar-to-ductal metaplasia through NF-κB and MMPs. J Cell Biol. 2013;202:563–577. doi: 10.1083/jcb.201301001. [DOI] [PMC free article] [PubMed] [Google Scholar]