Abstract
Background
Naturally occurring acute spinal cord injury (SCI) in pet dogs provides an important clinical animal model through which to confirm and extend findings from rodent studies; however, validated quantitative outcome measures for dogs are limited.
New method
We adapted the Basso Beattie Bresnahan (BBB) scale for use in a clinical dog model of acute thoracolumbar SCI. Based on observation of normal dogs, modifications were made to account for species differences in locomotion. Assessments of paw and tail position, and trunk stability were modified to produce a 19 point scale suitable for use in dogs, termed the canine BBB scale (cBBB). Pet dogs with naturally occurring acute SCI were assigned cBBB scores at 3, 10 and 30 days after laminectomy.
Results
Scores assigned via the cBBB were stable across testing sessions in normal dogs but increased significantly between days 3 and 30 in SCI-affected dogs (p = 0.0003). The scale was highly responsive to changes in locomotor recovery over a 30 day period, with a standardized response mean of 1.34.
Comparison with existing methods
Concurrent validity was good, with strong correlations observed between the cBBB and two other locomotor scales, the OSCIS (r = 0.94; p < 0.001) and the MFS (r = 0.85; p < 0.0001). cBBB scores inversely correlated with other assessments of recovery including mechanical sensory threshold (r = −0.68; p < 0.0001) and coefficient of variation of stride length (r = −0.49; p < 0.0001).
Conclusions
These results support the use of the cBBB to assess locomotor recovery in canine clinical translational models of SCI.
Keywords: Canine, Translational model, Clinical spinal cord injury, Intervertebral disc extrusion
1. Introduction
Naturally occurring acute spinal cord injury (SCI) in pet dogs has gained recent attention as a large animal clinical and translational model through which to confirm and extend findings of rodent studies of SCI interventions (Olby et al., 2004; Jeffery et al., 2005; Granger et al., 2012; Lim et al., 2014; McMahill et al., 2015; Tamura et al., 2015). Intervertebral disc extrusion (IVDE) is a common cause of SCI in pet dogs and is common in breeds such as the dachshund, beagle, and shih tzu (Bergknut et al., 2012). Severity of neurologic injury spans a spectrum up to and including sensorimotor complete injury, with predictable patterns of recovery across different severities (Olby et al., 2003, 2004; Bergknut et al., 2012). Primary and secondary mechanisms of SCI in dogs are consistent with those observed in humans, and include central gray matter hemorrhage, necrosis, cavitation, sparing of subpial axons, axonal degeneration, and variable degrees of demyelination (Smith and Jeffery, 2006; Moore and Oglesbee, 2014; Henke et al., 2014, 2015). Accordingly, clinical dog models can serve as a “bridge” between rodent models of SCI and human patients because they address many translational issues. Unlike rodents, which are anesthetized prior to laboratory-induced SCI, pet dogs sustain naturally occurring injuries thus avoiding potential confounding anesthetic factors that may occur with laboratory injury. Dogs also offer heterogeneity in lesion and patient-related factors that closely approximate the human condition, allowing investigators to quickly and economically conduct large-scale veterinary clinical trials to rigorously test an intervention before they introduce it to humans (Jeffery et al., 2011). While several well-publicized canine treatment trials have been recently completed, quantitative outcome measures to assess recovery in the canine model are currently limited (Granger et al., 2012; Levine et al., 2014). Quantitative locomotor scales are one of the most widely used behavioral outcome measures across species. Although a handful of canine locomotor scales currently exist, they have large ceiling effects, are ambiguous with respect to operational definitions, or rely on subjective assessments (Olby et al., 2003; Levine et al., 2009; Chung-Sheng et al., 2015). These characteristics introduce variability, limit translation between labs/clinics, and impede comparison of locomotor outcomes across studies.
The Basso–Beattie–Bresnahan (BBB) locomotor rating scale is a 21-point scale originally developed for use in rat models of thoracolumbar SCI (Basso et al., 1995). The BBB discriminates phases of recovery based on injury severity and/or time after injury, two important features of clinical recovery. It has high sensitivity, good test–retest and intra-rater reliability and strong validity (Basso et al., 1995, 1996a,b). It is the only locomotor scale that correlates with histopathologic changes observed after SCI (Basso et al., 1996a; Olby et al. 2001). It has been modified for use in several other species including mice (Basso et al., 2006), opossums (Wang et al., 1998) and cats (Basso, unpublished data). Additionally, highly specific operational definitions within this tool improve ease of administration and fidelity across settings by reducing subjectivity of scoring. This scale has not been validated for use in dogs, but surprisingly it has been used without modification in several canine SCI studies (Jeffery et al., 2005; Fukuda et al., 2005).
The goals of the current study were to assess the utility of the BBB locomotor rating scale for use in dogs and to evaluate the scale’s ability to quantify locomotor recovery in dogs with spontaneously occurring acute thoracolumbar SCI. Only dogs with incomplete injuries were evaluated in order to provide a rigorous test of scale sensitivity in a clinical population of dogs expected to experience substantial neurologic recovery. We hypothesized that differences in normal locomotor patterns between rats and dogs would prevent the use of the BBB scale in its original published form, necessitating modification. Additionally, we hypothesized that a modified version of the BBB scale would correlate with other published locomotor scales for canine SCI, demonstrating concurrent validity. We also hypothesized that the scale would be highly responsive to delineate locomotor recovery over a 30 day period after injury and that scores would progressively increase over the recovery period.
2. Materials and methods
2.1. Animals
This study was reviewed and approved by The Ohio State University (OSU) Institutional Animal Care and Use Committee (2012A00000149), and the Clinical Research Advisory Committee at the OSU Veterinary Medical Center (OSUVMC). Written owner consent was obtained prior to enrollment of all dogs. Healthy, client-owned neurologically normal pet dogs (n = 20) as well as client-owned pet dogs with spontaneously occurring acute thoracolumbar SCI caused by IVDE (n = 30) were enrolled. All dogs weighed <20 kg. For SCI-affected dogs, degree of neurologic injury was variable and ranged from mild weakness with maintenance of ambulation to paraplegia with preserved sensory function.
2.2. Spinal cord injury model
All SCI-affected dogs had naturally occurring acute IVDE causing clinical signs of incomplete SCI referable to the regions between the third thoracic and third lumbar spinal cord segments. Injury location was confirmed via computed tomography or magnetic resonance imaging, and surgical spinal decompression (laminectomy) was performed in all cases as is standard of care for canine IVDE. Post-operative pain management was provided in all cases, and specific drug protocols were at the discretion of the veterinary clinician managing each case.
2.3. Locomotor scoring-normal dogs
Normal dogs were placed in a 10-ft diameter open field and allowed to ambulate freely for 4 min. Dogs reluctant to move about the open field were encouraged with verbal cues and treats. To determine how canine locomotion differed from rodents, two raters (SAM and RBS) initially used the published 21-point BBB scale with all operational definitions applied as previously described (Basso et al., 1995). One investigator (SAM) had been trained to proficiency in BBB assessment of rat SCI by experts (LCF, DMB) as part of the NIH SCI Center of Excellence training program at Ohio State. Each normal dog was assigned a BBB locomotor score for both the left and right hind limb on three occasions at least 24 h apart. Locomotor patterns were observed and the original published operational definitions of stepping, coordination, paw position, trunk stability, and tail position were carefully applied to each normal dog (Basso et al., 1995). Observations in normal dogs indicated that modifications to the scale were necessary, and a canine BBB (cBBB) locomotor rating scale was developed.
2.4. Locomotor scoring-SCI-affected dogs
An identical protocol was used by the same raters to assign cBBB scores to SCI-affected dogs at 3, 10 and 30 days following surgical decompression. To assess concurrent validity, all SCI-affected dogs at each time point were also simultaneously assigned locomotor scores using two previously validated canine scales at each time point: the Olby spinal cord injury scale (OSCIS) (Olby et al., 2001) and the modified Frankel scale (MFS) (Levine et al., 2014). Both raters (SAM and RBS) have extensive experience applying these scoring systems in a veterinary clinical setting. Additionally, one investigator (SAM) was trained by the developer of the OSCIS scoring system.
2.5. Sensory testing
Mechanical sensory threshold values were determined for all SCI-affected dogs at each time point using an electronic von Frey anesthesiometer (IITC), as previously described by our group (Moore et al., 2013). Briefly, dogs were positioned in lateral recumbency, maintained using minimal restraint, and were prevented from visualizing the device during application to assure that behavioral responses were due to tactile stimulation. Testing order of the limbs was randomly determined. For testing of the hind limbs, the electronic von Frey probe was applied perpendicular to the dorsal surface of the metatarsus, halfway between the tarsometatarsal and metatarsophalangeal joints between digits IV and V and corresponding to the cutaneous autonomous zone of the fibular branch of the sciatic nerve. Increasing pressure was applied until the dog demonstrated a conscious behavioral response to the stimulus such as lip licking, turning to look at the investigator, vocalization, or attempted escape. The evaluator (RBS) was blinded to the pressure reading obtained during testing. The minimum pressure required to elicit a conscious behavioral response was recorded as the sensory threshold. The test was repeated five times per limb, with each test separated by 1 min. The highest and lowest sensory threshold value for each limb was excluded and the middle three values were averaged to assign a single sensory threshold for each hind limb. Detailed results of sensory thresholds obtained from the group of SCI-affected dogs described here has been previously reported elsewhere (Song et al., 2015).
2.6. Walking track analysis
Stride length (SL) and coefficient of variation in stride length (COV SL) of the hind limbs was determined for all SCI-affected dogs that were able to walk without assistance at each time point. A simplified method of walking track analysis was used to obtain this measurement, and a detailed account of these methods and results are described elsewhere (Song et al., 2016).
2.7. Statistical analysis
The association between hind limb cBBB scores and the following other outcome assessment tools were tested by Spearman correlation: OSCIS and MFS scores, sensory threshold, SL, and COV SL. Difference in cBBB scores between normal and SCI-affected dogs, and change in cBBB scores for SCI-affected dogs between days 3 and 30 were evaluated using the Wilcoxon signed-rank test. Internal responsiveness of the cBBB scale in dogs was assessed by calculating the standard response mean (SRM) between days 3 and 30 using the following formula: mean change divided by standard deviation of change (Bekkers et al., 2009). Subjective qualifiers of internal responsiveness based on effect size were applied using the following definitions: mild (<0.50), moderate (0.50–0.80) or high (>0.80) (Bekkers et al., 2009). P < 0.05 was considered significant for all statistical comparisons.
3. Results
3.1. BBB locomotor scale requires modification for use in dogs
Twenty normal dogs were each evaluated on three separate occasions. Ages ranged from 8 months to 6.5 years (median 3 years) and weight ranged from 3.7 to 17.2 kg (median 9.4 kg). There were eight spayed females and 12 castrated males. Breeds included mixed breed dog (n = 6), dachshund (n = 4), miniature schnauzer (n = 2), Sealyham terrier (n = 2), and one each of the following: beagle, bichon frise, Cocker spaniel, Pembroke Welsh corgi, miniature pinscher, and shih tzu. Median time between testing sessions for normal dogs was 6 days (range 2–27 days).
Through observational studies, we identified three gait parameters described for BBB in rats that were not applicable to dogs, even by modifying operational definitions and observational focus. Normally, rats locomote with the paw in a parallel position and internal or external rotation is only observed after SCI. However, in neurologically normal dogs, both internal rotation and parallel positioning of the paws were routinely observed. External rotation of the paw was not observed in any normal dog. Some normal dogs also exhibited a mild symmetrical sway of the trunk, consistent with what would be scored as trunk instability in a rat. Tail position was also highly variable related to behavior, breed-associated, and conformational factors. Based on these observations, the BBB scoring parameters were adapted to result in a modified 19-point cBBB appropriate for use in dogs (Tables 1 and 2). When assessed via the cBBB, all normal dogs scored a 19/19 for both right and left hind limbs. This modified scale was prospectively applied to the SCI-affected dogs.
Table 1.
The canine locomotor rating scale (cBBB) developed for use in dogs with thoracolumbar spinal cord injury. Notable modifications from the rat scale include acceptance of internal paw rotation as normal, removal of tail assessment, and allowance for mild symmetrical truncal sway. Scores are assigned during a 4 min open field assessment of the dog, conducted in a 10 ft diameter space. Two observers are positioned directly across the open field from each other and verbally communicate observations related to score over the course of the 4 min time period. At the end of four minutes, a score is assigned to each hind limb by consensus of both reviewers based on their collective observations.
| Score | Description |
|---|---|
| 0 | No observable hind limb (HL) movement |
| 1 | Slight movement of one or two joints |
| 2 | Extensive movement of one joint, or extensive movement of one joint and slight movement of one other joint |
| 3 | Extensive movement of two joints |
| 4 | Slight movement of all three joints of the HL |
| 5 | Slight movement of two joints and extensive movement of the third |
| 6 | Extensive movement of two joints and slight movement of the third |
| 7 | Extensive movement of all three joints in the HL |
| 8 | Plantar placement of the paw with no weight support |
| 9 | Plantar placement of the paw with weight support only when stationary, or occasional, frequent or consistent weight-supported dorsal stepping and no plantar stepping |
| 10 | Occasional weight-supported plantar steps; no FL–HL coordination |
| 11 | Frequent to consistent weight-supported plantar steps and no FL–HL coordination |
| 12 | Frequent to consistent weight-supported plantar steps and occasional FL–HL coordination |
| 13 | Frequent to consistent weight-supported plantar steps and frequent FL–HL coordination |
| 14 | Consistent weight-supported plantar steps, consistent FL–HL coordination, and predominant paw position is externally rotated when it makes initial contact as well as just before it is lifted off; or frequent plantar stepping, consistent FL–HL coordination, and occasional dorsal stepping |
| 15 | Consistent plantar stepping and consistent FL–HL coordination and no toe clearance or occasional toe clearance; predominant paw position is parallel to the body or internally rotated at initial contact |
| 16 | Consistent plantar stepping and consistent FL–HL coordination and toe clearance occurs frequently; predominant paw position is parallel or internally rotated at initial contact and externally rotated at liftoff |
| 17 | Consistent plantar stepping and consistent FL–HL coordination and toe clearance occurs frequently; predominant paw position is parallel or internal at initial contact and at liftoff |
| 18 | Consistent plantar stepping and consistent FL–HL coordination and toe clearance occurs consistently; predominant paw position is parallel or internal at initial contact and at liftoff. Trunk instability is present |
| 19 | Consistent plantar stepping and consistent FL–HL coordination and toe clearance occurs consistently during forward limb advancement; predominant paw position is parallel or internal at initial contact and at liftoff. Trunk instability is not observed |
FL = forelimb; HL = hindlimb.
Table 2.
Operational definitions applied for locomotor scoring using the canine BBB scale.
| Term | Definition |
|---|---|
| Joint movement | Active flexion of the joint. Assessed separately for three joints (hip, stifle, hock) in each HL. Movement is scored if it occurs one or more times during testing |
| Slight movement | Movement of a joint through less than or equal to 50% of its normal range of motion |
| Extensive movement | Movement of a joint through more than 50% of its normal range of motion |
| Plantar placement | The paw is actively placed with the plantar surface resting on the ground |
| Weight support | Paw is plantar placed and muscle contraction of the limb causes HL extension and elevation of the hindquarter off the ground |
| Stepping | Weight support is established, the limb is advanced in the forward direction, and weight support is re-established when the paw contacts the ground. Assessed separately for each HL |
| Plantar stepping | A step is taken with the paw in plantar placement at both lift off and initial contact |
| Dorsal stepping | Weight is supported through the dorsal surface of the paw at any point during the step cycle |
| Occasional stepping | Stepping occurs less than half the time the animal is moving forward |
| Frequent stepping | Stepping occurs more than half the time but less than 95% of the time the animal is moving forward |
| Consistent stepping | Stepping occurs 95–100% of the time the animal is moving forward and fewer than 5 dorsal steps are observed |
| Forelimb–hindlimb (FL–HL) coordination | For every FL step taken, a HL step is also taken and the hind limbs alternate in stepping. This parameter is assessed during forward passes |
| Forward pass | The animal ambulates in a forward trajectory for a distance equal or greater than 3× its body length |
| Occasional FL–HL coordination | FL–HL coordination is observed at least once but occurs less than or equal to 50% of the instances the animal performs a forward pass |
| Frequent FL–HL coordination | FL–HL coordination is observed more than half the instances the animal performs forward passes, but at least one pass was observed to be uncoordinated |
| Consistent FL–HL coordination | All observed forward passes displayed FL–HL coordination |
| Paw position | Evaluated at lift off and initial contact for each HL during weight supported plantar stepping |
| External rotation | The paw is rotated externally for the majority of steps |
| Internal rotation | The paw is rotated internally for the majority of steps |
| Parallel | The paw is parallel to the body for the majority of steps |
| Toe clearance | The toe does not drag or scuff against the floor during forward limb advancement. Assessed separately for each HL by listening for scratching or brushing sounds as the animal walks about the open field |
| Occasional toe clearance | Toe clearance is achieved occasionally during the open field test, but toe drags are heard for most of the steps |
| Frequent toe clearance | Toe clearance occurs for more than half of the steps but more than 4 toe drags are recorded during a session |
| Consistent toe clearance | ≤4 HL toe drags are heard during the duration of open field testing |
| Trunk instability | Lateral weight shifts causing an asymmetrical excursion of the trunk to one side, or excursion of the trunk to both sides in a range of motion greater than one trunk’ s width, partial collapse of the trunk on one side. Trunk instability is scored if the animal displays this behavior one or more times during a testing session |
Adapted from Basso et al. (1995).
3.2. The cBBB can be applied across a spectrum of injury severities
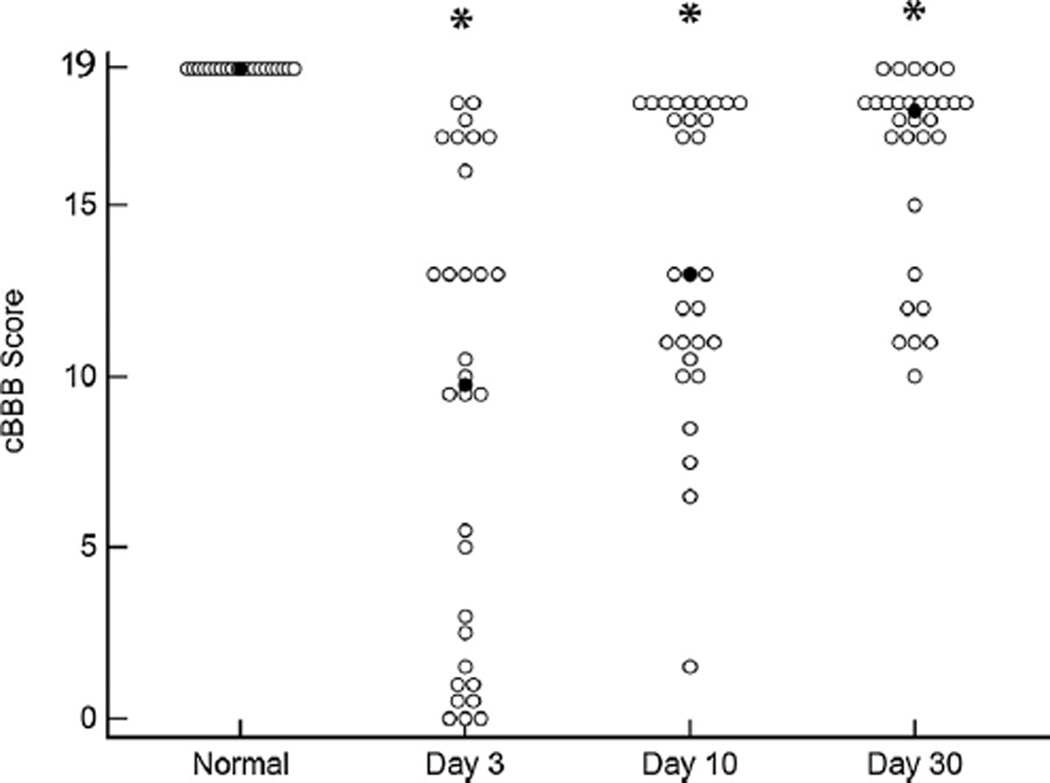
A total of 30 cases of acute clinical SCI caused by IVDE were observed using the locomotor scoring method described above. Dogs ranged in age from 2 to 11 years (median 5 years) and weighed between 3.9–17.0 kg (median 8.2 kg). There were 14 spayed females, 13 castrated males, and 2 intact males. Breeds were as follows: dachshunds (n = 12), mixed breed dog (n = 6), French bulldog (n = 4), beagle (n = 2), Pembroke Welsh corgi (n = 2), shih tzu (n = 2), and Cocker spaniel (n = 1). Each dog was assigned a cBBB score at days 3, 10, and 30 after laminectomy. Scores assigned to both left and right hind limb of each individual dog indicated that lesions were often symmetrical in nature; however, several dogs showed appreciable lateralization of neurologic deficits as measured by cBBB (Table 3). To facilitate statistical comparisons between locomotor scales, cBBB scores for the left and right hind limb were averaged to produce a combined single score at each time point. Median combined cBBB scores for the group were 9.5 at day 3 (range 0–18), 13 at day 10 (range 1.5–18), and 17.5 at day 30 (range 10–19). Scores for SCI-affected dogs differed from normal dogs at all three time points (p < 0.0001) (Fig. 1).
Table 3.
Individual cBBB scores for left and right hind limbs of dogs with spinal cord injury at days 3, 10, and 30 after surgery. In most cases, lesions were symmetrical; however, some dogs showed appreciable lateralization of neurologic deficits as measured by cBBB.
| Dog | Day 3 | Day 10 | Day 30 | |||
|---|---|---|---|---|---|---|
| LHL | RHL | LHL | RHL | LHL | RHL | |
| 1 | 17 | 17 | 18 | 18 | 19 | 19 |
| 2 | 19 | 16 | 19 | 17 | 19 | 19 |
| 3 | 18 | 18 | 19 | 17 | 19 | 19 |
| 4 | 1 | 1 | 7 | 8 | 10 | 10 |
| 5 | 10 | 11 | 17 | 18 | 17 | 18 |
| 6 | 13 | 13 | 18 | 18 | 18 | 18 |
| 7 | 5 | 0 | 12 | 12 | 18 | 17 |
| 8 | 10 | 9 | 11 | 11 | 13 | 13 |
| 9 | 10 | 9 | 13 | 13 | 19 | 17 |
| 10 | 0 | 0 | 4 | 9 | 11 | 11 |
| 11 | 1 | 10 | 18 | 17 | 17 | 17 |
| 12 | 17 | 17 | 18 | 18 | 18 | 18 |
| 13 | 13 | 13 | 17 | 17 | 19 | 19 |
| 14 | 5 | 1 | 10 | 10 | 12 | 12 |
| 15 | 0 | 0 | 11 | 11 | 19 | 17 |
| 16 | 17 | 17 | 17 | 17 | 17 | 17 |
| 17 | 14 | 18 | 17 | 19 | 19 | 19 |
| 18 | 0 | 0 | 10 | 11 | 18 | 18 |
| 19 | 9 | 10 | 12 | 12 | 17 | 19 |
| 20 | 1 | 1 | 11 | 11 | 18 | 17 |
| 21 | 2 | 8 | 10 | 10 | 18 | 17 |
| 22 | 1 | 0 | 2 | 1 | 11 | 11 |
| 23 | 17 | 17 | 18 | 18 | 18 | 18 |
| 24 | 1 | 0 | 11 | 11 | 13 | 13 |
| 25 | 1 | 2 | 8 | 9 | 11 | 11 |
| 26 | 13 | 13 | 18 | 18 | 18 | 18 |
| 27 | 13 | 13 | 13 | 13 | 17 | 17 |
| 28 | 19 | 17 | 18 | 18 | 19 | 17 |
| 29 | 10 | 10 | 13 | 13 | 15 | 15 |
| 30 | 13 | 13 | 18 | 17 | 17 | 17 |
| Median | 10 | 10 | 13 | 13 | 18 | 17 |
| Range | 0–19 | 0–18 | 2–19 | 1–19 | 10–19 | 10–19 |
LHL = left hind limb; RHL = right hind limb.
Fig. 1.
The cBBB can be applied across a spectrum of injury severities. Median (closed circle) and range of cBBB scores in normal dogs (n = 20) and dogs with clinical SCI (n = 30) at 3, 10, and 30 days after injury. Scores differ between normal and SCI-affected dogs at all three time points after injury. * denotes statistical difference from normal dogs, p < 0.0001.
3.3. Modifications of cBBB show preserved psychometric properties of original scale
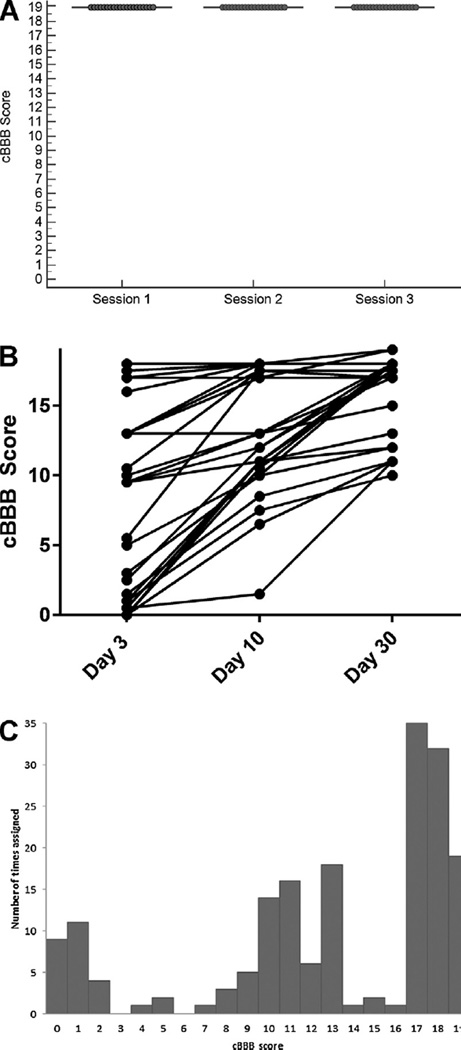
cBBB scores assigned to normal dogs were stable across three testing sessions with all dogs scoring 19 at each session (Fig. 2A). Scores for SCI-affected dogs showed an upward trajectory over time for each individual between sessions (Fig. 2B). Frequency with which each cBBB score was assigned to any limb of an SCI-affected dog at any time-point was visually evaluated, and scores were subjectively well distributed across the scale (Fig. 2C). The most common scores assigned to any limb were 17 (assigned 35 different times) and 18 (assigned 32 different times). Scores of 3 or 6 were not assigned to any limb at any testing session.
Fig. 2.
cBBB preserves psychometric properties of the original scale. cBBB scores for normal dogs are stable across three testing sessions (A). Scores for individual SCI-affected dogs show an upward trajectory in every case, consistent with expected improvement in locomotor function (B). Frequency of scores assigned to any limb at any time point in SCI-affected dogs are well-distributed across the scale over the 30 day recovery period (C).
3.4. cBBB detects locomotor recovery over time
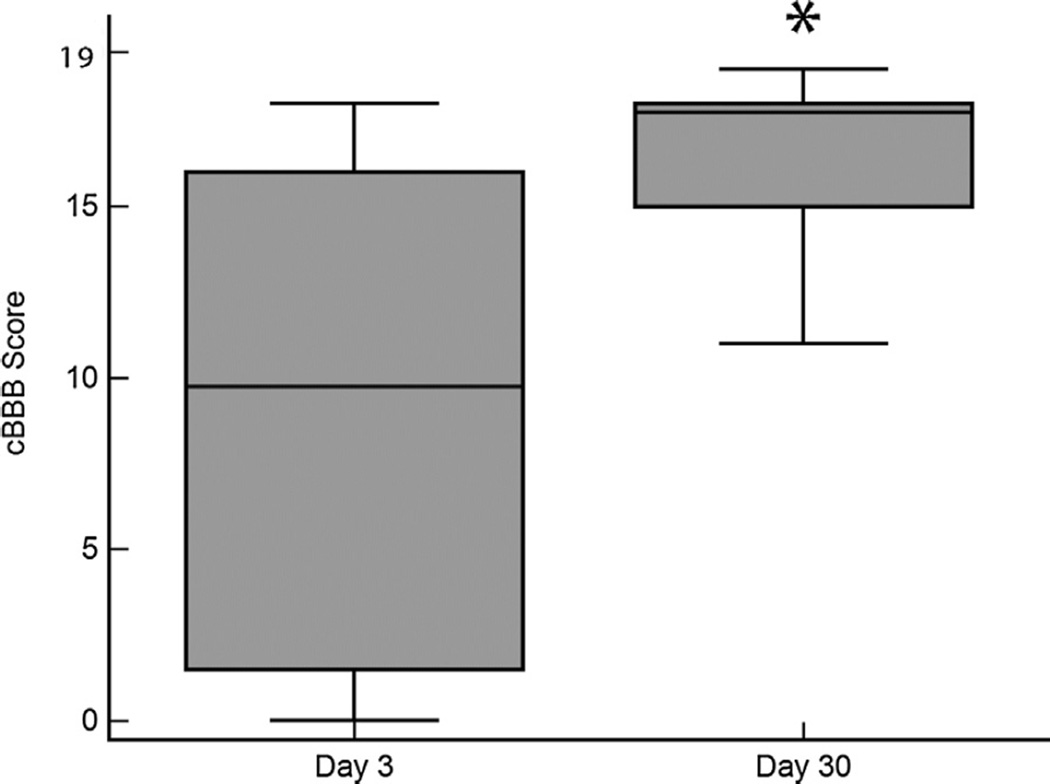
Scores for SCI-affected dogs increased with time, and scores on day 30 were significantly higher than scores on day 3, consistent with improved locomotor status (p = 0.0003) (Fig. 3). Responsiveness of the cBBB to detect locomotor improvement between days 3 and 30 was found to be high, with a calculated SRM of 1.34.
Fig. 3.
cBBB scores improve over time in SCI-affected dogs. Box plots for cBBB scores assigned at day 3 and day 30 after injury. cBBB scores are significantly higher on day 30 compared to day 3, consistent with expected improvement in locomotor status. *p = 0.0003.
3.5. cBBB shows concurrent validity with other canine locomotor scales
The median OSCIS for all SCI-affected dogs at days 3, 10 and 30 were 6.5 (range 1–11), 10 (range 4–13) and 11 (range 7–14), respectively. The median MFS score for SCI-affected dogs at days 3, 10 and 30 post-operatively were 3 (range 2–4), 4 (range 3–5), and 4 (range 4–5) respectively. A strong positive correlation was observed between cBBB scores and OSCIS scores (r = 0.94; p < 0.001) assigned to each dog across the three time points. A similar relationship was noted between cBBB and MFS scores (r = 0.85; p < 0.0001).
3.6. cBBB correlates with independent outcome assessment tools
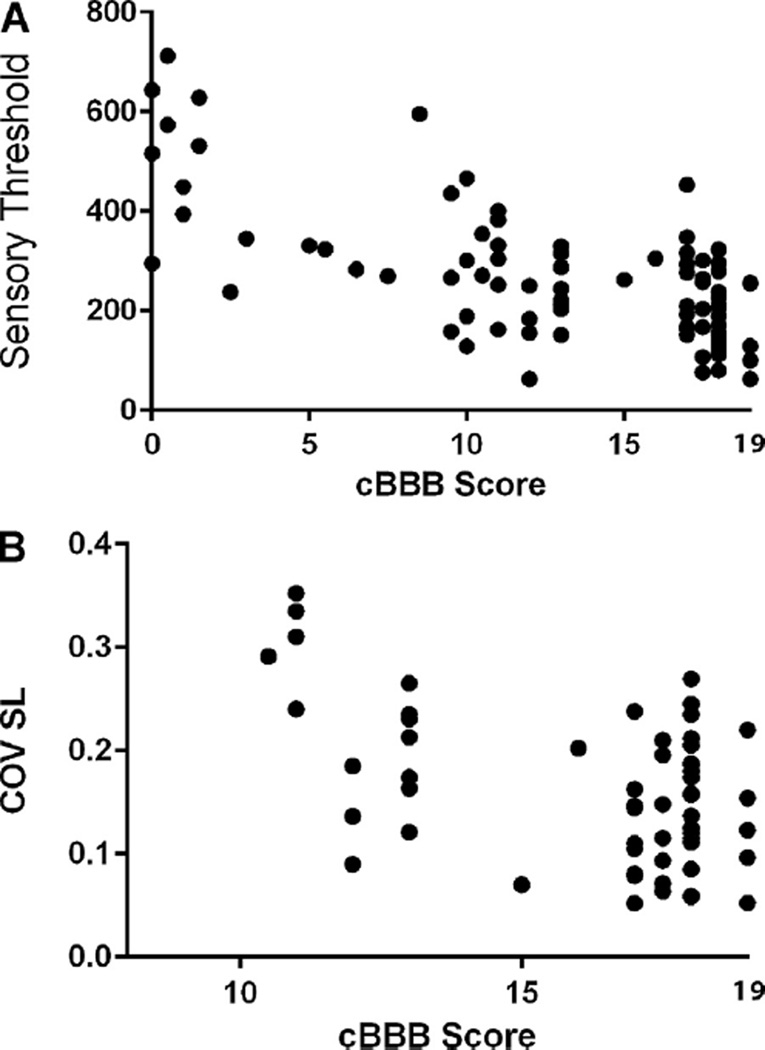
Electronic von Frey anesthesiometry was used to determine sensory values for the hind limbs of SCI-affected dogs at days 3, 10 and 30. Values obtained from the right and left hind limb were averaged to obtain a combined sensory threshold value for the hind limbs of each dog. A moderate inverse correlation was observed between hind limb sensory threshold and cBBB scores (r = −0.68; p <0.0001), indicating that as sensory threshold normalized, cBBB scores also improved (Fig. 4A). This relationship was similar when sensory threshold and cBBB scores were considered for each hind limb individually: left (r = −0.66; p < 0.0001) and right (r = −0.62; p < 0.0001). Similar inverse correlations were noted between sensory threshold and locomotor scores for MFS (r = −0.59; p < 0.0001) and OSCIS (r = −0.66; p < 0.0001).
Fig. 4.
cBBB scores correlate with other independent measures of recovery after SCI. Sensory threshold, as measured by an electronic von Frey anesthesiometer, is inversely correlated with cBBB score in SCI-affected dogs (r = −0.68; p < 0.0001), indicating that as acute hypoalgesia declines toward normal, locomotor status as measured by the cBBB also improves (A). For dogs who are able to ambulate unassisted (cBBB score ≥ 11), coefficient of variance of hind limb stride length (COV SL) is also inversely correlated with cBBB scores across a 30 day recovery period (r = −0.49; p ≤ 0.0001), such that SL becomes more consistent as locomotor score improves (B).
Stride length (cm) and COV SL of the hind limbs were measured for each SCI-affected dog that was ambulatory without assistance at days 3 (n = 13), 10 (n = 21) and 30 (n = 28). Hind limb SL was not correlated with cBBB score (r = 0.053; p = 0.68). A mild inverse correlation was observed between COV SL of the hind limbs and cBBB score (r = −0.49; p <0.0001) for dogs that were ambulatory without assistance at each time point, such that SL became more consistent as cBBB scores improved (Fig. 4B). This relationship was similar when each limb was evaluated individually: left hind limb (r = −0.49; p < 0.0001), right hind limb (r = −0.38; p = 0.002). The relationship between OSCIS and COV SL was weaker (r = −0.29; p = 0.04). A significant correlation was not observed between COV SL and MFS score (r = 0.03; p = 0.78). Because neither OSCIS nor MFS considers each hind limb individually, comparison between these scores and COV SL from individual limbs could not be performed.
4. Discussion
Our study is the first to assess the utility of the BBB locomotor rating scale to quantify recovery in dogs after SCI, despite the fact that this scale has been previously applied without modification to this species in pre-clinical studies. Careful observation of locomotor patterns in normal dogs necessitated several important modifications to the BBB in order to produce a scale appropriate for use in dogs with clinical SCI. We have termed this modified scale the cBBB. Awareness of differences in normal locomotive patterns among quadrupeds is crucial in creating an appropriate locomotor scale specific to each species. Specifically noted here, several parameters considered abnormal for rodent ambulation were observed frequently in normal dogs. These include internal rotation of the paw when stepping, and mild symmetrical truncal sway. Both of these may be related to conformation of canine breeds that typically experience spontaneous SCI. Accordingly, the original operational definitions for the BBB had to be adjusted for dogs such that internal rotation of the paw was scored as normal. The cBBB operational definition of trunk instability was modified such that this was scored only when there was asymmetry of truncal sway or when symmetrical truncal sway exceeded more than a “trunk’s width” toward either side of the body. Lastly, assessment of tail position was removed from the scale.
Acute SCI in pet dogs is most commonly caused by IVDE, an explosive rupture of the intervertebral disc leading to both compressive and concussive damage to the spinal cord (Olby et al., 2004). Degeneration of the nucleus pulposus of the intervertebral disc is a pre-requisite and occurs with the highest frequency in small chondrodystrophic breeds such as the miniature dachshund, shih tzu, and Pekingese (Bergknut et al., 2012). Angular limb deformities (particularly pes varus) of varying severity are also often observed in chondrodystrophic breeds and are in some breeds considered a standard finding (Johnson et al., 1989; Deruddere and Snelling, 2014). These breed-associated conformational differences may explain our observation that internal rotation of the paw was common in neurologically normal dogs in the present study. Additionally, chondrodystrophic dogs have elongated backs and shortened limbs, which may contribute to a mild symmetrical sway of the trunk in health. Tail position was removed from the cBBB as a parameter for recovery assessment due to several issues: some breeds have tails which are docked at birth preventing assessment of position, many dogs have tails too short to allow assessment of tail “up” or “down” as described in rats, and tail position was noted to be highly variable within an individual dog in our population related to behavioral factors such as whether the dog was happy or fearful.
During the development phase of our project, we assessed the cBBB in normal dogs at three separate testing sessions. Normal dogs scored 19/19 in both left and right hind limbs at all three sessions, indicating that scores in normal dogs are stable over time. We compared scores from normal dogs to scores from SCI-affected dogs at three time points after injury and found that scores from SCI-affected dogs were distinct from normal at 3, 10, and 30 days after injury (p < 0.0001). Using the cBBB, we detected recovery across a spectrum of injuries ranging from mild paresis to motor complete injuries, and scores were significantly higher on day 30 compared to day 3 (p = 0.0003), consistent with expected improvements in locomotor function over time. Additionally, the cBBB was highly responsive to change in locomotor recovery following SCI. Responsiveness of a scale is defined as its ability to detect changes in patient function over time (Kirshner and Guyatt, 1985). Various clinical measures of responsiveness exist, but SRM is commonly used when evaluating responsiveness of outcome assessment tools in other neuromuscular conditions (Beaton et al., 1997; Wallace et al., 2002). In the present study, the cBBB was highly responsive to detect improvements in locomotor function over a 30 day period following injury in a group of dogs intentionally selected to have a high rate of spontaneous recovery.
We evaluated concurrent validity of the cBBB as measured agreement between observed improvements on this scale and two other locomotor scales tested at the same time, the OSCIS and MFS. Scores assigned using the cBBB were highly correlated with scores assigned by both scales. We also compared the cBBB to other validated assessments of neurologic recovery: hind limb sensory threshold and COV SL in the hind limbs. Scores assigned via the cBBB showed an inverse correlation with measures of sensory threshold and COV SL of the hind limbs. This means that as sensory threshold values normalized, cBBB scores also improved toward normal. Additionally, as cBBB scores improved, stride length became more consistent (improvement toward normal). For COV SL, this inverse correlation was stronger for cBBB than for OSCIS. In the present study, we did not identify a correlation between MFS score and COV SL. This is likely because the median MFS score at both 10 and 30 days after injury was 4 out of 5, making it impossible to discriminate finer improvements in locomotor recovery for animals that are already able to walk unassisted. It should be noted that a perfect correlation between the cBBB and outcome assessment tools measuring sensory and quantitative gait parameters would not be expected, as these tools test markers of neurologic recovery not specifically assessed with the cBBB. A limitation to the current study is the lack of available data to correlate the cBBB scale with histopathologic changes, which would strengthen our assessment of predictive value. However, the present work constitutes a veterinary clinical study of client-owned dogs—all of which recovered significantly from their injuries. Thus, histopathologic correlations were not possible. Additionally, imaging performed in the present study was for diagnostic purposes and not acquired using ideal methods to quantify lesion severity.
In SCI-affected dogs, we identified two scores in the cBBB that were not assigned to any limb at any time point during the 30 day recovery period. A score of 3 is “extensive movement of two joints” and a score of 6 is “extensive movement of two joints and slight movement of a third.” In rodent models of SCI, a score of 6 on the BBB scale is a commonly assigned score; however, a score of 3 is assigned less frequently and is typically observed during the early phase of recovery (Kloos et al., 2005). It is unclear from the present study whether these points during the recovery process were not documented due to observations at only a single time point during early recovery, or whether these phases of recovery do not occur in this injury model. Future studies should focus on more frequent evaluation during the early phases of recovery (between days 3 and 10) to determine the utility of maintaining these scores within the scale.
The cBBB offers several advantages over other canine ordinal recovery scales such as the OSCIS, MFS, and the Texas spinal cord injury scale (TSCIS) (Olby et al., 2001; Levine et al., 2009). First, the cBBB is designed specifically to document locomotor recovery and does not directly assess sensory or proprioceptive function. It also provides precise operational definitions based on visual detection associated with each score and avoids asking the rater to subjectively determine whether limb strength appears reduced (OSCIS), or an animal exhibits an appropriate behavioral response to a nociceptive stimulus (OSCIS and TSCIS). Perhaps most importantly, the cBBB provides an assessment of forelimb–hindlimb coordination, an important component of long-track recovery that is not evaluated in other ordinal scales. A limitation of the cBBB is that, as with the rodent BBB, it is expected to require specific training for users to accurately apply the operational definitions correctly (Basso et al., 1996a,b). However, a previous study evaluating inter-rater reliability of the rodent BBB scale showed that inexperienced observers can quickly learn to assign consistent scores (Basso et al., 1996a,b).
In conclusion, the BBB locomotor rating scale required several modifications to allow use in a canine clinical and translational model of SCI. Once scale parameters were adapted, the cBBB was highly responsive to detect locomotor recovery over a 30 day period and correlated well with other assessment tools previously validated for use in canine models of SCI. Moreover, the cBBB out-performed other currently available canine locomotor rating scales in correlating with other measures of sensory and motor function. Given these results, use of the cBBB is recommended for canine translational studies in SCI. Additionally, precise operational definitions associated with the cBBB may ease administration of this scale compared to other available canine locomotor indices. Future studies should be directed at examining the utility of specific cBBB scores not assigned during the present study, comparing inter-rater agreement between the cBBB and other scales such as the OSCIS, and correlating cBBB with imaging and histopathological biomarkers of disease.
highlights.
We assessed the utility of the BBB locomotor scale for use in dogs.
Several key adaptations were required for use in dogs.
A modified canine BBB was developed (cBBB).
The cBBB was highly responsive to detect locomotor recovery.
cBBB showed strong concurrent validity with other outcome assessment tools.
Acknowledgments
The authors also gratefully acknowledge Mrs. Amanda Disher, Ms. Heather Myers, Ms. Tamra Mathie, Ms. Annie Adrian and Dr. Maureen Oldach for their assistance with data collection and Mr. Tim Vojt for his assistance with figure preparation. This study was funded by the Morris Animal FoundationD13CA-024 and NIHCCTS UL1TR001070. R01 NS074882 and P30-NS04758 supported DMB and LCF. The funding sources had no involvement in study design, data collection, analysis, interpretation, or reporting.
Abbreviations
- SCI
spinal cord injury
- BBB
Basso Beattie Bresnahan locomotor rating scale
- cBBB
canine BBB
- SRM
standard response mean
- OSU
The Ohio State University
- IVDE
intervertebral disc extrusion
- OSCIS
Olby spinal cord injury scale
- MFS
modified Frankel scale
- SL
stride length
- COV
coefficient of variation
- FL
forelimb
- HL
hindlimb
- TSCIS
Texas spinal cord injury scale.
Footnotes
Author disclosure
No competing financial interests exist.
References
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp. Neurol. 1996a;139:244–246. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC, et al. MASCIS evaluation of open field locomotor scores: effects of experience and teamwork on reliability. J. Neurotrauma. 1996b;13:343–359. doi: 10.1089/neu.1996.13.343. [DOI] [PubMed] [Google Scholar]
- Basso DM, Fisher LC, Anderson AJ, et al. Basso mouse scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J. Neurotrauma. 2006;23:635–659. doi: 10.1089/neu.2006.23.635. [DOI] [PubMed] [Google Scholar]
- Beaton DE, Hogg-Johnson S, Bombardier C. Evaluating changes in health status: reliability and responsiveness of five generic health status measures in workers with musculoskeletal disorders. J. Clin. Epidemiol. 1997;50:79–93. doi: 10.1016/s0895-4356(96)00296-x. [DOI] [PubMed] [Google Scholar]
- Bekkers JJ, de Windt TS, Raijmakers NJH, et al. Validation of the knee injury and osteoarthritis outcome score (KOOS) for the treatment of focal cartilage lesions. Osteoarthr. Cartil. 2009;17:1434–1439. doi: 10.1016/j.joca.2009.04.019. [DOI] [PubMed] [Google Scholar]
- Bergknut N, Egenvall A, Hagman R, et al. Incidence of intervertebral disk degeneration-related diseases and associated mortality rates in dogs. J. Am. Vet. Med. Assoc. 2012;240:1300–1309. doi: 10.2460/javma.240.11.1300. [DOI] [PubMed] [Google Scholar]
- Chung-Sheng L, Bentley RT, Weng HY, Breur GJ. A preliminary evaluation of the reliability of a modified functional scoring system for assessing neurologic function in ambulatory thoracolumbar myelopathy dogs. BMC Vet. Res. 2015;11:1–7. doi: 10.1186/s12917-015-0557-8. http://dx.doi.org/10.1186/s12917-015-0557-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deruddere KJ, Snelling SR. A retrospective review of antebrachial angular limb and rotational limb deformity correction in dogs using intraoperative alignment and type 1b external fixation. N. Z. Vet. J. 2014;62:290–296. doi: 10.1080/00480169.2014.910089. [DOI] [PubMed] [Google Scholar]
- Fukuda S, Nakamura T, Kishigami Y, et al. New canine spinal cord injury model free from laminectomy. Brain Res. Brain Res. Protoc. 2005;14:171–180. doi: 10.1016/j.brainresprot.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Granger N, Blamires H, Franklin RJ, Jeffery ND. Autologous olfactory mucosal cell transplants in clinical spinal cord injury: a randomized double-blinded trial in a canine translational model. Brain. 2012;135(Pt 11):3227–3237. doi: 10.1093/brain/aws268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke D, Vandevelde M, Doherr MG, et al. Correlations between severity of clinical signs and histopathologic changes in 60 dogs with spinal cord injury associated with acute thoracolumbar intervertebral disc disease. Vet. J. 2014;198:70–75. doi: 10.1016/j.tvjl.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Henke D, Gorgas B, Doherr MG, et al. Longitudinal extension of myelomalacia by intramedullary and subdural haemorrhage in a canine model of spinal cord injury. Spine J. 2015 doi: 10.1016/j.spinee.2015.09.018. http://dx.doi.org/10.1016/j.spinee.2015.09.018, pii: S1529-9430(15)01394-7. [DOI] [PubMed] [Google Scholar]
- Jeffery ND, Lakatos A, Franklin RJM. Autologous olfactory glial cell transplantation is reliable and safe in naturally occurring canine spinal cord injury. J. Neurotrauma. 2005;22:1282–1293. doi: 10.1089/neu.2005.22.1282. [DOI] [PubMed] [Google Scholar]
- Jeffery ND, Hamilton L, Granger N. Designing clinical trials in canine spinal cord injury as a model to translate successful laboratory interventions into the clinical setting. Vet. Rec. 2011;168:102–107. doi: 10.1136/vr.d475. [DOI] [PubMed] [Google Scholar]
- Johnson SG, Hulse DA, Vangundy TE, Green RW. Corrective osteotomy for pes varus in the dachshund. Vet. Surg. 1989;18:373–379. doi: 10.1111/j.1532-950x.1989.tb01103.x. [DOI] [PubMed] [Google Scholar]
- Kirshner B, Guyatt G. A methodological framework for assessing health indices. J. Chronic Dis. 1985;38:27–36. doi: 10.1016/0021-9681(85)90005-0. [DOI] [PubMed] [Google Scholar]
- Kloos AD, Fisher LC, Detloff MR, Hassenzahl DL, Basso DM. Stepwise motor and all-or-none sensory recovery is associated with nonlinear sparing after incremental spinal cord injury in rats. Exp. Neurol. 2005;191:251–265. doi: 10.1016/j.expneurol.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Levine GJ, Levine JM, Budke CM, et al. Description and repeatability of a newly developed spinal cord injury scale for dog. Prev. Vet. Med. 2009;89:121–127. doi: 10.1016/j.prevetmed.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Levine JM, Cohen ND, Heller M, et al. Efficacy of a metalloproteinase inhibitor in spinal cord injured dogs. PLoS One. 2014;9:e96408. doi: 10.1371/journal.pone.0096408. http://dx.doi.org/10.1371/journal.pone.0096408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JH, Muguet-Chanoit AC, Smith DT, Laber E, Olby NJ. Potassium channel antagonists 4-aminopyridine and the T-butyl carbamate derivative of 4-aminopyridine improve hind limb function in chronically non-ambulatory dogs; a blinded, placebo-controlled trial. PLoS One. 2014;9:e116139. doi: 10.1371/journal.pone.0116139. http://dx.doi.org/10.1371/journal.pone.0116139, eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahill BG, Fpriet M, Siso S, et al. Feasibility study of canine epidermal neural crest stem cell transplantation in the spinal cords of dogs. Stem Cells Transl. Med. 2015;4:1173–1186. doi: 10.5966/sctm.2015-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SA, Oglesbee MJ. Spinal cord ependymal responses to naturally occurring spinal cord injury in dogs. Vet. Pathol. 2014 doi: 10.1177/0300985814560235. pii: 0300985814560235. [DOI] [PubMed] [Google Scholar]
- Moore SA, Hettlich BF, Waln A. The use of an electronic von Frey device for evaluation of sensory threshold in neurologically normal dogs and those with acute spinal cord injury. Vet. J. 2013;197:216–219. doi: 10.1016/j.tvjl.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Olby NJ, De Risio L, Munana KR, et al. Development of a functional scoring system in dogs with acute spinal cord injuries. Am. J. Vet. Res. 2001;62:1624–1628. doi: 10.2460/ajvr.2001.62.1624. [DOI] [PubMed] [Google Scholar]
- Olby N, Levine J, Harris T, et al. Long-term functional outcome of dogs with severe injuries of the thoracolumbar spinal cord: 87 cases (1996–2001) J. Am. Vet. Med. Assoc. 2003;222:762–769. doi: 10.2460/javma.2003.222.762. [DOI] [PubMed] [Google Scholar]
- Olby N, Harris T, Burr J, et al. Recovery of hind limb function in dogs following acute intervertebral disc herniations. J. Neurotrauma. 2004;21:49–59. doi: 10.1089/089771504772695940. [DOI] [PubMed] [Google Scholar]
- Smith PM, Jeffery ND. Histological and ultrastructural analysis of white matter damage after naturally-occurring spinal cord injury. Brain Pathol. 2006;16:99–109. doi: 10.1111/j.1750-3639.2006.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song RB, Basso DM, da Costa RC, et al. von Frey anesthesiometry to assess sensory impairment after acute spinal cord injury caused by thoracolumbar intervertebral disc extrusion in dogs. Vet. J. 2015 doi: 10.1016/j.tvjl.2015.07.028. http://dx.doi.org/10.1016/j.tvjl.2015.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song RB, Basso DM, da Costa RC, et al. A simplified method of walking track analysis to assess short-term locomotor recovery after acute spinal cord injury caused by thoracolumbar intervertebral disc extrusion in dogs. Vet. J. 2016 doi: 10.1016/j.tvjl.2016.01.013. http://dx.doi.org/10.1016/j.tvjl.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Harada Y, Kunimi M, et al. Autologous bone marrow mononuclear cell transplant and surgical decompression in a dog with chronic spinal cord injury. Exp. Clin. Transplant. 2015;13:100–105. doi: 10.6002/ect.2013.0237. [DOI] [PubMed] [Google Scholar]
- Wallace D, Duncan PW, Lai SM. Comparison of the responsiveness of the Barthel Index and the motor component of the Functional Independence Measure in stroke: the impact of using different methods for measuring responsiveness. J. Clin. Epidemiol. 2002;55:922–928. doi: 10.1016/s0895-4356(02)00410-9. [DOI] [PubMed] [Google Scholar]
- Wang XM, Basso DM, Terman JR, et al. Adult opossums (Didelphis virginiana) demonstrate near normal locomotion after spinal cord transection as neonates. Exp. Neurol. 1998;151:50–69. doi: 10.1006/exnr.1998.6795. [DOI] [PubMed] [Google Scholar]