Abstract
The family of JADE proteins includes three paralogues encoded by individual genes and designated PHF17 (JADE1), PHF16 (JADE2), and PHF15 (JADE3). All three JADE proteins bear in tandem two Plant Homeo-domains (PHD) which are zinc finger domains. This review focuses on one member of the JADE family, JADE1. Studies addressing the biochemical, cellular and biological role of JADE1 are discussed. Recent discoveries of JADE1 function in the regulation of the epithelial cell cycle with potential relevance to disease are presented. Unresolved questions and future directions are formulated.
I. Introduction
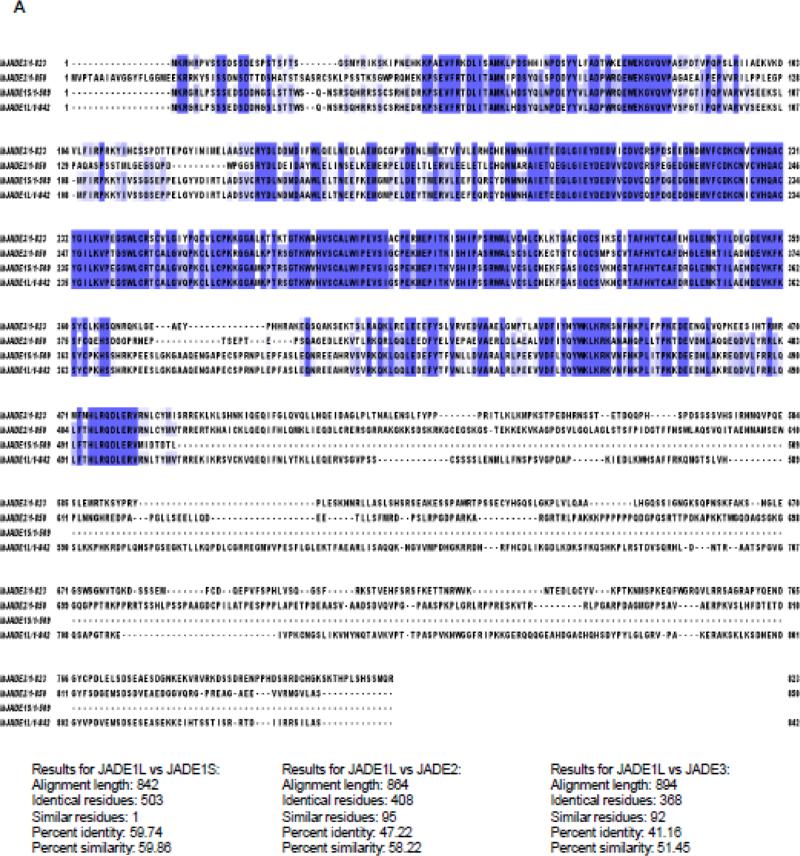
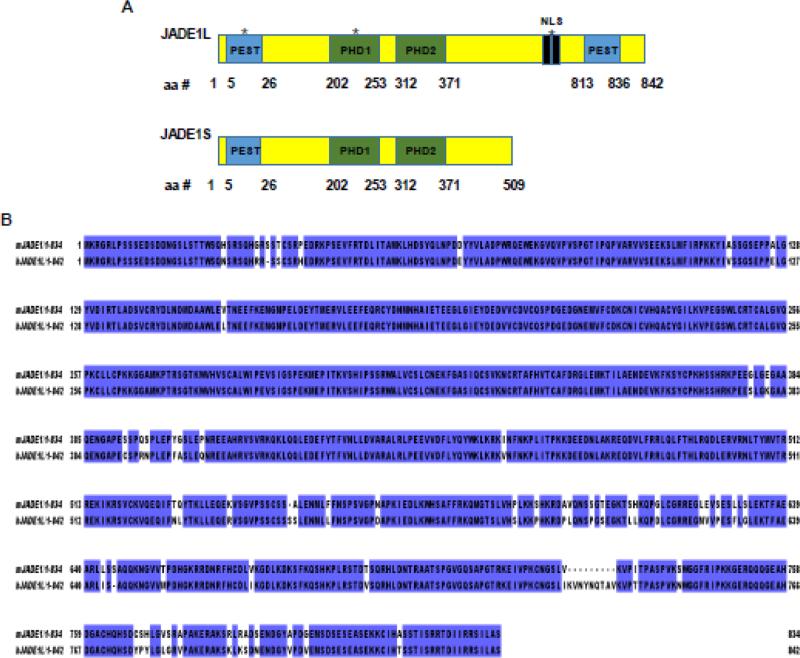
JADE family PHD zinc finger 1 (Gene for Apoptosis and Differentiation in Epithelia 1; JADE1, KIAA1807)1-3 is a member of the small JADE family which also includes JADE2 and JADE3 paralogs3. So far JADE1 is the most studied member of the JADE family proteins. JADE proteins are encoded by individual genes which are mapped to different chromosomes and designated as PHF17 (JADE1), PHF16 (JADE3), and PHF15 (JADE2) (http://www.ncbi.nlm.nih.gov) (Table 1). All three JADE1/2/3 proteins bear in tandem two mid-molecule Plant Homeodomain (PHD) zinc fingers. To date, about 90 proteins with one or more PHD zinc finger domains represent a PHD protein family. Amino acid sequences of all JADEs present a high degree of identity in the N-terminal and PHD finger domain regions but are variable in their C-terminal fragments (Fig 1, Identity and Similarity was calculated using “Ident and Sim”4). As a result of an alternative splicing, JADE1 mRNA gives rise to two protein products, the full length isoform JADE1L and the truncated version JADE1S which is missing the large C-terminal fragment3 (Fig 2). To date, this posttranscriptional modification leading to two experimentally detectable protein products appears to be a unique characteristic of JADE1 but not JADE2 or JADE3. The N-terminal fragment of JADE1 presents a degree of homology with the Enhancer of Polycomb Like (http://pfam.xfam.org/protein/Q6IE81). JADE1L has two putative protein degradation (PEST) and nuclear localization (NLS) signal peptides. JADE1S has N-terminal PEST signal peptide but is missing a putative NLS (Fig 2A). JADE1 proteins are subject to posttranslational modifications via serine and threonine phosphorylation (http://www.phosphosite.org). The phosphorylation of several specific functional sites have been demonstrated and proved experimentally by Mass Spectrometry 5-7.
Table 1.
The JADE family PHD finger genes and proteins from human and mouse
| Gene symbol | JADE1 human | Jade1 mouse | JADE2 human | Jade2 mouse | JADE3 human | Jade3 mouse |
|---|---|---|---|---|---|---|
| Chromosome number |
4 | 3 | 5 | 11 | X | X |
| Location | 128,809,623- 128,875,224 |
41,555,734- 41,616,864 |
134,524,312- 134,583,230 |
51,813,455- 51,857,653 |
20,425,688- 20,519,939 |
20,425,688- 20,519,939 |
| Protein product(s)- length |
JADE1L- 842 aa, 95.5K Da; JADE1S- 509 aa, 58.4 KDa |
Jade1L- 834 aa, 93.9 Kda; Jade1S- 510 aa, 58.1 KDa |
JADE2- 790 aa, 87.5 KDa |
Jade2- 829 aa 92.2 KDa |
JADE3- 823 aa 93.8 KDa |
Jade3- 823 aa 93.5 KDa |
| Ensemble gene ID |
ENSG000000 77684 |
ENSMUSG000000 25764 |
ENSG000000 43143 |
ENSMUSG000000 20387 |
ENSG000000 102221 |
ENSMUSG000000 37315 |
| UniProt ID | Q6IE81-1 (JADE1L) Q6IE81-3 (JADE1S) |
Q6ZPI0-1 (Jade1L) Q6ZPI0-2 (Jade1S) |
Q9NQC1 | Q6ZQF7 | Q92613 | Q6IE82 |
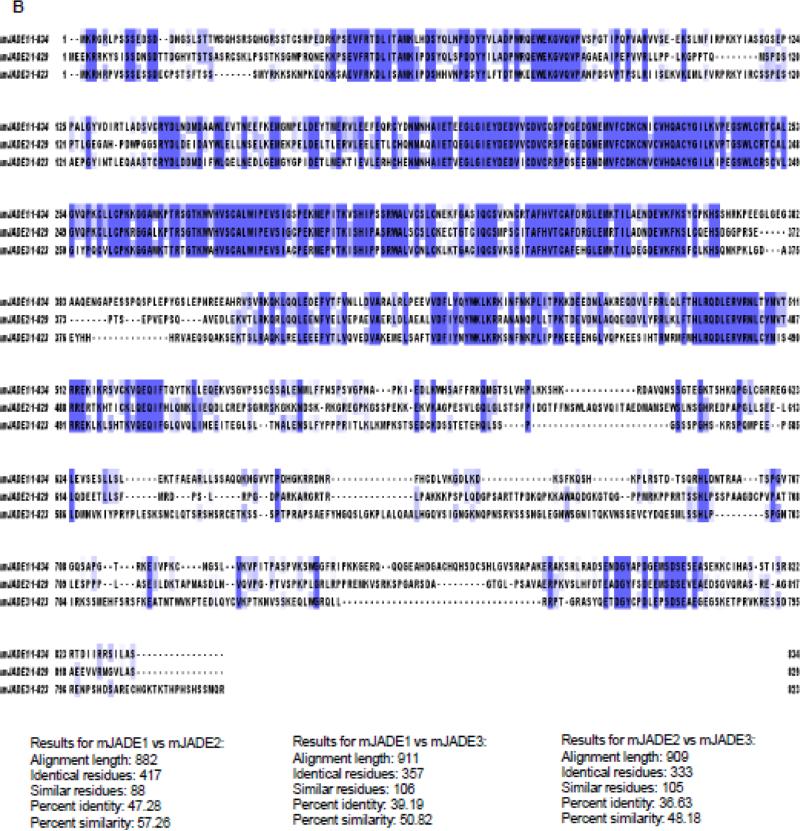
Figure 1. Sequence alignment of JADE family PHD finger proteins.
A, Human. B, Mouse. Protein sequences were obtained from UniProt, and aligned using Clustal Omega (http://www.ebi.ac.uk/Tools/msa/clustalo/). Identity and Similarity was calculated using “Ident and Sim” (see the text for reference).
Figure 2.
A, The domains of the human JADE1 protein isoforms. *PHD1 and PHD2 - Cys4HisCys3 plant homeo domain; NLS – nuclear localization signal; PEST - protein degradation amino acid sequence (proline, glutamic acid, serine, and threonine). B, Alignment of amino acid sequences of mouse and human JADE1. Sequences from the Uniprot database (Q6IE81-1 and Q6ZPI0-1) were aligned with Jalview software.
JADE1 has been mapped to chromosome 4 (4q26-q27). JADE1 is conserved and its orthologs have been found or predicted in most vertebrate and some invertebrate species. Analysis of similarity between human and mouse JADE1 proteins is presented in Fig 2B. Most information about gene structure, conservation, orthologs and paralogs, phylogenetic tree and large scale screening of JADE1 tissue expression are available in several extensive databases (http://www.genecards.org; http://useast.ensembl.org; see also3).
JADE1 is a multifunctional protein and interacts with several protein partners. Except for the function of JADE1S in cytokinesis, most of the insights about other JADE1 functions have originated from the analysis of protein partners of JADE1. As of now the role in histone acetyl transferase complex and the role in the regulation of the cell cycle progression have been studied the most and experimentally supported by different research groups. While the protein product of Von Hippel Lindau gene (pVHL) is the first confirmed protein partner of JADE1, the role in pVHL and beta-catenin signaling pathway is still under investigation and awaits further experimental analysis. Not much is known about the biological role of JADE1 as few in vivo studies in human subjects or in mice are available.
The function of JADE2 and JADE3 remains unexplored. There are only two full experimental papers describing the function of JADE2 and JADE3. There is a small number of reports as well as data base entries describing the expression of JADE2 and JADE3. These studies rely on screening and high through put approaches and require further confirmation.
II. JADE1 Identification and Structure
II-1. JADE1 Identification
Using the fetal brain cDNA library, Nagase et al. (2001) cloned and sequenced 100 individual cDNAs of which 50 were predicted to have protein products, including clone KIAA1807 which was designated PHF171. The deduced 702-amino acid protein product of KIAA1807 clone was found to be similar to the human zinc finger protein BR140 (BRPF1)8. Initial RT-PCR ELISA assays in the same study showed some tissue-specific expression. Based on in silico analysis PHF17 was attributed to a category of nucleic acid managing proteins. A study searching for proteins that physically interact with pVHL used the yeast two hybrid pull down approach and, among other candidates, identified a cDNA of a protein which was designated with the name JADE12. The cDNA sequence of JADE1 matched with 5’ fragment of the KIAA1807 clone. The deduced 509 amino acid protein product of JADE1 cDNA was described and characterized as a physical partner of the Von Hippel-Lindau protein (pVHL)8. Shortly after that, in a genetic screen study searching for genes involved in embryogenesis, the mouse ortholog of JADE1 was identified3. The study provided extensive insights about the JADE1 gene and defined the novel JADE family. It also yielded mice with knock out of JADE1.
Based on comparative analysis of the genomic and cDNA sequences a conclusion was made that primary Jade1 transcripts in both humans and mice undergo alternative splicing and polyadenylation, yielding the full length 6 kb mRNA and 3.6 kb mRNA3. Two resultant protein products of these transcripts were designated JADE1S for the short isoform (matching KIAA18072) and JADE1L for the full length protein. Moreover, the database analysis revealed two additional homologs (paralogs) of JADE1 which were named JADE2 and JADE3, thus identifying the new JADE subfamily of proteins. Interestingly, JADE3 turned out to be identical to the E9 protein identified in an earlier independent study which suggested a role in apoptosis for PHF16/JADE3/E9 in breast cancer cells9.
According to the Human Gene Nomenclature Database (www.genenames.org/hgnc-searches) the original symbol for all three genes (that is, PHF15/16/17) was substituted for the name “JADE Family PHD finger 1/2/3”. Therefore, in this review we use the abbreviation JADE1/2/3 for JADE family proteins.
II-2. PHD Zinc Fingers of JADE1
The PHD zing finger protein family includes a smaller subfamily characterized by the presence of two PHD zinc fingers: the canonical PHD1 followed by the non-canonical extended PHD2 finger. By virtue of the presence of PHD zinc fingers, all three proteins, JADE1, JADE2, and JADE3, belong to the extended PHD finger protein subfamily. Several other paralogs of JADE1, bearing the PHD finger tandem are known, including BRPF1, BRPF3, BRD1 and others (http://useast.ensembl.org). The crystal structure for JADE1 PHD domains has not been solved and its function is still under investigation. Canonical PHD zinc fingers (C4HC3) bind two zinc ions and are present in a number of factors that regulate chromatin-dependent transcription and nucleosome homeostasis10, 11. The PHDs are thought to mediate protein-protein interaction and appear to be important for the assembly and activity of multicomponent complexes. Additionally, the PHD fingers might be important for protein stability and folding. Early in vitro studies demonstrated that PHD fingers bind nucleosomes10. The canonical PHD finger motif represents a relatively small, stable structure and is distinct from the C3HC4 type RING finger. The extended PHD fingers have similarities with RING domains. According to several studies characterizing individual PHD finger proteins, the canonical PHD domains were able to recognize and bind specific methylated lysine of histone H3 (mostly H3K4me1/2/3) which defined these domains as novel epigenetic histone code readers12-14. A number of comprehensive reviews describe structure and properties of PHD fingers in depth15-18. Biochemical studies characterizing the binding and specificity of JADE1 PHD zinc fingers towards the N-terminal of histone H3 have been reported19-21. According to binding assays, the two PHD fingers of JADE1 appear to bind the N-terminal of histone H3 in a modification-independent manner, although the PHD1 finger alone seems to prefer H3K36me3 histone mark19, 20. The crystallization and X-ray analysis of these complexes would need to be done to define the precise JADE1 affinities towards histone marks.
III. Cellular and Biochemical Function
III-1. Histone Acetylation
The first demonstration of nuclear localization and JADE1 function in histone acetylation was reported a decade ago22. In cell free assays purified JADE1 protein did not promote acetylation of either soluble or nucleosomal histones. However, in live cell cultures JADE1 dramatically enhanced bulk histone H4 acetylation. This suggested JADE1 interaction with an H4-specific nuclear HAT, which are found only within the MYST family. Indeed, experiments showed that JADE1 physically interacted with TIP60 and via cooperation promoted histone H4 acetylation. The tethering of Gal4-JADE1 fusion protein to Gal4-responsive promoters in co-transfection experiments activated transcription by several fold, suggesting that JADE1 is a transcriptional activator. Thus, JADE1 was identified as a candidate transcription factor which functions in association with a MYST family of HAT22. In cultured cells the JADE1-mediated acetylation targets H4K5, H4K12 and most likely H4K8 within histone H4, although these specificities have not been thoroughly confirmed5, 20, 23. A correlation with these specificities for JADE1 was also found in vivo in a mice model of tubular epithelial regeneration (discussed in 7)23.
The deletion analysis of JADE1 polypeptide in cell free and live cell culture assays revealed the chromatin binding role of the second extended PHD zinc finger domain.22 Thus, the deletion of PHD2 resulted in a dominant-negative mutant of JADE1. This was evident from functional competition assays, demonstrating that PHD2 is required for global histone H4 acetylation in chromatin context. This was confirmed and dissected further by in vitro binding analysis showing that PHD2 of JADE1 binds histones within a chromatin context irrespective of histone H3 methylation status20. These results provided the first experimental evidence for the chromatin-targeting role of JADE1 PHD fingers in histone acetylation and transcription function. The study suggested that by synergizing with an endogenous HAT, JADE1 facilitates acetylation of bulk histone H4 in a nucleosome context. Moreover, results suggested that HAT TIP60 is a candidate for that role.
Consequently, a study characterizing native complexes of INhibitor of Growth (ING) PHD finger family of proteins found an association of JADE1 with a close homolog of TIP60 and a member of the MYST family, HBO124. According to the study, JADE1S, HAT HBO1, and Eaf6 proteins were present in the ING4/5 pull down complexes, while TIP60, EPC1, Eaf6 and several other proteins were present in the ING3 pull down complex. The striking similarities and identities between TIP60 and HBO1 HAT complexes further supported the role for JADE1 in bulk histone H4 acetylation. Thus, according to protein binding and sequence analysis, JADE1 is a close homolog of EPC1, HBO1 is highly homologous to TIP60, while Eaf6 is the shared member of both MYST complexes24. Further investigation of the role for JADE1 in histone acetylation, revealed that JADE1 and HBO1 interactions have striking structural and functional similarities with TIP6022, 25. Thus, similar to TIP60, JADE1 and HBO1 mutually stabilize each other25. Moreover, similar to TIP60, the ability of HBO1 to acetylate histone H4 in a chromatin context was dramatically enhanced by JADE1 and this required an intact PHD2 finger of JADE1. In contrast, the PHD protein ING4/5 did not potentiate histone acetylation activities of HBO1 or HBO1-JADE1 complex in either assay25. These interactions with two HATs raise a question: What are the mechanisms that ensure specificities of interactions between homologs in the cellular context. Sharing partners and a combinatorial mode of interaction has been described for other chromatin-associated complexes. It is possible that by switching the two known HAT partners JADE1 might perform distinct functions in different cellular compartments during the cell cycle (discussed in III-2).
Although nuclear JADE1 complex composition has been investigated and the role in HAT-dependent gene transcription proposed, neither the specific transcription targets nor the transcription function of JADE1 have been identified22. Potential transcription targets of JADE1 have been suggested from experiments using screening approaches, but so far not confirmed20, 21.
Evidence for biochemical, cellular, and in vivo differences between the two JADE1 isoforms have been demonstrated but remain puzzling (discussed throughout the review). For example, structurally, JADE1S lacks the C-terminal fragment and hence is incapable of binding ING4/5, which are well characterized tumor suppressors and PHD zinc finger proteins25. As a result, at a minimum, JADE1 isoforms assemble two different complexes, JADE1L-HBO1-ING4/5 and JADE1S-HBO1 complex25. A small less characterized protein Eaf6 might be another component of JADE1 complexes21. JADE1L complexes with TIP60 have never been studied.
III-2. Cell Cycle Regulation
III-2-1. DNA Replication
It has been reported that lysine acetylation of the N-terminal tails of bulk histone H4 correlates with DNA synthesis, suggesting a role in cell proliferation26-30. Importantly, JADE1 is required for the acetylation of bulk histone H4 in cultured cells22, 23, 25. In search of the cellular function of JADE1, studies were initiated to examine the JADE1 role in cell cycle progression5, 6, 23. While these studies established JADE1 function in cell cycle regulation, the mechanisms of action, the stages involved and, most importantly, the specific contribution of JADE1L and JADE1S isoforms are yet to be identified. As evident from the results, the two isoforms of JADE1 appear to accomplish different functions during epithelial cell division.
The following experimental observations support the cell cycle role for JADE1 in conjunction with the HBO1-mediated pathway5, 23, 31. The depletion of JADE1 proteins by RNA interference (siRNA) resulted in slower rates of DNA synthesis in cell cycle-synchronized epithelial cell lines and primary fibroblast cell cultures23. The siRNA reagent used in these experiments targeted both isoforms of JADE1. In addition, the depletion of JADE1 proteins resulted in decreased levels of the total and chromatin-bound HBO1, which nicely correlated with previously described effects of JADE1-HBO1 mutual upregulation23, 25. In dividing synchronized cultures, JADE1 depletion prevented time-dependent chromatin recruitment of MCM723. Agreeing with these results, JADE1L over-expression in asynchronously dividing cell cultures increased total chromatin-bound MCM3 protein32. The effect of JADE1 depletion on MCM7 chromatin recruitment recapitulates that of HBO1 protein depletion originally reported by Mitch Smith and colleagues33 and suggests a role for JADE1 in the HBO1 pathway. The JADE1 role in DNA licensing has been suggested but not proven. Based on data available so far it is more likely that JADE1 functions in DNA synthesis (S-phase) rather than replication licensing (G1-phase).
A recent report describing novel non-coding RNA lncRNA-JADE supports the role for JADE1 in DNA synthesis linked to histone H4 acetylation34. In addition, the study demonstrates that lncRNA-JADE provides a functional link between the DNA damage response (DDR) and bulk histone H4 acetylation. Knocking down of lncRNA-JADE in cultured cells increased cells sensitivity to DNA damaging drugs. In the mice tumor xenograft model the down regulation of lncRNA-JADE resulted in inhibition of xenograft mammary tumor growth. In a pilot human study, higher levels of lncRNA-JADE as well as JADE1 protein were detected in breast cancer tissues compared to normal tissues and the higher levels of JADE1 protein inversely correlated with survival rates of patients with breast cancer. The study suggests that lncRNA-JADE might contribute to breast tumorigenesis and that JADE1 protein mediates at least part of this effect34. It is unclear whether shRNA used in this study targeted JADE1S, JADE1L or both isoforms. In light of the recent evidence of differential effects of the two JADE isoforms5, 6, it would be very interesting to assess the contribution of individual proteins to DDR and tumor growth.
III-2-2. Cytokinesis
Recently, a novel and rather unexpected function of JADE1 was reported in the regulation of cytokinesis of the epithelial cell cycle which was specific to the small isoform, JADE1S6. The first hint that JADE1 operates between G2/M and G1 of the cell cycle came from the previous report which identified and characterized cell cycle-dependent dynamic properties of JADE15, 31. According to the study, during the late G2 phase, JADE1S undergoes phosphorylation linked to its dissociation from chromatin into the cytoplasm. Mass Spectral analysis identified that a total of six individual amino acid residues are phosphorylated by a mitotic kinase, including two novel residues5. Based on pharmacological analysis, JADE1 phosphorylation and compartmentalization is regulated by Aurora A and Aurora B pathways5, 6. Other kinases have been reported and may play a role7, 35. Upon completion of mitosis around the telophase, the main pool of the JADE1S protein undergoes dephosphorylation and re-associates with apparently condensing chromatin inside the reformed nuclei5. Strikingly, a minor pool of JADE1S follows the cleavage furrow and subsequently appears in the midbody of the cytokinetic bridge6. Interestingly, only JADE1S, but not JADE1L or HBO1 were found in the midbody of the cells undergoing cytokinesis. The cell cycle-dependent regulation of JADE1S suggested a role in G2/M to G1 transition, which includes cytokinesis and final abscission stages of the cell division6, 36.
Cytokinesis orchestrates several steps of cell division, including chromosome segregation, cell membrane furrowing, actin cytoskeleton remodeling, and the assembly of connecting bridge and midbody. The final abscission occurs near the midbody which may take up to 2 hours and has been actively studied in recent years. Cytokinesis and final abscission are tightly controlled by regulatory protein complexes, including checkpoint proteins. The number of breakthrough experimental studies and excellent reviews that cover the topic of cytokinesis control has been growing over the past decade37-41.
The evidence for the JADE1 role in cytokinesis came from experiments using several functional approaches and cell culture models6. A set of experiments using DNA profiling by FACS showed that JADE1S depletion facilitated rates of G1-cells accumulation in synchronously dividing HeLa cells. Further, JADE1S protein depletion in asynchronously dividing cells decreased the proportion of cytokinetic cells. These results show that JADE1 negatively controls cytokinesis, presumably by contributing to cytokinesis delay. Moreover, JADE1 down-regulation caused increased proportion of multi-nuclear cells, which is indicative of premature and failed cytokinesis. In contrast, moderate overexpression of JADE1S increased the number of cytokinetic cells in a time- and dose- dependent manner, indicating cytokinetic delay. The progression of mitosis and cytokinesis is controlled by the activities of checkpoint master kinases Aurora A and B. Pharmacological inhibition of NoCut checkpoint kinase Aurora B resulted in the release of JADE1S-mediated cytokinetic delay and the progression of abscission. Thus, JADE1S is likely to regulate cytokinesis at the abscission checkpoint control6, 36.
III-2-3. JADE1L versus JADE1S
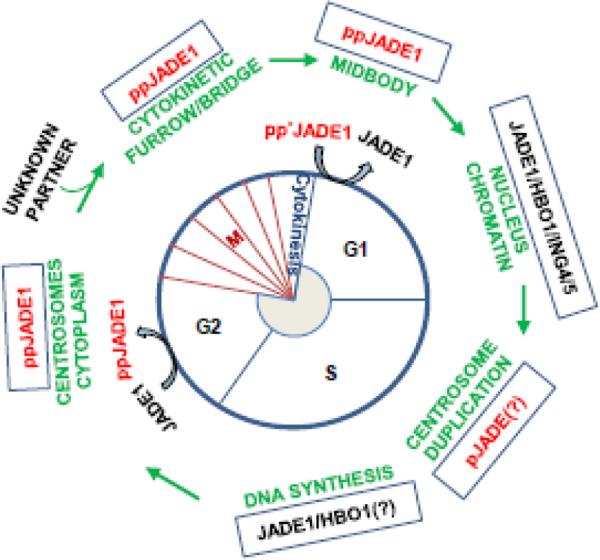
Interestingly that JADE1S but not JADE1L or HBO1 was localized to centrosomes of the dividing cells. These data further highlighted differences between JADE1 isoforms. None of these three proteins was found in cilia. Centrosomal localization of JADE1 was also reported earlier in another study. However, in this study the antibody used is of unknown specificity and therefore does not allow discrimination between JADE1 isoforms35. Centrosome signaling contributes to the definition of cell shape, motility, orientation, polarity, division plane and to the fidelity of sister chromosome separation during mitosis and cytokinesis42, 43. During the S phase of the cell cycle centrosomes undergo duplication. It is notable that centrosome duplication is tightly controlled by cdk-dependent kinases and that similar to DNA replication licensing, has to occur once and only once during the cell cycle. It has been suggested that as part of the coordination mechanism, shared pathways and factors would control spatial and temporal regulation of centrosome duplication, DNA replication, mitosis and cytokinesis42, 43. Based on data available so far, during the cell cycle, JADE1 is involved in a puzzling set of alternative activities that are hard to reconcile. The possible explanation is that the two JADE1 isoforms might be involved in spatial-temporal coordination of the DNA synthesis and cytokinesis and perhaps direct transcription regulation of cell cycle factors. In this case the phosphorylation of specific amino acid residues by cell cycle kinases (cdk's, Aurora A and B kinases, others) would target JADE1S and JADE1L to the appropriate cellular compartments to enable their function in a given phase of the cell cycle progression (see schematics of summaries in Fig 3 and Table 2).
Figure 3. JADE1 function in the cell cycle.
For details see the text. * indicates phosphorylation
Table 2.
Function and regulation of JADE1 protein isoforms in epithelial cells and tissues.
| G1/S | G2/M | Cytokinesis | ||||
|---|---|---|---|---|---|---|
| JADE1 isoform | JADE1L | JADE1S | ppJADE1L** | ppJADE1S** | JADE1L | pJADE1S** |
| JADE1 subcellular localization | Chromatin | Chromatin Centrosome | Cytoplasm | Cytoplasm Centrosome | Chromatin | Cleavage furrow, Midbody |
| JADE1 activities | Histone H4 bulk acetylation, homeostasis. DNA synthesis. Replication licensing(?*). Transcription(?*). | JADE1S is phosphorylated by Aurora A pathway. Bulk of pJADE1** is removed from chromatin to allow chromosome condensation(?*). | Specific fraction of pJADE1S** mediates NoCut in Aurora B pathway. Dephosphorylated JADE1S/L re-associates with relaxing chromatin | |||
| JADE1 dysregulation: predicted outcomes | DNA damage, amplification, impaired tissue homeostasis, degeneration, or cancer | Premature or delayed cytokinesis, cytokinesis failure with following aneuploidy, polyploidy, gene mutations, cell death or transformation. Impaired tissue homeostasis, degeneration, or cancer | ||||
The hypothetical scheme summarizes current literature about cellular activities of JADE1 protein isoform activities during the cell division.
predicted
phosphorylated form of JADE1 proteins
In addition to JADE1S, several transcription factors, histone and DNA modifiers with predominantly nuclear localization such as Brd4 and WDR5, have been found to regulate cytokinesis and abscission44, 45. Recently, it has been reported that the novel Abscission/NoCut Checkpoint Regulator (ANCHR) protein functions in Aurora-B-dependent abscission checkpoint control in HeLa cells46. Interestingly, ANCHR polypeptide includes FYVE and RING finger domains, while PHD2 finger of JADE1S may have structural similarities with the RING finger. PHD fingers of JADE1S are required for chromatin association and are candidate epigenetic code readers19, 20, 22, 25. It would be interesting to find out whether PHD zinc fingers play a role in the novel JADE1S activities during cytokinesis and potentially abscission.
It has been shown that similar to phosphorylation, lysine acetylation of proteins is involved in centrosome-mediated cytokinesis control47-49. The function of JADE1S in cytokinesis raises a question whether this activity is dependent on protein acetylation and interactions with HATs (HBO1, TIP60, others). We have, thus far, not detected HBO1 in the midbody and centrosomes and assessments of TIP60 have not been completed yet6. Alternatively, JADE1 protein might operate in complex with other identified or as yet unknown protein partners.
III-3. JADE1 and pVHL
The von Hippel–Lindau tumor suppressor (pVHL) is a protein product of the human VHL gene which is mutated in von Hippel–Lindau hereditary disease and in the majority of sporadic clear cell renal carcinomas50-54. The pVHL is the first protein partner of JADE1S isoform which was identified in 20022. Consequently, a few more studies characterized JADE1-pVHL interactions and suggested a role in apoptosis and tumor suppression55-57.
The properties and function of pVHL have been investigated for many decades and an extensive literature is available. One of the better known functions of the pVHL protein is the contribution to the protein ubiquitination and degradation pathway. As a component of the ubiquitin ligase E3 complex, pVHL binds and targets several factors, including HIF1a and HIF2a for proteolytic degradation53. An elegant study unraveling the molecular mechanism of oxygen sensing and describing pVHL-dependent HIF1a activation by hypoxia was reported over a decade ago58. At least two isoforms of pVHL protein are known: the 19 kDa short and 30 kDa long isoforms. Both isoforms have been intensely studied and the link of naturally occurring pVHL mutations to cancers established. While cancer associated mutations affect ubiquitination and proteosome targeting activities of pVHL, the mere disruption of this function does not fully explain the mechanisms of pVHL-mediated cancer pathogenesis and sporadic renal cancer disease - a phenomenon which has been challenging scientists in the field. Defects with other pVHL cellular activities and pathways have been proposed and considered59. In a study searching for new pVHL partners, JADE1S has been identified by use of the yeast two hybrid screening analysis2. The pVHL-JADE1S physical interaction was further confirmed biochemically. Interestingly co-transfection of pVHL increased protein half-life and JADE1S protein abundance, suggesting a potential positive relationship2. Certain pVHL cancer-derived truncations but not point mutations diminished the pVHL-JADE1 stabilization function, suggesting a link to cancer57.
Although some aspects of pVHL-JADE1 interactions have been characterized, the molecular mechanisms and more importantly physiological role of such interactions remain unclear. One study reported that JADE1S has intrinsic ubiquitin-ligase activity and is capable of binding and ubiquitination of beta-catenin55. Based on that study, a model has been proposed that pVHL regulates beta-catenin abundance through JADE1 and that PHD zinc fingers are required for this activity. Additional experimental proof and a follow up study would need to support this possibility.
III-4. Is There Function in Apoptosis?
The role of JADE1S in apoptosis was proposed in early studies which were often reliant on overexpression approaches2, 21, 55-57. Due to the nature of the cell cycle the ectopic expression of high, non-physiological levels of cell cycle regulatory proteins often results in deleterious cellular effects. In various cell cultures, we have repeatedly observed that the introduction of JADE1 protein into the growing cells by conventional methods renders toxic consequences and morphological changes that do not resemble apoptosis6. Transient transfections of JADE1S using cDNA in quantities suggested by the manufacturers typically results in apparently dying cells with abnormal shapes and large, multi-lobular nuclei6. This diminishes the apparent transfection efficiency, most likely due to cell death and negative self-selection. Several earlier attempts to establish dependable cell lines stably expressing JADE1S protein have failed. In light of current findings, the cell death that has been observed after JADE1S overload may have been caused by the prolonged NoCut and stalled cytokinesis rather than direct transcription activation of apoptotic drivers by JADE1S. While the role in signaling of apoptosis is not excluded, these studies and their interpretations may need to be re-visited. A reliable approach to investigate JADE1S cellular function, including proposed apoptosis, must include controlled isoform-specific knockdown of JADE1 or ectopic expression of physiologically relevant protein quantities. At this point such experiments examining JADE1 apoptosis-signaling function have not been done.
IV. Biological Function
IV-1. Murine Models
A possible in vivo role of JADE1 in epithelial cell proliferation was addressed in a murine model of acute kidney injury and regeneration5, 23. The results of the study strongly suggest a role for JADE1 in the tubular cell proliferation regeneration phase as well as differential roles for JADE1S and JADE1L. Expression patterns and dynamics of HBO1-JADE1S/L were examined in regenerating tubular epithelial cells23. Ischemia and reperfusion injury resulted in an initial decrease in JADE1S, JADE1L, and HBO1 protein levels, which returned to the baseline during renal recovery. Expression levels of HBO1 and JADE1S recovered as cell the proliferation rate reached maximum, whereas JADE1L recovered after bulk proliferation had diminished. The temporal expression of JADE1 correlated with the acetylation of histone H4 on lysines 5 and 12, but not with acetylation of histone H3 on lysine 14, suggesting that in vivo the JADE1-HBO1 complex specifically marks H4 during epithelial cell proliferation. The results of the study implicate JADE1-HBO1 complex in acute kidney injury and yet again suggest distinct roles for JADE1 isoforms during epithelial cell recovery23.
The biological role of JADE1 has not been established. There is a limited number of publications that attempt to address this question using adult and embryonic mice models. The most informative remains the earlier study which identified the mouse ortholog of human JADE1, Jade1, and described its expression during mouse embryogenesis3. Searching for developmentally regulated genes expressed in the primitive streak and tail bud, the authors of the study used the gene trap screen and identified mouse Jade1 as the gene strongly regulated during embryogenesis. Insertion of the vector occurred in the third intron of the mJade1 gene, leading to the production of a 47-amino-acid truncated protein. The gene trap insertional mutation resulted in JADE1-beta-galactosidase reporter fusion product and JADE1 null allele. While the homozygotes for the gene trap integration did not produce a strong developmental phenotype, the fusion product allowed detailed and precise examination of Jade1 gene spatial-temporal expression in mouse embryonic cells and tissues of the developing embryo up to 15.5-d.p.c. In addition, the study reports experimental and in silico comparative analysis of JADE1 mRNA transcripts, JADE1 gene structure, and JADE1 protein orthologs from mouse, human and zebra fish3.
According to the study, strong mJade1 expression was detected in extraembryonic ectoderm and trophoblast, which are placental components important for vasculogenesis, as well as in sites enriched with multipotent or tissue-specific progenitors, including neural progenitors3. The dynamics of mJade1-b-Gal expression in these areas indicates that JADE1 may be involved in determination and elongation of the anterior posterior axis, which was one of the important suggestions of the study3. The potential role for JADE1 in the renewal of embryonic stem cells and embryonal carcinoma cells was suggested in another screening study which used cultured cell models. According to that study, the activation of stem cell transcription factor OCT4 pathway upregulated JADE1 gene expression along with stem cell factors NANOG, PHC1, USP44 and SOX260. It would be interesting to investigate whether there is a biological relevance between JADE1 functions in the cell cycle and JADE1 activities communicated in these two studies.
IV-2. Human and Diseases
The potential role of JADE1 in human disease is under investigation. As a novel cell cycle regulator and candidate transcription factor, JADE1 represents an interesting pathway to explore the pathogenesis of epithelial cancers and regenerative disorders. In fact, two of the JADE1 partners and several proteins closely related to JADE1 are mutated in cancers and are tumor suppressors50, 61. An interesting recent study was set out to search for submicroscopic genetic changes in myelofibrosis which is a bone marrow cancer62. The study identified seven novel deletions and translocations in five out of a total of 15 patients with primary myelofibrosis. JADE1 along with the adjacent gene called Sodium channel and clathrin linker 1 (SCLT1) were found to be significantly modified. The resultant mutated JADE1 gene has deletions of intron 5-6 and exons 6-11 which would result in mutated JADE1 missing a large chunk of protein starting from the PHD zinc finger. The relevance to pathogenesis is under investigation.
In a handful of pilot studies JADE1 expression was examined in colon cancers and renal carcinomas. The results of these studies do not always reconcile perhaps due to the fact that they were generated predominantly from the histochemical analysis of tumor specimens using JADE1 antibody with uncharacterized specificities towards JADE1 in general, and JADE1S or JADE1L in particular63, 64. Results of a study using in silico microarray algorithm analysis show that PHF17 mRNA may play a role in the development of pancreatic cancer65. While promising, these lines of investigation require further controls and additional assessments to warrant any conclusions.
IV-3. Challenges and Future Directions
The understanding of the mechanism of cell cycle regulation by JADE1 is a necessary prerequisite for the clarification of the JADE1 cellular and biological function. The puzzling relationship between JADE1S and JADE1L isoforms and their specific functions appears to be especially important. Major points to tackle would be centrosome- and midbody- associated activities of JADE1 in cytokinesis as well as chromatin-dependent regulation of DNA synthesis. JADE1 transcription activities and targets remain to be determined. The cell cycle activities of JADE1, including phosphorylation and shuttling, HAT-dependent epigenetic control of DNA synthesis, and cytokinesis control presumably via NoCut final abscission check point suggest the in vivo role of JADE1 in epithelial tissue regeneration, homeostasis and in proliferative diseases. The role of cytokinesis control in cancer pathogenesis has been recently appreciated and is under active investigation. Therefore, JADE1 might represent one of the new key pharmacological targets for anticancer drug development. Use of various mice models, including JADE1 knock out will be instrumental to address these questions. Controlled human studies examining potential disease-relevant mutations of the JADE1 gene and the expression of JADE1 isoform-specific mRNA and protein will help to determine their role in cancer. Very little is known about the two other members of the JADE family, JADE2 and JADE3. Except for the variable C-terminal tail, the amino acid sequences of JADE family proteins are highly homologous. Hence, the similarity suggests an interchangeable compensatory role, while the variability could define tissue distribution or specific pathways involved. Future investigations of JADE2 and JADE3 will facilitate our understanding of the cellular and biological role of JADE1 as well as the JADE family.
Highlights.
PHD zinc finger proteins JADE1, JADE2, and JADE3 make up a small family of proteins.
JADE1 facilitates bulk histone H4 acetylation via interaction with an endogenous HAT.
JADE1S and JADE1L isoforms of JADE1 appear to play different cellular roles.
JADE1 proteins directly regulate cell cycle progression.
JADE1S is a negative regulator of cytokinesis and possibly the NoCut checkpoint.
Acknowledgements
This review and the corresponding Gene Wiki article are written as part of the Gene Wiki Review series--a series resulting from a collaboration between the journal GENE and the Gene Wiki Initiative. The Gene Wiki Initiative is supported by National Institutes of Health (GM089820). Additional support for Gene Wiki Reviews is provided by Elsevier, the publisher of GENE. The corresponding Gene Wiki entry for this review can be found here: <https://en.wikipedia.org/wiki/JADE1>
This work was supported by National Institutes of Health Grant RO1 DK087910 (MVP).
The author would like to thank Martin Steffen for help in generating quantitation results presented in Figure 1.
Abbreviations
- JADE1/2/3
Gene for Apoptosis and Differentiation 1/2/3
- PHF15/16/17
Plant Homeodomain Factor 15/16/17
- PHD
Plant Homeo Domain
- RING finger
Really Interesting New Gene
- pVHL
Von Hippel Lindau protein
- MYST family
the name of the HAT family is abbreviated from the names of the original members (MOZ, Ybf2 (Sas3), Sas2, and Tip60). Other members of MYST family are HBO1, MOF, MORF and Esa1.
- HBO1
HAT Binding Origin of Replication Complex1
- TIP60
HIV1 Tat interacting protein
- ING4, ING5
Inhibitor of Growth 4 and 5
- MCM7
Mini-chromosome maintenance protein 7
- BRD4
Bromodomain-containing protein 4
- BR140/BRPF1
bromodomain and PHD finger-containing protein 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nagase T, Nakayama M, Nakajima D, Kikuno R, Ohara O. Prediction of the coding sequences of unidentified human genes. XX. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro. DNA research : an international journal for rapid publication of reports on genes and genomes. 2001;8:85–95. doi: 10.1093/dnares/8.2.85. [DOI] [PubMed] [Google Scholar]
- 2.Zhou MI, Wang H, Ross JJ, Kuzmin I, Xu C, Cohen HT. The von Hippel-Lindau tumor suppressor stabilizes novel plant homeodomain protein Jade-1. J Biol Chem. 2002;277:39887–39898. doi: 10.1074/jbc.M205040200. [DOI] [PubMed] [Google Scholar]
- 3.Tzouanacou E, Tweedie S, Wilson V. Identification of Jade1, a gene encoding a PHD zinc finger protein, in a gene trap mutagenesis screen for genes involved in anteroposterior axis development. Mol Cell Biol. 2003;23:8553–8552. doi: 10.1128/MCB.23.23.8553-8562.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stothard P. The sequence manipulation suite: JavaScript programs for analyzing and formatting protein and DNA sequences. Biotechniques. 2000;28:1102–1104. doi: 10.2144/00286ir01. [DOI] [PubMed] [Google Scholar]
- 5.Siriwardana NS, Meyer R, Havasi A, Dominguez I, Panchenko MV. Cell cycle-dependent chromatin shuttling of HBO1-JADE1 histone acetyl transferase (HAT) complex. Cell Cycle. 2014;13:1885–1901. doi: 10.4161/cc.28759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siriwardana NS, Meyer RD, Panchenko MV. The novel function of JADE1S in cytokinesis of epithelial cells. Cell Cycle. 2015;14:2821–2834. doi: 10.1080/15384101.2015.1068476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borgal L, Rinschen MM, Dafinger C, Hoff S, Reinert MJ, Lamkemeyer T, Lienkamp SS, Benzing T, Schermer B. Casein kinase 1 alpha phosphorylates the Wnt regulator Jade-1 and modulates its activity. J Biol Chem. 2014;289:26344–26356. doi: 10.1074/jbc.M114.562165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson KA, Wang B, Argraves WS, Giancotti FG, Schranck DP, Ruoslahti E. BR140, a novel zinc-finger protein with homology to the TAF250 subunit of TFIID. Biochem Biophys Res Commun. 1994;198:1143–1152. doi: 10.1006/bbrc.1994.1162. [DOI] [PubMed] [Google Scholar]
- 9.Szelei J, Soto AM, Geck P, Desronvil M, Prechtl NV, Weill BC, Sonnenschein C. Identification of human estrogeninducible transcripts that potentially mediate the apoptotic response in breast cancer. J Steroid Biochem Mol Biol. 2000;72:89–102. doi: 10.1016/s0960-0760(00)00025-x. [DOI] [PubMed] [Google Scholar]
- 10.Aasland R, Gibson TJ, Stewart AF. The PHD finger: implications for chromatin-mediated transcriptional regulation. Trends Biochem Sci. 1995;20:56–59. doi: 10.1016/s0968-0004(00)88957-4. [DOI] [PubMed] [Google Scholar]
- 11.Ragvin A, Valvatne H, Erdal S, Arskog V, Tufteland KR, Breen K, AM OY, Eberharter A, Gibson TJ, Becker PB, Aasland R. Nucleosome binding by the bromodomain and PHD finger of the transcriptional cofactor p300. J Mol Biol. 2004;337:773–788. doi: 10.1016/j.jmb.2004.01.051. [DOI] [PubMed] [Google Scholar]
- 12.Wysocka J, Swigut T, Xiao H, Milne TA, Kwon SY, Landry J, Kauer M, Tackett AJ, Chait BT, Badenhorst P, Wu C, Allis CD. A PHD finger of NURF couples histone H3 lysine 4 trimethylation with chromatin remodelling. Nature. 2006;442:86–90. doi: 10.1038/nature04815. [DOI] [PubMed] [Google Scholar]
- 13.Shi X, Hong T, Walter KL, Ewalt M, Michishita E, Hung T, Carney D, Pena P, Lan F, Kaadige MR, Lacoste N, Cayrou C, Davrazou F, Saha A, Cairns BR, Ayer DE, Kutateladze TG, Shi Y, Cote J, Chua KF, Gozani O. ING2 PHD domain links histone H3 lysine 4 methylation to active gene repression. Nature. 2006;442:96–99. doi: 10.1038/nature04835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taverna SD, Ilin S, Rogers RS, Tanny JC, Lavender H, Li H, Baker L, Boyle J, Blair LP, Chait BT, Patel DJ, Aitchison JD, Tackett AJ, Allis CD. Yng1 PHD finger binding to H3 trimethylated at K4 promotes NuA3 HAT activity at K14 of H3 and transcription at a subset of targeted ORFs. Mol Cell. 2006;24:785–796. doi: 10.1016/j.molcel.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanchez R, Zhou MM. The PHD finger: a versatile epigenome reader. Trends Biochem Sci. 2011;36:364–372. doi: 10.1016/j.tibs.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwan AH, Gell DA, Verger A, Crossley M, Matthews JM, Mackay JP. Engineering a protein scaffold from a PHD finger. Structure (Camb) 2003;11:803–813. doi: 10.1016/s0969-2126(03)00122-9. [DOI] [PubMed] [Google Scholar]
- 17.Mansfield RE, Musselman CA, Kwan AH, Oliver SS, Garske AL, Davrazou F, Denu JM, Kutateladze TG, Mackay JP. Plant homeodomain (PHD) fingers of CHD4 are histone H3-binding modules with preference for unmodified H3K4 and methylated H3K9. J Biol Chem. 2011;286:11779–11791. doi: 10.1074/jbc.M110.208207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matthews JM, Bhati M, Lehtomaki E, Mansfield RE, Cubeddu L, Mackay JP. It takes two to tango: the structure and function of LIM, RING, PHD and MYND domains. Current pharmaceutical design. 2009;15:3681–3696. doi: 10.2174/138161209789271861. [DOI] [PubMed] [Google Scholar]
- 19.Lalonde ME, Avvakumov N, Glass KC, Joncas FH, Saksouk N, Holliday M, Paquet E, Yan K, Tong Q, Klein BJ, Tan S, Yang XJ, Kutateladze TG, Cote J. Exchange of associated factors directs a switch in HBO1 acetyltransferase histone tail specificity. Genes Dev. 2013;27:2009–2024. doi: 10.1101/gad.223396.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saksouk N, Avvakumov N, Champagne KS, Hung T, Doyon Y, Cayrou C, Paquet E, Ullah M, Landry AJ, Cote V, Yang XJ, Gozani O, Kutateladze TG, Cote J. HBO1 HAT complexes target chromatin throughout gene coding regions via multiple PHD finger interactions with histone H3 tail. Mol Cell. 2009;33:257–265. doi: 10.1016/j.molcel.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Avvakumov N, Lalonde ME, Saksouk N, Paquet E, Glass KC, Landry AJ, Doyon Y, Cayrou C, Robitaille GA, Richard DE, Yang XJ, Kutateladze TG, Cote J. Conserved molecular interactions within the HBO1 acetyltransferase complexes regulate cell proliferation. Mol Cell Biol. 2012;32:689–703. doi: 10.1128/MCB.06455-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panchenko MV, Zhou MI, Cohen HT. von Hippel-Lindau partner Jade-1 is a transcriptional co-activator associated with histone acetyltransferase activity. J Biol Chem. 2004;279:56032–56041. doi: 10.1074/jbc.M410487200. [DOI] [PubMed] [Google Scholar]
- 23.Havasi A, Haegele JA, Gall JM, Blackmon S, Ichimura T, Bonegio RG, Panchenko MV. Histone acetyl transferase (HAT) HBO1 and JADE1 in epithelial cell regeneration. Am J Pathol. 2013;182:152–162. doi: 10.1016/j.ajpath.2012.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doyon Y, Cayrou C, Ullah M, Landry AJ, Cote V, Selleck W, Lane WS, Tan S, Yang XJ, Cote J. ING tumor suppressor proteins are critical regulators of chromatin acetylation required for genome expression and perpetuation. Mol Cell. 2006;21:51–64. doi: 10.1016/j.molcel.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 25.Foy RL, Song IY, Chitalia VC, Cohen HT, Saksouk N, Cayrou C, Vaziri C, Cote J, Panchenko MV. Role of Jade-1 in the histone acetyltransferase (HAT) HBO1 complex. J Biol Chem. 2008;283:28817–28826. doi: 10.1074/jbc.M801407200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Megee PC, Morgan BA, Smith MM. Histone H4 and the maintenance of genome integrity. Genes Dev. 1995;9:1716–1727. doi: 10.1101/gad.9.14.1716. [DOI] [PubMed] [Google Scholar]
- 27.Maki N, Tsonis PA, Agata K. Changes in global histone modifications during dedifferentiation in newt lens regeneration. Molecular vision. 2010;16:1893–1897. [PMC free article] [PubMed] [Google Scholar]
- 28.Jasencakova Z, Meister A, Walter J, Turner BM, Schubert I. Histone H4 acetylation of euchromatin and heterochromatin is cell cycle dependent and correlated with replication rather than with transcription. Plant Cell. 2000;12:2087–2100. doi: 10.1105/tpc.12.11.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clarke AS, Lowell JE, Jacobson SJ, Pillus L. Esa1p is an essential histone acetyltransferase required for cell cycle progression. Mol Cell Biol. 1999;19:2515–2526. doi: 10.1128/mcb.19.4.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choy JS, Tobe BT, Huh JH, Kron SJ. Yng2p-dependent NuA4 histone H4 acetylation activity is required for mitotic and meiotic progression. J Biol Chem. 2001;276:43653–43662. doi: 10.1074/jbc.M102531200. [DOI] [PubMed] [Google Scholar]
- 31.Calvi BR. HBO1:JADE1 at the cell cycle chromatin crossroads. Cell Cycle. 2014;13 doi: 10.4161/cc.29832. [DOI] [PubMed] [Google Scholar]
- 32.Miotto B, Struhl K. HBO1 histone acetylase activity is essential for DNA replication licensing and inhibited by Geminin. Mol Cell. 2010;37:57–66. doi: 10.1016/j.molcel.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iizuka M, Matsui T, Takisawa H, Smith MM. Regulation of replication licensing by acetyltransferase Hbo1. Mol Cell Biol. 2006;26:1098–1108. doi: 10.1128/MCB.26.3.1098-1108.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wan G, Hu X, Liu Y, Han C, Sood AK, Calin GA, Zhang X, Lu X. A novel non-coding RNA lncRNA-JADE connects DNA damage signalling to histone H4 acetylation. Embo J. 2013;32:2833–2847. doi: 10.1038/emboj.2013.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borgal L, Habbig S, Hatzold J, Liebau MC, Dafinger C, Sacarea I, Hammerschmidt M, Benzing T, Schermer B. The ciliary protein nephrocystin-4 translocates the canonical Wnt regulator Jade-1 to the nucleus to negatively regulate beta-catenin signaling. J Biol Chem. 2012;287:25370–25380. doi: 10.1074/jbc.M112.385658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prekeris R. Cut or NoCut: the Role of JADE1S in Regulating Abscission Checkpoint. Cell Cycle. 2015;0 doi: 10.1080/15384101.2015.1089074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Agromayor M, Martin-Serrano J. Knowing when to cut and run: mechanisms that control cytokinetic abscission. Trends Cell Biol. 2013;23:433–441. doi: 10.1016/j.tcb.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 38.Elia N, Sougrat R, Spurlin TA, Hurley JH, Lippincott-Schwartz J. Dynamics of endosomal sorting complex required for transport (ESCRT) machinery during cytokinesis and its role in abscission. Proc Natl Acad Sci U S A. 2011;108:4846–4851. doi: 10.1073/pnas.1102714108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fabbro M, Zhou BB, Takahashi M, Sarcevic B, Lal P, Graham ME, Gabrielli BG, Robinson PJ, Nigg EA, Ono Y, Khanna KK. Cdk1/Erk2- and Plk1-dependent phosphorylation of a centrosome protein, Cep55, is required for its recruitment to midbody and cytokinesis. Developmental cell. 2005;9:477–488. doi: 10.1016/j.devcel.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 40.Green RA, Paluch E, Oegema K. Cytokinesis in animal cells. Annual review of cell and developmental biology. 2012;28:29–58. doi: 10.1146/annurev-cellbio-101011-155718. [DOI] [PubMed] [Google Scholar]
- 41.Hu CK, Coughlin M, Mitchison TJ. Midbody assembly and its regulation during cytokinesis. Mol Biol Cell. 2012;23:1024–1034. doi: 10.1091/mbc.E11-08-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nigg EA, Stearns T. The centrosome cycle: Centriole biogenesis, duplication and inherent asymmetries. Nat Cell Biol. 2011;13:1154–1160. doi: 10.1038/ncb2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sluder G, Khodjakov A. Centriole duplication: analogue control in a digital age. Cell biology international. 2010;34:1239–1245. doi: 10.1042/CBI20100612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bailey JK, Fields AT, Cheng K, Lee A, Wagenaar E, Lagrois R, Schmidt B, Xia B, Ma D. WD repeat-containing protein 5 (WDR5) localizes to the midbody and regulates abscission. J Biol Chem. 2015;290:8987–9001. doi: 10.1074/jbc.M114.623611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.You J, Li Q, Wu C, Kim J, Ottinger M, Howley PM. Regulation of aurora B expression by the bromodomain protein Brd4. Mol Cell Biol. 2009;29:5094–5103. doi: 10.1128/MCB.00299-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thoresen SB, Campsteijn C, Vietri M, Schink KO, Liestol K, Andersen JS, Raiborg C, Stenmark H. ANCHR mediates Aurora-B-dependent abscission checkpoint control through retention of VPS4. Nature cell biology. 2014;16:550–560. doi: 10.1038/ncb2959. [DOI] [PubMed] [Google Scholar]
- 47.Fu J, Hagan IM, Glover DM. The centrosome and its duplication cycle. Cold Spring Harbor perspectives in biology. 2015;7:a015800. doi: 10.1101/cshperspect.a015800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ling H, Peng L, Seto E, Fukasawa K. Suppression of centrosome duplication and amplification by deacetylases. Cell Cycle. 2012;11:3779–3791. doi: 10.4161/cc.21985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sakai H, Urano T, Ookata K, Kim MH, Hirai Y, Saito M, Nojima Y, Ishikawa F. MBD3 and HDAC1, two components of the NuRD complex, are localized at Aurora-A-positive centrosomes in M phase. J Biol Chem. 2002;277:48714–48723. doi: 10.1074/jbc.M208461200. [DOI] [PubMed] [Google Scholar]
- 50.Crossey PA, Richards FM, Foster K, Green JS, Prowse A, Latif F, Lerman MI, Zbar B, Affara NA, Ferguson-Smith MA, et al. Identification of intragenic mutations in the von Hippel-Lindau disease tumour suppressor gene and correlation with disease phenotype. Hum Mol Genet. 1994;3:1303–1308. doi: 10.1093/hmg/3.8.1303. [DOI] [PubMed] [Google Scholar]
- 51.Foster K, Prowse A, van den Berg A, Fleming S, Hulsbeek MM, Crossey PA, Richards FM, Cairns P, Affara NA, Ferguson-Smith MA, et al. Somatic mutations of the von Hippel-Lindau disease tumour suppressor gene in non-familial clear cell renal carcinoma. Hum Mol Genet. 1994;3:2169–2173. doi: 10.1093/hmg/3.12.2169. [DOI] [PubMed] [Google Scholar]
- 52.Duan DR, Humphrey JS, Chen DY, Weng Y, Sukegawa J, Lee S, Gnarra JR, Linehan WM, Klausner RD. Characterization of the VHL tumor suppressor gene product: localization, complex formation, and the effect of natural inactivating mutations. Proc Natl Acad Sci U S A. 1995;92:6459–6463. doi: 10.1073/pnas.92.14.6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 54.Latif F, Tory K, Gnarra J, Yao M, Duh FM, Orcutt ML, Stackhouse T, Kuzmin I, Modi W, Geil L, et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993;260:1317–1320. doi: 10.1126/science.8493574. [DOI] [PubMed] [Google Scholar]
- 55.Chitalia VC, Foy RL, Bachschmid MM, Zeng L, Panchenko MV, Zhou MI, Bharti A, Seldin DC, Lecker SH, Dominguez I, Cohen HT. Jade-1 inhibits Wnt signalling by ubiquitylating beta-catenin and mediates Wnt pathway inhibition by pVHL. Nat Cell Biol. 2008;10:1208–1216. doi: 10.1038/ncb1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou MI, Foy RL, Chitalia VC, Zhao J, Panchenko MV, Wang H, Cohen HT. Jade-1, a candidate renal tumor suppressor that promotes apoptosis. Proc Natl Acad Sci U S A. 2005;102:11035–11040. doi: 10.1073/pnas.0500757102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou MI, Wang H, Foy RL, Ross JJ, Cohen HT. Tumor Suppressor von Hippel-Lindau (VHL) Stabilization of Jade-1 Protein Occurs through Plant Homeodomains and Is VHL Mutation Dependent. Cancer Res. 2004;64:1278–1286. doi: 10.1158/0008-5472.can-03-0884. [DOI] [PubMed] [Google Scholar]
- 58.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 59.Gossage L, Eisen T, Maher ER. VHL, the story of a tumour suppressor gene. Nat Rev Cancer. 2015;15:55–64. doi: 10.1038/nrc3844. [DOI] [PubMed] [Google Scholar]
- 60.Jung M, Peterson H, Chavez L, Kahlem P, Lehrach H, Vilo J, Adjaye J. A data integration approach to mapping OCT4 gene regulatory networks operative in embryonic stem cells and embryonal carcinoma cells. PLoS One. 2010;5:e10709. doi: 10.1371/journal.pone.0010709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Soliman MA, Riabowol K. After a decade of study-ING, a PHD for a versatile family of proteins. Trends Biochem Sci. 2007;32:509–519. doi: 10.1016/j.tibs.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 62.Lasho T, Johnson SH, Smith DI, Crispino JD, Pardanani A, Vasmatzis G, Tefferi A. Identification of submicroscopic genetic changes and precise breakpoint mapping in myelofibrosis using high resolution mate-pair sequencing. American journal of hematology. 2013;88:741–746. doi: 10.1002/ajh.23495. [DOI] [PubMed] [Google Scholar]
- 63.Lian X, Duan X, Wu X, Li C, Chen S, Wang S, Cai Y, Weng Z. Expression and clinical significance of von Hippel-Lindau downstream genes: Jade-1 and beta-catenin related to renal cell carcinoma. Urology. 2012;80:485, e487–413. doi: 10.1016/j.urology.2012.02.024. [DOI] [PubMed] [Google Scholar]
- 64.Lim SR, Gooi BH, Singh M, Gam LH. Analysis of differentially expressed proteins in colorectal cancer using hydroxyapatite column and SDS-PAGE. Applied biochemistry and biotechnology. 2011;165:1211–1224. doi: 10.1007/s12010-011-9339-3. [DOI] [PubMed] [Google Scholar]
- 65.Liu PF, Jiang WH, Han YT, He LF, Zhang HL, Ren H. Integrated microRNA-mRNA analysis of pancreatic ductal adenocarcinoma. Genetics and molecular research : GMR. 2015;14:10288–10297. doi: 10.4238/2015.August.28.14. [DOI] [PubMed] [Google Scholar]