Abstract
Type 1 diabetes (T1D) is an autoimmune disorder characterized by the destruction of insulin-producing pancreatic β cells. Immune modulators have achieved some success in modifying the course of disease progression in T1D. However, there are parallel declines in C-peptide levels in treated and control groups after initial responses. In this review, we discuss mechanisms of β cell death in T1D that involve necrosis and apoptosis. New technologies are being developed to enable visualization of insulitis and β cell mass involving positron emission transmission that identifies β cell ligands and magnetic resonance imaging that can identify vascular leakage. Molecular signatures that identify β cell derived insulin DNA that is released from dying cells have been described and applied to clinical settings. We also consider changes in β cells that occur during disease progression including the induction of DNA methyltransferases that may affect the function and differentiation of β cells. Our findings from newer data suggest that the model of chronic long standing β cell killing should be reconsidered. These studies indicate that the pathophysiology is accelerated in the peridiagnosis period and manifest by increased rates of β cell killing and insulin secretory impairments over a shorter period than previously thought. Finally, we consider cellular explanations to account for the ongoing loss of insulin production despite continued immune therapy that may identify potential targets for treatment. The progressive decline in β cell function raises the question as to whether β cell failure that is independent of immune attack may be involved.
Keywords: Type 1 diabetes, β cell death, Biomarkers, Immune therapy, Imaging, Neogenesis, autoimmunity, regeneration
1. Introduction
Despite the success of immune therapies in modifying the short term course of Type 1 diabetes (T1D), these treatments have not achieved long-term retention of insulin production, and their ability to improve clinical outcomes is uncertain. Therapies such as teplizumab and otelixizumab, that target the ε chain of the CD3 molecule on T cells, abatacept, that blocks CD28 costimulation by binding to B7.1 and B7.2, alefacept (soluble LFA3Ig) that binds CD2 and depletes T cells, and rituximab, that binds CD20 and depletes B cells have all significantly improved C-peptide responses and even glucose control with reduced use of exogenous insulin for 1 to 4 years compared to control groups(1-12). However, 6 months to a year after treatment, a decline in C-peptide responses occurred despite continuous administration of abatacept or re-administration of teplizumab. One possible explanation for the decline is that intrinsic β cellular factors that activate β cell death are involved. Previous notions of complete β cell death/ablation have become uncertain since a number of recent clinical studies have identified significant residual β cell function in individuals with long standing T1D(13; 14). This indicates that in many individuals, either β cell killing ceases or β cell recovery occurs. In this review, we consider the mechanisms of β cell death in T1D, and changes to the cells that occur during disease progression. These mechanisms are relevant to the long term function and survival of β cells.
2. Mechanisms of β cell death in T1D
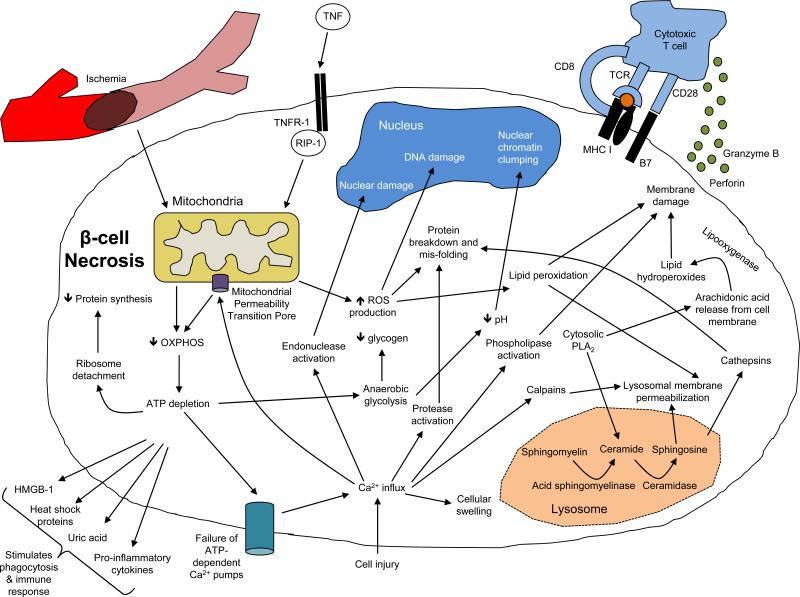
The ways in which β cells die may determine whether immune responses are activated. Necrotic cell death is considered to be a likely mechanism whereby cytolytic T cells, including those reactive with diabetes antigens, cause killing (Figure 1). Findings from pancreatic biopsies and post-mortem studies of whole organs from islet donors are consistent with this mechanism, showing a predominance of infiltrating CD8+ T cells and macrophages which mediate necrosis(15). Necrosis can occur following the release of cytolytic granules from T cells that contain granzymes and perforin, which act on the β cell membrane. Ischemia resulting from impaired vascularity may also lead to necrotic cell death, and impaired vascular supply occurs in the islets of prediabetic NOD mice(16). Common mediators of necrotic cell death include calcium and reactive oxygen species (ROS). In particular, ROS results in mitochondrial injury as well as loss of both cell membrane integrity and ion balance through protein damage, lipid peroxidation and oxidative DNA damage(17; 18). These factors lead to opening of mitochondrial permeability transition pores, which in turn disrupts oxidative phosphorylation and ATP production. DNA damage leads to poly (ADP-ribose) polymerase activation and increased NAD consumption and thus reduced ATP production (19). The serine/threonine kinase receptor-interacting protein 1 (RIP1) is another initiator of necrotic cell death that is induced by ligand-receptor interactions. ATP depletion plays a crucial role as an initiating factor in this process(20; 21). Other factors including the lyososmal enzyme acid-sphingomyelinase, cytosolic phospholipase A(2) and calpains, a Ca2-dependent cysteine protease family, all of which contribute to cell membrane destabilization. Necrotic cells release cellular contents including factors, such as high mobility group box 1 proteins (HMGB-1), heat shock proteins, uric acid and pro-inflammatory cytokines, which lead to recognition and engulfment by phagocytes and immunologic activity. Ultimately, necrotic death of β cells may lead to a “feed-forward” mechanism whereby the released factors stimulate immune responses even after tolerance has been established or the immune response has been treated(22; 23).
Figure 1.
Pathways of necrotic β cell death. Please see text for details
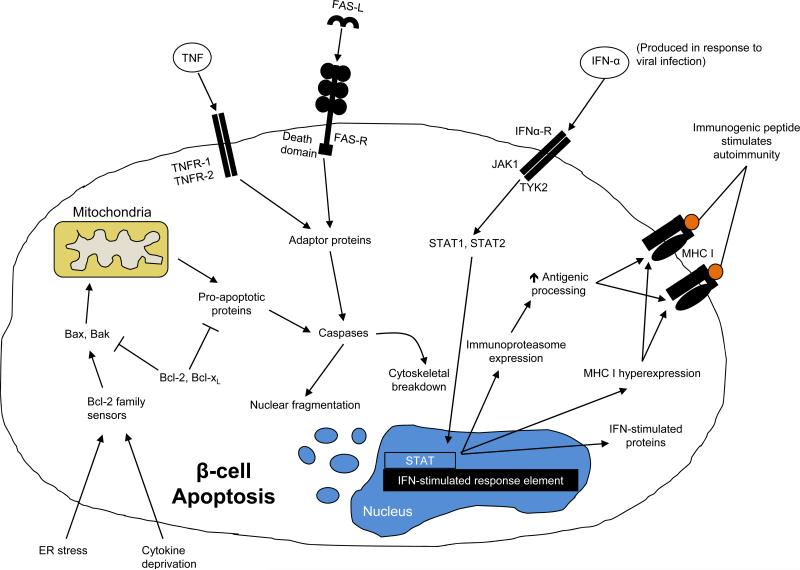
In addition to necrosis, genetic pathway studies have implicated roles for JAK1, JAK2, and TYK2, suggesting that apoptosis may also be involved in β cell death (24)(Figure 2). Apoptosis of β cells may be initiated by cytokines such as TNF that bind and activate TNFR1, TNFR2, or by Fas/FasL activation. When apoptosis is initiated through the BCL-2 pathway by cytokine deprivation or endoplasmic reticulum stress, BIM, PUMA, and BID inhibit the pro-survival proteins of the pathway and activate both BAX and BAK, which in turn lead to caspase activation. Apoptosis due to the activity of caspases leads to apoptotic cell morphology, nuclear fragmentation, and chromatin condensation. In histologic sections from diabetic patients and pre-diabetic NOD mice, evidence for both necrosis and apoptosis have been suggested by expression of caspase 3 and TUNEL+ cells. Caspase-induced nuclear fragmentation may eliminate nucleic acid trails of β cell destruction (see below).
Figure 2.
Pathways of apoptotic β cell death. Please see text for details.
β cell death most likely involves both mechanisms. Apoptosis and necrosis have been observed in cytokine-induced islet cell death, as well as following activation of the death receptors FasL and TNF. TNFR1 is expressed by β cells from NOD mice before the onset of insulitis and there is a significant increase in Fas and TNFR2 expression with cellular infiltrates (25; 26). This apoptotic pathway is active in the islets of NOD mice following ligation of Fas/FasL or TNFR1. A dominant negative Fas-associating protein with death domain (dnFADD) could prevent apoptosis induced by TNF and IFNγ in NIT-1 cells. In addition, studies in non-obese diabetes (NOD) mice suggest that β cells undergo necrotic cell death. Cytokines such as IFN-γ and IL-1β can induce nitric oxide, which contributes to β cell necrosis(15; 27-29).
3. Visualization of β cell mass and inflammation
The pathologic progression and killing of β cells cannot be appreciated with metabolic studies alone. A number of environmental factors are known to modify β cell function, including fatty acids, glucose, as well as insulin sensitivity, which changes with adolescence(30-32). The acute changes in β cells after onset and with immune therapy have been difficult to identify because of the absence of direct measures of inflammation and β cell mass. In a study of the islets in NOD mice at the time of onset of hyperglycemia, we found that there were many degranulated β cells that could recover their granularity and the ability to produce insulin after immune therapy(33).
Islet inflammation has been visualized using MRI-detectable magnetic nanoparticles (MNP) as vascular probes(34; 35). Accumulation of the probe in the pancreas was correlated with the microvascular changes, including leakage with uptake of the particles by macrophages, in addition to monocyte recruitment and activation. When NOD mice were treated with anti-CD3 mAb, MNP-MRI imaging of pancreatic inflammation, showed reduced leakiness of the microvasculature and was able to predict clinical responses in female NOD mice (34). These investigators showed a similar proof-of-concept in humans, showing that the accumulation of ferumoxytol, an FDA-approved nanoparticle in local macrophages in the inflamed pancreatic lesions. They then used high-resolution 3-dimensional maps to clearly visualize differences in whole pancreas nanoparticle accumulation in 11 auto-antibody positive, recent-onset T1D patients and 10 healthy controls. The results showed a high degree of variability but a statistically significantly higher ΔR2 value in T1D patients relative to healthy controls, a metric representing pancreas-wide quantification of nanoparticle uptake.
Accurate quantification of β cell mass that distinguishes between anatomic and functional losses is also important. PET imaging of the vesicular monamine transporter (VMAT), which is selectively expressed on β cell granules containing insulin, represents one such approach(36-39). VMAT2 is stably transcribed in vitro under a range of glucose and lipid concentrations. The density of VMAT2 can be quantified using PET imaging, using PET tracer ligands such as 18F-FP-(+)-DTBZ that bind to VMAT2 with high affinity. PET quantitation of VMAT2 in the pancreas with the tracer 18F-FP-(+)-DTBZ was shown to effectively differentiate β cell mass between T1D patients and healthy controls. In addition, PET imaging with the dopamine type 2 receptor (D2R), which like VMAT2 is selectively expressed on β cells in a pattern that overlaps with insulin staining, has been shown to serve as a biomarker of β cell mass in rodents.
Other strategies in development include a radiotracer imaging method for measuring β cell mass in mice based on a near-infrared fluorescent imaging agent using a neopeptide (4x12-VT750), which has binding properties of exendin-4(40). Finally, a distinct approach employs magnetic resonance imaging (MRI), which requires the abundant uptake of a β cell-specific, non-toxic and stable contrast agent with high intensity. MRI has the potential to differentiate pancreatic islets from the surrounding exocrine parenchyma, and this has been tested using manganese (Mn2+) as a contrast agent, which enters pancreatic β cells through voltage gated Ca2+ channels in a glucose-dependent manner(41).
4. Identifying β cell killing with molecular signatures
Unlike cells that do not transcribe insulin, CpG sites in the INS gene in β cells are generally unmethylated(42). We took advantage of this epigenetic feature to identify β cells that had died and released their unmethylated INS DNA into the serum. A nested PCR reaction was performed in which a sequence from the Ins1 or INS genes was first amplified with primers non-specific for CpG sites. Subsequently, the products of this reaction were used as template in a second reaction with primers specific for methylated or unmethylated CpG sites. By real-time PCR, the relative abundance of the two forms of the DNA was measured.
There was a 45-fold enrichment in the abundance of unmethylated CpG sites in bisulfite-treated DNA isolated from β cells purified by FACS compared to islet-derived non-β cells. The assay was used to measure unmethylated Ins1 DNA in the serum at NOD mice and a significant increase was found prior to the onset of hyperglycemia (at 11 and 14 weeks). Unmethylated INS DNA was also increased in human islets and in serum from patients with new-onset T1D relative to age-matched healthy control subjects (p<0.02). Hussein et al developed a similar assay for unmethylated INS DNA that targeted 5 differentially methylated sites in the promoter of the human INS gene and showed an increase in unmethylated INS DNA both 1 and 14 days after transplantation of human islets(43; 44), when compared to healthy control subjects.
The original nested PCR method was replaced with droplet digital PCR (ddPCR) to improve the specificity and sensitivity, and the relative abundance of unmethylated INS DNA was expressed as a ratio to methylated INS DNA(45). The probe targeted two methylation sensitive sites of the human gene at nucleotides the region +396 and +399 from the transcriptional start site. A significantly higher ratio of unmethylated INS DNA to methylated INS DNA was found in patients with recent-onset T1D compared to 39 non-diabetic subjects (P<0.0001). Using a ddPCR assay to target a region of CpG sites in preproinsulin, Fisher et al found that the absolute level of unmethylated and methylated INS DNA differed between patients with new onset T1D and controls. The levels of methylated INS DNA remained elevated at 8 weeks post-onset, but the levels of unmethylated INS DNA fell to levels that were similar to controls at 1year post-onset (46).
With these assays, β cell killing during disease development could be studied. The ddPCR assay was used to study euglycemic at-risk autoantibody+ individuals, relatives of patients with T1D, who did and did not develop T1D (47). Interestingly, while the median ratios were increased in those who progressed to T1D compared to non-diabetic control subjects, the differences between the at-risk progressors and non-progressors were modest. In addition, less than ¼ of the levels in the at-risk progressors were increased above the 95th percentile for control subjects over the 3-4 yr observation period. In spite of the low frequency, increased levels of unmethylated INS DNA were associated with impaired insulin secretion in the at-risk progressors but not the non-progressors.
Additionally, 30 individuals at very high risk were studied. This high-risk status was based on the presence of at least 2 autoantibodies and dysglycemia during an OGTT. (Approximately 75% of these subjects will develop diabetes within 5 years). The levels of unmethylated INS DNA were significantly higher in this group than the levels that had been found in individuals studied at earlier time points prior to diagnosis. In these subjects, rapid progression to overt diabetes was associated with a functional impairment in insulin secretion but the levels of unmethylated INS DNA did not discriminate between those who did and did not rapidly progress to overt diabetes suggesting that there was β cell dysfunction in the absence of increased β cell killing was involved in the appearance of hyperglycemia during the OGTT.
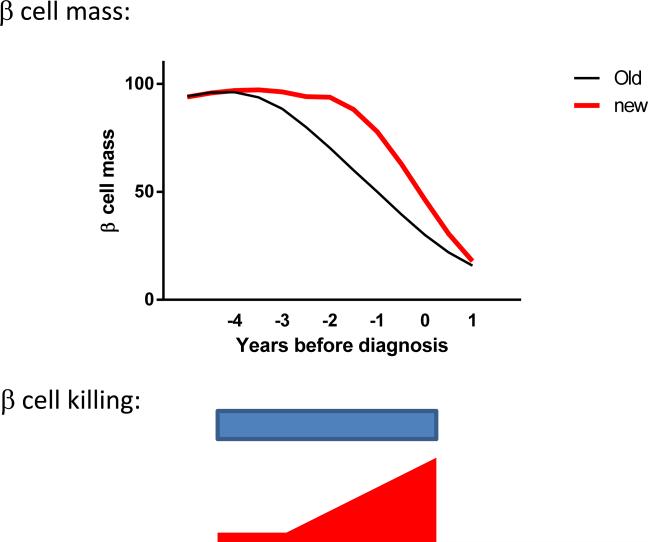
These findings suggest that previous models of diabetes progression may not accurately reflect the pathophysiology that is measured with newer techniques. β cell destruction is a relatively late event during diabetes progression after the first appearance of autoantibodies. Moreover, β cell killing is closely associated with deterioration of glucose tolerance although an impaired functional response is the final event leading to overt hyperglycemia (Figure 3).
Figure 3.
Modified model of the natural history of T1D. Studies of β cell death and metabolic studies indicate that the timing of β cell killing and metabolic decompensation may be more closely associated with the time of diagnosis than appreciated from the initial descriptions of the natural history of the disease(69-71)(47).
In addition, this method of detecting unmethylated INS DNA enabled us to determine whether successful immune therapies reduce β cell killing. In a study of patients with recent onset T1D who had been treated with anti-CD3 mAb (teplizumab), we compared the levels of unmethylated INS DNA to those randomized to placebo (48). In the sample of 37 patients, there was a significant improvement in C-peptide responses in the drug vs placebo treated subjects and a decline in the levels of β cell death. This study suggested that successful immune therapy does decrease the rates of β cell killing one year after therapy and that the recovery of β cells is not exclusively a functional improvement.
There are limitations to this approach to measure cell death. First, it can only be detected if DNA is released into the circulation. Necrotic cell death, which is associated with release of cellular contents and activation of an inflammatory response that may further increase cell killing, is likely to be detected. However, apoptosis, which does not involve the release of DNA into the circulation, may not be detectable. Second, the half-life of DNA in the circulation after its release from dying cells is relatively short. In a study of the clearance of a region of fetal DNA containing the Y gene, the DNA from the male offspring was undetectable by day 1 after delivery. Seven of 12 women had undetectable fetal DNA 2 hours postpartum. These investigators estimated that the half-life for circulating fetal DNA was 16.3 min (49). In our studies, based on the clearance of INS DNA following islet autotransplants, we estimated that the half life of the DNA was approximately 120 minutes indicating that the measurement of epigenetically modified INS DNA captures ongoing events over a brief period of time. Third, the sensitivity of the assay is an important factor since histologic studies have repeatedly shown that human insulitis is modest and does not have the robust cellular infiltrates and cell killing that characterizes insulitis in the NOD model. The ddPCR can measure about 210 copies in an aliquot of 300 μl of serum. Assuming a single cell DNA mass of 6.2 ng and that only 1 of the 2 copies of INS DNA are unmethylated and available for analysis, there are about 16,500 copies of DNA from all cell sources available for analysis (from 33,000 cells) in a 300μl aliquot of serum. Therefore, about 1.3% of the total DNA would need to be derived from β cells to be detected in this assay. Of note, in our studies of recipients of autologous islet transplants the dilution curve suggested that a positive signal could likely be detected from 355 islets but there may be INS DNA that is released from β cells that have died in the preparation prior to infusion.
Alternative approaches have been developed. Measurement of microRNAs (miRNA), have been studied as potential biomarker for progression of diabetes due both to their differential expression in diabetic patients and their stability in circulation. miR-375, which is important for normal β and α cell maintenance was investigated as a biomarker in vivo to detect β cell death using quantitative real-time PCR in both streptozotocin (STZ)-induced diabetic and NOD mouse models(50). In rats with STZ-induced diabetes, protein phosphatase 1 regulatory inhibitor subunit 1A (PPP1R1A) was depleted from injured insulin-producing β cells hours after treatment, which coincided with a maximal peak plasma PPP1R1A level at 4 hours(51). In 4 human diabetic patients receiving intraportal islet infusion, there was a post-transplant increase in plasma levels of PPP1R1A in 3 patients relative to control patients with acute damage to exocrine pancreas, brain or kidney. However, a concern with these approaches are their relative lack of selectivity, for example, PPP1R1A is also detectable at low levels in brain and muscle cells.
Circulating insulin mRNA has also been measured to detect islet cell death after transplantation(52; 53). Immediately after islet infusion, all (19) recipients of islet allografts showed an initial peak of insulin mRNA, with a mean duration of 4.2 days and amplitude of 510 copies/2.5 mL, indicating a release of the mRNA from β cells. However, the amplitude and duration of the primary peak neither correlated with the graft size nor the subsequent graft function. Secondary peaks of circulating insulin mRNA were observed in the follow-up period for 17 islet transplantations correlated with metabolic events. These events reflected increasing islet graft dysfunction, including significant increases in HbA1c or doubling of exogenous insulin requirements.
Changes in β cells under immune assault
The β cell dysfunction during the progression of T1D implies that there are acquired functional changes in the cells, possibly as a result of immunologic stressors or metabolic demand. Islet cells from individuals with T1D show a partial ER stress response with induction of some components of the unfolded protein response (54). Cytokines such as IL-1β, together with TNF and IFNγ have been found to inhibit insulin secretion in vitro(55-57). In addition, cytokines released by infiltrating immune cells may affect function of β cells. Our studies of insulitis in NOD mice have shown that cytokine transcription increased with age, and these cytokines can induce methylation marks in the insulin DNA by enhancing expression of DNA methyltransferases (submitted). There is reduced insulin gene expression with methylation marks, particularly, in mice, within Ins2exon1. Similar changes are induced in human β cells in vitro, with enhanced expression of DNMT3a by cytokines. Thus in addition to killing of cells, metabolic disturbances may also be a manifestation of functional impairments. Despite these strong preclinical findings, the results from trials of IL-1RA and anti-IL-1β mAb (Canakinumab), failed to show improvement in C-peptide responses in patients with new onset T1D(58). However, the timing of the intervention with the anti-IL-1 agents may have been too late to arrest killing that may be in an aggressive phase at onset.
Changes in β cells following immune therapy
Unfortunately, there is relatively little data concerning the mechanisms that are involved in the ongoing losses after immune therapy. However, apoptosis has been observed in patients with long standing T1D, which, these investigators speculate, is due to ongoing autoimmunity or possibly toxicity from external factors such as glucose (59). They also point out that in view of the ongoing cell death, there must be ongoing β cell replication. These and other observations from clinical trials suggest three hypotheses to explain the failure after immune therapy:
1) Loss of the immune responses to treatment
Recurrence of autoimmunity has been identified using Class I MHC tetramers in failing islet allografts suggesting that immune therapies may not be able to completely and permanently suppress autoreactive responses(60). Likewise, following treatment with rituximab and repopulation of peripheral immune cells, both autoreactive T and B cells were found(61). In this regard, immune suppression with more potent agents for longer duration may be considered but even with extensive immune depletion, autoreactive cells may reappear (62). In addition, the risk:benefit ratio may not be acceptable. Rather than depleting effector cells, agents that can restore immune tolerance may be preferable. Recent studies that enhance the number and function of regulatory T cells are examples of that strategy(63; 64).
2) Individual differences in responses to immune therapies
Post-hoc analyses of the clinical responses in trial have identified subgroups of individuals who do and do not respond to therapies. In our studies with teplizumab, C-peptide levels in the responders were maintained for 2 years after diagnosis. While the differences in effects in responders compared to non-responders or controls continued up to 9 years (unpublished observations) there was a decline in C-peptide levels in the responder group. Although not certain, early evidence suggests that the differences between the subgroups are immunologic in nature and identified soon after drug administration(65).
3) β cell failure that is independent of immune attack
The parallel track of decline in C-peptide in clinical trials in drug and placebo treated subjects after the initial immune response raises the possibility that ongoing β cell loss proceeds independent of the immune assault. The cell loss may occur through pathways that have been associated with aging and senescence including mitochondrial dysfunction, oxidative stress, kinase pathways, calcium dysregulation, inflammation, and protein handling or even involve the unfolding of mechanisms leading to cell death that began at the time that the disease was initiated(66). The impaired vasculature and other sequelae of the inflammatory events in insulitis may contribute to the failure of these normal reparative mechanisms. Studies of tissues from the time of diagnosis and afterwards would shed light on these processes. In addition to actual cell death, stressors may result in dedifferentiation of β cells. Preclinical data from mice lacking FoxO1 in β cells has shown that following physiologic stressors such as multiparity and aging, the loss of β cell mass was due to β cell dedifferentiation rather than death(67). Similar results have been found in conditions of β cell hypoxia or oxidative stress, indicating that loss of β cell identity is a common phenomenon following diverse cellular injury. Dedifferentiated β cells reverted to progenitor-like cells and in some cases adopted an α cell fate resulting in hyperglucagonemia. These changes have been found in islets from patients with T2D in which a decrease in MAFA, NKX6.1, and PDX1 expression was seen in comparison to islets from non-diabetic donors(55; 68). It is not clear whether similar changes occur in β cells in Type 1 diabetes that are under immunologic stress or in humans and whether dedifferentiated β cells are susceptible to the same immune response as those targeted in the process that is responsible for hyperglycemia. While these “dedifferentiated” cells are a potential source of new β cells evidence for their differentiation into functional β cells is lacking.
5. Conclusions
Despite the successes of immune therapies in improving C-peptide responses, permanent or even long term maintenance of C-peptide has not been achieved. Our understanding of the reasons for this failure has been hampered by the absence of tools to assess the destructive process that causes the disease. Novel tools for visualizing and quantifying β mass and killing may be useful in determining β cell changes that lead to the disease and following immune therapy. Future studies will require analysis of human materials and trials that combine agents that can modulate immune responses with those that directly interfere with β cell killing as well as augment their responses.
Highlights for “Life and death of β cells in Type 1 diabetes: a comprehensive review”.
During progression of Type 1 diabetes beta cell most likely die from a combination of necrosis and apoptosis
Methods to visualize beta cell inflammation and beta cell mass are being developed
Molecular techniques have been applied to identify beta cell killing in vivo
Use of these methods suggests revision of previous models of the pathogenesis of the disease
The basis for the long term loss of beta cell remains unclear. It is possible that ongoing beta cell destruction is intrinsic.
Acknowledgements
Supported by grants U01 AI102011, DP3 DK10122, R01 DK057846, UC4 DK104205, R43 DK104522 from the NIH and grants 2014-158 from the Juvenile Diabetes Research Foundation and support from the Brehm Coalition and the Howalt family.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hagopian W, Ferry RJ, Jr., Sherry N, Carlin D, Bonvini E, Johnson S, Stein KE, Koenig S, Daifotis AG, Herold KC, Ludvigsson J. Teplizumab preserves C-peptide in recent-onset type 1 diabetes: two-year results from the randomized, placebo-controlled Protege trial. Diabetes. 2013;62:3901–3908. doi: 10.2337/db13-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herold KC, Gitelman SE, Ehlers MR, Gottlieb PA, Greenbaum CJ, Hagopian W, Boyle KD, Keyes-Elstein L, Aggarwal S, Phippard D, Sayre PH, McNamara J, Bluestone JA. Teplizumab (anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: Metabolic and immunologic features at baseline identify a subgroup of responders. Diabetes. 2013 doi: 10.2337/db13-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herold KC, Gitelman SE, Masharani U, Hagopian W, Bisikirska B, Donaldson D, Rother K, Diamond B, Harlan DM, Bluestone JA. A Single Course of Anti-CD3 Monoclonal Antibody hOKT3{gamma}1(Ala-Ala) Results in Improvement in C-Peptide Responses and Clinical Parameters for at Least 2 Years after Onset of Type 1 Diabetes. Diabetes. 2005;54:1763–1769. doi: 10.2337/diabetes.54.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herold KC, Gitelman SE, Willi SM, Gottlieb PA, Waldron-Lynch F, Devine L, Sherr J, Rosenthal SM, Adi S, Jalaludin MY, Michels AW, Dziura J, Bluestone JA. Teplizumab treatment may improve C-peptide responses in participants with type 1 diabetes after the new-onset period: a randomised controlled trial. Diabetologia. 2013;56:391–400. doi: 10.1007/s00125-012-2753-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, Gitelman SE, Harlan DM, Xu D, Zivin RA, Bluestone JA. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346:1692–1698. doi: 10.1056/NEJMoa012864. [DOI] [PubMed] [Google Scholar]
- 6.Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, Gorus F, Goldman M, Walter M, Candon S, Schandene L, Crenier L, De Block C, Seigneurin JM, De Pauw P, Pierard D, Weets I, Rebello P, Bird P, Berrie E, Frewin M, Waldmann H, Bach JF, Pipeleers D, Chatenoud L. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med. 2005;352:2598–2608. doi: 10.1056/NEJMoa043980. [DOI] [PubMed] [Google Scholar]
- 7.Keymeulen B, Walter M, Mathieu C, Kaufman L, Gorus F, Hilbrands R, Vandemeulebroucke E, Van de Velde U, Crenier L, De Block C, Candon S, Waldmann H, Ziegler AG, Chatenoud L, Pipeleers D. Four-year metabolic outcome of a randomised controlled CD3-antibody trial in recent-onset type 1 diabetic patients depends on their age and baseline residual beta cell mass. Diabetologia. 2010;53:614–623. doi: 10.1007/s00125-009-1644-9. [DOI] [PubMed] [Google Scholar]
- 8.Orban T, Bundy B, Becker DJ, Dimeglio LA, Gitelman SE, Goland R, Gottlieb PA, Greenbaum CJ, Marks JB, Monzavi R, Moran A, Raskin P, Rodriguez H, Russell WE, Schatz D, Wherrett D, Wilson DM, Krischer JP, Skyler JS. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet. 2011 doi: 10.1016/S0140-6736(11)60886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pescovitz MD, Greenbaum CJ, Bundy B, Becker DJ, Gitelman SE, Goland R, Gottlieb PA, Marks JB, Moran A, Raskin P, Rodriguez H, Schatz DA, Wherrett DK, Wilson DM, Krischer JP, Skyler JS. B-lymphocyte depletion with rituximab and beta-cell function: two-year results. Diabetes Care. 2014;37:453–459. doi: 10.2337/dc13-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, Becker DJ, Gitelman SE, Goland R, Gottlieb PA, Marks JB, McGee PF, Moran AM, Raskin P, Rodriguez H, Schatz DA, Wherrett D, Wilson DM, Lachin JM, Skyler JS. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med. 2009;361:2143–2152. doi: 10.1056/NEJMoa0904452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rigby M, DiMeglio L, Rendell M, Felner E, Dostou J, Gitelman S, Patel C, Griffin K, Tsalikian E, Gottlieb P, Greenbaum C, Sherry N, Moore W, Roshanak M, Willi S, Raskin P, Moran A, Russell W, Pinckney A, Keyes-Elstein L, Howell M, Aggarwal S, Lim N, Phippard D, Nepom G, McNamara J, Ehlers MR. Targeting effector memory T cells with alefacept in new onset type 1 diabetes: 12 month results from the TIDAL study. Lancet Endocrinology and Metabolism. 2013 doi: 10.1016/S2213-8587(13)70111-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rigby MR, Harris KM, Pinckney A, DiMeglio LA, Rendell MS, Felner EI, Dostou JM, Gitelman SE, Griffin KJ, Tsalikian E, Gottlieb PA, Greenbaum CJ, Sherry NA, Moore WV, Monzavi R, Willi SM, Raskin P, Keyes-Elstein L, Long SA, Kanaparthi S, Lim N, Phippard D, Soppe CL, Fitzgibbon ML, McNamara J, Nepom GT, Ehlers MR. Alefacept provides sustained clinical and immunological effects in new-onset type 1 diabetes patients. J Clin Invest. 2015;125:3285–3296. doi: 10.1172/JCI81722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keenan HA, Sun JK, Levine J, Doria A, Aiello LP, Eisenbarth G, Bonner-Weir S, King GL. Residual insulin production and pancreatic ss-cell turnover after 50 years of diabetes: Joslin Medalist Study. Diabetes. 2010;59:2846–2853. doi: 10.2337/db10-0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oram RA, Jones AG, Besser RE, Knight BA, Shields BM, Brown RJ, Hattersley AT, McDonald TJ. The majority of patients with long-duration type 1 diabetes are insulin microsecretors and have functioning beta cells. Diabetologia. 2014;57:187–191. doi: 10.1007/s00125-013-3067-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eizirik DL, Darville MI. beta-cell apoptosis and defense mechanisms: lessons from type 1 diabetes. Diabetes. 2001;50(Suppl 1):S64–69. doi: 10.2337/diabetes.50.2007.s64. [DOI] [PubMed] [Google Scholar]
- 16.Akirav EM, Baquero MT, Opare-Addo LW, Akirav M, Galvan E, Kushner JA, Rimm DL, Herold KC. Glucose and inflammation control islet vascular density and beta-cell function in NOD mice: control of islet vasculature and vascular endothelial growth factor by glucose. Diabetes. 2011;60:876–883. doi: 10.2337/db10-0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jorns A, Arndt T, Meyer zu Vilsendorf A, Klempnauer J, Wedekind D, Hedrich HJ, Marselli L, Marchetti P, Harada N, Nakaya Y, Wang GS, Scott FW, Gysemans C, Mathieu C, Lenzen S. Islet infiltration, cytokine expression and beta cell death in the NOD mouse, BB rat, Komeda rat, LEW.1AR1-iddm rat and humans with type 1 diabetes. Diabetologia. 2014;57:512–521. doi: 10.1007/s00125-013-3125-4. [DOI] [PubMed] [Google Scholar]
- 18.Zhao Y, Scott NA, Fynch S, Elkerbout L, Wong WW, Mason KD, Strasser A, Huang DC, Kay TW, Thomas HE. Autoreactive T cells induce necrosis and not BCL-2-regulated or death receptor-mediated apoptosis or RIPK3-dependent necroptosis of transplanted islets in a mouse model of type 1 diabetes. Diabetologia. 2015;58:140–148. doi: 10.1007/s00125-014-3407-5. [DOI] [PubMed] [Google Scholar]
- 19.Kawasaki E, Abiru N, Eguchi K. Prevention of type 1 diabetes: from the view point of beta cell damage. Diabetes Res Clin Pract. 2004;66(Suppl 1):S27–32. doi: 10.1016/j.diabres.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 20.Thomas HE, Kay TW. Intracellular pathways of pancreatic beta-cell apoptosis in type 1 diabetes. Diabetes Metab Res Rev. 2011;27:790–796. doi: 10.1002/dmrr.1253. [DOI] [PubMed] [Google Scholar]
- 21.Wali JA, Rondas D, McKenzie MD, Zhao Y, Elkerbout L, Fynch S, Gurzov EN, Akira S, Mathieu C, Kay TW, Overbergh L, Strasser A, Thomas HE. The proapoptotic BH3-only proteins Bim and Puma are downstream of endoplasmic reticulum and mitochondrial oxidative stress in pancreatic islets in response to glucotoxicity. Cell death & disease. 2014;5:e1124. doi: 10.1038/cddis.2014.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vanlangenakker N, Vanden Berghe T, Krysko DV, Festjens N, Vandenabeele P. Molecular mechanisms and pathophysiology of necrotic cell death. Curr Mol Med. 2008;8:207–220. doi: 10.2174/156652408784221306. [DOI] [PubMed] [Google Scholar]
- 23.Dorn GW., 2nd Molecular mechanisms that differentiate apoptosis from programmed necrosis. Toxicologic pathology. 2013;41:227–234. doi: 10.1177/0192623312466961. [DOI] [PubMed] [Google Scholar]
- 24.Marroqui L, Dos Santos RS, Floyel T, Grieco FA, Santin I, Op de Beeck A, Marselli L, Marchetti P, Pociot F, Eizirik DL. TYK2, a Candidate Gene for Type 1 Diabetes, Modulates Apoptosis and the Innate Immune Response in Human Pancreatic beta-Cells. Diabetes. 2015;64:3808–3817. doi: 10.2337/db15-0362. [DOI] [PubMed] [Google Scholar]
- 25.Walter U, Franzke A, Sarukhan A, Zober C, von Boehmer H, Buer J, Lechner O. Monitoring gene expression of TNFR family members by beta-cells during development of autoimmune diabetes. Eur J Immunol. 2000;30:1224–1232. doi: 10.1002/1521-4141(200004)30:4<1224::AID-IMMU1224>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 26.Stephens LA, Thomas HE, Ming L, Grell M, Darwiche R, Volodin L, Kay TW. Tumor necrosis factor-alpha-activated cell death pathways in NIT-1 insulinoma cells and primary pancreatic beta cells. Endocrinology. 1999;140:3219–3227. doi: 10.1210/endo.140.7.6873. [DOI] [PubMed] [Google Scholar]
- 27.Cieslak M, Wojtczak A, Cieslak M. Role of pro-inflammatory cytokines of pancreatic islets and prospects of elaboration of new methods for the diabetes treatment. Acta biochimica Polonica. 2015;62:15–21. doi: 10.18388/abp.2014_853. [DOI] [PubMed] [Google Scholar]
- 28.Cnop M, Welsh N, Jonas JC, Jorns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes. 2005;54(Suppl 2):S97–107. doi: 10.2337/diabetes.54.suppl_2.s97. [DOI] [PubMed] [Google Scholar]
- 29.Irawaty W, Kay TW, Thomas HE. Transmembrane TNF and IFNgamma induce caspase-independent death of primary mouse pancreatic beta cells. Autoimmunity. 2002;35:369–375. doi: 10.1080/0891693021000024834. [DOI] [PubMed] [Google Scholar]
- 30.Kjorholt C, Akerfeldt MC, Biden TJ, Laybutt DR. Chronic hyperglycemia, independent of plasma lipid levels, is sufficient for the loss of beta-cell differentiation and secretory function in the db/db mouse model of diabetes. Diabetes. 2005;54:2755–2763. doi: 10.2337/diabetes.54.9.2755. [DOI] [PubMed] [Google Scholar]
- 31.Steil GM, Trivedi N, Jonas JC, Hasenkamp WM, Sharma A, Bonner-Weir S, Weir GC. Adaptation of beta-cell mass to substrate oversupply: enhanced function with normal gene expression. Am J Physiol Endocrinol Metab. 2001;280:E788–796. doi: 10.1152/ajpendo.2001.280.5.E788. [DOI] [PubMed] [Google Scholar]
- 32.Vernier S, Chiu A, Schober J, Weber T, Nguyen P, Luer M, McPherson T, Wanda PE, Marshall CA, Rohatgi N, McDaniel ML, Greenberg AS, Kwon G. beta-cell metabolic alterations under chronic nutrient overload in rat and human islets. Islets. 2012;4:379–392. doi: 10.4161/isl.22720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sherry NA, Kushner JA, Glandt M, Kitamura T, Brillantes AM, Herold KC. Effects of autoimmunity and immune therapy on beta-cell turnover in type 1 diabetes. Diabetes. 2006;55:3238–3245. doi: 10.2337/db05-1034. [DOI] [PubMed] [Google Scholar]
- 34.Gaglia JL, Guimaraes AR, Harisinghani M, Turvey SE, Jackson R, Benoist C, Mathis D, Weissleder R. Noninvasive imaging of pancreatic islet inflammation in type 1A diabetes patients. J Clin Invest. 2011;121:442–445. doi: 10.1172/JCI44339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gaglia JL, Harisinghani M, Aganj I, Wojtkiewicz GR, Hedgire S, Benoist C, Mathis D, Weissleder R. Noninvasive mapping of pancreatic inflammation in recent-onset type-1 diabetes patients. Proc Natl Acad Sci U S A. 2015;112:2139–2144. doi: 10.1073/pnas.1424993112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Freeby M, Goland R, Ichise M, Maffei A, Leibel R, Harris P. VMAT2 quantitation by PET as a biomarker for beta-cell mass in health and disease. Diabetes Obes Metab. 2008;10(Suppl 4):98–108. doi: 10.1111/j.1463-1326.2008.00943.x. [DOI] [PubMed] [Google Scholar]
- 37.Goland R, Freeby M, Parsey R, Saisho Y, Kumar D, Simpson N, Hirsch J, Prince M, Maffei A, Mann JJ, Butler PC, Van Heertum R, Leibel RL, Ichise M, Harris PE. 11C-dihydrotetrabenazine PET of the pancreas in subjects with long-standing type 1 diabetes and in healthy controls. J Nucl Med. 2009;50:382–389. doi: 10.2967/jnumed.108.054866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maffei A, Liu Z, Witkowski P, Moschella F, Del Pozzo G, Liu E, Herold K, Winchester RJ, Hardy MA, Harris PE. Identification of tissue-restricted transcripts in human islets. Endocrinology. 2004;145:4513–4521. doi: 10.1210/en.2004-0691. [DOI] [PubMed] [Google Scholar]
- 39.Souza F, Simpson N, Raffo A, Saxena C, Maffei A, Hardy M, Kilbourn M, Goland R, Leibel R, Mann JJ, Van Heertum R, Harris PE. Longitudinal noninvasive PET-based beta cell mass estimates in a spontaneous diabetes rat model. J Clin Invest. 2006;116:1506–1513. doi: 10.1172/JCI27645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reiner T, Thurber G, Gaglia J, Vinegoni C, Liew CW, Upadhyay R, Kohler RH, Li L, Kulkarni RN, Benoist C, Mathis D, Weissleder R. Accurate measurement of pancreatic islet beta-cell mass using a second-generation fluorescent exendin-4 analog. Proc Natl Acad Sci U S A. 2011;108:12815–12820. doi: 10.1073/pnas.1109859108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Antkowiak PF, Tersey SA, Carter JD, Vandsburger MH, Nadler JL, Epstein FH, Mirmira RG. Noninvasive assessment of pancreatic beta-cell function in vivo with manganese-enhanced magnetic resonance imaging. Am J Physiol Endocrinol Metab. 2009;296:E573–578. doi: 10.1152/ajpendo.90336.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akirav EM, Lebastchi J, Galvan EM, Henegariu O, Akirav M, Ablamunits V, Lizardi PM, Herold KC. Detection of beta cell death in diabetes using differentially methylated circulating DNA. Proc Natl Acad Sci U S A. 2011;108:19018–19023. doi: 10.1073/pnas.1111008108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Husseiny MI, Kaye A, Zebadua E, Kandeel F, Ferreri K. Tissue-specific methylation of human insulin gene and PCR assay for monitoring beta cell death. PLoS One. 2014;9:e94591. doi: 10.1371/journal.pone.0094591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Husseiny MI, Kuroda A, Kaye AN, Nair I, Kandeel F, Ferreri K. Development of a quantitative methylation-specific polymerase chain reaction method for monitoring beta cell death in type 1 diabetes. PLoS One. 2012;7:e47942. doi: 10.1371/journal.pone.0047942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Usmani-Brown S, Lebastchi J, Steck AK, Beam C, Herold KC, Ledizet M. Analysis of beta-cell death in type 1 diabetes by droplet digital PCR. Endocrinology. 2014;155:3694–3698. doi: 10.1210/en.2014-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fisher MM, Watkins RA, Blum J, Evans-Molina C, Chalasani N, DiMeglio LA, Mather KJ, Tersey SA, Mirmira RG. Elevations in Circulating Methylated and Unmethylated Preproinsulin DNA in New-Onset Type 1 Diabetes. Diabetes. 2015 doi: 10.2337/db15-0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Herold KC, Usmani-Brown S, Ghazi T, Lebastchi J, Beam CA, Bellin MD, Ledizet M, Sosenko JM, Krischer JP, Palmer JP. beta Cell death and dysfunction during type 1 diabetes development in at-risk individuals. J Clin Invest. 2015;125:1163–1173. doi: 10.1172/JCI78142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lebastchi J, Deng S, Lebastchi AH, Beshar I, Gitelman S, Willi S, Gottlieb P, Akirav EM, Bluestone JA, Herold KC. Immune therapy and beta-cell death in type 1 diabetes. Diabetes. 2013;62:1676–1680. doi: 10.2337/db12-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lo YM, Zhang J, Leung TN, Lau TK, Chang AM, Hjelm NM. Rapid clearance of fetal DNA from maternal plasma. Am J Hum Genet. 1999;64:218–224. doi: 10.1086/302205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Poy MN, Hausser J, Trajkovski M, Braun M, Collins S, Rorsman P, Zavolan M, Stoffel M. miR-375 maintains normal pancreatic alpha- and beta-cell mass. Proc Natl Acad Sci U S A. 2009;106:5813–5818. doi: 10.1073/pnas.0810550106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiang L, Brackeva B, Ling Z, Kramer G, Aerts JM, Schuit F, Keymeulen B, Pipeleers D, Gorus F, Martens GA. Potential of protein phosphatase inhibitor 1 as biomarker of pancreatic beta-cell injury in vitro and in vivo. Diabetes. 2013;62:2683–2688. doi: 10.2337/db12-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berney T, Mamin A, James Shapiro AM, Ritz-Laser B, Brulhart MC, Toso C, Demuylder-Mischler S, Armanet M, Baertschiger R, Wojtusciszyn A, Benhamou PY, Bosco D, Morel P, Philippe J. Detection of insulin mRNA in the peripheral blood after human islet transplantion predicts deterioration of metabolic control. Am J Transplant. 2006;6:1704–1711. doi: 10.1111/j.1600-6143.2006.01373.x. [DOI] [PubMed] [Google Scholar]
- 53.Ritz-Laser B, Oberholzer J, Toso C, Brulhart MC, Zakrzewska K, Ris F, Bucher P, Morel P, Philippe J. Molecular detection of circulating beta-cells after islet transplantation. Diabetes. 2002;51:557–561. doi: 10.2337/diabetes.51.3.557. [DOI] [PubMed] [Google Scholar]
- 54.Marhfour I, Lopez XM, Lefkaditis D, Salmon I, Allagnat F, Richardson SJ, Morgan NG, Eizirik DL. Expression of endoplasmic reticulum stress markers in the islets of patients with type 1 diabetes. Diabetologia. 2012;55:2417–2420. doi: 10.1007/s00125-012-2604-3. [DOI] [PubMed] [Google Scholar]
- 55.Puri S, Akiyama H, Hebrok M. VHL-mediated disruption of Sox9 activity compromises beta-cell identity and results in diabetes mellitus. Genes & development. 2013;27:2563–2575. doi: 10.1101/gad.227785.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Puri S, Cano DA, Hebrok M. A role for von Hippel-Lindau protein in pancreatic beta-cell function. Diabetes. 2009;58:433–441. doi: 10.2337/db08-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Corbett JA, Sweetland MA, Wang JL, Lancaster JR, Jr., McDaniel ML. Nitric oxide mediates cytokine-induced inhibition of insulin secretion by human islets of Langerhans. Proc Natl Acad Sci U S A. 1993;90:1731–1735. doi: 10.1073/pnas.90.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moran A, Bundy B, Becker DJ, Dimeglio LA, Gitelman SE, Goland R, Greenbaum CJ, Herold KC, Marks JB, Raskin P, Sanda S, Schatz D, Wherrett DK, Wilson DM, Krischer JP, Skyler JS, Pickersgill L, de Koning E, Ziegler AG, Boehm B, Badenhoop K, Schloot N, Bak JF, Pozzilli P, Mauricio D, Donath MY, Castano L, Wagner A, Lervang HH, Perrild H, Mandrup-Poulsen T. Interleukin-1 antagonism in type 1 diabetes of recent onset: two multicentre, randomised, double-blind, placebo-controlled trials. Lancet. 2013;381:1905–1915. doi: 10.1016/S0140-6736(13)60023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meier JJ, Bhushan A, Butler AE, Rizza RA, Butler PC. Sustained beta cell apoptosis in patients with long-standing type 1 diabetes: indirect evidence for islet regeneration? Diabetologia. 2005 doi: 10.1007/s00125-005-1949-2. [DOI] [PubMed] [Google Scholar]
- 60.Pinkse GG, Tysma OH, Bergen CA, Kester MG, Ossendorp F, van Veelen PA, Keymeulen B, Pipeleers D, Drijfhout JW, Roep BO. Autoreactive CD8 T cells associated with beta cell destruction in type 1 diabetes. Proc Natl Acad Sci U S A. 2005;102:18425–18430. doi: 10.1073/pnas.0508621102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Herold KC, Pescovitz MD, McGee P, Krause-Steinrauf H, Spain LM, Bourcier K, Asare A, Liu Z, Lachin JM, Dosch HM. Increased T Cell Proliferative Responses to Islet Antigens Identify Clinical Responders to Anti-CD20 Monoclonal Antibody (Rituximab) Therapy in Type 1 Diabetes. J Immunol. 2011;187:1998–2005. doi: 10.4049/jimmunol.1100539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Couri CE, Oliveira MC, Stracieri AB, Moraes DA, Pieroni F, Barros GM, Madeira MI, Malmegrim KC, Foss-Freitas MC, Simoes BP, Martinez EZ, Foss MC, Burt RK, Voltarelli JC. C-peptide levels and insulin independence following autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2009;301:1573–1579. doi: 10.1001/jama.2009.470. [DOI] [PubMed] [Google Scholar]
- 63.Bluestone JA, Buckner JH, Fitch M, Gitelman SE, Gupta S, Hellerstein MK, Herold KC, Lares A, Lee MR, Li K, Liu W, Long SA, Masiello LM, Nguyen V, Putnam AL, Rieck M, Sayre PH, Tang Q. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl Med. 2015;7:315ra189. doi: 10.1126/scitranslmed.aad4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marek-Trzonkowska N, Mysliwiec M, Dobyszuk A, Grabowska M, Techmanska I, Juscinska J, Wujtewicz MA, Witkowski P, Mlynarski W, Balcerska A, Mysliwska J, Trzonkowski P. Administration of CD4+CD25highCD127- regulatory T cells preserves beta-cell function in type 1 diabetes in children. Diabetes Care. 2012;35:1817–1820. doi: 10.2337/dc12-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tooley JE, Vudattu N, Choi J, Cotsapas C, Devine L, Raddassi K, Ehlers MR, McNamara JG, Harris KM, Kanaparthi S, Phippard D, Herold KC. Changes in T-cell subsets identify responders to FcR non-binding anti-CD3 mAb (teplizumab) in patients with Type 1 diabetes. Eur J Immunol. 2015 doi: 10.1002/eji.201545708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jaberi-Douraki M, Schnell S, Pietropaolo M, Khadra A. Unraveling the contribution of pancreatic beta-cell suicide in autoimmune type 1 diabetes. Journal of theoretical biology. 2015;375:77–87. doi: 10.1016/j.jtbi.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic beta Cell Dedifferentiation as a Mechanism of Diabetic beta Cell Failure. Cell. 2012;150:1223–1234. doi: 10.1016/j.cell.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guo S, Dai C, Guo M, Taylor B, Harmon JS, Sander M, Robertson RP, Powers AC, Stein R. Inactivation of specific beta cell transcription factors in type 2 diabetes. J Clin Invest. 2013 doi: 10.1172/JCI65390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sosenko JM, Palmer JP, Greenbaum CJ, Mahon J, Cowie C, Krischer JP, Chase HP, White NH, Buckingham B, Herold KC, Cuthbertson D, Skyler JS. Patterns of metabolic progression to type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care. 2006;29:643–649. doi: 10.2337/diacare.29.03.06.dc05-1006. [DOI] [PubMed] [Google Scholar]
- 70.Sosenko JM, Skyler JS, Herold KC, Palmer JP. The metabolic progression to type 1 diabetes as indicated by serial oral glucose tolerance testing in the Diabetes Prevention Trial-type 1. Diabetes. 2012;61:1331–1337. doi: 10.2337/db11-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Walker M, Mari A, Jayapaul MK, Bennett SM, Ferrannini E. Impaired beta cell glucose sensitivity and whole-body insulin sensitivity as predictors of hyperglycaemia in non-diabetic subjects. Diabetologia. 2005;48:2470–2476. doi: 10.1007/s00125-005-0004-7. [DOI] [PubMed] [Google Scholar]