Abstract
As interest in the gut microbiome has grown in recent years, attention has turned to the impact of our diet on our brain. The benefits of a high fiber diet in the colon have been well documented in epidemiological studies, but its potential impact on the brain has largely been understudied. Here, we will review evidence that butyrate, a short-chain fatty acid (SCFA) produced by bacterial fermentation of fiber in the colon, can improve brain health. Butyrate has been extensively studied as a histone deacetylase (HDAC) inhibitor but also functions as a ligand for a subset of G protein-coupled receptors and as an energy metabolite. These diverse modes of action make it well suited for solving the wide array of imbalances frequently encountered in neurological disorders. In this review, we will integrate evidence from the disparate fields of gastroenterology and neuroscience to hypothesize that the metabolism of a high fiber diet in the gut can alter gene expression in the brain to prevent neurodegeneration and promote regeneration.
Keywords: Gut-brain axis, Neuroepigenetics, Butyrate, High fiber diet, Gut microbiome
Introduction
The relationship between our gut microbiota and nervous system is a large part of the gut-brain axis that has attracted increasing interest in recent years. It is estimated that 90% of the cells in the human body are of microbial origin, and the vast majority of these microbiota are comprised of 15,000–36,000 species of commensal and symbiotic bacteria that reside within the lumen of the gut [1,2]. A diverse microbial community is crucial for our health and disease prevention based on microbiome studies (i.e., metagenomic sequence analyses) and perturbed energy homeostasis that has been observed in germ free mice [3]. Although it is not yet clear how gut microbiota positively and negatively affect brain function, multiple mechanisms are likely to be involved. Gut bacteria, have a prodigious metabolic capacity and some microbe-derived metabolites enter the circulation and can cross the blood-brain barrier. There is growing evidence that these microbes produce neurotransmitters, such as GABA and serotonin, modulate the immune system, alter epigenetic markers and produce bioactive food components and energy metabolites [2,4,5]. Thus, dietary manipulation to achieve a symbiosis that can improve the health of the microbiome and our brains is an attractive idea currently under investigation.
In this review, we will focus on the short chain fatty acid (SCFA), butyrate, which is most commonly produced by bacteria in the colon, and its role as a potential therapeutic for neurological diseases. Butyrate is an attractive therapeutic molecule because of its wide array of biological functions, such as its ability to serve as a histone deacetylase (HDAC) inhibitor, an energy metabolite to produce ATP and a G protein-coupled receptor (GPCR) activator. Pharmacologically, butyrate has had a profoundly beneficial effect on brain disorders ranging from neurodegenerative diseases to psychological disorders. In this review, we will discuss how butyrate is made and the pharmacological effects of butyrate in neurological disorders. Finally, we will summarize the current evidence that high fiber, butyrate-producing diets are capable of improving the health of our brains.
Sources of Butyrate
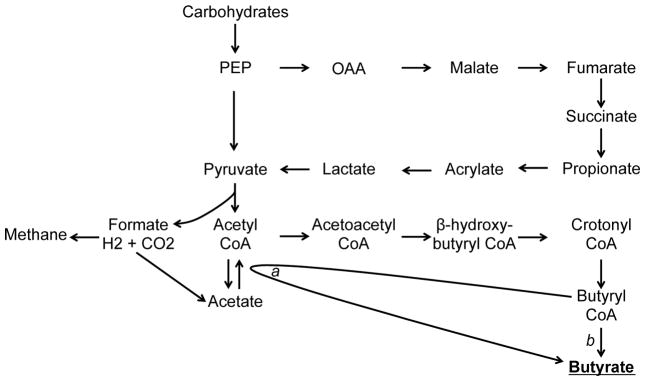
Butyrate is synthesized via the fermentation of otherwise non-digestible fiber by bacteria in the colon. Two acetyl CoA molecules are condensed into acetoacetyl CoA, which is converted through L(+)-beta-hydroxybutyryl CoA and crotonyl CoA intermediates to butyryl CoA. Butyryl CoA is then converted to butyrate either by butyrate kinase or butyryl CoA and acetate CoA transferase (as shown in Figure 1) [6,7].
Figure 1.
Schematic representation of the carbohydrate fermentation pathways that lead to butyrate production in the large intestine. The final enzymes involved in the formation of butyrate are: a. Butyryl CoA:acetate CoA transferase b. Phosphotransbutyrylase/butyrate kinase. Adapted from Pryde et al. 2002 [7].
Bacteria, such as those from the Clostridium, Eubacterium, and Butyrivibrio genera, are able produce butyrate in the gut lumen at mM levels [7,8]. In addition to producing butyrate as an endpoint, bacteria produce fermentation intermediates, including lactate, succinate or formate, which are used by the bacteria themselves to proliferate and survive [9,10]. Butyrate is also utilized by microbiota and serves as the primary energy source of colonocytes (as discussed below), making this a vital and mutually beneficial relationship. High fiber foods, summarized in Table 1, that enable these butyrate-producing bacteria to thrive include resistant starches (e.g. whole grain and legumes) and fructo-oligosaccharides (FOS) (e.g. bananas, onions, and asparagus). In fact, within two weeks of a high FOS diet, rats showed increase butyrate in the large intestine without changing the total number of anaerobic bacteria [11]. Similar results were seen in another study with diets containing FOS as well as resistant starches, but were not observed in the starch free wheat bran diet, which produces less butyrate [12]. This study, as well as many others, demonstrates that different sources of fiber yield different levels of butyrate so care must be used in selecting the appropriate fiber diets to increase butyrate levels.
Table 1.
Summary of the proportions of SCFA produced with different forms of carbohydrates. Adapted from Smith et al. 1998 [102].
| Proportion of SCFA energy (kJ) produced | |||
|---|---|---|---|
| Carbohydrate | Butyric Acid | Propionic Acid | Acetic Acid |
| Cellulose | 0.33 | 0.24 | 0.43 |
| Gum arabic | 0.17 | 0.28 | 0.56 |
| Lactulose | 0.36 | 0.16 | 0.48 |
| Oat bran | 0.38 | 0.24 | 0.38 |
| Pectic substances | 0.32 | 0.17 | 0.51 |
| Resistant starch | 0.55 | 0.21 | 0.24 |
| Wheatbran | 0.34 | 0.23 | 0.42 |
| Xylan | 0.06 | 0.23 | 0.71 |
Aside from being produced by bacterial fermentation, butyrate can also be produced in much lower concentrations by mammalian cells through fatty acid oxidation and glucose metabolism [13,14] and can be found in plant oils and animal fats [4]. Butyric acid (the acid form of butyrate) is also present in the milk of ruminant animals, such as cows. Butter contains 3–4% butyric acid, in the form of tributyrin (butyryl triglyceride), making it the richest dietary source of butyrate [15]. Interestingly, the term butyrate originates from the Greek word for butter [16,17]. One molecule of tributyrin is metabolized into three butyrate molecules by intestinal enzymes. Tributyrin (1 g/kg) was able to elevate portal vein concentrations of butyrate to 2.4 mM after 1 hour in rats [18].
The Functions of Butyrate
Histone Deacetylase Inhibitor
Histone acetylation is a post-translational modification by an epigenetic protein, which are proteins that bind to chromatin and influence chromatin structure to change the propensity that a gene is transcribed or repressed. Acetylated histones cause the chromatin structure to loosen by weakening electrostatic attraction between the histone proteins and the DNA backbone. This process enables transcription factors and the basal transcriptional machinery to bind and increases transcription. Acetyl groups are added to highly conserved N-terminal L-lysine residues by histone acetyltransferases (HATs) and removed by histone deacetylases (HDACs). Reduced HAT activity, lower global histone acetylation and transcriptional dysfunction are characteristics of many neurodegenerative diseases [19]. Thus, HDAC inhibitors have become attractive therapeutic candidates due to their ability to increase histone acetylation and promote the expression of prosurvival, proregenerative and proplasticity genes.
Sodium butyrate (NaB), the sodium salt form of butyrate commonly used in pharmacological studies, is a well-known HDAC inhibitor that results in increased histone acetylation when applied to cells in culture in the high micromolar range [20,21]. Studies from our own lab demonstrated that NaB treatment could resist oxidative stress in vitro and in vivo [22–25]. These salutary effects were highly correlated with HDAC inhibition as a mechanism of protection. In multiple models of Huntington’s disease, we have shown that NaB and phenylbutyrate, a structurally similar analog, rescues histone acetylation, prevents neuronal cell death and extends the lifespan of mice [23,26].
Numerous subsequent studies have shown that NaB’s salutary effects span many neurological disease models and aspects of the pathophysiology of disease. For example, NaB can protect neurons from cell death in models of Parkinson’s disease [27–29] and in cisplatin-induced hearing loss [30], where NaB was able to reverse the disease-associated reduction in histone acetylation. Similarly, NaB was able to reduce the infarct size in models of ischemic stroke, limiting the damage to the brain and improving behavioral outcomes [24,31–33]. In vitro and in vivo (via intraperitoneal injection) data from our own laboratory also suggests that butyrate can induce resistance to oxidative stress and increase histone acetylation and enhance gene expression of a number of genes in the high micromolar range [24,34]. Altogether, these observations are consistent with the idea that NaB can modulate the expression of a large number of genes to affect numerous pathophysiological pathways. The prospect of accomplishing this goal with a single, naturally occurring small molecule is exciting.
NaB has also demonstrated a profound effect on improving learning and memory, particularly in cases of disease-associated or toxicity-induced dementia. In mouse models of Alzheimer’s disease, histone acetylation is restored and expression of learning-associated genes is increased with NaB treatment [35,36]. While NaB had no effect on the contextual memory on wild-type mice, contextual memory in the transgenic mouse model showed significant improvements, even at late stages of Alzheimer’s disease. These improvements in learning and memory have also been demonstrated in models of memory impairment from a toxic overload of metals [37,38], traumatic brain injury [39] or neurological infections [40,41].
As HDAC inhibitors influence the transcription of numerous genes, it seems unlikely that a single gene is responsible for its neurotrophic effects. However, many studies have shown that at least some of these beneficial effects can be attributed NaB’s ability to increase acetylation around the promoters of neurotrophic factors, such as BDNF, GDNF and NGF and thus increasing their transcription [41–48]. Other studies have demonstrated the importance of immediate early genes, including c-Fos and Homer1a, which are activity dependent genes involved in plasticity [44,49–52]. Based on these data, NaB is capable of upregulating a suite of genes that promote survival, plasticity and regeneration.
It should also be noted that the effects of HDAC inhibitors are not specific to histone proteins alone. In fact, a growing list of over 1700 proteins can be acetylated on their lysine residues, and HDAC inhibitors block their deacetylation as well [53]. Thus, the effect of HDAC inhibitors on non-histone proteins should not be overlooked. Acetylation plays an important role that affects the enzymatic and metabolic activity of many proteins. For example, our group has shown the transcription factor Sp1 is acetylated and activated by oxidative stress, and butyrate, as well as other HDAC inhibitors, can enhance this protective adaptive response to promote cell survival [22,23,54].
Metabolism and Mitochondria
Butyrate plays two major roles in metabolism and mitochondrial activity. First, butyrate can serve as an energy substrate. In fact, colonocytes have adapted to use butyrate as their primary source of energy, which accounts for approximately 70% of ATP produced [55]. Given overwhelming use of butyrate as an energy substrate in the colon and the microbiome, much of the research to date has focused on its metabolic effects in the colonic epithelium. It was recently shown that germ free mice, which lack a microbiome, had a significantly reduced NADH/NAD+ ratio and reduced ATP levels in the colon compared to conventionally raised mice with a normal microbiome [56]. Butyrate was able to rescue the diminished mitochondrial respiration in the germ free mice, which demonstrates its importance in energy homeostasis. It was also found that cancerous colonocytes undergoing the Warburg effect, defined by their preferential use of glycolysis rather than mitochondrial respiration, allows butyrate to accumulate in the nucleus where it can act as an HDAC inhibitor [57]. However, in normal coloncytes the cells rapidly utilize butyrate for energy via mitochondrial β-oxidation. Interestingly, mitochondrial metabolism of butyrate does produce more acetyl-CoA, which can be used by HATs to add acetyl groups to proteins so under either condition butyrate could in theory increase acetylation and affect gene transcription.
While the metabolic events in the colon may appear disconnected from that of the brain, it’s important to consider the immense energy demands of the brain and the energy dyshomeostasis that occurs in the brain in many neurological diseases. Perhaps the best cited example is the reduced glucose utilization in the Alzheimer’s brain, which occurs at the earliest stages of the disease and well before memory loss [58,59]. This reduction in glucose utilization has prompted a number of studies to identify therapeutic strategies to provide alternative sources of energy to fulfill the brain’s energy requirements. For example, the use of caprylic triglycerides as a medical food to enhance ketogenesis via increasing β-hydroxybutyrate has been shown to improve cognitive scores in Apo-E4 negative Alzheimer’s disease patients after 45 days [60]. Longer time points were less successful, but it is likely that these treatments would need to begin before significant cognitive symptoms to achieve maximum effectiveness. Currently no studies have examined the metabolic effect of gut-derived butyrate on the brain. However, we hypothesize that if sufficient butyrate levels could be reached in the brain, butyrate could stand in as an energy substrate, as in the colon, and restore energy homeostasis, but the precise concentration to affect brain physiology via changes in the diet, gut microbiome or through pharmacological supplementation remain undefined.
Reduced brain glucose availability is believed to contribute to mitochondrial dysfunction in acute and chronic neurological diseases but could, in theory, be aided by the presence of butyrate’s direct and indirect effects on energy metabolism. In this context, butyrate can: 1) directly affect energy metabolism by acting as a substrate for beta-oxidation; 2) can upregulate genes involved in mitochondrial biogenesis (e.g. PGC1α) via its effects as a selective HDAC inhibitor; and 3) via its ability to affect the acetylation of a wide number of metabolic proteins. This last effect of butyrate and other HDAC inhibitors on metabolism was brought to light by the studies demonstrating that nearly every enzyme involved in glycolysis, gluconeogenesis, the TCA cycle, fatty acid metabolism and glycogen metabolism is acetylated [61]. The potent HDAC inhibitor, TSA, was able to significantly increase acetylation of these proteins. In the case of malate dehydrogenase, acetylation was increased by both glucose and TSA to lead to increased activity levels. To a large degree, these studies have been replicated with NaB by the lab of João Quevedo, which showed that NaB can restore Complex I–IV, as well as the TCA cycle activity, after being repressed by amphetamines and ouabain in animal models of mania [62–64]. These studies demonstrate that butyrate is also capable of increasing mitochondrial activity, which can help to rectify the disease-associated mitochondrial dysfunction in the brain.
G Protein-Coupled Receptor Activator
G protein-coupled receptors (GPCR) are the largest and most diverse family of transmembrane proteins. They are comprised of 7 transmembrane α-helices, which bind extracellular signals, such as light-sensitive compounds, hormones, growth factors and neurotransmitters, and activate signal transduction pathways inside of the cell, primarily the cAMP and phosphatidylinositol signaling pathways [65]. With the role in detecting extracellular signals, GPCRs are critical for a number of physiological functions including regulation of immune system, autonomic nervous system regulation, sensory (taste and smell) function, and maintaining energy homeostasis. Dysfunction of GPCRs is associated with a number of diseases, which has made them the target for more than 40% of prescribed drugs [4].
In 2003, two previously orphan GPCRs, with no known ligands (GPR41 and GPR43), were identified as receptor targets for SCFA and aptly renamed free fatty acid receptors (FFAR) 3 and 2, respectively. Although GPR41 and GPR43 are related showing 52% sequence similarity and identified as tandemly encoded genes in chromosome 19, they differ on their preference in chain length of the SCFA ligands. FFAR2 is more specific to the shorter aliphatic chains of acetate and propionate, while FFAR3 preferentially binds propionate, butyrate and valerate [66].
FFAR3 appears to play a significant role in balancing energy metabolism through intestinal gluconeogenesis (IGN) and the sympathetic nervous system [67]. FFAR3 is highly expressed in the sympathetic nervous system and knockout of FFAR3 in mice show reduced sympathetic ganglion activity. What is most intriguing is that while it has been demonstrated that SCFA (propionate) activate the ganglia activity via FFAR3, the ketone body, β-hydroxybutyrate, which is produced under conditions of starvation or ketosis but structurally similar to butyrate, inhibits ganglia activity by functioning as an antagonist to FFAR3. Although both butyrate and propionate are agonists of FFAR3 in the nervous system, only propionate uptake in the IGN is dependent on FFAR3. This finding demonstrates a novel interaction between metabolic substrates and sympathetic nervous system activity [68].
Butyrate also signals through GPR109a, which is widely expressed in the in colonocytes and T cells, but has also been found in microglia [69–72]. The expression of GPR109a is reduced in human colon cancer cells, and the forced expression of GPR109a in these cancer cells induces apoptosis [69]. While it requires millimolar concentrations of butyrate to activate these receptors, the concentration of butyrate in the colon is more than sufficient to enhance activity the GPR109a. This has the potentially protect the healthy colon tissue through its anti-inflammatory signaling. Indeed, butyrate was shown to suppress colonic inflammation by inducing apoptosis in T cells residing in the colon [70]. Interestingly, knocking down GPR109a (or its transporter Slc5a8) in these cells increased the inflammatory response. More recently, it was shown that GPR109a is upregulated in the substantia nigra in Parkinson’s disease patients, where immunostaining for GPR109a was colocalized with microglia [71]. Treatment with β-hydroxybutyrate induced anti-inflammatory effects in both in vitro and in vivo models of Parkinson’s disease through GPR109a activation and down regulating NF-κB activation [72]. The neurons were also protected from LPS-induced injury and improved behavioral outcomes in the animal models. In contrast to the study above, where β-hydroxybutyrate was shown to be an FFAR3 antagonist, here β-hydroxybutyrate is acting as a GPR109a agonist. However, the effect of β-hydroxybutyrate with respect to GPCRs appears to be under significant debate, as other groups have identified β-hydroxybutyrate as a FFAR3 agonist [73]. However, the Parkinson’s disease studies demonstrate that the activation of GPR109a leads to anti-inflammatory effects in the brain and suggest that it is a good target for therapeutics in PD.
High fiber diets and the brain
High fiber diets have numerous reported health benefits in reducing risk of type 2 diabetes, colon cancer, obesity, stroke and cardiovascular disease, making it a widely recommended healthy diet. Many of the reported effects have been associated with the microbiome and its ability to produce SCFA, like butyrate. Much of the butyrate produced in the colon is used as an energy source by the colonocytes, but some butyrate can also exit the colon through the portal vein, where the liver absorbs another large portion [74,75]. However, the distal colon is not connected to the portal vein, allowing for some systemic butyrate to be circulated. Indeed, there are many reports of high fiber diets increasing blood levels of circulating butyrate [75–77]. These later reports raise the possibility that increases in circulating butyrate could affect CNS function directly.
To date, only a handful of studies have probed the mechanistic basis surrounding the beneficial neurological effects of the high fiber diet and butyrate. A recent study demonstrated that germ free mice have increased blood brain barrier (BBB) permeability, when compared with specific pathogen free mice containing a healthy microbiota [78]. The increased permeability was associated with decreased levels of tight junction proteins, claudin 5 and occludin in the frontal cortex, hippocampus and striatum. Colonizing the germ free mice with the butyrate-producing bacteria, Clostridium tyrobutyricum, or an oral gavage (for 3 days) of NaB restored BBB permeability to the healthy levels of the pathogen free mice, while simultaneously increasing brain histone acetylation and expression of occludin and claudin 5. This study demonstrated the strong and important connection between the microbiota, butyrate and the brain. Another study found significant immune benefits in the brain of mice fed a diet high in fermentable (soluble) fiber and found that they recovered faster from endotoxin-induced sickness [79]. Not only did the diet successfully increase all SCFAs in the colon, but they also found significant benefits by attenuating neuroinflammation resulting from the diet. Mice fed the soluble fiber diet showed an increase in IL-1RA, a cytokine and inhibitor of the pro-inflammatory, IL-1β, in the brain after exposure to the endotoxin, lipopolysaccharide (LPS) and a decrease in IL-1β and TNF-α. IL-4 is a cytokine shown to increase IL-1RA and as expected IL-4 mRNA was also increased in the brains of mice on the soluble fiber diet after LPS treatment. IL-4 expression is enhanced by increased histone acetylation. Thus, the authors hypothesized that the elevated butyrate from the dietary fiber fermentation may contribute to the immune response. In contrast to the soluble fiber diets, splenocytes from mice on the insoluble fiber diet did not increase IL-4 production when exposed to butyrate in vitro.
The microbiome and cognition
Several studies have examined the beneficial effects of a high fiber diet on memory and cognition. In these studies the diet and/or microbiome are manipulated to enhance brain function. For example, children on a high fiber diet demonstrate better cognitive control (e.g. multitasking, working memory and maintaining focus) than children who typically ate a lower fiber diet [80]. Other studies have examined the effects of probiotics that would increase butyrate-producing bacteria. These studies showed that the probiotics reduced anxiety in rats and lowered psychological stress in human subjects [81]. A similar study in subjects with chronic fatigue syndrome showed reduced anxiety, a common symptom of the disease, with the use of probiotics [82]. Another study provided healthy subjects with a fermented milk product and used fMRI to asses changes in the brain [83]. However, no significant difference was observed in fecal microbiota samples in patients who received the fermented milk product.
Perhaps one of the most interesting brain-microbiome connections lies in autism, where an overwhelming 70% of autistic children suffer from gastrointestinal (GI) symptoms [84]. The degree of GI symptoms, most commonly diarrhea and bloating, is often positively correlated with the severity of autism. This correlation has launched numerous studies to determine whether the microbiome of autistic patients differ from those without autism. Indeed these studies have found evidence of decreased Bifidobacteria and Prevotella and higher levels of Lactobacillus, Sutterella and Firmicutes based on cultures from fecal samples, and these differences appear to be independent of diet [84–87]. Some studies have even reported an alleviation of autism symptoms in children with late-onset autism with the use of vancomycin, a poorly absorbed antibiotic; though the effects quickly diminished after treatment ended [88]. Since many of the bacteria altered in autistic patients are important in the fermentation process that produces SCFAs, like butyrate, additional studies measured the SCFA content in autistic children. However, there are conflicting data as to whether or not this change in microbiota result in an increase or decrease in SCFAs in fecal samples [84,89]. Regardless, more studies would be necessary to determine if a change in SCFAs in the fecal matter is the result of poor absorption based on the increased gut permeability seen in autistic patients or excessive fermentation.
Interestingly, it has been hypothesized that elevated SCFAs in the circulatory system due to increased gut permeability or abnormal microbiota, may actually be detrimental to children with autism. Recent studies have shown that intracerebroventricular and peripheral injections of propionic acid (one carbon shorter than butyrate) during development results in autistic like behavior, including repetitive dystonic behaviors and object preference [90–94]. These behavioral changes were also observed to a lesser extent with butyric acid and acetic acid. However, this phenomenon has led to a rodent model of autism using propionic acid to induce autistic symptoms [95,96]. In agreement with these studies is the increased risk of autism with prenatal exposure to valproate, an anti-seizure and mood-stabilizing drug that is a carboxylic acid HDAC inhibitor, like butyrate [97–100]. Given the large increase in autism diagnoses in recent years, there is a significant interest in understanding the etiology of the disease. While there are certainly many factors at play, propionic acid has come under some scrutiny. It has become an increasingly common food preservative due to its antimicrobial properties, which has increased our exposure to propionic acid [101]. In general, there is overwhelming evidence of the beneficial effects of butyrate and other SCFAs, but based on the autism literature some caution is warranted when considering prenatal exposure and the developing brain.
Conclusion
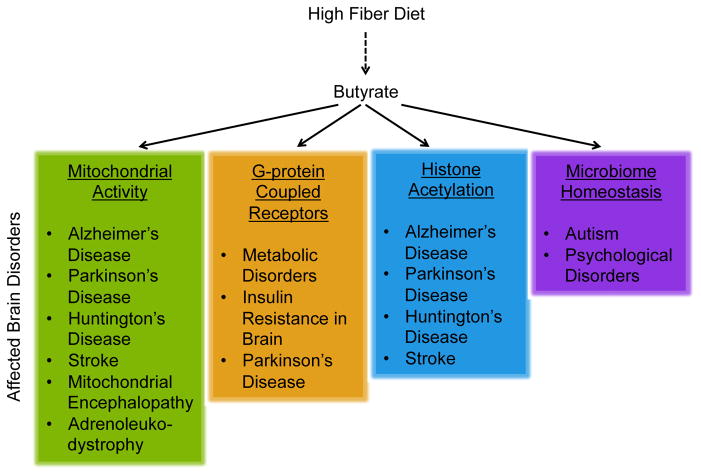
Butyrate is multi-functional molecule that has significant potential as a therapeutic for the brain, both in its pharmacologic and dietary form. Figure 2 summaries the various proposed mechanisms in which butyrate may influence brain health in a number of different neurological disorders. Pharmacologically, butyrate is capable of targeting many pathways with multiple mechanisms of action that are disease specific. The dietary sources of butyrate through a high fiber diet or a diet rich in natural sources of butyrate is a highly appealing approach, as it presents a simple and relatively low risk method to potentially improve outcomes in patients with brain disorders. Though much more research is needed to understand the effectiveness of these dietary interventions, they remain promising interventions that, if validated, may be used in the future in conjunction with traditional pharmacological treatments. As the current literature suggests, we can no longer overlook the importance of the gut-brain axis and nutrition in disease pathogenesis and treatment.
Figure 2.
The proposed mechanisms for the neuroprotective effects of butyrate and the diseases which may benefit from butyrate treatment or a high fiber diet.
Highlights.
Interest in how diet influences brain function via the gut microbiome is growing
Butyrate can protect the brain and enhance plasticity in neurological disease models
Gut microbiota produce butyrate by fermenting carbohydrates in a high fiber diet
Hypothesis: A high fiber diet can elevate butyrate to prevent/treat brain disorders
Acknowledgments
This work was supported by the NINDS of the National Institutes of Health under award number F32NS090819 and the Dr. Miriam and Sheldon G. Adelson Program in Neurorehabilitation and Neural Repair.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104:13780–5. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stilling RM, Dinan TG, Cryan JF. Microbial genes, brain & behaviour - epigenetic regulation of the gut-brain axis. Genes Brain Behav. 2014;13:69–86. doi: 10.1111/gbb.12109. [DOI] [PubMed] [Google Scholar]
- 3.Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. 2004;558:263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Layden BT, Angueira AR, Brodsky M, Durai V, Lowe WL. Short chain fatty acids and their receptors: new metabolic targets. Transl Res. 2013;161:131–140. doi: 10.1016/j.trsl.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Selkrig J, Wong P, Zhang X, Pettersson S. Metabolic tinkering by the gut microbiome: Implications for brain development and function. Gut Microbes. 2014;5:369–380. doi: 10.4161/gmic.28681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gottschalk G. Bacterial Metabolism. Springer-Verlag; New York: 1979. [Google Scholar]
- 7.Pryde SE, Duncan SH, Hold GL, Stewart CS, Flint HJ. The microbiology of butyrate formation in the human colon. FEMS Microbiol Lett. 2002;217:133–139. doi: 10.1111/j.1574-6968.2002.tb11467.x. [DOI] [PubMed] [Google Scholar]
- 8.Barcenilla A, Pryde SE, Martin JC, Duncan SH, Stewart CS, Henderson C, et al. Phylogenetic relationships of butyrate-producing bacteria from the human gut. Appl Environ Microbiol. 2000;66:1654– 1661. doi: 10.1128/aem.66.4.1654-1661.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cummings JH, Rombeau JL, Sakata T. Physiological and Clinical Aspects of Short-Chain Fatty Acids. Cambridge University Press; 2004. [Google Scholar]
- 10.Topping DL, Clifton PM. Short-Chain Fatty Acids and Human Colonic Function: Roles of Resistant Starch and Nonstarch Polysaccharides. Physiol Rev. 2001;81:1031–1064. doi: 10.1152/physrev.2001.81.3.1031. [DOI] [PubMed] [Google Scholar]
- 11.Le Blay G, Michel C, Blottiere HM, Cherbut C. Prolonged Intake of Fructo-Oligosaccharides Induces a Short-Term Elevation of Lactic Acid-Producing Bacteria and a Persistent Increase in Cecal Butyrate in Rats. J Nutr. 1999;129:2231–2235. doi: 10.1093/jn/129.12.2231. [DOI] [PubMed] [Google Scholar]
- 12.Perrin P. Only fibres promoting a stable butyrate producing colonic ecosystem decrease the rate of aberrant crypt foci in rats. Gut. 2001;48:53–61. doi: 10.1136/gut.48.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pouteau E, Nguyen P, Ballèvre O, Krempf M. Production rates and metabolism of short-chain fatty acids in the colon and whole body using stable isotopes. Proc Nutr Soc. 2003;62:87–93. doi: 10.1079/PNS2003208. [DOI] [PubMed] [Google Scholar]
- 14.Wolever TMS, Josse RG, Leiter LA, Chiasson JL. Time of day and glucose tolerance status affect serum short-chain fatty concentrations in humans. Metabolism. 1997;46:805–811. doi: 10.1016/s0026-0495(97)90127-x. [DOI] [PubMed] [Google Scholar]
- 15.Newmark HL, Lupton JR, Young CW. Butyrate as a differentiating agent: pharmacokinetics, analogues and current status. Cancer Lett. 1994;78:1–5. doi: 10.1016/0304-3835(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 16.Parodi PW. Conjugated linoleic acid and other anticarcinogenic agents of bovine milk fat. J Dairy Sci. 1999;82:1339–1349. doi: 10.3168/jds.S0022-0302(99)75358-0. [DOI] [PubMed] [Google Scholar]
- 17.Leonel AJ, Alvarez-Leite JI. Butyrate: implications for intestinal function. Curr Opin Clin Nutr Metab Care. 2012;15:474–479. doi: 10.1097/MCO.0b013e32835665fa. [DOI] [PubMed] [Google Scholar]
- 18.Miyoshi M, Sakaki H, Usami M, Iizuka N, Shuno K, Aoyama M, et al. Oral administration of tributyrin increases concentration of butyrate in the portal vein and prevents lipopolysaccharide-induced liver injury in rats. Clin Nutr. 2011;30:252–258. doi: 10.1016/j.clnu.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Sleiman SF, Basso M, Mahishi L, Kozikowski AP, Donohoe ME, Langley B, et al. Putting the “HAT” back on survival signalling: the promises and challenges of HDAC inhibition in the treatment of neurological conditions. Expert Opin Investig Drugs. 2009;18:573–584. doi: 10.1517/13543780902810345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sealy L. The effect of sodium butyrate on histone modification. Cell. 1978;14:115–121. doi: 10.1016/0092-8674(78)90306-9. [DOI] [PubMed] [Google Scholar]
- 21.Candido E. Sodium butyrate inhibits histone deacetylation in cultured cells. Cell. 1978;14:105–113. doi: 10.1016/0092-8674(78)90305-7. [DOI] [PubMed] [Google Scholar]
- 22.Ryu H, Lee J, Olofsson BA, Mwidau A, Dedeoglu A, Escudero M, et al. Histone deacetylase inhibitors prevent oxidative neuronal death independent of expanded polyglutamine repeats via an Sp1-dependent pathway. Proc Natl Acad Sci U S A. 2003;100:4281–4286. doi: 10.1073/pnas.0737363100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrante RJ, Kubilus JK, Lee J, Ryu H, Beesen A, Zucker B, et al. Histone Deacetylase Inhibition by Sodium Butyrate Chemotherapy Ameliorates the Neurodegenerative Phenotype in Huntington’s Disease Mice. J Neurosci. 2003;23:9418–9427. doi: 10.1523/JNEUROSCI.23-28-09418.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langley B, D’Annibale MA, Suh K, Ayoub I, Tolhurst A, Bastan B, et al. Pulse inhibition of histone deacetylases induces complete resistance to oxidative death in cortical neurons without toxicity and reveals a role for cytoplasmic p21(waf1/cip1) in cell cycle-independent neuroprotection. J Neurosci. 2008;28:163–176. doi: 10.1523/JNEUROSCI.3200-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Camelo S, Iglesias AH, Hwang D, Due B, Ryu H, Smith K, et al. Transcriptional therapy with the histone deacetylase inhibitor trichostatin A ameliorates experimental autoimmune encephalomyelitis. J Neuroimmunol. 2005;164:10–21. doi: 10.1016/j.jneuroim.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 26.Gardian G, Browne SE, Choi DK, Klivenyi P, Gregorio J, Kubilus JK, et al. Neuroprotective effects of phenylbutyrate in the N171-82Q transgenic mouse model of Huntington’s disease. J Biol Chem. 2005;280:556–563. doi: 10.1074/jbc.M410210200. [DOI] [PubMed] [Google Scholar]
- 27.Sharma S, Taliyan R, Singh S. Beneficial effects of sodium butyrate in 6- OHDA induced neurotoxicity and behavioral abnormalities: Modulation of histone deacetylase activity. Behav Brain Res. 2015;291:306–314. doi: 10.1016/j.bbr.2015.05.052. [DOI] [PubMed] [Google Scholar]
- 28.Kidd SK, Schneider JS. Protection of dopaminergic cells from MPP+-mediated toxicity by histone deacetylase inhibition. Brain Res. 2010;1354:172–178. doi: 10.1016/j.brainres.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.St Laurent R, O’Brien LM, Ahmad ST. Sodium butyrate improves locomotor impairment and early mortality in a rotenone-induced Drosophila model of Parkinson’s disease. Neuroscience. 2013;246:382–390. doi: 10.1016/j.neuroscience.2013.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drottar M, Liberman MC, Ratan RR, Roberson DW. The histone deacetylase inhibitor sodium butyrate protects against cisplatin-induced hearing loss in guinea pigs. Laryngoscope. 2006;116:292–6. doi: 10.1097/01.mlg.0000197630.85208.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim HJ, Chuang DM. HDAC inhibitors mitigate ischemia-induced oligodendrocyte damage: potential roles of oligodendrogenesis, VEGF, and anti-inflammation. Am J Transl Res. 2014;6:206–223. [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Z, Leng Y, Tsai LK, Leeds P, Chuang DM. Valproic acid attenuates blood-brain barrier disruption in a rat model of transient focal cerebral ischemia: the roles of HDAC and MMP-9 inhibition. J Cereb Blood Flow Metab. 2011;31:52–57. doi: 10.1038/jcbfm.2010.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim HJ, Rowe M, Ren M, Hong JS, Chen PS, Chuang DM. Histone deacetylase inhibitors exhibit anti-inflammatory and neuroprotective effects in a rat permanent ischemic model of stroke: multiple mechanisms of action. J Pharmacol Exp Ther. 2007;321:892–901. doi: 10.1124/jpet.107.120188. [DOI] [PubMed] [Google Scholar]
- 34.Sleiman SF, Berlin J, Basso M, Karuppagounder SS, Rohr J, Ratan RR. Histone Deacetylase Inhibitors and Mithramycin A Impact a Similar Neuroprotective Pathway at a Crossroad between Cancer and Neurodegeneration. Pharmaceuticals (Basel) 2011;4:1183–1195. doi: 10.3390/ph4081183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Govindarajan N, Agis-Balboa RC, Walter J, Sananbenesi F, Fischer A. Sodium butyrate improves memory function in an Alzheimer’s disease mouse model when administered at an advanced stage of disease progression. J Alzheimers Dis. 2011;26:187–197. doi: 10.3233/JAD-2011-110080. [DOI] [PubMed] [Google Scholar]
- 36.Kilgore M, Miller CA, Fass DM, Hennig KM, Haggarty SJ, Sweatt JD, et al. Inhibitors of class 1 histone deacetylases reverse contextual memory deficits in a mouse model of Alzheimer’s disease. Neuropsychopharmacology. 2010;35:870–880. doi: 10.1038/npp.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharma B, Sharma PM. Arsenic toxicity induced endothelial dysfunction and dementia: pharmacological interdiction by histone deacetylase and inducible nitric oxide synthase inhibitors. Toxicol Appl Pharmacol. 2013;273:180–188. doi: 10.1016/j.taap.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 38.da Silva PF, Garcia VA, da Dornelles AS, da Silva VK, Maurmann N, Portal BCD, et al. Memory impairment induced by brain iron overload is accompanied by reduced H3K9 acetylation and ameliorated by sodium butyrate. Neuroscience. 2012;200:42–49. doi: 10.1016/j.neuroscience.2011.10.038. [DOI] [PubMed] [Google Scholar]
- 39.Dash PK, Orsi SA, Moore AN. Histone deactylase inhibition combined with behavioral therapy enhances learning and memory following traumatic brain injury. Neuroscience. 2009;163:1–8. doi: 10.1016/j.neuroscience.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steckert AV, Comim CM, Igna DMD, Dominguini D, Mendonça BP, Ornell F, et al. Effects of sodium butyrate on aversive memory in rats submitted to sepsis. Neurosci Lett. 2015;595:134–138. doi: 10.1016/j.neulet.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 41.Barichello T, Generoso JS, Simões LR, Faller CJ, Ceretta RA, Petronilho F, et al. Sodium Butyrate Prevents Memory Impairment by Reestablishing BDNF and GDNF Expression in Experimental Pneumococcal Meningitis. Mol Neurobiol. 2015;52:734–740. doi: 10.1007/s12035-014-8914-3. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki-Mizushima Y, Gohda E, Okamura T, Kanasaki K, Yamamoto I. Enhancement of NGF- and cholera toxin-induced neurite outgrowth by butyrate in PC12 cells. Brain Res. 2002;951:209–217. doi: 10.1016/s0006-8993(02)03163-3. [DOI] [PubMed] [Google Scholar]
- 43.Wu X, Chen PS, Dallas S, Wilson B, Block ML, Wang CC, et al. Histone deacetylase inhibitors up-regulate astrocyte GDNF and BDNF gene transcription and protect dopaminergic neurons. Int J Neuropsychopharmacol. 2008;11:1123–1134. doi: 10.1017/S1461145708009024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mahan AL, Mou L, Shah N, Hu JH, Worley PF, Ressler KJ. Epigenetic modulation of Homer1a transcription regulation in amygdala and hippocampus with pavlovian fear conditioning. J Neurosci. 2012;32:4651–4659. doi: 10.1523/JNEUROSCI.3308-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wei Y, Melas PA, Wegener G, Mathé AA, Lavebratt C. Antidepressant-like effect of sodium butyrate is associated with an increase in TET1 and in 5-hydroxymethylation levels in the Bdnf gene. Int J Neuropsychopharmacol. 2015;18:1–10. doi: 10.1093/ijnp/pyu032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Varela RB, Valvassori SS, Lopes-Borges J, Mariot E, Dal-Pont GC, Amboni RT, et al. Sodium butyrate and mood stabilizers block ouabain-induced hyperlocomotion and increase BDNF, NGF and GDNF levels in brain of Wistar rats. J Psychiatr Res. 2015;61:114–121. doi: 10.1016/j.jpsychires.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 47.Valvassori SS, Varela RB, Arent CO, Dal-Pont GC, Bobsin TS, Budni J, et al. Sodium butyrate functions as an antidepressant and improves cognition with enhanced neurotrophic expression in models of maternal deprivation and chronic mild stress. Curr Neurovasc Res. 2014;11:359–366. doi: 10.2174/1567202611666140829162158. [DOI] [PubMed] [Google Scholar]
- 48.Intlekofer KA, Berchtold NC, Malvaez M, Carlos AJ, McQuown SC, Cunningham MJ, et al. Exercise and sodium butyrate transform a subthreshold learning event into long-term memory via a brain-derived neurotrophic factor-dependent mechanism. Neuropsychopharmacology. 2013;38:2027–2034. doi: 10.1038/npp.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kumar A, Choi KH, Renthal W, Tsankova NM, Theobald DEH, Truong HT, et al. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48:303–314. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 50.Pascual M, Do Couto BR, Alfonso-Loeches S, Aguilar MA, Rodriguez-Arias M, Guerri C. Changes in histone acetylation in the prefrontal cortex of ethanol-exposed adolescent rats are associated with ethanol-induced place conditioning. Neuropharmacology. 2012;62:2309–2319. doi: 10.1016/j.neuropharm.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 51.Ji M, Dong L, Jia M, Liu W, Zhang M, Ju L, et al. Epigenetic enhancement of brain-derived neurotrophic factor signaling pathway improves cognitive impairments induced by isoflurane exposure in aged rats. Mol Neurobiol. 2014;50:937–944. doi: 10.1007/s12035-014-8659-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhong T, Qing QJ, Yang Y, Zou WY, Ye Z, Yan JQ, et al. Repression of contexual fear memory induced by isoflurane is accompanied by reduction in histone acetylation and rescued by sodium butyrate. Br J Anaesth. 2014;113:634–643. doi: 10.1093/bja/aeu184. [DOI] [PubMed] [Google Scholar]
- 53.Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, et al. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- 54.Gensert JM, Baranova OV, Weinstein DE, Ratan RR. CD81, a cell cycle regulator, is a novel target for histone deacetylase inhibition in glioma cells. Neurobiol Dis. 2007;26:671–680. doi: 10.1016/j.nbd.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 55.Roediger WE. Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut. 1980;21:793–798. doi: 10.1136/gut.21.9.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Donohoe DR, Garge N, Zhang X, Sun W, O’Connell TM, Bunger MK, et al. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13:517–526. doi: 10.1016/j.cmet.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Donohoe DR, Collins LB, Wali A, Bigler R, Sun W, Bultman SJ. The Warburg effect dictates the mechanism of butyrate-mediated histone acetylation and cell proliferation. Mol Cell. 2012;48:612–626. doi: 10.1016/j.molcel.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Friedland RP, Budinger TF, Ganz E, Yano Y, Mathis CA, Koss B, et al. Regional cerebral metabolic alterations in dementia of the Alzheimer type: positron emission tomography with [18F]fluorodeoxyglucose. J Comput Assist Tomogr. 1983;7:590–598. doi: 10.1097/00004728-198308000-00003. [DOI] [PubMed] [Google Scholar]
- 59.Mosconi L, Pupi A, De Leon MJ. Brain glucose hypometabolism and oxidative stress in preclinical Alzheimer’s disease. Ann N Y Acad Sci. 2008;1147:180–195. doi: 10.1196/annals.1427.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Henderson S, Vogel J, Barr L. Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer’s disease: a randomized, double-blind, placebo-controlled, multicenter trial. Nutr Metab. 2009;6:1–25. doi: 10.1186/1743-7075-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao S, Xu W, Jiang W, Yu W, Lin Y, Zhang T, et al. Regulation of cellular metabolism by protein lysine acetylation. Science. 2010;327:1000–1004. doi: 10.1126/science.1179689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Valvassori SS, Calixto KV, Budni J, Resende WR, Varela RB, de Freitas KV, et al. Sodium butyrate reverses the inhibition of Krebs cycle enzymes induced by amphetamine in the rat brain. J Neural Transm. 2013;120:1737–1742. doi: 10.1007/s00702-013-1056-3. [DOI] [PubMed] [Google Scholar]
- 63.Moretti M, Valvassori SS, Varela RB, Ferreira CL, Rochi N, Benedet J, et al. Behavioral and neurochemical effects of sodium butyrate in an animal model of mania. Behav Pharmacol. 2011;22:766–772. doi: 10.1097/FBP.0b013e32834d0f1b. [DOI] [PubMed] [Google Scholar]
- 64.Lopes-Borges J, Valvassori SS, Varela RB, Tonin PT, Vieira JS, Gonçalves CL, et al. Histone deacetylase inhibitors reverse manic-like behaviors and protect the rat brain from energetic metabolic alterations induced by ouabain. Pharmacol Biochem Behav. 2015;128:89–95. doi: 10.1016/j.pbb.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 65.Vassart G, Costagliola S. G protein-coupled receptors: mutations and endocrine diseases. Nat Rev Endocrinol. 2011;7:362–372. doi: 10.1038/nrendo.2011.20. [DOI] [PubMed] [Google Scholar]
- 66.Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278:11312–11319. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- 67.Kimura I, Inoue D, Maeda T, Hara T, Ichimura A, Miyauchi S, et al. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41) Proc Natl Acad Sci U S A. 2011;108:8030–8035. doi: 10.1073/pnas.1016088108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, et al. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156:84–96. doi: 10.1016/j.cell.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 69.Thangaraju M, Cresci GA, Liu K, Ananth S, Gnanaprakasam JP, Browning DD, et al. GPR109A is a G-protein-coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Res. 2009;69:2826–2832. doi: 10.1158/0008-5472.CAN-08-4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zimmerman MA, Singh N, Martin PM, Thangaraju M, Ganapathy V, Waller JL, et al. Butyrate suppresses colonic inflammation through HDAC1-dependent Fas upregulation and Fas-mediated apoptosis of T cells. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1405–G1415. doi: 10.1152/ajpgi.00543.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wakade C, Chong R, Bradley E, Thomas B, Morgan J. Upregulation of GPR109A in Parkinson’s disease. PLoS One. 2014;9:e109818. doi: 10.1371/journal.pone.0109818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fu SP, Wang JF, Xue WJ, Liu HM, Liu B, Zeng YL, et al. Anti-inflammatory effects of BHBA in both in vivo and in vitro Parkinson’s disease models are mediated by GPR109A-dependent mechanisms. J Neuroinflammation. 2015;12:1–14. doi: 10.1186/s12974-014-0230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Won YJ, Lu VB, Puhl HL, Ikeda SR. β-Hydroxybutyrate modulates N-type calcium channels in rat sympathetic neurons by acting as an agonist for the G-protein-coupled receptor FFA3. J Neurosci. 2013;33:19314–19325. doi: 10.1523/JNEUROSCI.3102-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tarini J, Wolever TMS. The fermentable fibre inulin increases postprandial serum short-chain fatty acids and reduces free-fatty acids and ghrelin in healthy subjects. Appl Physiol Nutr Metab. 2010;35:9–16. doi: 10.1139/H09-119. [DOI] [PubMed] [Google Scholar]
- 75.Wolever TMS, Chiasson JL. Acarbose raises serum butyrate in human subjects with impaired glucose tolerance. Br J Nutr. 2000;84:57–61. [PubMed] [Google Scholar]
- 76.Priebe MG, Wang H, Weening D, Schepers M, Preston T, Vonk RJ. Factors related to colonic fermentation of nondigestible carbohydrates of a previous evening meal increase tissue glucose uptake and moderate glucose-associated inflammation. Am J Clin Nutr. 2010;91:90–97. doi: 10.3945/ajcn.2009.28521. [DOI] [PubMed] [Google Scholar]
- 77.Robertson MD, Bickerton AS, Dennis AL, Vidal H, Frayn KN. Insulin-sensitizing effects of dietary resistant starch and effects on skeletal muscle and adipose tissue metabolism. Am J Clin Nutr. 2005;82:559–567. doi: 10.1093/ajcn.82.3.559. [DOI] [PubMed] [Google Scholar]
- 78.Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6:263ra158. doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sherry CL, Kim SS, Dilger RN, Bauer LL, Moon ML, Tapping RI, et al. Sickness behavior induced by endotoxin can be mitigated by the dietary soluble fiber, pectin, through up-regulation of IL-4 and Th2 polarization. Brain Behav Immun. 2010;24:631–640. doi: 10.1016/j.bbi.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Khan NA, Raine LB, Drollette ES, Scudder MR, Kramer AF, Hillman CH. Dietary fiber is positively associated with cognitive control among prepubertal children. J Nutr. 2015;145:143–149. doi: 10.3945/jn.114.198457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Messaoudi M, Lalonde R, Violle N, Javelot H, Desor D, Nejdi A, et al. Assessment of psychotropic-like properties of a probiotic formulation ( Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br J Nutr. 2010;105:755–764. doi: 10.1017/S0007114510004319. [DOI] [PubMed] [Google Scholar]
- 82.Rao AV, Bested AC, Beaulne TM, Katzman MA, Iorio C, Berardi JM, et al. A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut Pathog. 2009;1:1–6. doi: 10.1186/1757-4749-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tillisch K, Labus J, Kilpatrick L, Jiang Z, Stains J, Ebrat B, et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology. 2013;144:1394–1401. doi: 10.1053/j.gastro.2013.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Adams JB, Johansen LJ, Powell LD, Quig D, Rubin RA. Gastrointestinal flora and gastrointestinal status in children with autism--comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 2011;11:1–13. doi: 10.1186/1471-230X-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kang DW, Park JG, Ilhan ZE, Wallstrom G, Labaer J, Adams JB, et al. Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS One. 2013;8:e68322. doi: 10.1371/journal.pone.0068322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Finegold SM, Dowd SE, Gontcharova V, Liu C, Henley KE, Wolcott RD, et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe. 2010;16:444–453. doi: 10.1016/j.anaerobe.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 87.Williams BL, Hornig M, Parekh T, Lipkin WI. Application of novel PCR-based methods for detection, quantitation, and phylogenetic characterization of Sutterella species in intestinal biopsy samples from children with autism and gastrointestinal disturbances. MBio. 2012;3:e00261–11. doi: 10.1128/mBio.00261-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sandler RH, Finegold SM, Bolte ER, Buchanan CP, Maxwell AP, Vaisanen ML, et al. Short-Term Benefit From Oral Vancomycin Treatment of Regressive-Onset Autism. J Child Neurol. 2000;15:429–435. doi: 10.1177/088307380001500701. [DOI] [PubMed] [Google Scholar]
- 89.Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA. Elevated fecal short chain fatty acid and ammonia concentrations in children with autism spectrum disorder. Dig Dis Sci. 2012;57:2096–2102. doi: 10.1007/s10620-012-2167-7. [DOI] [PubMed] [Google Scholar]
- 90.MacFabe DF, Cain DP, Rodriguez-Capote K, Franklin AE, Hoffman JE, Boon F, et al. Neurobiological effects of intraventricular propionic acid in rats: possible role of short chain fatty acids on the pathogenesis and characteristics of autism spectrum disorders. Behav Brain Res. 2007;176:149–169. doi: 10.1016/j.bbr.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 91.MacFabe DF, Cain NE, Boon F, Ossenkopp KP, Cain DP. Effects of the enteric bacterial metabolic product propionic acid on object-directed behavior, social behavior, cognition, and neuroinflammation in adolescent rats: Relevance to autism spectrum disorder. Behav Brain Res. 2011;217:47–54. doi: 10.1016/j.bbr.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 92.Thomas RH, Foley KA, Mepham JR, Tichenoff LJ, Possmayer F, MacFabe DF. Altered brain phospholipid and acylcarnitine profiles in propionic acid infused rodents: further development of a potential model of autism spectrum disorders. J Neurochem. 2010;113:515–529. doi: 10.1111/j.1471-4159.2010.06614.x. [DOI] [PubMed] [Google Scholar]
- 93.El-Ansary AK, Ben Bacha A, Kotb M. Etiology of autistic features: the persisting neurotoxic effects of propionic acid. J Neuroinflammation. 2012;9:1–14. doi: 10.1186/1742-2094-9-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brusque A, Mello C, Buchanan D, Terracciano S, Rocha M, Vargas C, et al. Effect of Chemically Induced Propionic Acidemia on Neurobehavioral Development of Rats. Pharmacol Biochem Behav. 1999;64:529–534. doi: 10.1016/s0091-3057(99)00127-6. [DOI] [PubMed] [Google Scholar]
- 95.MacFabe DF, Rodriguez K, Hoffman JE, Franklin AE, Mohammad-A Y, Taylor AR, et al. A Novel Rodent Model of Autism: Intraventricular Infusions of Propionic Acid Increase Locomotor Activity and Induce Neuroinflammation and Oxidative Stress in Discrete Regions of Adult Rat Brain. Am J Biochem Biotechnol. 2008;4:146–166. [Google Scholar]
- 96.Shultz SR, MacFabe DF, Martin S, Jackson J, Taylor R, Boon F, et al. Intracerebroventricular injections of the enteric bacterial metabolic product propionic acid impair cognition and sensorimotor ability in the Long-Evans rat: further development of a rodent model of autism. Behav Brain Res. 2009;200:33–41. doi: 10.1016/j.bbr.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 97.Schneider T, Przewłocki R. Behavioral alterations in rats prenatally exposed to valproic acid: animal model of autism. Neuropsychopharmacology. 2005;30:80–89. doi: 10.1038/sj.npp.1300518. [DOI] [PubMed] [Google Scholar]
- 98.Ingram JL, Peckham SM, Tisdale B, Rodier PM. Prenatal exposure of rats to valproic acid reproduces the cerebellar anomalies associated with autism. Neurotoxicol Teratol. 2000;22:319–324. doi: 10.1016/s0892-0362(99)00083-5. [DOI] [PubMed] [Google Scholar]
- 99.Christensen J, Grønborg TK, Sørensen MJ, Schendel D, Parner ET, Pedersen LH, et al. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA. 2013;309:1696–1703. doi: 10.1001/jama.2013.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cohen OS, Varlinskaya EI, Wilson CA, Glatt SJ, Mooney SM. Acute prenatal exposure to a moderate dose of valproic acid increases social behavior and alters gene expression in rats. Int J Dev Neurosci. 2013;31:740–750. doi: 10.1016/j.ijdevneu.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Davidson MP, Sofos JN, Branen AL. Antimicrobials in Food. 3. CRC Press; 2005. [Google Scholar]
- 102.Smith JG, Yokoyama WH, German JB. Butyric acid from the diet: actions at the level of gene expression. Crit Rev Food Sci Nutr. 1998;38:259–297. doi: 10.1080/10408699891274200. [DOI] [PubMed] [Google Scholar]