Abstract
Background
Combination therapy using cyclosporine A (CsA) together with low-dose corticosteroids has adequate efficacy with little toxicity for the treatment of severe alopecia areata (AA).
Objective
We wanted to evaluate the clinical efficacy of combination therapy using CsA with low-dose corticosteroid for the treatment of severe AA and we also wanted to determine the safe therapeutic concentration of CsA in the peripheral blood.
Methods
We treated 34 cases of severe AA with combination therapy for 24 weeks and we evaluated the efficacy at 12 and 24 weeks. We monitored the peripheral blood concentration of CsA to determine the therapeutic range of CsA that has the fewest side effects.
Results
Of the patients, 77.4% (n=24) and 22.6% (n=10) were classified in the responder and poor-responder groups, respectively. The mean trough concentration of CsA was 95.1 and 101.2 ng/ml in the responder and poor-responder groups, respectively. For the patients with side effects associated with CsA, the mean CsA concentration was 195.8 ng/ml.
Conclusion
We found that combination therapy with systemic CsA and low-dose corticosteroids effectively treats severe AA and this therapy results in a safe, therapeutic concentration of CsA in the peripheral blood.
Keywords: Alopecia areata, Cyclosporine A, Low-dose corticosteroids
INTRODUCTION
Treating patients with alopecia areata (AA) remains difficult, and many treatment modalities have been used, including intralesional, topical and systemic corticosteroids, ultraviolet light and contact sensitizers1,2,3,4,5,6,7,8. Of these, systemic corticosteroids are effective for the treatment of severe AA3,4,5, but to maintain hair regrowth, an increase in the dosage is inevitable. A high dose of systemic corticosteroids poses a major concern because of the side effects of systemic steroid use, such as diabetic mellitus, hypertension, acne, psychological changes, osteoporosis and the suppression of the adrenocorticotropic axis.
Cyclosporine A (CsA) is a cyclic, lipophilic undecapeptide that selectively and reversibly inhibits the T cell-mediated immune response by suppressing the phosphatase activity of calcineurin. Calcineurin regulates the nuclear factor of activated T cells (NFAT) and it also induces T cell activation by regulating the interleukin-2 (IL-2) gene expression and increasing the production of IL-2, which stimulates the secretion of interferon-γ (IFN-γ)9,10. The immunologic cascade leading to AA is believed to involve these events because IL-2 and IFN-γ are involved in the pathogenesis of AA. Therefore, CsA is thought to be effective for treating AA because it eliminates immune cells from the hair follicles.
Corticosteroids are nonselective immunosuppressive agents that inhibit the late-phase antigen-independent inflammatory reaction. In addition, they decrease the availability of nuclear factor kappa B and IL-2 gene transcription11. Since CsA acts as an early immune modulator at the induction phase, combination therapy with CsA and corticosteroids may have synergistic effects on immunosuppression 11. Although several studies have used systemic CsA in combination with corticosteroids for the treatment of severe AA12,13,14, the results have been variable. Many studies that used CsA as a treatment modality recommended monitoring the peripheral blood concentration of CsA to evaluate the efficacy of treatment and avoid CsA-related toxicity15,16,17.
In this study, we evaluated the efficacy of a combination therapy using CsA with low-dose corticosteroids for treating patients with severe AA and we estimated the therapeutic range of the trough concentration of CsA for this treatment.
MATERIALS AND METHODS
Patients
Thirty-four patients (16 men and 18 women) with severe or refractory AA were enrolled in this study. Their mean age was 28.1 years (range: 6~54 years) and the duration of the disease ranged from 6 months to 16 years.
The extent of AA was evaluated in accordance with the National Alopecia Areata Foundation guidelines18: S0: no hair loss, S1: <25% hair loss, S2: 26~50% hair loss, S3: 51~75% hair loss, S4: 76~99% hair loss, alopecia totalis (AT): total scalp hair loss and alopecia universalis (AU): total scalp and body hair loss. Of the 34 patients, eight had AU and seven had alopecia totalis/universalis (AT/AU), which is defined as alopecia totalis with various degrees of body hair loss, 16 patients had patchy AA involving more than 50% of the scalp (S3: 8, S4: 7), one had involvement of less than 50% of the scalp (S2: 2) and two had ophiasis. None of the patients had previously responded to conventional treatments at least 6 months follow-up.
Treatment regimens
CsA was started at a dose of 2.5 to 5 mg/kg/day and methyl prednisone was started at a dose of 20 to 24 mg/day. The dose of methyl prednisone was subsequently tapered to 4 mg/day for 3 weeks, and then the methyl prednisone was maintained at a dose of 2 to 4 mg/day, according to the treatment response.
Evaluation of efficacy
All the patients were monitored on a monthly basis during the 24 weeks. Each patient was photographed at baseline and at 1, 3 and 6 months of treatment.
The responders were defined as the patients who recovered more than 50% of hair regrowth, while poor responders had less than 50% of regrowth. The responders were subclassified into cosmetically acceptable responders, and these were defined as patients with 75 to 100% hair regrowth, and satisfactory responders had 50 to 74% hair regrowth.
Monitoring of the trough CsA concentration and the laboratory data
To estimate the therapeutic concentration of CsA, blood samples were collected immediately before the oral administration of CsA and then they were analyzed. The initial trough concentration of CsA was measured 2 weeks after initiating the treatment, and the maintenance trough concentration was measured 1, 3 and 6 months after beginning the treatment. Blood pressure, complete blood counts, serum transaminase, blood urea nitrogen, creatinine, direct bilirubin and urinalysis were checked at 1, 3, and 6 months to evaluate the side effects associated with CsA and corticosteroid combination therapy.
Statistical analysis
Statistical analysis was performed using SAS version 9.1.3 (SAS Institute, Cary, NC, USA). The chi-square test was used to compare the trough concentrations of CsA between the responders and poor responders and between the groups with and without side effects.
RESULTS
Response
Of all the patients, 77.4% (n=24) were classified into the responder group (Table 1). Of these 24 patients, 66.6% (n=16) were cosmetically acceptable responders, and 33.3% (n=8) were satisfactory responders (Fig. 1). The initial response time ranged from 4 to 9.5 weeks with a mean of 5.4 weeks. The mean dose of methyl prednisone during the maintenance phase was 2.9 mg/day and the dose ranged from 2 to 6 mg/day. The type with the best response was ophiasis, followed in order by patchy AA affecting more than 50% of the scalp, AU and AT/AU. The least responsive type of AA was patchy AA affecting less than 50% of the scalp.
Table 1. Demographic data of 24 patients of the clinical responders.
*Extent of AA: in accordance with the National Alopecia Areata Foundation guidelines: S0: no hair loss, S1: <25% hair loss, S2: 26~50%, S3: 51~75%, S4: 76~99%, Alopecia totalis (AT): total scalp hair loss, Alopecia universalis (AU): total scalp and body hair loss, Alopecia totalis/universalis (AT/AU): alopecia totalis with variable degrees of loss of body hair, †MTC: the mean trough concentration of cyclosporine A, ‡CsA: cyclosporin A, §MPDS: methylprednisone
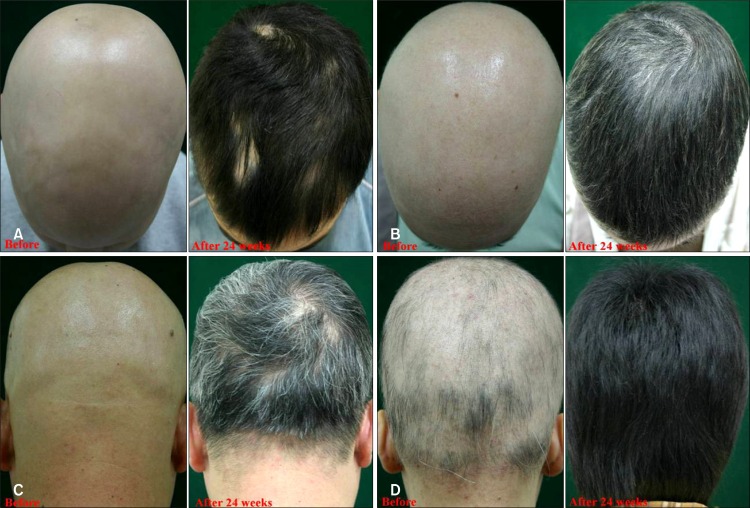
Fig. 1. (A) A 10-year-old boy with alopecia universalis before and after 24 weeks of treatment. (B) A 23-year-old women with alopecia universalis before and after 24 weeks of treatment. (C) A 35-year-old man with alopecia universalis before and after 24 weeks of treatment. (D) A 16-year-old women with alopecia areata affecting more than 75% of hair loss before and after 24 weeks of treatment.
Of the patients, 22.6% (n=10) were classified into the poor-responder group (Table 2).
Table 2. Demographic data of 10 patients of the clinical poor responders.
*Extent of AA: in accordance with the guidelines of National Alopecia Areata Foundation guidelines: S0: no hair loss, S1: <25% hair loss, S2: 26~50%, S3:51~75%, S4: 76~99%, Alopecia totalis (AT): total scalp hair loss, Alopecia universalis (AU): total scalp and body hair loss, Alopecia totalis/universalis (AT/AU): alopecia totalis with variable degrees of loss of body hair, †MTC: the mean trough concentration of cyclosporine A, ‡CsA: cyclosporin A, §MPDS: methylprednisone
Trough CsA concentrations in the responders and poor responders
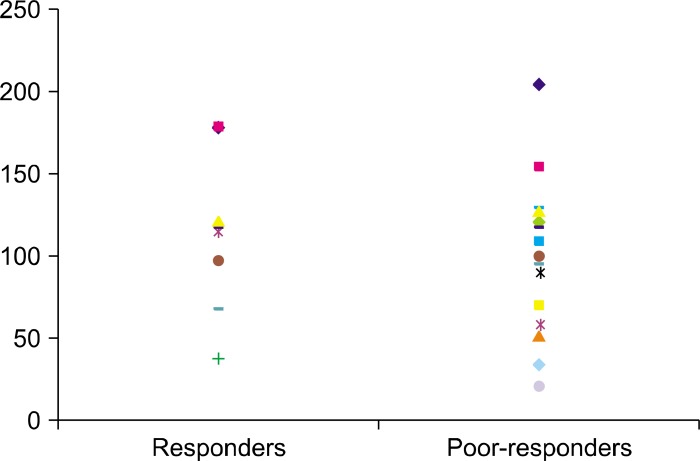
The trough CsA concentrations were determined for all the patients. For the responders, the mean CsA dose for maintenance was 3.0 mg/kg/day (2.5~3.9 mg/kg/day) and the mean trough concentration for an initial response was 88.4 ng/ml (range: 33.4~141.4 ng/ml). The mean trough concentration of CsA for maintenance was 94.2 ng/ml (range: 22.1~156.2 ng/ml). For the poor responders, the mean CsA dose for maintenance was 3.7 mg/kg/day (range: 2.2~6.0 mg/kg/day) and the mean trough concentration was 101.2 ng/ml (range: 39.2~178.4 ng/ml). The mean dose of CsA for these two groups showed no statistical differences (p>0.05). For the responders, most patients maintained their hair regrowth with trough CsA concentrations between 50 and 150 ng/ml (Fig. 2). The responders had a lower mean trough concentration than did the poor responders (p<0.05).
Fig. 2. Trough concentration of cyclosporine A in the responders and poor-responders. The mean trough concentration of cyclosporine A in the responders was 94.2 ng/ml (range: 22.1~162.1 ng/ml) and 101.2 ng/ml (range: 39.2~178.4 ng/ml) in the poor-responders. The responders displayed a lower mean trough concentration than did the poor-responders (p<0.05).
In addition, the mean trough concentration was not related to either the duration of AA (p=0.33) or the extent of AA (p=0.60) for both groups.
Trough CsA concentration in the patients with and without side effects
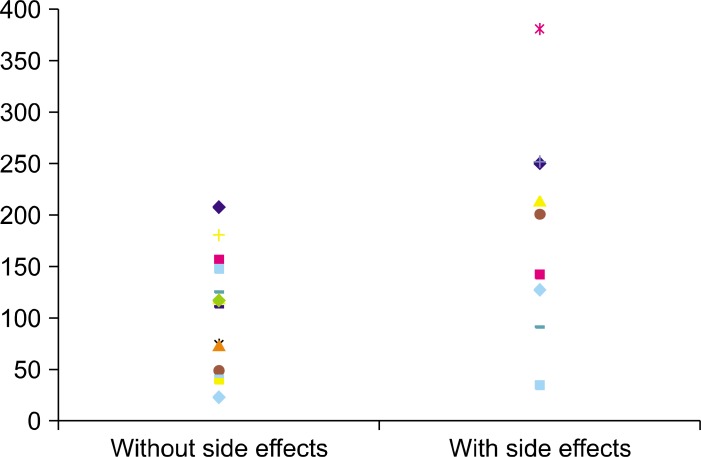
In this study, 12 patients showed side effects associated with CsA (Fig. 3), including 4 with hyperlipidemia, 2 with hypertension, 2 with hypertrichosis, 2 with hyperbilirubinemia, 1 with weakness in the lower legs and 1 with hematuria (Table 1, 2). The mean dose of CsA that was used for these two groups showed no statistical differences (p=0.23). For the group with side effects, the mean CsA concentration measured 195.8 ng/ml (range: 34.8~381.2 ng/ml). The mean concentration of CsA for the patients without side effects was 91.2 ng/ml (range: 22.1~204.8 ng/ml). The difference in the trough CsA concentrations between these two groups was statistically significant (p<0.05).
Fig. 3. Trough concentration of cyclosporine A in the patients with and without side effects. In the group with side effects, the mean concentration of cyclosporine A was 195.8 ng/ml (34.8~381.2 ng/ml). The mean concentration of cyclosporine A in the patients without side effects was 91.2 ng/ml (22.1~204.8 ng/ml). The difference of the trough concentration of cyclosporine A between these two groups was significant (p<0.05).
DISCUSSION
The effects of CsA for the treatment of AA have been assessed in various in vitro studies. Oliver et al9 demonstrated that oral CsA restored hair growth in the Dundee Experimental Bald Rat Model of AA by reducing the mononuclear cell infiltrate around the hair follicles and renewing hair growth. Gafter-Gvili et al10 reported that CsA-induced hair growth in mice was associated with the inhibition of the calcineurin-dependent activation of NFAT in the follicular keratinocytes. They found that CsA delays the duration of the terminal differentiation of hair follicular keratinocytes and it retards catagen induction. They further demonstrated that CsA induced hair regrowth through delaying the regression of hair follicles by inhibiting the apoptosis of follicular keratinocytes19.
However, the use of systemic CsA is also limited because its side effect profile is similar to that of systemic corticosteroids. CsA has a narrow therapeutic window and many side effects, including nephrotoxicity, neurotoxicity and hyperglycemia. Because of these problems, therapeutic drug monitoring (TDM) is commonly used to optimize the efficacy and safety of CsA and TDM can provide a more optimal dosing strategy.
Combination therapy using systemic CsA and low-dose corticosteroid to treat AA has been previously reported on12,13,14, and TDM has helped to reduce the toxicity of these drugs, such as by preventing life-threatening infections via administering concentration-oriented therapy rather than dose-oriented therapy20,21. However, the results of previous studies have shown variable success rates13,14,22 and the therapeutic range of the trough CsA concentration varied from study to study12,18. Teshima et al12 treated six AU patients using oral CsA at 2.5 mg/kg/day and prednisolone was started at a dose of 5 mg/day for 7 months. The CsA concentration was maintained at 30 to 50 ng/ml. An initial response appeared within 1 month and all the patients showed hair regrowth after 7 months of treatment. Ferrando et al18 treated 15 patients with severe AA by using CsA monotherapy; they administered CsA at a dose of 5 mg/kg/day for 6 to 12 months and they maintained the CsA concentration at 100 to 350 ng/ml. One patient discontinued treatment due to hypertension. Seven of the 14 patients had more than 70% hair regrowth, two had complete hair regrowth and five showed no response. CsA induced severe side effects such as gingival hypertrophy and hyperlipidemia. The authors recommended using CsA for 4 months duration for treating refractory AA.
In our study, 77.4% of the patients had hair regrowth exceeding 50%. They maintained the hair regrowth with trough concentrations between 50 and 150 ng/ml and they had lower mean trough concentrations than did the poor responders. Despite administering a higher dose of CsA to the poor responders, they had poorer hair regrowth rates.
In this study, 12 patients showed side effects associated with CsA. However, the side effects were either temporary or well managed. The hyperlipidemia and hypertension were controlled using hyrdoxymethylglutaryl-coenzyme A reductase inhibitors or antihypertensive agents, and the hyperbilirubinemia and hematuria cleared after tapering the dose of CsA. The weakness in the lower legs disappeared spontaneously within a few weeks. The mean CsA concentration was 195.8 ng/ml in the patients with side effects and 91.2 ng/ml in those patients without side effects. Interestingly, except one patient, most of the patients without side effects maintained trough concentration under 200 ng/ml, and two-third of the patients with side effects showed side effects when the trough concentration was over 200 ng/ml. In the light of these results, we recommend that it is helpful for minimizing side effects to maintain the CsA trough concentration under 200 ng/ml. A further study with a larger number of AA patients is required to establish a more effective, safe treatment strategy with using oral CsA and low dose corticosteroids combination therapy.
Recent studies have reported on the immunologic aspects associated with the severity and prognosis of AA21,23, and so further studies are needed to identify the genetic and immunologic factors that may play a role for the inter-individual variations in the pharmacokinetics of CsA or the prognosis of AA.
This study demonstrated that systemic CsA and low-dose corticosteroid combination therapy for patients suffering with severe AA led to a high response rate with steroid-sparing effects, and there were no severe side effects. The therapeutic range of the trough CsA concentration for maintenance was 50~150 ng/ml.
Further concentration-oriented studies with more patients are required to establish the optimal dose regimen of oral CsA and low dose corticosteroids combination therapy for treating patients with AA.
Footnotes
This work was supported by a 2005 Inje University research grant.
References
- 1.Tosti A, Piraccini BM, Pazzaglia M, Vincenzi C. Clobetasol propionate 0.05% under occlusion in the treatment of alopecia totalis/universalis. J Am Acad Dermatol. 2003;49:96–98. doi: 10.1067/mjd.2003.423. [DOI] [PubMed] [Google Scholar]
- 2.Feldmann KA, Kunte C, Wollenberg A, Wolfe H. Is topical tacrolimus effective in alopecia areata universalis? Br J Dermatol. 2002;147:1031–1032. doi: 10.1046/j.1365-2133.2002.500210.x. [DOI] [PubMed] [Google Scholar]
- 3.Kurosawa M, Nakagawa S, Mizuashi M, Sasaki Y, Kawamura M, Saito M, et al. A comparison of the efficacy, relapse rate and side effects among three modalities of systemic corticosteroid therapy for alopecia areata. Dermatology. 2006;212:361–365. doi: 10.1159/000092287. [DOI] [PubMed] [Google Scholar]
- 4.Kar BR, Handa S, Dogra S, Kumar B. Placebo-controlled oral pulse prednisolone therapy in alopecia areata. J Am Acad Dermatol. 2005;52:287–290. doi: 10.1016/j.jaad.2004.10.873. [DOI] [PubMed] [Google Scholar]
- 5.Alabdulkareem AS, Abahussein AA, Okoro A. Severe alopecia areata treated with systemic corticosteroids. Int J Dermatol. 1998;37:622–624. doi: 10.1046/j.1365-4362.1998.00422.x. [DOI] [PubMed] [Google Scholar]
- 6.Rokhsar CK, Shupack JL, Vafai JJ, Washenik K. Efficacy of topical sensitizers in the treatment of alopecia areata. J Am Acad Dermatol. 1998;39:751–761. doi: 10.1016/s0190-9622(98)70048-9. [DOI] [PubMed] [Google Scholar]
- 7.Yoshizawa Y, Izaki S, Kitamura K, Kawana S. Systemic immunotherapy with topical dinitrochlorobenzene as additional treatment of alopecia areata. Acta Derm Venereol. 2002;82:136–138. doi: 10.1080/00015550252948202. [DOI] [PubMed] [Google Scholar]
- 8.Behrens-Williams SC, Leiter U, Schiener R, Weidmann M, Peter RU, Kerscher M. The PUVA-turban as a new option of applying a dilute psoralen solution selectively to the scalp of patients with alopecia areata. J Am Acad Dermatol. 2001;44:248–252. doi: 10.1067/mjd.2001.110060. [DOI] [PubMed] [Google Scholar]
- 9.Oliver RF, Lowe JG. Oral cyclosporin A restores hair growth in the DEBR rat model for alopecia areata. Clin Exp Dermatol. 1995;20:127–131. doi: 10.1111/j.1365-2230.1995.tb02669.x. [DOI] [PubMed] [Google Scholar]
- 10.Gafter-Gvili A, Sredni B, Gal R, Gafter U, Kalechman Y. Cyclosporin A-induced hair growth in mice is associated with inhibition of calcineurin-dependent activation of NFAT in follicular keratinocytes. Am J Physiol Cell Physiol. 2003;284:C1593–C1603. doi: 10.1152/ajpcell.00537.2002. [DOI] [PubMed] [Google Scholar]
- 11.Ferron GM, Pyszczynski NA, Jusko WJ. Gender-related assessment of cyclosporine/prednisolone/sirolimus interactions in three human lymphocyte proliferation assays. Transplantation. 1998;65:1203–1209. doi: 10.1097/00007890-199805150-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teshima H, Urabe A, Irie M, Nakagawa T, Nakayama J, Hori Y. Alopecia universalis treated with oral cyclosporine A and prednisolone: immunologic studies. Int J Dermatol. 1992;31:513–516. doi: 10.1111/j.1365-4362.1992.tb02706.x. [DOI] [PubMed] [Google Scholar]
- 13.Shapiro J, Lui H, Tron V, Ho V. Systemic cyclosporine and low-dose prednisone in the treatment of chronic severe alopecia areata: a clinical and immunopathologic evaluation. J Am Acad Dermatol. 1997;36:114–117. doi: 10.1016/s0190-9622(97)70342-6. [DOI] [PubMed] [Google Scholar]
- 14.Park HH, Sim WY. Cyclosporine combination therapy in alopecia areata. Korean J Dermatol. 2002;40:1311–1315. [Google Scholar]
- 15.Umezawa Y, Ozawa A. Optimal time for therapeutic drug monitoring of cyclosporine microemulsion in patients with psoriasis. Int J Dermatol. 2007;46:763–766. doi: 10.1111/j.1365-4632.2007.03136.x. [DOI] [PubMed] [Google Scholar]
- 16.Wang CH, Chou NK, Wu FL, Ko WJ, Tsao CI, Chi NH, et al. Therapeutic drug monitoring of cyclosporine Neoral in de novo Chinese cardiac transplant recipients treated with an everolimus-cyclosporine immunosuppressive regimen. Transplant Proc. 2006;38:2132–2134. doi: 10.1016/j.transproceed.2006.06.050. [DOI] [PubMed] [Google Scholar]
- 17.Rinaldi S, Sesto A, Barsotti P, Faraggiana T, Sera F, Rizzoni G. Cyclosporine therapy monitored with abbreviated area under curve in nephrotic syndrome. Pediatr Nephrol. 2005;20:25–29. doi: 10.1007/s00467-004-1618-6. [DOI] [PubMed] [Google Scholar]
- 18.Ferrando J, Grimalt R. Partial response of severe alopecia areata to cyclosporine A. Dermatology. 1999;199:67–69. doi: 10.1159/000018184. [DOI] [PubMed] [Google Scholar]
- 19.Gafter-Gvili A, Kalechman Y, Sredni B, Gal R, Gafter U. Cyclosporin A-induced hair growth in mice is associated with inhibition of hair follicle regression. Arch Dermatol Res. 2004;296:265–269. doi: 10.1007/s00403-004-0516-x. [DOI] [PubMed] [Google Scholar]
- 20.Grevel J. Area-under-the-curve versus trough level monitoring of cyclosporine concentration: critical assessment of dosage adjustment practices and measurement of clinical outcome. Ther Drug Monit. 1993;15:488–491. doi: 10.1097/00007691-199312000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Armstrong VW, Oellerich M. New developments in the immunosuppressive drug monitoring of cyclosporine, tacrolimus, and azathioprine. Clin Biochem. 2001;34:9–16. doi: 10.1016/s0009-9120(00)00175-2. [DOI] [PubMed] [Google Scholar]
- 22.Kim BJ, Min SU, Park KY, Choi JW, Park SW, Youn SW, et al. Combination therapy of cyclosporine and methylprednisolone on severe alopecia areata. J Dermatolog Treat. 2008;19:216–220. doi: 10.1080/09546630701846095. [DOI] [PubMed] [Google Scholar]
- 23.Masuda S, Inui K. An up-date review on individualized dosage adjustment of calcineurin inhibitors in organ transplant patients. Pharmacol Ther. 2006;112:184–198. doi: 10.1016/j.pharmthera.2006.04.006. [DOI] [PubMed] [Google Scholar]