Abstract
Malignant peripheral nerve sheath tumor (MPNST) is a term encompassing tumors previously diagnosed as malignant schwannoma, malignant neurilemmoma, neurogenic sarcoma, and neurofibrosarcoma The occurrence rate of MPNST in neurofibromatosis type 1 patients is known to be about 4.6%. Tumors occurring in this particular group have a worse prognosis in that they occur at an earlier age, are more centrally located, tend to be of a larger size and show more metastases and recurrences. We present a typical case of MPNST in a 36-year-old man with NF type 1, which occurred on the left buttock. A PET-CT showed findings of possible inguinal lymph node metastasis and a lymph node biopsy confirmed the diagnosis. The patient was treated with wide surgical resection and is undergoing adjuvant radiation therapy.
Keywords: Malignant peripheral nerve sheath tumor, Neurofibromatosis type 1
INTRODUCTION
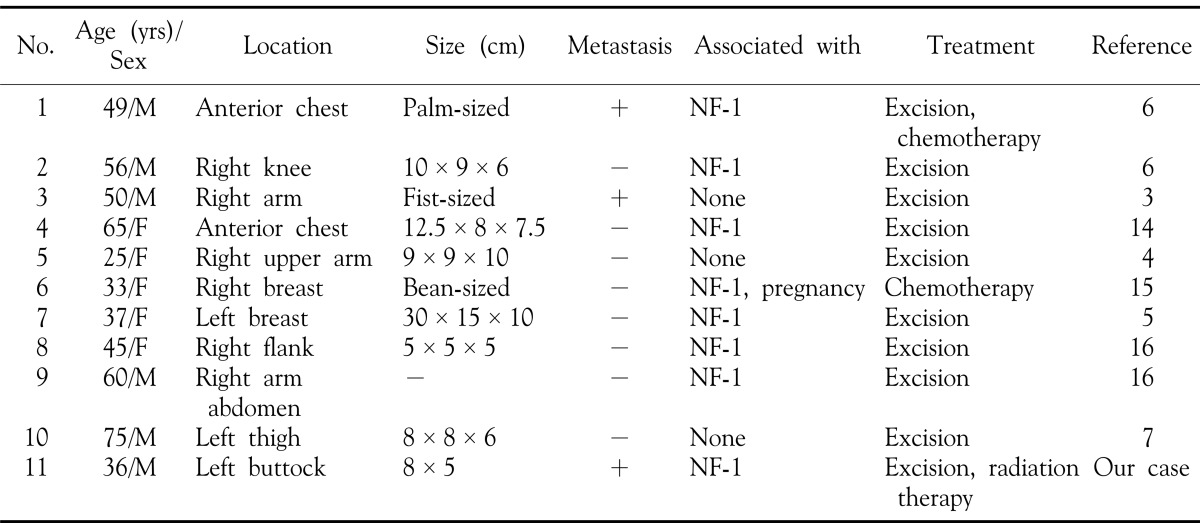
Malignant peripheral nerve sheath tumor (MPNST) is a malignant mesenchymal tumor that accounts for 5-10% of all soft tissue mesenchymal tumors1. While the incidence of MPNST is estimated at 0.001% in the general population, it is increased in patients with neurofibromatosis (NF) type 1, occurring in as much as 4.6% of all patients2. To our knowledge, there have been 10 cases of MPNST reported in Korean dermatological literature to date (Table 1), out of which 7 cases were associated with NF type 13,4,5,6,7. In this report, we present a case of MPNST that occurred on the left buttock and was accompanied with inguinal lymph node metastasis, of a NF type 1 patient, which was accompanied with inguinal lymph node metastasis, along with a brief review of the relevant literature and a summary of the cases reported in Korea to date.
Table 1. Summary of the reported cases of malignant peripheral nerve sheath tumor in Korean dermatologic literature.
NF-1: neurofibromatosis type 1
CASE REPORT
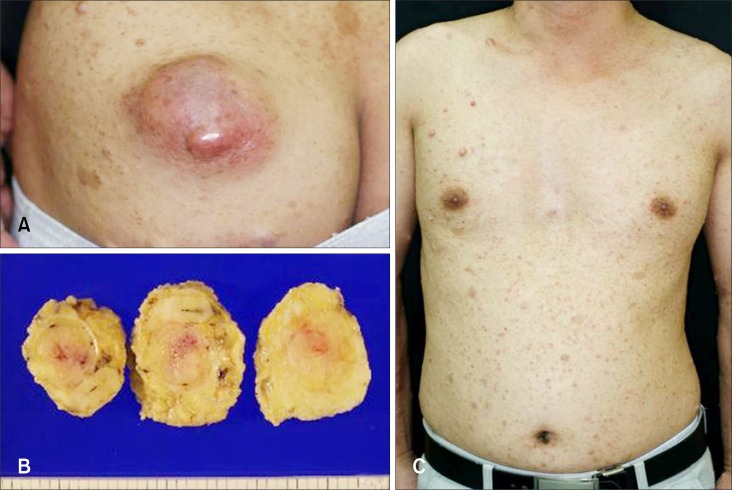
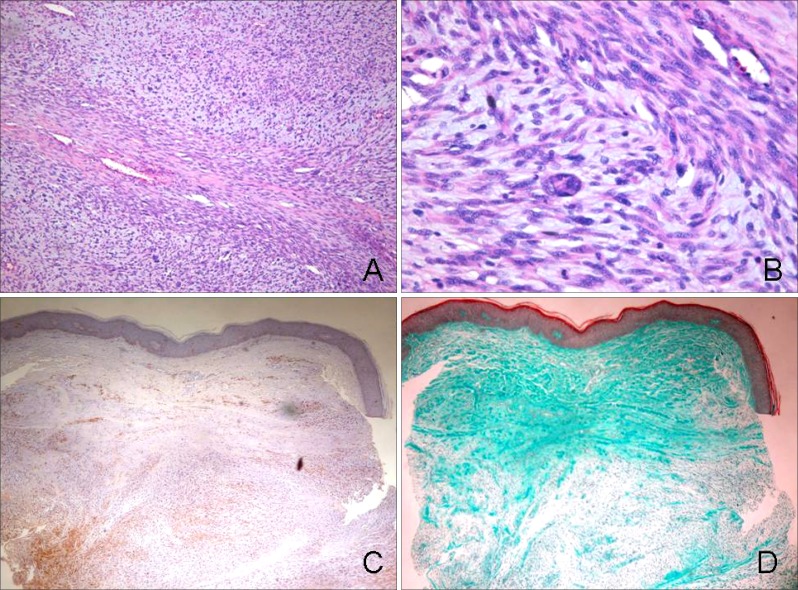
A 36-year-old man presented with an asymptomatic, large, movable mass on the left buttock, which began as a small nodule, but progressively enlarged to the present size over a period of 2 years (Fig. 1A). The mass was erythematous to brownish in color and measured 8 × 5 cm in size. There was no apparent ulceration or hemorrhage. Physical examination revealed multiple, variable-sized, skin-colored or tan, soft nodules, along with several hyperpigmented patches that were distributed on the entire body (Fig. 1C). There was no history of NF in the patient's family; nevertheless, he had been diagnosed as NF type 1 when he was a child. The results of routine laboratory investigations were within normal limits. Punch biopsy of the lesion revealed a neurogenic tumor that was possibly malignant. A wide excision of the mass was subsequently performed. The tumor measured 8 × 5 cm in size. It was soft to rubbery in consistency, with no encapsulation. The cut surface of the mass was yellowish in color and several hemorrhagic and necrotic foci were observed (Fig. 1B). Histopathologic findings revealed densely compacted bundles and whorls of spindle cells that were arranged in a fascicular pattern (Fig. 2A). Under higher power magnification, the tumor cells showed elongated and wavy nuclei and occasional atypism with nucleic pleomorphism (Fig. 2B). A few weakly cyanophilic tumor cells were found upon Masson trichrome staining (Fig. 2D) and immunohistochemistry showed focal positivity of the tumor cells to S-100 protein (Fig. 2C).
Fig. 1. A 8 × 5 cm-sized, erythematous to brownish mass was observed on the left buttock. The mass was soft to firm upon palpation (A). The cut-surface of the excised specimen exhibited focal areas of hemorrhage and necrosis, on a yellowish background (B). There were multiple neurofibromas and café-au-lait spots on the patient's trunk (C).
Fig. 2. The biopsy specimen showed a large mass in the dermis, showing a dense cellularity of spindle cells in a fascicular pattern (A: H&E, × 100). Upon magnification, compact bundles of spindle cells with pleomorphic, atypical nuclei were seen arranged in a whorling pattern. An anaplastic tumor giant cell is also seen (B: H&E, × 400). The tumor cells showed focal positivity to S-100 protein (C: S-100 protein, × 100), and a few weakly cyanophilic tumor cells were found upon Masson trichrome staining (D: Masson trichrome, × 100).
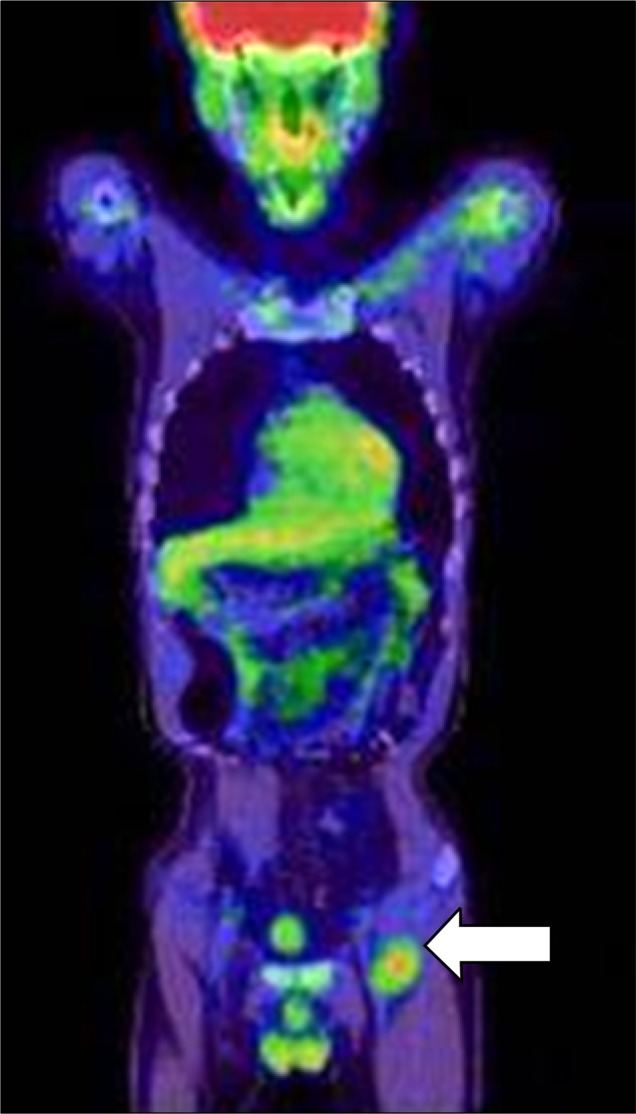
After excision of the tumor, a PET-CT examination of the whole body was performed in order to rule out any possible metastases or additional lesions. Findings showed an increase in fluorine-18-fluorodeoxyglucose (18F-FDG) uptake in the left inguinal region (Fig. 3), which was suggestive of lymph node metastasis or the occurrence of an additional soft tissue tumor. Sonogram-guided biopsy of the inguinal mass showed results consistent with a diagnosis lymph node metastasis. The patient was admitted to the surgical ward for extensive excision of the metastatic mass found in the left inguinal area, as well as residual lesions of the left buttock. An MRI of the pelvis was performed 1 month after surgery to check for recurrences or any residual tumor masses. The results were negative.
Fig. 3. PET-CT of the whole body showed an increase in FDG uptake in the left inguinal region (arrow), findings suggestive of lymph node metastasis, which was confirmed with biopsy.
The patient is currently undergoing adjuvant radiation therapy and is being followed up on a regular basis. After nine months of follow up, there is no evidence of local recurrence and no appearance of similar lesions elsewhere on the body.
DISCUSSION
MPNST is a malignant spindle cell tumor of the soft tissues that is thought to be derived from nerve sheath components such as Schwann cells or perineural fibroblasts8. They may occur de novo, but are mostly associated with long-standing neurofibromas in patients with NF type 1. The transformation rate of such neurofibromas is estimated to be at about 4.6%9. Tumors associated with NF type 1 are generally associated with a poor prognosis, in that they occur at a younger age, are more centrally located, are larger, and have more common metastases and recurrences, as well as being more poorly differentiated histologically8.
The histogenesis of MPNST is still unclear, and at times the features show overlap with other soft tissue sarcomas. Clinically, MPNSTs occur in adults 20 to 50 years of age as huge masses, at times arising from preexisting neurofibromas, and are most commonly located on the proximal limbs, head and neck, and trunk, as well as the retroperitoneum, mediastinum, and viscera10. The diagnosis of MPNST has always remained a challenge due to the lack of standardized diagnostic criteria, and its tendency to show overlap with over fibrous or neural crestderived tumors9. MRI can be very useful in identifying the extent of the tumor before surgery, as well as in detecting metastases or recurrences during follow-up. Electromyography may help localize small tumors of the plexus or roots11.
Histopathologically, the tumors are composed of spindle cells arranged in fascicular or whorling patterns with hemorrhagic or necrotic foci. There are hyperchromatic nuclei and widespread, multiple mitotic figures. The differential diagnosis of MPNST and benign schwannoma can be confusing; necrotic foci, atypical mitoses, and the lack of differentiated cells all indicate the former diagnosis10. These three features determine the histologic grade of the tumor, which in our case was grade 3. Immunohistochemistry can be of help in identifying nerve sheath differentiation. Markers such as S-100, vimentin, Leu7, and myelin basic protein can be used. S-100 protein, which was positive in our case, is a calcium-binding protein present in Schwann cells, and stains 60% of all MPNSTs12. Fukushima, et al.10 reported that the expression of Ki67 and p16 may be helpful in distinguishing between MPNST and benign peripheral nerve sheath tumor (BPNST). He states that the upregulation of Ki67 and the downregulation of p16 are highly indicative of MPNST.
The treatment of choice for MPNST is wide surgical excision with adjuvant radiotherapy11. The effect of chemotherapy is still questionable, but may be used in some cases13. Our patient was treated with both surgery and radiotherapy, and is considering chemotherapy as a precautionary measure. Prognosis remains quite poor, with more aggressive disease in patients with tumors larger than 5 cm, with a histological grade of 2 or 3, with associated NF or metastases8. Our patient was not only positive for all three factors, but showed regional lymph node metastasis as well; thus, the prognosis, though yet to be determined, seems quite poor.
There have been 10 cases of MPNST reported in Korean dermatological literature, all of which are summarized in Table 1. Of these, 7 cases occurred in patients with underlying NF-1. The location of the lesions were quite variable, some occurring on the chest, others on the arms or legs. While most of the lesions were large in size, most cases showed no signs of metastasis, and were thus treated with excision. Metastases were found in only two cases3,6, one of which was treated by excision and lymphadenectomy. The other was given chemotherapy along with excision of the primary tumor. Despite the fact that wide surgical excision with adjuvant radiotherapy11 is the treatment of choice, our case is the only reported case of MPNST that has been treated with radiation therapy.
We herein report a case of MPNST arising in a patient with NF type 1, which, although rare, must be considered in the differential diagnosis of similar lesions.
References
- 1.McCormack LJ, Hazard JB, Dickson JA. Malignant epithelioid neurilemoma (schwannoma) Cancer. 1954;7:725–728. doi: 10.1002/1097-0142(195407)7:4<725::aid-cncr2820070412>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 2.Ducatman BS, Scheithauer BW, Piepgras DG, Reiman HM, Ilstrup DM. Malignant peripheral nerve sheath tumors. A clinicopathologic study of 120 cases. Cancer. 1986;57:2006–2021. doi: 10.1002/1097-0142(19860515)57:10<2006::aid-cncr2820571022>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 3.Choi DY, Park JW, Son SJ. A case of solitary malignant schwannoma with regional lymph node metastasis. Korean J Dermatol. 1981;19:347–351. [Google Scholar]
- 4.Chon HJ, Lee DK, Kim DJ, Son SJ. A case of purely epithelioid peripheral nerve sheath tumor. Korean J Dermatol. 2000;38:518–521. [Google Scholar]
- 5.Lee JH, Park CJ, Yi JY, Kim JS, Jeon HM. A case of malignant peripheral nerve sheath tumor in neurofibromatosis. Korean J Dermatol. 1999;37:1535–1537. [Google Scholar]
- 6.Lee JY, Suh JI, Ihm CW. Two cases of neurofibrosarcoma associated with multiple neurofibromatois. Korean J Dermatol. 1988;26:110–115. [Google Scholar]
- 7.Park HJ, Cinn YW. Malignant schwannoma. Korean J Dermatol. 1986;24:159–163. [Google Scholar]
- 8.Garg A, Gupta V, Gaikwad SB, Mishra NK, Ojha BK, Chugh M, et al. Scalp malignant peripheral nerve sheath tumor (MPNST) with bony involvement and new bone formation: case report. Clin Neurol Neurosurg. 2004;106:340–344. doi: 10.1016/j.clineuro.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Al Akloby O, Bukhari IA, El-Shawarby M, Mulhim FA. Malignant peripheral nerve sheath tumor of the skin: case report. Am J Clin Dermatol. 2006;7:201–203. doi: 10.2165/00128071-200607030-00007. [DOI] [PubMed] [Google Scholar]
- 10.Fukushima S, Kageshita T, Wakasugi S, Matsushita S, Kaguchi A, Ishihara T, et al. Giant malignant peripheral nerve sheath tumor of the scalp. J Dermatol. 2006;33:865–868. doi: 10.1111/j.1346-8138.2006.00197.x. [DOI] [PubMed] [Google Scholar]
- 11.Baehring JM, Betensky RA, Batchelor TT. Malignant peripheral nerve sheath tumor: the clinical spectrum and outcome of treatment. Neurology. 2003;61:696–698. doi: 10.1212/01.wnl.0000078813.05925.2c. [DOI] [PubMed] [Google Scholar]
- 12.Dabski C, Reiman HM, Muller SA. Neurofibrosarcoma of skin and subcutaneous tissues. Mayo Clin Proc. 1990;65:164–172. doi: 10.1016/s0025-6196(12)65011-3. [DOI] [PubMed] [Google Scholar]
- 13.Hruban RH, Shiu MH, Senie RT, Woodruff JM. Malignant peripheral nerve sheath tumors of the buttock and lower extremity. A study of 43 cases. Cancer. 1990;66:1253–1265. doi: 10.1002/1097-0142(19900915)66:6<1253::aid-cncr2820660627>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 14.Ha SJ, Park YM, Yi JY, Kim TY, Kom CW, Lee JY, et al. Malignant peripheral nerve sheath tumor arising from neurofibromatosis. Ann Dermatol. 1996;8:153–157. [Google Scholar]
- 15.Kim HD, Shin HJ, Park YL, Whang KU, Baik SH, Park JS. A case of malignant peripheral nerve sheath tumor arising from neurofibromatosis during pregnancy. Korean J Dermatol. 2006;44:75–78. [Google Scholar]
- 16.Lee JH, Kim YC, Park HJ, Cinn YW. Two cases of neurofibrosarcoma arising from cutaneous neurofibroma in patients with neurofibromatosis. Korean J Dermatol. 1998;36:924–927. [Google Scholar]