Abstract
Background
A disruption of the balance between the water content of the stratum corneum (SC) and skin surface lipids may lead to the clinical manifestation of dryness of skin in patients with atopic dermatitis (AD).
Objective
To determine whether supplementation of gromwell (Lithospermum erythrorhizon), one of herbs used in East Asia in remedies for various abnormal skin conditions, may improve the SC level of hydration and ceramides, major lipid in SC in patients with AD.
Methods
A total of 28 subjects with AD were randomly assigned into two groups: either gromwell group received dextrose contained capsules with 1.5 g of gromwell extracts or placebo group received only dextrose contained capsules for 10 weeks.
Results
In contrast to no alteration of SC hydration and ceramides in placebo group, the SC hydration in gromwell group was significantly increased in parallel with an increase of SC ceramides. Furthermore, % increase of SC hydration in gromwell group bore a positive correlation with the clinical severity, which suggests that the increase of SC hydration in gromwell group was more effective as AD was more severe.
Conclusion
Supplementation of gromwell improves SC hydration in parallel with an increase of ceramides in part.
Keywords: Atopic dermatitis, Ceramide, Gromwell, Hydration
INTRODUCTION
Atopic dermatitis (AD) is a chronic inflammatory skin disease characterized by dryness, pruritic and eczematous lesions. Although the complex interrelationship between genetic, environmental, pharmacological, psychological and immunological factors contributes to the pathogenesis of AD, the disruption of epidermal barrier is of considerable importance and has been extensively studied.
Ceramides are the main lipids of the stratum corneum (SC), the outermost layer of epidermis1,2. During keratinization, almost all of the epidermal phospholipids, found abundantly in the basal layer, disappear and ceramides, synthesized de novo from phospholipids intermediates, remain within the SC2. Ceramide-rich intercellular lipid lamellae are thought to be of particular importance in maintaining the structural integrity of epidermal barrier3,4. Depletion of ceramides in the SC has been suggested as an etiological factor for dryness and barrier disruption in skin conditions such as AD5,6. In an animal model and patients with AD, reduction of ceramides in epidermis is shown to bear correlation to disruption of epidermal barrier7,8. Furthermore, a negative correlation between SC ceramide content and transepidermal water loss (TEWL) has been reported in AD patients7.
Gromwell (Lithospermum erythrorhizon) has been used as an herbal medicine for treatment of various abnormal skin conditions including wounds, inflammation and healing burns9. When the systemic efficacy of gromwell is examined in hyperproliferative epidermis of essential fatty acid (EFA) deficient guinea pigs in our previous study, gromwell supplementation was effective in reversing epidermal hyperproliferation in parallel with a marked increase of ceramides10. Although the systemic efficacy of gromwell on skin was reported in a few studies including our previous study10,11, most of efficacies of gromwell in skin such as antiproliferative, anti-microbial and anti-inflammatory activities have been reported mostly based on topical application11,12 and only limited information is available for the systemic efficacy of gromwell on skin. In this study, we investigated whether supplementation of gromwell may improve the epidermal level of hydration and ceramides in patients with AD.
MATERIALS AND METHODS
Preparation of gromwell extracts
Gromwell (the dried root of Lithospermum erythrorhizon Sieb et Zucc) were prepared as dried powder from 70% ethanol extracts, and provided by Amorepacific Corporation R&D Center (Gyeonggi-do, Republic of Korea) and Nutrex Co., Ltd (Seoul, Republic of Korea). Gromwell (100 g) was cut into small pieces and extracted with 500 ml of 70% ethanol at room temperature for 12 hours with stirring. The extraction procedure was repeated twice. Each of the combined extracts was filtered through Whatman No. 2 paper and lyophilized to yield 30.0 g of 70% ethanol extract of gromwell. For the preparation of gromwell capsule, lyophilized powder of 70% ethanol extract of gromwell was mixed with dextrose in the ratio of 50:50 (w/w).
Patients and administration of gromwell extract
Subjects were recruited via advertisements from an existing pool of outpatients visiting Dermatology clinic in KyungHee Medical Hospital Center in Seoul, Korea. Initially acceptable volunteers, ie, those who were nonsmokers; not suffering from acute or serious chronic diseases other than AD; not taking prescription of systemic medication for at least 10 weeks prior to this study; not taking vitamin, mineral, or lipid supplements for the past month took part in the present study. All of the subjects, who had given their informed consent, had AD as identified through clinical assessment. The study protocol was approved by he KyungHee Medical Hospital Center Human Investigation Review Committee. All measurements in this study were performed from November 10th 2004 to August 12th 2005.
In this double-blind, placebo-controlled protocol, subjects were randomly assigned to either the placebo (14 subjects) or the gromwell (14 subjects) group. Each group received 3 placebo or gromwell capsules per day. Each gromwell capsule contained 1.0 g of mixture with 70% ethanol extract powder of gromwell and dextrose (50:50, w/w; 0.5 g gromwell extract/capsule) and each placebo capsule contained 1.0 g of dextrose only. Total amount of gromwell provided by gromwell capsules per day was 1.5 g. The subjects consumed the capsules daily for 10 weeks while continuing their typical food intake, dietary habits and lifestyle. An adequate number of capsules were given to subjects on weekly basis. Compliance was monitored by capsule count. During the period of this study, subjects were required to abstain from taking any other supplements or any other systemic medications except two kinds of H1-antihistamines (Azeptin® and Ebastel®), and all subjects used the same dosage and frequency of these two antihistamine medications. All subjects (both placebo and gromwell group) also applied weak topical steroid agents (1% hydrocortisone lotion). Also, all subjects were not allowed to use any other moisturizers except these agents during the period of this study.
Assessment of clinical severity of AD
The objective SCORAD (SCORing Atopic Dermatitis: score range 0-83) combines an assessment of disease extent using the rule of nines with six clinical features of disease intensity: erythema/darkening, edema/papulation, oozing/crust, excoriation, lichenification and dryness. Dryness is evaluated on noninflammed skin. The other features are assessed on an average representative area for a given intensity item, each on a scale of 0-3 (0- absent, 1- mild, 2- moderate, 3- severe). The SCORAD score is calculated as follows: SCORAD =A/5+7(B/2), where A=The extent score based on body surface area calculated using the 'Rule of 9'; B= Intensity score based on 6 clinical findings, graded on a scale of 0-3. A higher score indicates more severe disease. The following cut-off points for objective SCORAD have been suggested for classification of disease severity: mild AD, score <15; moderate AD, score 15~40; and severe AD, score >4013. Only patients with mild to moderate severity of AD (SCORAD <40) were admitted in this study in order to determine whether the alterations in the SC level of either hydration or ceramides bear a close-enough correlation to the clinical severity in mild to moderate status of AD.
Assessment of SC hydration
Under the standardized room conditions of 22~24℃ and 55~60% humidity, SC hydration was measured on the lesional forearms of subjects at 0 and 10 weeks using a corneometer (Corneometer MPA-5, Courage-Khazaka, Cologne, Germany). The degree of epidermal skin humidity is indicated in a system-specific unit. One unit corresponds to a water content of stratum corneum of 0.02 mg/m2, for a measurement depth of 20 nm. Data are expressed as capacitance (au).
Analysis of SC ceramides
SC was stripped from the lesional forearms using 10 tape strips (14 mm D-SQUAME® Tape: CuDerm corporation, Dallas, Texas, USA). All tapes were stored at −20℃ until required. Corneocytes were removed from the tapes by sonication in methanol and the lipids were extracted in folch solution (CHCl3: MeOH (2:1, v/v) as reported previously14. The extracted lipids (lower phase) were subjected to high performance thin layer chromatography (HPTLC) on 0.20-mm silicagel 60-coated plates (Whatman, Clifton, NJ) according to the modified method reported by Uchida et al15. Specifically, the samples applied on the plates were first eluted up to 1.0 cm and then up to 3.5 cm using a CHCl3: MeOH: acetone (76: 20: 4, v/v/v) solution, then up to 7.5 cm using a CHCl3: Acetone: MeOH (80: 10: 10, v/v/v) solution, and finally up to the top using a CHCl3: Ethylacetate: Ether: MeOH (76: 20: 6: 2, v/v/v/v) solution. Each stage of elution was carried out after the plates were completely air-dried. The fraction containing ceramides that comigrated with respective standards were scanned at 420 nm with a TLC III scanner (CAMAG; Muttenz, Switzerland). The level of ceramides in each sample was quantified by calibration curves with the various concentrations of external standards of ceramides, and expressed as µg ceramides/µg protein.
Anthropometric evaluation
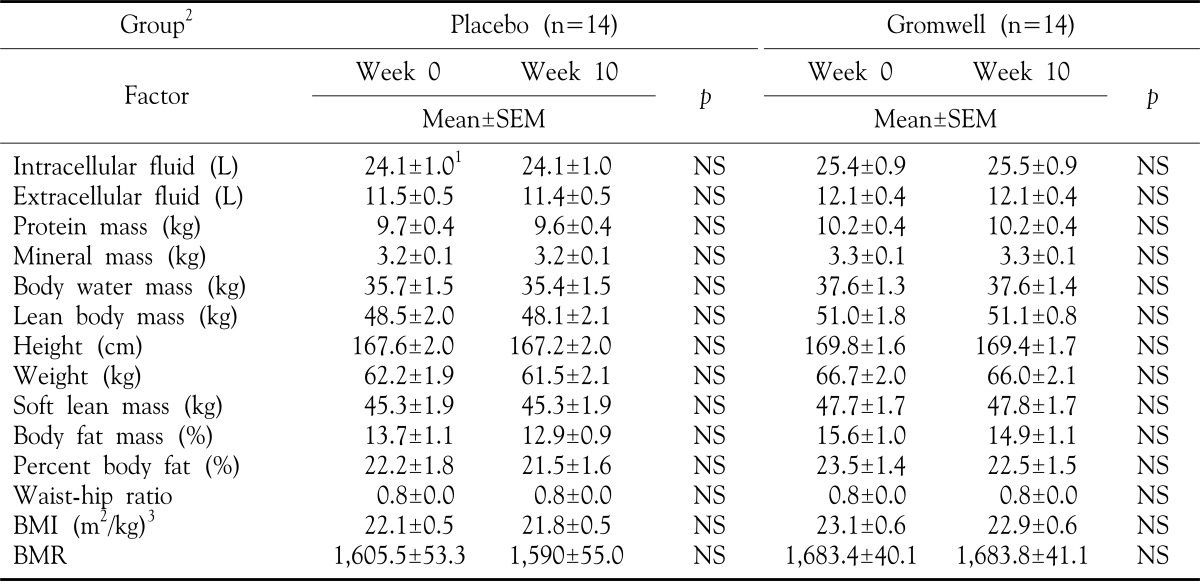
To evaluate the adverse systemic effects of long- term intake of gromwell extracts, anthropometric measurements were taken at 0 and 10 weeks. Standing height (wall mounted Harpenden stadio-meter) and weight (digital electronic scales) were measured. Body mass index (BMI) was calculated from the ratio of weight to height. Protein mass, mineral mass and fat mass were assessed according to Murgatroyd et al16 and lean body mass and body water mass were assessed according to Durnin et al17. Body water is known to broken down into three fluid compartments: Intracellular fluid (2/3 of Body Water), Plasma (1/15 of Body Water), and Intercellular fluid (4/15 of Body Water), thus, we calculated the quantities of intracellular fluid and extracellular fluid through these formulas. The basal metabolic rate (BMR) is measured in Calories and is primarily accounted for by the activity of the brain, heart, liver, and kidneys. The Harris-Benedict equations are commonly used for calculation of the BMR in adults. BMR for men (kcal)=66+13.7 (weight in kg)+5 (height in cm) 6.8 (age in years), BMR for women (kcal)=655+9.6 (weight in kg)+1.85 (height in cm) 4.7 (age in years).
Biochemical analysis of nutritional status
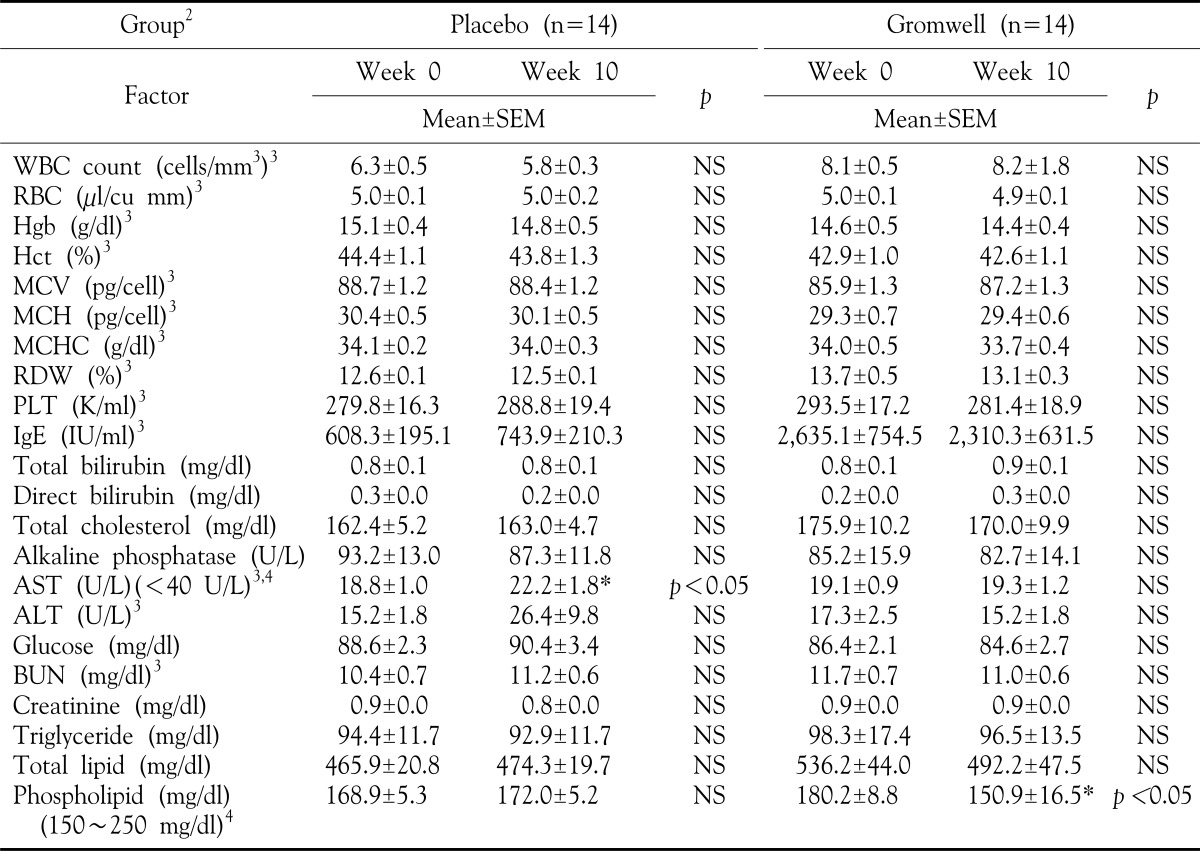
After fasting overnight, blood was collected and biochemical analysis of nutritional status was determined at weeks 0 and 10 in all subjects. The plasma was separated within 30 minutes, and samples were kept frozen at −70℃, if not analyzed immediately. All parameters were measured by routine methods at the Department of Laboratory medicine, KyungHee Medical Hospital Center, Seoul, Korea.
Patient assessments of pruritus at 10 weeks after treatment
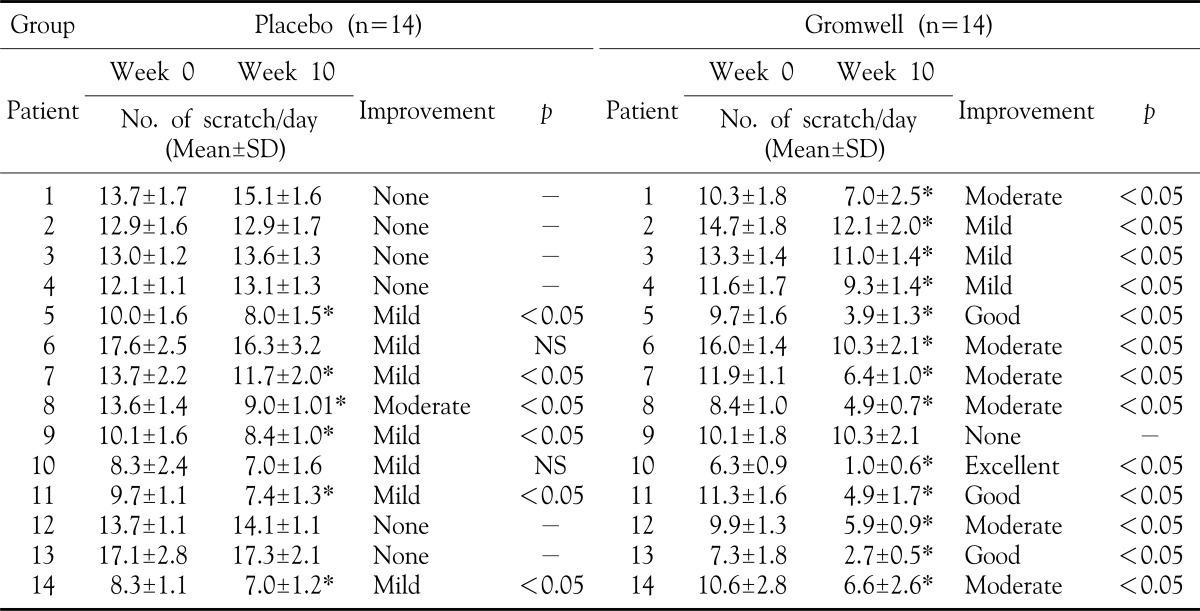
The cardinal feature of AD is significant pruritus or itching that often interferes with sleep and daily activities. Pruritus leads to scratching, which can result in skin changes such as lichenification, excoriation, and breakdown of the skin barrier, eventually leading to infection. Patient's self-assessment of pruritus was obtained by recording the number of scratch per day before and after in all subjects18. Each patient recorded the number of scratch per day for 1 week (both 7 days before treatment and 7 days at 10 weeks). Improvement was evaluated by the average reduced number of scratch per day: none (not improved), mild (less than 25% of change in number of scratch), moderate (25 to 50% of reduced number of scratch), good (50 to 75% of reduced number of scratch) and excellent (more than 75% of reduced number of scratch).
Statistical analysis
Statistical analysis was carried out with SAS statistical software package (version 9.13; SAS institute, Cary, NC) and Peism 4.0 software (Graph Pad, San Diego, CA). The homogeneity of the treatment groups with respect to age, baseline SCORAD were tested descriptively at the significance level of p-value<0.05 using the independent sample t-test. Data within each group were analyzed by paired student's t-tests. The comparison of change between groups was conducted by using student's t-test. Correlation between the % increase of SC hydration and the SCORAD scores was determined using Pearson correlation test. p-values<0.05 were considered to be statistical significance.
RESULTS
General characteristics and clinical improvement in subjects
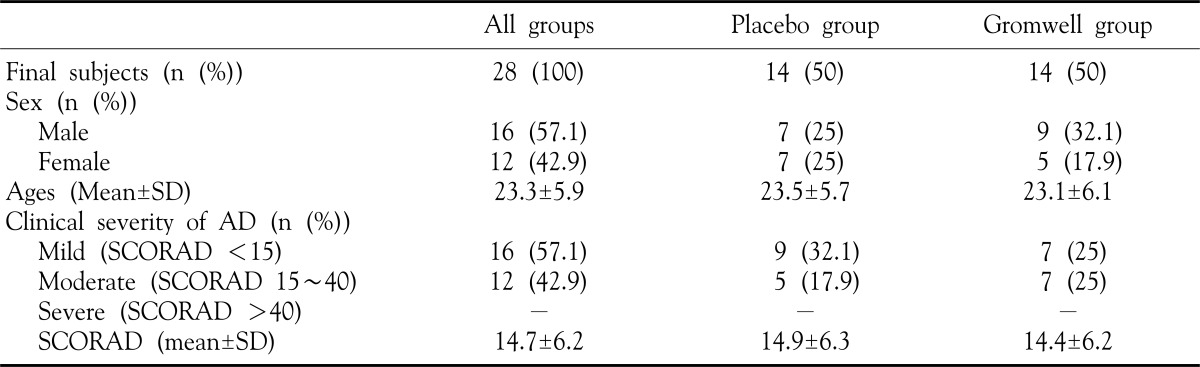
A total of 28 subjects entered the study, with 28 completing 10 weeks of the study. The 28 patients (16 male, 12 female) ranging in the age from 14 to 39 years randomized into either placebo or gromwell groups. No statistically significant differences were noted in mean ages, male/female ratio and clinical severity of atopic dermatitis (SCORAD) between the two treatment groups (Table 1). The mean SCORAD of these two groups are not significantly different (SCORAD mean in placebo group: 14.9±6.3; SCORAD mean in gromwell group: 14.4±6.2). In gromwell group, SCORAD at week 10 (10.2±4.2) was significantly decreased compared to at week 0 (p-value=0.0005), but in placebo group, SCORAD was not significantly decreased at week 10 (13.5±5.6) compared to at week 0 (p-value=0.08).
Table 1. General characteristics of the subjects.
Effect of gromwell on SC hydration
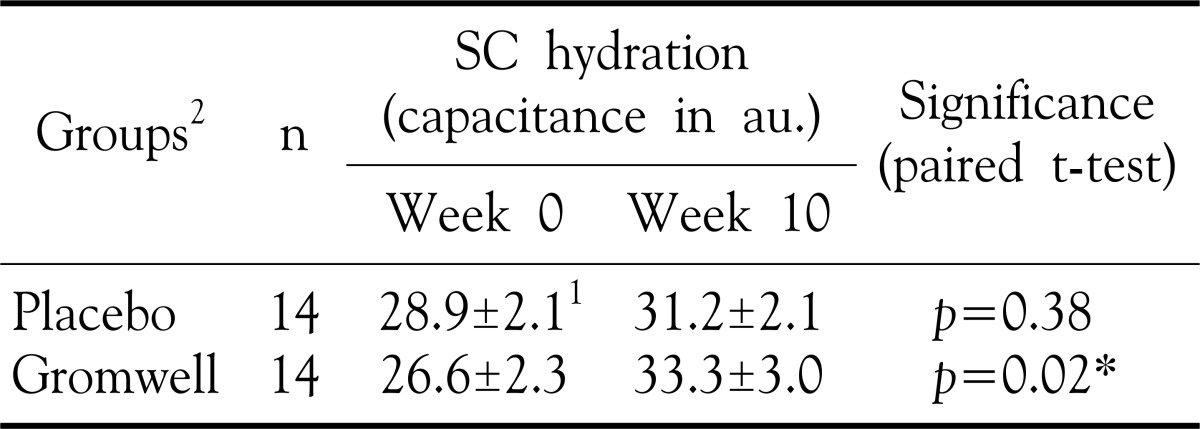
Contrast to no significant difference of epidermal hydration at 0 and 10 weeks in placebo group, SC hydration at week 10 was significantly increased compared to at 0 week in gromwell group (p-value =0.02 by paired T-test) (Table 2). These data, coupled with no difference of SC hydration at 0 week in placebo and gromwell groups, demonstrated that gromwell supplementation for 10 weeks sig-nificantly enhanced SC hydration in patient of AD.
Table 2. Effect of gromwell on SC hydration.
1Values are mean±SEM, 2Double blind type of supplement for either placebo or gromwell *p-value<0.05 by paired T-test
Effect of gromwell on SC ceramide levels
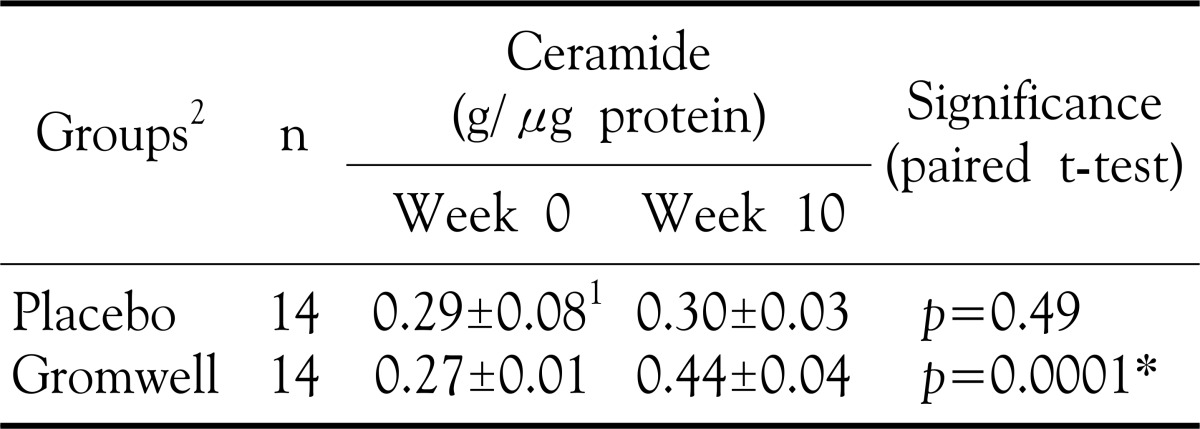
Analysis of ceramides in SC revealed that the level of ceramide in the epidermis of gromwell group was significantly increased at 10 weeks (p-value= 0.0001) (Table 3). In contrast, the levels of ceramides at 0 and 10 weeks in placebo group were not significantly different. These results indicate that gromwell supplementation induced the accumulation of ceramides in epidermis, ultimately enhancing SC hydration.
Table 3. Effect of gromwell on SC ceramide levels.
1Values are mean±SEM, 2Double blind type of supplement for either placebo or gromwell *p-value<0.05 by paired T-test
Correlation between SC hydration and SCORAD score of subjects
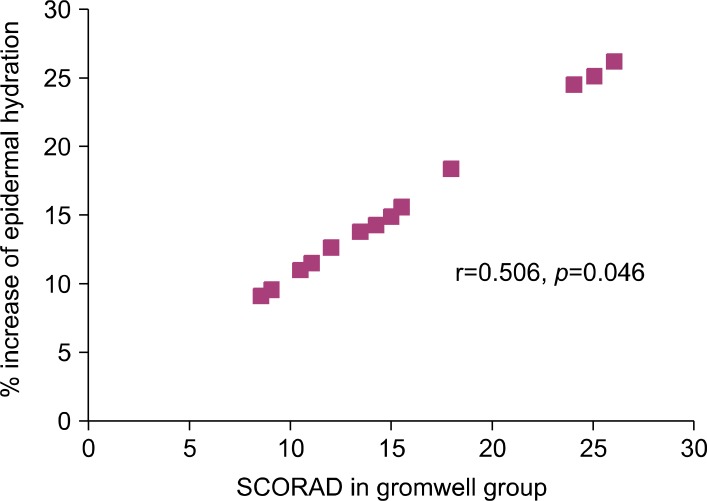
Although enhanced SC hydration (Table 2) was associated with increased level of ceramides in SC of gromwell group (Table 3), there was no significant correlation between either the absolute level or % increase of ceramide and SCORAD score (data not shown) in gromwell group. However, a highly significantly positive correlation between the % increase of SC hydration and SCORAD score was observed in the gromwell group (r=0.506, p= 0.046) (Fig. 1).
Fig. 1. Correlation between epidermal hydration and SCORAD score in the gromwell group. A significant positive correlation was observed (r=0.506, p=0.046 by Pearson correlation test). % Increase of epidermal hydration was calculated as {(the level of SC hydration at 10 weeks the level of SC hydration at 0 weeks)/ the level of SC hydration at 0 weeks}×100.
Anthropometric analysis and biochemical analysis of nutritional status
There were not significant differences in all parameters of anthropometric analysis between at 0 and at 10 weeks (Table 4). In routine biochemical analysis of nutritional status, several factors such as levels of AST in placebo group, and phospholipid in gromwell group were significantly elevated after 10 weeks (Table 5). However, the levels of these two factors were still within the normal ranges, therefore regarding as no inducement of adverse effects on biochemical analysis. These results suggest that gromwell is well tolerated as a supplement and does not have adverse effects in patients with AD.
Table 4. Anthropometric analysis in groups.
1Values are mean±SEM, 2Double blind type of supplement for either placebo or gromwell, 3BMI: body mass index, BMR: basal metabolic rate
Table 5. Biochemical analysis of nutritional status in groups.
1Values are mean±SEM, 2Double blind type of supplement for either placebo or gromwell, 3WBC: white blood cell, RBC: red blood cell, Hgb: hemoglobin, Hct: hematocrit, MCV: mean corpuscular volume, MCH: mean corpuscular hemoglobin, MCHC: mean corpuscular hemoglobin concentration, RDW: red cell distribution width, PLT: platelet count, IgE: immunoglobulin E, 4Values in brackets indicate the normal range of factor. *p-value<0.05 by paired T-test
The effect of gromwell extract on pruritus of AD
Patient assessment of pruritus at 10 weeks after treatment were as follow : In gromwell group, one patient reported no improvement, three patients reported mild improvement, six patients reported moderate improvement, three patients reported good improvement, and one patient reported excellent improvement. On the other hand, in placebo group, six patients reported no improvement, seven patients reported mild improvement, one patient reported moderate improvement (Table 6). Thus, we concluded that the gromwell extract was effective for pruritus of AD compared to plcebo group.
Table 6. The effect of gromwell extract on pruritus of AD.
*The statistical significance of the difference in the number of scratch per day between before and after treatment. p-value<0.05 by paired T-test
DISCUSSION
AD skin tends to be easily irritated and appears dry. AD has been shown to be accompanied by impairement in the epidermal barrier function including reduced water content and an augmentation in TEWL due to the alteration of SC lipid profile. In mild and moderate AD, moisturizing and emollient creams are known to be effective through an increase of skin hydration and improvement of barrier function19,20,21,22. Tabata et al21 indeed demonstrated that repeated daily applications of moisturizers, without any pharmacologically active agent, can induce long-lasting hydration effects in AD patients.
In the epidermis, ceramides combined with cholesterol and free fatty acids, are particular important in maintaining the extracellular lamellar membrane structure of epidermal barrier23. Depletion of ceramides has been suggested to be an etiological factor for epidermal barrier disruption, the decrease of SC hydration, increased TEWL and epidermal hyperproliferation10. We focused on reduced water content rather than TEWL for evaluating the effect of gromwell extract on SC ceramide.
Ceramides bearing the moieties of amide-linked non-hydroxy acid, α-hydroxy acid, or ω-hydroxy acid and ester-linked fatty acids on sphingosine provide the driving force for lamellar assembly24, and these structural moieties are thought to be important in maintaining the structural integrity of epidermal barrier against water loss through the skin3,4. Correspondingly, decreases in the level of ceramides have been associated with skin conditions involving dryness and barrier disruption, such as AD5,6, essential fatty acid deficiency (EFAD)25,26, xerosis27 and psoriasis15,28,29,30,31,32,33,34. Extremely high activities of ceramidase7 and sphingomyelin deacylase35, which hydrolyze sphingomyelin into sphingophosphorylcholine and free fatty acid, have been demonstrated to be the metabolic features leading to low levels of ceramides in the SC in AD. The patients with AD in preclinical stages have altered proportions of ceramides in their skin36,37 as a result of alterations in the activity of enzymes involved in the biosynthetic pathways of ceramide production38,39. Previously, Imokawa et al.40 demonstrated that there is a marked decrease in ceramide level within the SC of both uninvolved and involved skin of patients with AD. Yamamoto et al.41 observed that the relative amounts of all the stratum corneum lipid classes including squalene, cholesterol esters, wax esters, triglycerides, free fatty acids, cholesterol, ceramides, cholesterol sulphate and phospholipids did not differ statistically between AD patients and controls but, a significant decrease in proportion of ceramide 1. On the other hand, the fatty acid compositions as well as the proportions of C16:1 straight-chain component in sebum wax esters of AD patients were very similar to those of controls.Di Nardo A et al.36 analyzed the relationship between epidermal lipids and barrier impairment in the skin of patients with AD. In this study, patients with AD, the levels of ceramide 1 and 3 were significantly lower and values of cholesterol signi-ficantly higher with respect to healthy subjects. Thus, SC ceramide deficiency can be thought to play a major role in barrier impairement of AD lesional skin. Therefore, we only measured the level of SC ceramide, but not the other SC lipid contents. Thus, ceramide-containing, or ceramide-enhancing in the epidermis products may be of value in preventing the expression of eczema and decreasing the need for topical steroids and immunomodulators.
Gromwell, a plant species of the Boraginaceae family, referred to as zicao in China, shikon in Japan, and jacho in Korea, has been used as an herbal medicine for the treatment of wounds and inflammation and the healing burns9, and has also been used as a colorant in cosmetics, fabrics, and foods in diverse cultures42,43. Additionally, it has been used for the production of traditional liquor (Jindo hongju) in Korea44,45. As for bioactive components, naphthaquinones such as shikonin and its derivatives have been characterized mostly in organic extracts of gromwell12,46,47 which have anti- proliferative activities in various cancer cells with several mechanisms of action, such as inhibition of DNA topoisomerase, induction of apoptosis by caspase-3 activation, and anti-proliferative activity with down-regulation of activated extracellular signal-regulated kinase (ERK) and activation of phosphorylated Jun N-terminal kinase (JNK)48,49,50,51. In previous study, both the organic extract and the water extract of gromwell showed anti-proliferative activity, which suggests that well-characterized bioactive components such as napthaquinones or fatty acids might be extracted with water as well as with organic solvents10.
In this study, to examine the localization and distribution of substances within the SC, skin surface tape stripping with adhesive tape is a widely accepted and used method. The measurement of skin surface hydration state was evaluated by Corneometer. We definitely found that the SC hydration value in gromwell group was much higher than in placebo group, and also SC ceramide composition was increased significantly in gromwell group. We could suggest that increased SC ceramide composition after 10-week intake of gromwell could contribute to the improvement of skin hydration. The results of clinical studies suggest that these deficits can be addressed through the judicious use of appropriate moisturizers, which have been shown to improve skin hydration, reduce susceptibility to irritation, and restore the integrity of the SC. And also, we could find that significant decrease of SCORAD was shown in gromwell group and significant positive correlation was observed between the level of SC hydration and SCORAD score. We could demonstrate that supplementation of gromwell improved SC hydration with a marked increase of SC ceramides, and could relieve pruritic symp-tom. However, there was no significant correlation between the level of SC ceramide and SCORAD score (data not shown). Since SC hydration is maintained also with complex mixtures of water soluble compounds named as natural moisturizing factor (NMF) as much as with ceramides52, no correlation between SC ceramides and SCORAD score could be explained possibly by the requirement of either increase of NMF only or synergism of NMF and ceramides for enhancing SC hydration of patients with in moderate clinical severity status. Although the enhanced SC hydration in gromwell group could not be explained solely by the increase of SC ceramide in case of moderate clinical severity status of AD, our data in Fig. 1 clearly demonstrated that supplementation of gromwell was more effective in improving SC hydration of patients with AD in moderate clinical severity status rather than in mild severity. The influence of gromwell extracts on other moisturizing factors rather than ceramide would need more study.
Nowadays, many people are interested in functional foods, and the benefits of functional foods are overrated and excessively publicized in comparison with the actual established clinical effects. Although, moisturizing agents are not the definite treatment for AD, the repairement of skin barrier is important in patients with AD. The Gromwell (Lithospermum erythrorhizon) is thought to the safe functional food that can be used for improving SC hydration in dry skin condition of AD.
Footnotes
This study was supported by a grant of the Korean Health 21 R & D project of the Ministry of Health and Welfare of the Republic of Korea (no. 0405-FS00-0501-0014).
References
- 1.Gray GM, White RJ, Williams RH, Yardley HJ. Lipid composition of the superficial stratum corneum cells of the epidermis. Br J Dermatol. 1982;106:59–63. doi: 10.1111/j.1365-2133.1982.tb00902.x. [DOI] [PubMed] [Google Scholar]
- 2.Hedberg CL, Wertz PW, Downing DT. The time course of lipid biosynthesis in pig epidermis. J Invest Dermatol. 1988;91:169–174. doi: 10.1111/1523-1747.ep12464438. [DOI] [PubMed] [Google Scholar]
- 3.Grubauer G, Feingold KR, Harris RM, Elias PM. Lipid content and lipid type as determinants of the epidermal permeability barrier. J Lipid Res. 1989;30:89–96. [PubMed] [Google Scholar]
- 4.Wertz PW, Cho ES, Downing DT. Effect of essential fatty acid deficiency on the epidermal sphingolipids of the rats. Biochim Biophys Acta. 1983;753:350–355. doi: 10.1016/0005-2760(83)90058-9. [DOI] [PubMed] [Google Scholar]
- 5.Imokawa G, Abe A, Jin K, Higaki Y, Kawashima M, Hidano A. Decreased levels of ceramides in stratum corneum of atopic dermatitis: an etiological factor in atopic dry skin. J Invest Dermatol. 1991;96:523–526. doi: 10.1111/1523-1747.ep12470233. [DOI] [PubMed] [Google Scholar]
- 6.Matsumoto M, Umemoto N, Sugiura H, Uehara M. Difference in ceramide composition between "dry" and normal skin in patients with atopic dermatitis. Acta Derm Venereol. 1999;79:246–247. doi: 10.1080/000155599750011183. [DOI] [PubMed] [Google Scholar]
- 7.Aioi A, Tonogaito H, Suto H, Hamada K, Ra CR, Ogawa H, et al. Impairment of skin barrier function in NC/Nga Tnd mice as a possible model for atopic dermatitis. Br J Dermatol. 2001;144:12–18. doi: 10.1046/j.1365-2133.2001.03946.x. [DOI] [PubMed] [Google Scholar]
- 8.Elias PM. Stratum corneum defensive functions: an integrated view. J Invest Dermatol. 2005;125:183–200. doi: 10.1111/j.0022-202X.2005.23668.x. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka S, Tajima M, Tsujada M, Tabata MA. Comparative study on anti-inflammatory activities of the enantiomers, shikonin and alkanin. J Nat Prod. 1986;49:466–469. doi: 10.1021/np50045a014. [DOI] [PubMed] [Google Scholar]
- 10.Kim J, Jeong DH, Kim SH, Park SK, Cho Y. Comparative effect of Gromwell (Lithospermum erytheorhizon) extract and Borage oil reversing epidermal proliferation in Guinea Pig. Biosci Biotechnol Biochem. 2006;70:2086–2095. doi: 10.1271/bbb.60038. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi M. Pharmacological studies of shikon and tooki. Effect of topical application of the ether extracts and shiunko on inflammatory reactions. Nippon Yakurigaku Zasshi. 1977;63:205–214. [PubMed] [Google Scholar]
- 12.Fujita N, Sakaguchi I, Kobayashi H, Ikeda N, Kato Y, Minamin M, et al. An extract of the root of Lithospermum erytheorhizon accelerates wound healing in diabetic mice. Biol Pharm Bull. 2003;26:329–335. doi: 10.1248/bpb.26.329. [DOI] [PubMed] [Google Scholar]
- 13.Kunz B, Oranje AP, Labreze L, Stalder JF, Ring J, Taieb A. Clinical validation and guidelines for the SCORAD index: consensus report of the European Task Force on Atopic Dermatitis. Dermatology. 1997;195:10–19. doi: 10.1159/000245677. [DOI] [PubMed] [Google Scholar]
- 14.Gary GM, Yardley HJ. Lipid composition of cells isolated from pig, human and rat epidermis. J Lipid Res. 1975;16:434–440. [PubMed] [Google Scholar]
- 15.Uchida Y, Hara M, Nishio H, Sidransky E, Inoue S, Otsuka F, et al. Epidermal sphingomyelins are precursors for selected stratum corneum ceramides. J Lipid Res. 2000;41:2071–2082. [PubMed] [Google Scholar]
- 16.Murgatroyd PR, Coward W. An improved method for estimating changes in whole-body fat and protein mass in man. Br J Nutr. 1989;62:311–314. doi: 10.1079/bjn19890032. [DOI] [PubMed] [Google Scholar]
- 17.Durnin JV, Rahaman MM. The assessment of the amount of fat in the human body from measurements of skinfold thickness. Br J Nutr. 2003;89:147–155. [PubMed] [Google Scholar]
- 18.Arkwright PD, Fujisawa C, Tanaka A, Matsuda H. Mycobacterium vaccae reduces scratching behavior but not the rash in NC mice with eczema: a randomized, blinded, placebo-controlled trial. J Invest Dermatol. 2005;124:140–143. doi: 10.1111/j.0022-202X.2004.23561.x. [DOI] [PubMed] [Google Scholar]
- 19.Grassi A, Palermi G, Paradisi M. Study of tolerance and efficacy of cosmetic preparations with lenitive action in atopic dermatitis in children. Clin Ter. 2000;151:77–80. [PubMed] [Google Scholar]
- 20.Lodén M. The increase in skin hydration after application of emollients with different amounts of lipids. Acta Derm Venereol. 1992;72:327–330. [PubMed] [Google Scholar]
- 21.Tabata N, O'Goshi K, Zhen YX, Kligman AM, Tagami H. Biophysical assessment of persistent effects of moisturizers after their daily applications: evaluation of corneotherapy. Dermatology. 2000;200:308–313. doi: 10.1159/000018393. [DOI] [PubMed] [Google Scholar]
- 22.Lodén M, Andersson A-C, Lindberg M. Improvement in skin barrier function in patients with atopic dermatitis after treatment with a moisturizing cream (Canoderm1) Br J Dermatol. 1999;140:264–267. doi: 10.1046/j.1365-2133.1999.02660.x. [DOI] [PubMed] [Google Scholar]
- 23.Elias PM, Menon GK. Structural and lipid biochemical correlates of the epidermal permeability barrier. Adv Lipid Res. 1991;24:1–26. doi: 10.1016/b978-0-12-024924-4.50005-5. [DOI] [PubMed] [Google Scholar]
- 24.Wertz PW, Swartzendruber DC, Abraham W, Madison KC, Downing DT. Essential fatty acids and epidermal integrity. Arch Dermatol. 1987;123:1381–1384. [PubMed] [Google Scholar]
- 25.Burr GO, Burr MM. A new deficiency disease produced by rigid exclusion of fat from the diet. J Biol Chem. 1929;82:345–367. [Google Scholar]
- 26.Chung S, Kong S, Seong K, Cho Y. γ-Linolenic acid in borage oil reverses epidermal hyperproliferation in guinea pigs. J Nutr. 2002;132:3090–3097. doi: 10.1093/jn/131.10.3090. [DOI] [PubMed] [Google Scholar]
- 27.Akimoto K, Yoshikawa N, Higaki Y, Kawashima M, Imokawa G. Quantitative analysis of stratum corneum lipids in xerosis and asteatotic eczema. J Dermatol. 1993;20:1–6. doi: 10.1111/j.1346-8138.1993.tb03820.x. [DOI] [PubMed] [Google Scholar]
- 28.Motta S, Monti M, Sesana S, Mellesi L, Ghidoni R, Caputo R. Abnormality of water barrier function in psoriasis. Role of ceramide functions. Arch Dermatol. 1994;130:452–456. [PubMed] [Google Scholar]
- 29.Williams Ml. Lipids in normal and pathological desquamation. Adv Lipid Res. 1991;24:211–262. doi: 10.1016/b978-0-12-024924-4.50012-2. [DOI] [PubMed] [Google Scholar]
- 30.Serup J, Blichmann C. Epidermal hydration of psoriasis plaques and the relation to scaling. Acta Derm Venereol. 1987;67:357–366. [PubMed] [Google Scholar]
- 31.Macheleidt O, Kaiser HW, Sandhoff K. Deficiency of epidermal protein-bound ω-hydroxyceramides in atopic dermatitis. J Invest Dermatol. 2002;119:166–173. doi: 10.1046/j.1523-1747.2002.01833.x. [DOI] [PubMed] [Google Scholar]
- 32.Tohyama J, Oya Y, Ezoe T, Vanier MT, Nakayasu H, Fujita N, et al. Ceramide accumulation is associated with increased apoptotic cell death in cultured fibroblasts of sphingolipid activator protein- deficient mouse but not in fibroblasts of patients with Farber disease. J Inher Metab Dis. 1999;22:649–662. doi: 10.1023/a:1005590316064. [DOI] [PubMed] [Google Scholar]
- 33.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 34.Cho Y, Lew BL, Seong K, Kim NI. An inverse relationship between ceramide synthesis and clinical severity in patients with psoriasis. J Korean Med Sci. 2004;19:859–863. doi: 10.3346/jkms.2004.19.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hara J, Hoguchi K, Okamoto R, Kawashima M, Imokawa G. High expression of sphingomyelin deacylase is an important determinant of ceramide deficiency leading to barrier disruption in atopic dermatitis. J Invest Dermatol. 2000;115:406–413. doi: 10.1046/j.1523-1747.2000.00072.x. [DOI] [PubMed] [Google Scholar]
- 36.Di Nardo A, Wertz P, Giannetti A, Seidenari S. Ceramide and cholesterol composition of the skin of patients with atopic dermatitis. Acta Derm Venereol. 1998;78:27–30. doi: 10.1080/00015559850135788. [DOI] [PubMed] [Google Scholar]
- 37.Melnik B, Hollmann J, Hofmann U, Yuh MS, Plewig G. Lipid composition of outer stratum corneum and nails in atopic and control subjects. Arch Dermatol Res. 1990;282:549–551. doi: 10.1007/BF00371952. [DOI] [PubMed] [Google Scholar]
- 38.Murata Y, Ogata J, Higaki Y, Kawashima M, Yada Y, Higuchi K, et al. Abnormal expression of sphingomyelin acylase in atopic dermatitis: an etiologic factor for ceramide deficiency? J Invest Dermatol. 1996;106:1242–1249. doi: 10.1111/1523-1747.ep12348937. [DOI] [PubMed] [Google Scholar]
- 39.Yamamura T, Tezuka T. Change in sphingomyelinase activity in human epidermis during aging. J Dermatol Sci. 1990;1:79–83. doi: 10.1016/0923-1811(90)90219-4. [DOI] [PubMed] [Google Scholar]
- 40.Imokawa G, Hattori M. A possible function of structural lipid in water-holding properties by the stratum corneum. J Invest Dermatol. 1985;84:282–284. doi: 10.1111/1523-1747.ep12265365. [DOI] [PubMed] [Google Scholar]
- 41.Yamamoto A, Serizawa S, Ito M, Sato Y. Stratum corneum lipid abnormalities in atopic dermatitis. Arch Dermatol Res. 1991;283:219–223. doi: 10.1007/BF01106105. [DOI] [PubMed] [Google Scholar]
- 42.Ballantine JA. The isolation of two esters of the naphthalquinone alcohol, shikonin, from the shrub Jatropha glandulifera. Phytochemistry. 1969;8:1587–1590. [Google Scholar]
- 43.Tabata M, Fujita Y. Production of shikonin by plant cell cultures. In: Zaitilin M, Day P, Hollaender A, editors. Biotechnology in Plant Science. San Diego: Academic Press; 1985. pp. 207–218. [Google Scholar]
- 44.Kim YS, Kang SH, Jung JH. Studies on the processing of Korean traditional so-ju, Jindo-Hongju. Korean J Dietary Culture. 1991;6:245–249. [Google Scholar]
- 45.Kim SJ, Jung JH, Park KH. Studies on the standard method of Jindo Hongju pigments. Korean J Dietary Culture. 1991;7:19–23. [Google Scholar]
- 46.Cho MH, Paik YS, Hann TR. Physical stability of shikonin derivatives from the roots of Lithospermum erythrorhizon cultivated in Korea. J Agric Food Chem. 1999;47:4117–4120. doi: 10.1021/jf9902853. [DOI] [PubMed] [Google Scholar]
- 47.Staniforth V, Wang SY, Shyur LF, Yang NS. Shikonins, phytocompounds from Lithospermum erythrorhizon, inhibit the transcriptional activation of human tumor necrosis factor α promoter in vivo. J Biol Chem. 2004;279:5877–5885. doi: 10.1074/jbc.M309185200. [DOI] [PubMed] [Google Scholar]
- 48.Lu G, Liao J. Detection of the anticancer biological effect of naphtaquinone pigment-LIII. Zhong Xi Yi Jie He Za Zhi. 1990;10:422–425. [PubMed] [Google Scholar]
- 49.Yoon Y, Kim YO, Lim NY, Jeon WK, Sung HJ. Shikonin, an ingredient of Lithospermum erythrorhizon-induced apoptosis in HL-60 human premyelocytic leukemia cell line. Planta Med. 1999;65:532–535. doi: 10.1055/s-1999-14010. [DOI] [PubMed] [Google Scholar]
- 50.Fujii N, Yamashita Y, Arima Y, Nagashima M, Nakano H. Induction of topoisomerase II-mediated DNA cleavage by the plant naphtaquinones plumbagin and shikonin. Antimicrob Agents Chemother. 1992;36:2589–2594. doi: 10.1128/aac.36.12.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hashimoto S, Xu M, Masuda Y, Aiuchi T, Nakajo S, Cao J, et al. beta-Hydroxyisovalery lshikonin inhibits the cell growth of various cancer cell lines and induces apoptosis in leukemia HL-60 cells through a mechanism different from those of Fas and etoposide. J Biochem. 1999;125:17–23. doi: 10.1093/oxfordjournals.jbchem.a022255. [DOI] [PubMed] [Google Scholar]
- 52.Rawlings AV, Harding CR. Moisturization and skin barrier function. Dermatol Ther. 2004;17(Suppl.1):43–48. doi: 10.1111/j.1396-0296.2004.04s1005.x. [DOI] [PubMed] [Google Scholar]