Abstract
Background
Studies of Malawi’s Option B+ programme for HIV-positive pregnant and breastfeeding women have reported high loss to follow-up during pregnancy and at the start of antiretroviral therapy (ART), but few data exist about retention during breastfeeding and after weaning. We examined loss to follow-up and retention in care in patients in the Option B+ programme during their first 3 years on ART.
Methods
We analysed two data sources: aggregated facility-level data about patients in Option B+ who started ART between Oct 1, 2011, and June 30, 2012, at 546 health facilities; and patient-level data from 20 large facilities with electronic medical record system for HIV-positive women who started ART between Sept 1, 2011, and Dec 31, 2013, under Option B+ or because they had WHO clinical stages 3 or 4 disease or had CD4 counts of less than 350 cells per μL. We used facility-level data to calculate representative estimates of retention and loss to follow-up. We used patient- level data to study temporal trends in retention, timing of loss to follow-up, and predictors of no follow-up and loss to follow-up. We defined patients who were more than 60 days late for their first follow-up visit as having no follow-up and patients who were more than 60 days late for a subsequent visit as being lost to follow-up. We calculated proportions and cumulative probabilities of patients who had died, stopped ART, had no follow-up, were lost to follow-up, or were retained alive on ART for 36 months. We calculated odds ratios and hazard ratios to examine predictors of no follow-up and loss to follow-up.
Findings
Analysis of facility-level data about patients in Option B+ who had not transferred to a different facility showed retention in care to be 76·8% (20 475 of 26 658 patients) after 12 months, 70·8% (18 306 of 25 849 patients) after 24 months, and 69·7% (17 787 of 25 535 patients) after 36 months. Patient-level data included 29 145 patients. 14 630 (50·2%) began treatment under Option B+. Patients in Option B+ had a higher risk of having no follow-up and, for the first 2 years of ART, higher risk of loss to follow-up than did patients who started ART because they had CD4 counts less than 350 cells per μL or WHO clinical stage 3 or 4 disease. Risk of loss to follow-up during the third year was low and similar for patients retained for 2 years. Retention rates did not change as the Option B+ programme matured.
Interpretation
Our data suggest that pregnant and breastfeeding women who start ART immediately after they are diagnosed with HIV can be retained on ART through the Option B+ programme, even after many have stopped breastfeeding. Interventions might be needed to improve retention in the first year on ART in Option B+.
Funding
Bill & Melinda Gates Foundation, Partnerships for Enhanced Engagement in Research Health, and National Institute of Allergy and Infectious Diseases.
Introduction
In 2011, the Malawian Ministry of Health implemented the Option B+ programme for HIV-positive pregnant and breastfeeding women, which increases coverage of prevention of mother-to-child transmission (PMTCT) services, improves maternal health, and reduces transmission to serodiscordant sexual partners. Option B+ provides lifelong antiretroviral therapy (ART) for all HIV-infected pregnant and breastfeeding women, irrespective of CD4 count or WHO clinical stage.1–3 In Malawi, women in Option B+ receive a fixed dose combination of tenofovir, lamivudine, and efavirenz.1,2 PMTCT services are integrated into antenatal care, maternity, and postnatal clinics, clinics for children younger than 5 years, and specialised HIV clinics.2,4 HIV testing and PMTCT services are offered in primary, secondary, and tertiary care facilities.5 When the Option B+ programme was implemented on a national scale, the number of pregnant women who initiated ART quickly rose5 and PMTCT coverage increased substantially, from 49% in 2011,3 to 85% in 2014.6
Option B+ has greatly improved coverage of PMTCT services, but women must stay on ART for their whole lives if they7–10 and their communities11 are to receive the full individual and public health benefits of treatment. Qualitative evidence suggests that women are strongly motivated to protect their children from prenatal and postnatal HIV transmission by staying on ART while pregnant and breastfeeding. However, they might see less need for treatment after they stop breastfeeding. Many patients in Option B+ are asymptomatic when diagnosed with HIV, so they might be less motivated to stay on ART to protect their own health than to protect their children’s health.12,13
Studies of Option B+ have shown high rates of loss to follow-up during the first 6–12 months of ART,5,14 but data about long-term retention are very scarce: aggregated facility-level data from Malawi’s HIV programme reports6 show that retention in the Option B+ programme at 3 years is about 70%, but no analyses of patient-level data have been published.
We used country-wide facility-level data to calculate representative estimates of retention and loss to follow-up and patient-level data from 20 large health-care facilities to study temporal trends in retention and exact timing and predictors of loss to follow-up. We followed up women from the start of ART for up to 3 years to describe retention during pregnancy and breastfeeding and after breastfeeding.
Methods
Study design and participants
We did cohort analysis of facility-level aggregated data from 546 health facilities in the national Option B+ programme and patient-level data from 20 large health facilities. The National Health Sciences Research Committee (number 962) and the Cantonal Ethics Committee of Bern (number IRB00003905 FW A00005976) granted ethical approval for the study.
In our analysis of facility-level data, we included women registered between Oct 1, 2011, and June 30, 2012, who had Option B+ as their indication for starting ART. We chose the study period to ensure that all patients had started ART at least 3 years before the database closed on June 30, 2015. We restricted the analysis to health facilities with available data for 6, 12, 24, and 36 months to ensure that the ART outcomes were comparable over time. We excluded patients who transferred to another facility before their ART outcome was assessed. In a sensitivity analysis, we included data from all facilities, irrespective of the completeness of the data.
To examine temporal trends in retention and exact timing and predictors of loss to follow-up, we analysed patient-level data from women who started ART between Sept 1, 2011, and Dec 31, 2013, at 20 large health facilities in central and southern Malawi which used an electronic medical record system when the Option B+ programme was implemented (Figure S1, appendix). We included treatment-naive women who started with Option B+ as their indication for ART and women who started ART because they had WHO clinical stage 3 or 4 disease or CD4 counts less than 350 cells per μL.
Procedures
The medical records of patients taking ART at facilities with fewer than 2500 patients are noted on paper-based treatment cards and entered in paper-based clinical registers. Facility-level data are collected from all integrated ART and PMTCT facilities during quarterly supervision visits by representatives from the Ministry of Health. Key outcomes, including the number of patients who initiated ART, the number of patients who were lost to follow-up, died, transferred-out, stopped ART, or were retained on ART at 6, 12, 24, and 36 months, are extracted from treatment cards and aggregated at each facility.2,6 To collect patient-level data at large facilities (ie, those with >2500 patients), health-care workers use an electronic medical record system operated by the Baobab Health Trust (Lilongwe, Malawi). Barcode scanners and touchscreen displays are used to collect patient-level data at the point of care.15 Baseline and longitudinal measurements of clinical staging, ART history, laboratory results, clinic visits, pharmacy claims, and ART outcomes (loss to follow-up, death, transfer out, stopped ART, or retained alive on ART) are recoded in the electronic medical record system. We extracted patient-level data from the electronic medical record system, and facility-level data from the central database of the HIV Department at the Ministry of Health of Malawi.
We categorised patients according to their eligibility for ART and then compared outcomes between groups of patients. We classified patients as eligible for ART for their own health if they were not pregnant or breastfeeding and had reached WHO clinical stage 3 or 4 disease or had a CD4 count less than 350 cells per μL. We classified patients as eligible for Option B+ because of pregnancy if they began ART while pregnant, or as eligible for Option B+ because of breastfeeding if they were breastfeeding when they started ART, irrespective of their clinical or immunological stage.
We based our definition of patients’ cumulative medication adherence on data from pharmacy claims recorded in the electronic medical record system and we decided that cumulative medication adherence would be the percentage of days pharmacy records showed that drugs were available to a patient (ie, days covered by the prescriptions patients had collected).
Outcomes
We defined patients who stopped taking ART and were known to be alive, as having stopped ART. Patients were defined as dead if the health facility received a reliable report that they had died from any cause. Patients who had not died, transferred out, or stopped ART, and who were more than 60 days late for their first follow-up visit were classified as having no follow-up. Patients who were more than 60 days late for a subsequent follow-up visit were counted as lost to follow-up.2 We judged loss to follow-up of less than 5% per year on ART to be low, 5–10% to be moderate, and more than 10% to be high. Patients who were not classified as having no follow-up or being lost to follow-up and who had not died, transferred out, or stopped ART were deemed to be retained alive on ART. In an analysis of temporal trends in retention, we adapted our standard definition of loss to follow-up. In the other analyses, retained patients included those who were classified as lost to follow-up, but who later returned to care. We could not use this definition to analyse temporal trends in retention because transient treatment interruptions differentially affect patients enrolled in different time periods. If ART is interrupted in a recently enrolled patient (ie, an individual who enrolled near the end of the study period), the patient has less time to return to care than do patients enrolled earlier, making it more likely that recently enrolled patients who temporarily interrupt care will be classified as lost.16 To avoid bias in our analyses of temporal trends of retention, we classed patients as lost to follow-up if they were more than 150 days late for an appointment, and counted them as lost to follow-up, even if they later returned. We chose a longer time window because classification of those patients absent from care for more than 150 days after their last missed appointment (about 180 days after last visit) as lost to follow-up minimises misclassification.17
Statistical analysis
We analysed facility-level data to calculate representative estimates for Option B+ programme retention. We used descriptive statistics and calculated the proportions of Option B+ patients who had died, stopped ART, were lost to follow-up, or who were retained in care at 6, 12, 24, and 36 months.
We used survival analysis methods to analyse the patient-level data from 20 large health facilities. Patients were followed up from the beginning of ART for up to 36 months. We censored patients at the date at which an ART outcome was recorded or when they were no longer at risk of loss to follow-up (60 or 150 days before closing date, depending on the time window that defined loss to follow-up). We viewed no follow-up, loss to follow-up, and death to be competing events. In a time-to-event analysis, we estimated the cause-specific cumulative incidence of ART outcomes (no-follow-up, loss to follow- up, transferred-out, stopped ART, died, or alive on ART) to compare the proportions of patients who had died, stopped ART, were lost to follow-up, or who were retained at 6, 12, 24 and 36 months in patient-level and facility-level data. We plotted cause-specific cumulative incidence curves for all ART outcomes and stratified the analysis by indication for ART to examine the exact timing of loss to follow-up.18 We used logistic regressions with a random effect for the facilities to examine predictors of no follow-up (yes or no) and loss to follow- up during the first year on ART (yes or no), generating odds ratios (OR) and 95% CIs. In time-to-event analysis, we used competing-risk regression to examine factors associated with loss to follow-up during years 2 and 3 on ART, generating hazard ratios (HR) and 95% CIs.19 We adjusted analyses for age (15–19, 20–24, 25–29, and ≥30 years), type of facility (health centre, district hospital, faith-based hospital, or central hospital), and reason for starting ART (pregnancy, breastfeeding, or ART for own health). For patients whose cumulative medication a dherence at 6 months was more than 95%, 85–95%, and less than 85%, we plotted cause-specific cumulative incidence curves for loss to follow-up between months 7 and 36 on ART to compare the risk of loss to follow-up between patients with good and poor adherence. In a multivariable competing-risk regression, we calculated subdistribution HRs for risk of loss to follow-up for patients whose 6 month cumulative medication adherence was more than 95%, 85–95%, and less than 85%. We adjusted for indication for ART, age, and facility. We plotted Kaplan-Meier curves for retention in care for pregnant and breastfeeding patients who started ART in the years 2011, 2012, and 2013, to examine time trends in retention. We used Stata version 13 for all statistical analyses.
Role of the funding source
The funder had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication.
Results
We analysed facility-level data to calculate representative estimates for loss to follow-up and Option B+ programme retention. We included data for 29 768 women from 546 facilities (all facilities with available data), of whom 6648 (22·3%) started ART at the 20 large health facilities included in the patient-level analysis. Patients who transferred to another facility were excluded (Table 1, Figure S1, appendix). At 12 months after the start of ART, of 26 658 patients, 5746 (21·6%) were lost to follow-up and 20 475 (76·8%) were retained in care. At 24 months, of 25 849 patients, 7053 (27·3 %) were lost to follow-up and 18 306 (70·8%) were retained. At 36 months, of 25 535 patients, 7186 (28·1%) were lost to follow-up, and 17 787 (69·7%) were retained in care, 427 (1·7%) had died, and 135 (0·5%) had stopped ART (Table 1). In a sensitivity analysis, we included all available data from 584 facilities, including those excluded from the main analysis because of missing data, and our results were similar (Table S1, appendix).
Table 1.
Treatment outcomes of patients in Option B+ by time since start of ART
| Number of patients | Number (%) of patients transferred1 | Number of patients not transferred2 | Percentage of patients not transferred (confidence interval) |
||||
|---|---|---|---|---|---|---|---|
| Lost to follow-up3 | Stopped ART4 | Dead5 | Retained in care6 | ||||
|
|
|
|
|
|
|
|
|
| 6 months | 29,768 | 1,685 (5.7) | 28,083 | 17.0 (16.5–17.4) |
0.2 (0.2–0.3) |
0.7 (0.6–0.8) |
82.1 (81.7–82.6) |
| 12 months | 29,007 | 2,349 (8.1) | 26,658 | 21.6 (21.1–22.1) |
0.5 (0.5–0.6) |
1.1 (1.0–1.2) |
76.8 (76.3–77.3) |
| 24 months | 29,121 | 3,272 (11.2) | 25,849 | 27.3 (26.7–27.8) |
0.6 (0.5–0.7) |
1.3 (1.2–1.5) |
70.8 (70.3–71.4) |
| 36 months | 29,313 | 3,778 (12.9) | 25,535 | 28.1 (27.6–28.7) |
0.5 (0.4–0.6) |
1.7 (1.5–1.8) |
69.7 (69.1–70.2) |
Data are n (%) or % (95% CI) unless indicated otherwise. Results from analysis of aggregated facility-level data for patients starting ART between October, 2011, and June, 2012, from 546 health facilities in Malawi. We included cohorts with available outcome data at 6, 12, 24, and 36 months. ART=antiretroviral therapy.
Transferred to another facility before the end of the reporting period (6, 12, 24, or 36 months after start of ART).
Started ART and not transferred to another facility before the end of reporting period.
More than 60 days late for a follow-up visit and did not return to care thereafter.
Stopped taking ART.
Known to be dead.
Retained alive on ART and under active follow-up.
We analysed patient-level data from 20 large health facilities to examine timing of loss to follow-up, predictors of no follow-up and loss to follow-up, and temporal trends in retention. In total, 29 145 patients were eligible for analysis (Figure S1, appendix). Patients were followed up for a total of 48 445 person-years, for a median duration of 1·8 years (IQR 0·5–2·8). Overall, 14 630 started ART for Option B+ and 14 515 women started ART for their own health. 11 151 women starting for Option B+ (76·2%) started ART while pregnant. At the start of ART, patients in Option B+ were younger than were those who started ART for their own health. 14 200 women in Option B+ (97·1%) and 3705 starting ART for their own health (25·5%) were initiated on tenofovir, lamivudine, and efavirenz. 10 743 (74·0%) women who started ART for their own health were initiated on a first-line regimen of stavudine, lamivudine, and nevirapine (Table 2).
Table 2.
Patients’ characteristics at start of ART
| Reason for ART initiation
|
Total | |||||||
|---|---|---|---|---|---|---|---|---|
| Option B+ because of pregnancy | Option B+ because of breastfeeding | For own health | ||||||
| Number of patients (%) | 11151 | (38·3%) | 3479 | (11·9%) | 14515 | (49·8%) | 29145 | (100·0%) |
| Age (in years) | ||||||||
| 15–19 (%) | 828 | (7·4%) | 188 | (5·4%) | 329 | (2·3%) | 1345 | (4·6%) |
| 20–24 (%) | 2697 | (24·2%) | 750 | (21·6%) | 1200 | (8·3%) | 4647 | (15·9%) |
| 25–29 (%) | 4330 | (38·8%) | 1374 | (39·5%) | 3270 | (22·5%) | 8974 | (30·8%) |
| 30+ (%) | 3296 | (29·6%) | 1167 | (33·5%) | 9716 | (66·9%) | 14179 | (48·6%) |
| Median (IQR) | 27 | (23–31) | 28 | (24–32) | 34 | (29–42) | 30 | (25–36) |
| Year of ART initiation (%) | ||||||||
| 2011 | 1541 | (13·8%) | 1165 | (33·5%) | 2768 | (19·1%) | 5474 | (18·8%) |
| 2012 | 5083 | (45·6%) | 1434 | (41·2%) | 6459 | (44·5%) | 12976 | (44·5%) |
| 2013 | 4527 | (40·6%) | 880 | (25·3%) | 5288 | (36·4%) | 10695 | (36·7%) |
| WHO clinical stage (%) | ||||||||
| Stage 1 | 10937 | (98·1%) | 3301 | (94·9%) | 5623 | (38·7%) | 19861 | (68·1%) |
| Stage 2 | 106 | (1·0%) | 178 | (5·1%) | 1541 | (10·6%) | 1825 | (6·3%) |
| Stage 3 | 88 | (0·8%) | 0 | (0·0%) | 6337 | (43·7%) | 6425 | (22·0%) |
| Stage 4 | 20 | (0·2%) | 0 | (0·0%) | 1014 | (7·0%) | 1034 | (3·5%) |
| First-line ART | ||||||||
| TDF 3TC EFV | 10943 | (98·1%) | 3257 | (93·6%) | 3705 | (25·5%) | 17905 | (61·4%) |
| d4T 3TC NVP | 182 | (1·6%) | 217 | (6·2%) | 10743 | (74·0%) | 11142 | (38·2%) |
| Other | 26 | (0·2%) | 5 | (0·1%) | 67 | (0·5%) | 98 | (0·3%) |
Data are n (%) unless indicated otherwise and are from women who attended 20 large health facilities with patient-level data.
ART=antiretroviral therapy.
In the 20 large facilities with patient-level data, retention in care was about 10% less than the national average. Retention in the Option B+ programme at 12 months after ART initiation, was 68·5% (95% CI 67·7–69·2), at 24 months it was 61·1% (60·3–61·9%), and at 36 months it was 56·3% (55·4–57·2; Table S2, appendix). Most patients who defaulted from care had no follow-up or were lost to follow-up in the first 12 months (Figure 1).
Figure 1. Cumulative incidence of ART outcomes for patients at 20 large.
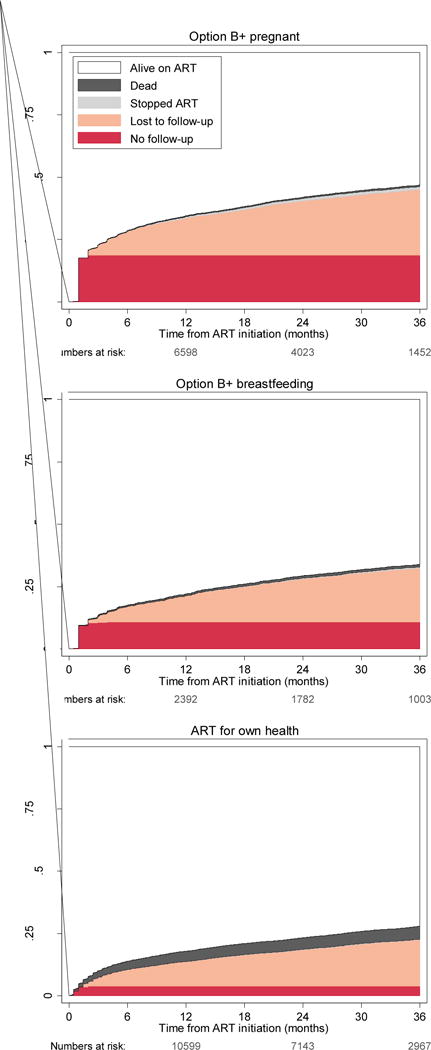
Treatment outcomes are compared between three groups on the basis of indication for ART. Option B+ indication and ART started during pregnancy (top panel), Option B+ indication and ART started while breastfeeding (middle panel), and WHO stage 3 or 4 disease or CD4 cell count meeting the ART eligibility threshold (bottom panel). Estimates derived from competing-risk regression. ART=antiretroviral therapy.
We compared the risk of no-follow-up or loss to follow-up during years 1, 2, and 3 between groups of patients. Women in Option B+ who started ART while breastfeeding had more than twice the risk of no follow-up (adjusted OR [AOR] 2·47, 95% CI 2·14–2·85) compared with patients who started ART for their own health. Patients who started ART during pregnancy were almost five times more likely to have no follow-up visit (4·73, 4·27–5·24) than were patients who started for their own health (Table 3).
Table 3.
Predictors of no follow-up and loss to follow-up
| Univariable analysis
|
Multivariable analysis
|
|||||||
|---|---|---|---|---|---|---|---|---|
| No-follow-up after ART start1
|
LTF during year 1 on ART
|
LTF during year 2 on ART
|
LTF during year 3 on ART
|
No-follow-up after ART start
|
LTF during year 1 on ART
|
LTF during year 2 on ART
|
LTF during year 3 on ART
|
|
| OR (95% CI) [p-value] |
OR (95% CI) [p-value] |
SHR (95% CI) [p-value] |
SHR (95% CI) [p-value] |
aOR (95% CI) [p-value] |
aOR (95% CI) [p-value] |
aSHR (95% CI) [p-value] |
aSHR (95% CI) [p-value] |
|
| ART eligibility | ||||||||
| Own health | 1.00 [<0.0001] |
1.00 [<0.0001] |
1.00 [<0.0001] |
1.00 [<0.0001] |
1.00 [<0.0001] |
1.00 [<0.0001] |
1.00 [<0.0001] |
1.00 [0.1809] |
| B+ pregnant | 5.65 (5.13–6.23) |
2.00 (1.84–2.16) |
1.81 (1.61–2.03) |
1.53 (1.28–1.82) |
4.73 (4.27–5.24) |
1.61 (1.48–1.76) |
1.35 (1.19–1.53) |
1.15 (0.95–1.40) |
| B+ breastfeeding | 2.89 (2.52–3.31) |
1.19 (1.05–1.35) |
1.49 (1.27–1.76) |
1.17 (0.92–1.48) |
2.47 (2.14–2.85) |
1.05 (0.92–1.20) |
1.18 (1.00–1.40) |
0.94 (0.73–1.21) |
| Age (years) | ||||||||
| ≥30 | 1.00 [<0.0001] |
1.00 [<0.0001] |
1.00 [<0.0001] |
1.00 [<0.0001] |
1.00 [<0.0001] |
1.00 [<0.0001] |
1.00 [<0.0001] |
1.00 [<0.0001] |
| 25–29 | 2.01 (1.83–2.21) |
1.55 (1.42–1.69) |
1.67 (1.47–1.90) |
1.60 (1.32–1.93) |
1.32 (1.19–1.45) |
1.33 (1.21–1.46) |
1.51 (1.32–1.73) |
1.54 (1.26–1.88) |
| 20–24 | 2.83 (2.56–3.14) |
2.23 (2.02–2.47) |
2.73 (2.37–3.14) |
2.37 (1.90–2.94) |
1.64 (1.47–1.83) |
1.83 (1.64–2.03) |
2.37 (2.03–2.75) |
2.23 (1.76–2.81) |
| 15–19 | 3.41 (2.93–3.97) |
2.79 (2.39–3.26) |
2.87 (2.30–3.57) |
3.06 (2.21–4.24) |
1.92 (1.64–2.25) |
2.21 (1.88–2.60) |
2.43 (1.94–3.05) |
2.82 (2.02–3.94) |
| Facility type | ||||||||
| Health Centre | 1.00 [<0.0001] |
1.00 [<0.0001] |
1.00 [<0.0001] |
1.00 [0.0007] |
1.00 [0.0743] |
1.00 [0.0001] |
1.00 [<0.0001] |
1.00 [0.0113] |
| District Hospital | 1.15 (1.01–1.30) |
0.83 (0.74–0.93) |
0.79 (0.68–0.93) |
0.77 (0.61–0.97) |
1.21 (0.81–1.81) |
0.79 (0.54–1.15) |
0.84 (0.72–0.98) |
0.81 (0.64–1.03) |
| Mission Hospital | 0.49 (0.40–0.61) |
0.35 (0.29–0.44) |
0.40 (0.31–0.53) |
0.45 (0.30–0.66) |
0.75 (0.45–1.25) |
0.42 (0.26–0.69) |
0.49 (0.37–0.64) |
0.52 (0.35–0.76) |
| Central Hospital | 0.71 (0.60–0.84) |
1.01 (0.87–1.17) |
0.67 (0.54–0.83) |
0.70 (0.51–0.96) |
0.96 (0.57–1.64) |
1.19 (0.72–1.96) |
0.81 (0.65–1.02) |
0.79 (0.57–1.10) |
Patient-level data from 20 large facilities. Multivariable analyses are adjusted for all variables shown in the table. We defined patients as having no follow-up if they were >60 days late for a follow-up visit and did not return to care thereafter. We defined patients as lost to follow-up (LTF) if they attended at least one follow-up visit and were more than 60 days late for a subsequent visit (during time period) and did not return to care thereafter. p-value shows whether a variables is a significant predictor of outcome overall.
OR=odds ratio. aOR=adjusted odds ratio. HR=subdistribution hazard ratio. aHR=adjusted subdistribution hazard ratio, CI=confidence interval.
Among patients who attended their first follow-up visit, risk of loss to follow-up in the first year of ART was slightly higher in pregnant women (AOR 1·61, 95% CI 1·48–1·76) than in women who started for their own health. Breastfeeding women had the same risk of loss to follow- up in the first year of ART as did patients who started for their own health (1·05, 0·92–1·20). The pattern in year 2 was similar. In year 3, patients in all groups had a similar risk of loss to follow-up (p=0·2). Patients aged 24 years or younger were about twice as likely to default from care at any time during follow-up compared with patients aged 30 years or older at the start of ART (Table 3). Risk of loss to follow-up in years 1, 2, and 3 was reduced in faith-based hospitals compared with other types of facilities.
Patients with poor adherence (cumulative medication adherence <85%) had the highest risk of loss to follow-up. Risk decreased in a linear fashion as adherence improved (Figure S2). Adjusted subdistribution HRs were 1·35 (95% CI 1·21–1·52) for patients with cumulative medication adherence of 85–95% and 1·94 (1·78–2·13) for patients with cumulative medication adherence of less than 85% (p<0·0001) compared with patients with a 6 month cumulative medication adherence of more than 95%.
Retention rates were similar between pregnant women who started ART in 2011, 2012, and 2013. Retention in care decreased with time in women who started ART while breastfeeding (Figure 2).
Figure 2. Retention in care by indication for Option B+ and year of ART initiation.
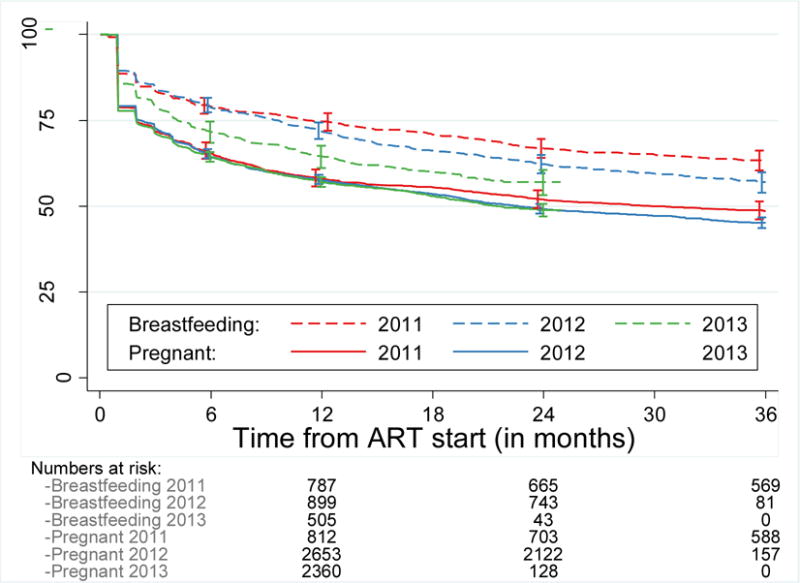
Data are for women who attended 20 large facilities with patient-level data. Kaplan-Meier curves show retention in care for pregnant or breastfeeding women in Option B+ who started ART in 2011, 2012, or 2013. In this analysis of temporal trends in retention, patients were classified as lost to follow-up if they were more than 150 days late for an appointment, even if they returned to care thereafter. ART=antiretroviral therapy.
Discussion
69·7% of all Malawian women who started ART under Option B+ were in care 3 years later. Loss to follow-up was high (21·6%) during the first year of ART, moderate (5·7%) in year 2, and, low (0·8%) in year 3. Analysis of patient-level data from large facilities showed that most patients who defaulted from care failed to return to the clinic and had no follow-up. Women in Option B+ were at much higher risk of having no follow-up than were women who were not pregnant and began ART for their own health. The risk of loss to follow-up was higher for women in Option B+ than for those who started ART for their own health in years 1 and 2, but was not higher in year 3. Younger age was associated with an increased risk of defaulting from care at any point during follow-up. Women who had poor adherence to treatment during early ART were twice as likely to be lost to follow-up compared with patients who had optimum adherence. Risk of loss to follow-up in years 1, 2, and 3 was reduced in faith-based hospitals, which generally have better resources than do other types of health-care facilities. Retention rates were similar over time and did not improve as the programme matured.
This study is likely to underestimate programme retention. We relied on data routinely collected during standard care, and health-care workers do not usually actively trace patients lost to follow-up. Patients who self- transferred to another facility without telling their former health-care providers are likely to be misclassified as lost to follow-up in routine data. A systematic review20 noted that almost one in five patients classified as being lost to follow-up in routine ART programmes had self-transferred to another facility, and were retained in care. A tracing study21 from Malawi showed that one in three women in Option B+ classified as being lost to follow-up from a large, urban district hospital had self-transferred or used ART from other sources. Only 54% of women no longer took their antiretroviral drugs.21 A cohort study from the Karonga district in Malawi accounted for self- transfer and followed up patients across all clinics in the district, reporting that 85% of patients in Option B+ were retained in care after 6 months,22 even though routine health facility records noted their retention as 78%. Analyses of routine data without active defaulter tracing tend to underestimate programme retention. Patient- level data might be especially prone to bias from unaccounted self-transfer because patient-level data were mainly collected in district and central hospitals and patients who initiate ART at these hospitals are more likely to self-transfer to lower level facilities that are closer to home than are patients who start ART at primary care facilities.20,22
Our results support reports of high attrition rates during early ART from previous studies of Option B+ from Malawi.5,14 Many women might be reluctant to initiate ART, and high proportions of women with no follow-up might represent women who have not started ART despite being given the first monthly dose. Often cited barriers to successful PMTCT uptake and retention include distance to clinics, having no money for transport, other difficulties in travelling, illness or weakness, side-effects of ART, negative attitudes of health facility staff, insufficient treatment support, depression, denial of HIV infection, absence of male involvement, initiation of ART on the day of HIV diagnosis, the model of care implemented at the health facility, and fear that partners and community members will react badly, including fear of domestic violence.12,21,23 Health-care workers should pay special attention to patients with early adherence problems because they are more likely to default from care. These patients might benefit from targeted adherence interventions.
In pregnant women, retention rates were similar over time and did not change as the programme matured, but breastfeeding women who started ART in 2013 had worse retention than did breastfeeding women who started ART in earlier calendar years. Deterioration of retention in breastfeeding mothers might result from the selection of a non-representative sample of non-compliant or marginalised breastfeeding mothers who started ART during later calendar years. Breastfeeding mothers who started ART after the Option B+ roll-out in late 2012 and 2013 were those who either were not diagnosed during pregnancy or were reluctant to initiate treatment during pregnancy. By contrast, breastfeeding mothers who started ART in 2011 and early 2012 are a representative sample of all childbearing women who happened to be breastfeeding during the B+ roll-out (ie, Option B+ was not yet available to them during pregnancy).
We assessed ART outcomes for up to 3 years after the start of ART and our study is the first to use patient-level data to assess retention in care among women initiating ART for Option B+ who have passed the formal MTCT risk period. Although we have no data about the timing of weaning, few women in Malawi are likely to be breastfeeding after 2 years; the median duration of breastfeeding is 23 months.24 Our data suggest that if women are retained in care throughout breastfeeding, they are likely to continue treatment afterwards. In Malawi, Option B+ is deliberately presented as providing lifelong treatment to prevent MTCT and improve maternal health. Qualitative data show that patients in Option B+ understand the maternal health benefits of ART and are motivated to continue with it beyond the MTCT risk period.12 However, we could not assess the number of women lost to the programme along the full PMTCT cascade because electronic data are only collected for patients who start ART. Many women are not tested for HIV during pregnancy25 or are lost between HIV testing and counselling and ART uptake.4,26 Clinical and immunological stage at ART initiation was often missing. CD4 cell counts are rarely measured for pregnant and breastfeeding women in Malawi, and although WHO clinical staging is mandatory for all HIV patients,2 WHO stage-defining conditions at ART initiation are often not reported.
Our data show that the Option B+ programme, in which all HIV-positive pregnant and breastfeeding women are initiated on lifelong ART, is feasible and acceptable. Women retained in care after breastfeeding were generally motivated to continue ART. This adds weight to the argument that Option B+ is superior to prescription of ART for women with high CD4 cell counts only during pregnancy and breastfeeding (Option B). If HIV-positive women stop ART after breastfeeding, their CD4 cell counts rapidly decline9 and the maternal health benefits of early ART10 diminish. Because fertility rates are high and breastfeeding is prolonged in Malawi and other sub- Saharan African countries, a start-and-stop approach to treatment might not save money, especially in view of the fact that many women will need ART for their own health soon after they discontinue ART for PMTCT.9
Randomised controlled trials have provided evidence for the benefit of immediate initiation of ART for all HIV-infected individuals.10,27 Evidence from these studies retrospectively supports the aims of the Malawi Ministry of Health when it introduced Option B+ in 2011. Retention in the first year remains the big challenge facing the Option B+ programme, but loss to follow-up during year 2 is moderate and risk of loss to follow-up is low during year 3, when women stop breastfeeding.
Supplementary Material
Research in context.
Evidence before the study
We searched PubMed from Sep 1, 2011 to Aug, 25, 2015 with the following query without any language restrictions: retention[tiab] OR attrition[tiab] OR adherence[tiab] OR mortality[tiab] OR loss to follow-up[tiab] OR lost to follow-up[tiab] OR ART outcomes [tiab] OR evaluation [tiab]) AND (Option B+[tiab]. We aimed to find observational studies that describe retention in care among women who started ART under Option B+ indications. We also used the term “Option B+ to search IAS conference and Conference on Retroviruses and Opportunistic Infections abstracts from 2015. We identified 16 eligible studies from low- and lower middle-income countries. Most described short-term outcomes and reported retention at 3, 6 and 12 months after ART initiation. A study from Haiti reported longer-term retention (24 months). Estimates varied widely between studies and countries. Two studies from Mozambique and two studies from Haiti reported very high early loss to follow-up and poor retention. In Mozambique, retention was 50% after the first visit, and 33% after 12 months. In Haiti, retention was around 70% at 6 months, 50% at 12 months, and 30% at 24 months. One study in Uganda reported six-month retention of 88%, and a study in Cameroon found that six-month retention improved from 85% to 91%. Eleven studies in Malawi described retention among Option B+ patients. Six of these reported 6-month retention rates; results ranged from 72%–92%. Seven studies reported retention at 12 months; estimates ranged between 50% and 94%. A country-wide analysis of facility-level data showed that 77% of the women who started ART on Option B+ were still in care a year later.
Added value of this study
Our study is the first patient-level data analysis to describe retention in care among Option B+ patients throughout the course of breastfeeding and after its cessation. Over 70% of women who started ART on Option B+ were still in care three years later. For the first two years of ART, Option B+ women were more likely to be lost to follow-up than women who were not pregnant and who began ART for their own health. By year three, their risk of loss to follow-up was similar. Retention in care did not change as the Option B+ programme matured.
Implication of all the available evidence
Randomised controlled trials (eg, START and TEMPRANO) have provided evidence of the benefits immediate initiation of ART. Although long-term studies are needed to provide definative answers, our data suggest that pregnant and breastfeeding women who start ART immediately after they are diagnosed with HIV can be retained on ART.
Acknowledgments
Financial disclosure
This work was supported by the Bill & Melinda Gates Foundation (Global Health Grant Number OPP1090200), the USAID-NIH initiative Partnerships for Enhanced Engagement in Research (PEER) Health (NIH/PEER; grant number AID-OAA-A-11-00012), and by the National Institute Of Allergy And Infectious Diseases (NIAID), the Eunice Kennedy Shriver National Institute Of Child Health & Human Development (NICHD), and the National Cancer Institute (NCI) under Award Number U01AI069924. The content is solely the responsibility of the authors and does not necessarily represent the official views of the sponsors. OK was supported by a PROSPER fellowship grant from the Swiss National Science Foundation (grant number 3233B_150934). The Malawi Ministry of Health HIV/AIDS Programme is funded by The Global Fund and President’s Emergency Plan for AIDS Relief (PEPFAR). Baobab Health Trust is funded by the Centers for Disease Control and Prevention (CDC). We thank all patients, doctors, and study nurses at the participating facilities.
Footnotes
Contributions
ADH wrote the first draft of the study protocol; all authors contributed to the final version of the protocol. ADH did the statistical analyses, with interpretation of results by OK, JJvO, LT, AJ, AS, and ADH. ADH wrote the first draft of the report, which was revised by JJvO, OK, and KT; all authors commented on earlier drafts of the report. MTM and ADH extracted the data and did the data management. AJ coordinates monitoring and evaluation of the Malawian ART/PMTCT programme and implemented data collection. OJG coordinates electronic data collection. FC and OK are the principal investigators of the study. All authors reviewed and approved the final version for submission. MTM, ADH, LT, and OK had full access to all the data in the study. ADH takes responsibility for the integrity of the data and the accuracy of the data analysis.
Declaration of interests
We declare no competing interests.
References
- 1.Schouten EJ, Jahn A, Midiani D, et al. Prevention of mother-to-child transmission of HIV and the health-related Millennium Development Goals: time for a public health approach. Lancet. 2011;378:282–4. doi: 10.1016/S0140-6736(10)62303-3. [DOI] [PubMed] [Google Scholar]
- 2.Ministry of Health Malawi. Clinical Management of HIV In Children and Adults. 2014 http://cms.medcol.mw/cms_uploaded_resources/18381_16.pdf.
- 3.World Health Organization. Global update on HIV treatment 2013: results, impact and opportunities. 2013 http://apps.who.int/iris/bitstream/10665/85326/1/9789241505734_eng.pdf.
- 4.van Lettow M, Bedell R, Mayuni I, et al. Towards elimination of mother-to-child transmission of HIV: performance of different models of care for initiating lifelong antiretroviral therapy for pregnant women in Malawi (Option B+) J Int AIDS Soc. 2014;17:18994. doi: 10.7448/IAS.17.1.18994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Impact of an innovative approach to prevent mother-to-child transmission of HIV–Malawi, July 2011–September 2012. MMWR Morb Mortal Wkly Rep. 2013;62:148–51. [PMC free article] [PubMed] [Google Scholar]
- 6.Ministry of Health Malawi. Integrated HIV Program Report: October –December 2014. 2014 https://www.hiv.health.gov.mw/cha_repository/file.php/92/92.pdf.
- 7.The Kesho Bora Study Group. Maternal HIV-1 disease progression 18–24 months postdelivery according to antiretroviral prophylaxis regimen (triple-antiretroviral prophylaxis during pregnancy and breastfeeding vs zidovudine/single-dose nevirapine prophylaxis): The Kesho Bora randomized. Clin Infect Dis. 2012;55:449–60. doi: 10.1093/cid/cis461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hargrove JW, Humphrey JH. Mortality among HIV-positive postpartum women with high CD4 cell counts in Zimbabwe. AIDS. 2010;24:F11–4. doi: 10.1097/qad.0b013e328335749d. [DOI] [PubMed] [Google Scholar]
- 9.Minniear TD, Girde S, Angira F, et al. Outcomes in a Cohort of Women Who Discontinued Maternal Triple-Antiretroviral Regimens Initially Used to Prevent Mother-to-Child Transmission during Pregnancy and Breastfeeding-Kenya, 2003–2009. PLoS One. 2014;9:e93556. doi: 10.1371/journal.pone.0093556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The INSIGHT START Study Group. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med. 2015;373:795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.ICW, GNP+ Understanding the perspectives and/or experiences of women living with HIV regarding Option B+ in Uganda and Malawi. 2013 http://www.gnpplus.net/assets/2013-Option-B+-Report-GNP-and-ICW.pdf.
- 13.Ngarina M, Tarimo EaM, Naburi H, et al. Women’s Preferences Regarding Infant or Maternal Antiretroviral Prophylaxis for Prevention of Mother-To-Child Transmission of HIV during Breastfeeding and Their Views on Option B+ in Dar es Salaam, Tanzania. PLoS One. 2014;9:e85310. doi: 10.1371/journal.pone.0085310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tenthani L, Haas AD, Tweya H, et al. Retention in care under universal antiretroviral therapy for HIV-infected pregnant and breastfeeding women (‘Option B+’) in Malawi. AIDS. 2014;28:589–98. doi: 10.1097/QAD.0000000000000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Douglas GP, Gadabu OJ, Joukes S, et al. Using touchscreen electronic medical record systems to support and monitor national scale-up of antiretroviral therapy in Malawi. PLoS Med. 2010;7 doi: 10.1371/journal.pmed.1000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson LF, Estill J, Keiser O, et al. Do increasing rates of loss to follow-up in antiretroviral treatment programs imply deteriorating patient retention? Am J Epidemiol. 2014;180:1208–12. doi: 10.1093/aje/kwu295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chi BH, Yiannoutsos CT, Westfall AO, et al. Universal definition of loss to follow-up in HIV treatment programs: a statistical analysis of 111 facilities in Africa, Asia, and Latin America. PLoS Med. 2011;8:e1001111. doi: 10.1371/journal.pmed.1001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 19.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 20.Wilkinson LS, Skordis-Worrall J, Ajose O, Ford N. Self-transfer and mortality amongst adults lost to follow-up in ART programmes in low- and middle-income countries: systematic review and meta-analysis. Trop Med Int Heal. 2015;20:365–79. doi: 10.1111/tmi.12434. [DOI] [PubMed] [Google Scholar]
- 21.Tweya H, Gugsa S, Hosseinipour M, et al. Understanding factors, outcomes and reasons for loss to follow-up among women in Option B+ PMTCT programme in Lilongwe, Malawi. Trop Med Int Health. 2014;00:1360–6. doi: 10.1111/tmi.12369. [DOI] [PubMed] [Google Scholar]
- 22.Koole O, Houben RM, Mzembe T, et al. Improved retention of patients starting antiretroviral treatment in Karonga District, northern Malawi, 2005–2012. J Acquir Immune Defic Syndr. 2014;67:2005–12. doi: 10.1097/QAI.0000000000000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clouse K, Schwartz S, Van Rie A, Bassett J, Yende N, Pettifor A. What They Wanted Was to Give Birth; Nothing Else. JAIDS J Acquir Immune Defic Syndr. 2014;67:e12–8. doi: 10.1097/QAI.0000000000000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Statistical Office (NSO), ICF Macro. Malawi Demographic and Health Survey. Zomba, Malawi, and Calverton, Maryland, USA: NSO and ICF Macro; 2010. [Google Scholar]
- 25.Tenthani L, Haas AD, Egger M, et al. Brief Report. JAIDS J Acquir Immune Defic Syndr. 2015;69:610–4. doi: 10.1097/QAI.0000000000000669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim MH, Ahmed S, Hosseinipour MC, et al. Implementation and Operational Research. JAIDS J Acquir Immune Defic Syndr. 2015;68:e77–83. doi: 10.1097/QAI.0000000000000517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.TEMPRANO ANRS 12136 Study Group. A Trial of Early Antiretrovirals and Isoniazid Preventive Therapy in Africa. N Engl J Med. 2015;373:808–22. doi: 10.1056/NEJMoa1507198. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


