Abstract
Background: Emergency departments (EDs) are seeing more patients with palliative care (PC) needs, but evidence on best practice is scarce.
Objectives: To examine the effectiveness of ED-based PC interventions on hospital admissions (primary outcome), length of stay (LOS), symptoms, quality of life, use of other health care services, and PC referrals for adults with advanced disease.
Methods: We searched five databases until August 2014, checked reference lists/conference abstracts, and contacted experts. Eligible studies were controlled trials, pre-post studies, cohort studies, and case series reporting outcomes of ED-based PC.
Results: Five studies with 4374 participants were included: three case series and two cohort studies. Interventions included a screening tool, traditional ED-PC, and integrated ED-PC. Two studies reported on hospital admissions: in one study there was no statistically significant difference in 90-day readmission rates between patients who initiated integrated PC at the ED (11/50 patients, 22%) compared to those who initiated PC after hospital admission (179/1385, 13%); another study showed a high admission rate (90%) in 14 months following ED-PC, but without comparison. One study showed an LOS reduction (mean 4.32 days in ED-initiated PC group versus 8.29 days in postadmission-initiated group; p < 0.01). There was scarce evidence on other outcomes except for conflicting findings on survival: in one study, ED-PC patients were more likely to experience an interval between ED presentation and death >9 hours (OR 2.75, 95% CI 2.21–3.41); another study showed increased mortality risk in the intervention group; and a case series described a higher in-hospital death rate when PC was ED-initiated (62%), compared to ward (16%) or ICU (50%) (unknown p-value).
Conclusions: There is yet no evidence that ED-based PC affects patient outcomes except for indication from one study of no association with 90-day hospital readmission but a possible reduction in LOS if integrated PC is introduced early at ED rather than after hospital admission. There is an urgent need for trials to confirm these findings alongside other potential benefits and survival effects.
Introduction
Since the mid-twentieth century the world population has been rapidly aging, potentiated by decreasing birth cohorts and increases in life expectancy.1 This phenomenon has particular impact in the older population (aged 60 years or over). People who survive to age 60 can expect to live 20 additional years, and the global share of older people is predicted to continue to grow, reaching 21.1% by 2050.1 The aging of populations has major social and economic consequences,1 which include changes in the use of health care services and costs,2 such as an exponential increase of health resources utilization towards the end of life (EOL).3 As the number of deaths is predicted to continue to rise in the future, high numbers of hospital deaths become difficult to sustain and an “expansion of palliative care (PC) provision will need to happen in all settings.”4
Emergency departments (EDs) are highly vulnerable to this demographic transition. They are not only seen as an available option to seek relief from pain and other burdensome symptoms,5 but as an accessible entry point to a high-technology health care system.2 While not originally considered an ideal environment to deliver PC, EDs face the challenge of receiving growing numbers of patients at the EOL.2 Many patients with serious and life-threatening illness present to EDs, because symptoms cannot be controlled in the community setting.2,5,6 Emergency medicine (EM) providers may have limited training and resources to manage and respond well to patients in the ED who have PC needs, nor to fully respect these patients' preferences and expectations.7–10 There is scarce evidence about how ED services can best manage patients that are both clinically and socially complex, promoting continuity of care and preventing unnecessary admissions.2
Interest in the interface between EM and PC is recent but has been growing—supported by preliminary data11 that suggest these interventions might help to identify PC needs and reduce acute hospital admissions by promoting admission to a PC unit instead, reduce length of stay (LOS), and reduce costs.5 Reducing hospital admissions is key to ensure that patients stay for as long as possible at home in their last months of life. There is extensive evidence showing that this preference is shared by the majority of patients with advanced illness.12 Therefore, in this systematic review we aim to examine and compare the effectiveness of ED-based PC interventions on hospital admissions (primary outcome), LOS, symptoms, quality of life (QoL), use of other health care services, and PC referrals for adults with advanced disease.
Methods
The review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement13 and followed a predesigned protocol.
Identification of studies
Search strategy
We searched five databases (MEDLINE, EMBASE, CENTRAL, PsycINFO, and NHSEED) up to August 2014 using search terms “Palliative” and “Emergency” or their equivalents combined with the operator AND. In addition, we hand searched recent issues of relevant journals that were not fully indexed in databases, screened the references of relevant reviews and all included studies, screened proceedings of conferences in the field, and contacted 29 experts to identify further eligible studies.
Study selection
Studies were first screened by one reviewer (DS) who read titles and abstracts and then by two reviewers (DS and CN) who independently read the full text. Studies were included if they provided original data evaluating any type of PC intervention or service at the ED for adult patients (18 years or older) with advanced disease. This was aligned with Beynon et al.'s broad criteria for PC needs in an ED setting:14 diagnosis of cancer, or chronic obstructive pulmonary disease (COPD), or heart failure (HF), or renal failure, or liver failure, or neurological disease (multiple sclerosis, Parkinson's, dementia, or motor neuron disease), or >2 comorbidities on the Charlson Index.
We included interventional controlled studies (experimental and quasi-experimental studies), pre-post studies, cohort studies, and case series. We considered any type of PC intervention that was described as such by the authors and that was provided at the ED, with the requirement of being provided by at least one member of a PC team (e.g., social worker, nurse, or physician). Studies not written in English, Spanish, Portuguese, French, or Italian were excluded due to translation limits. Studies were also excluded if full publications were not available.
Data extraction
A data extraction form was developed specifically for the review. This included items recording study identification, methods, participants, intervention, outcome measurement, and results.
Quality assessment
Studies were independently assessed for methodological quality by two reviewers (DS and CN), using tools according to the study design. Three of the included studies were case series, for which we used the National Institute for Health and Care Excellence (NICE) quality assessment tool for case series.15 Two studies were cohort studies; for these, we used the Scottish Intercollegiate Guidelines Network (SIGN) checklist for cohort studies.16
Analysis
The characteristics and results of the studies were narratively reported in synthesis tables. For the primary outcome (hospital admissions), we have reported quantitative data when available from the papers or through the authors. For secondary outcomes (LOS, symptoms, QoL, use of other health care services, PC referrals), we reported the results according to the measure used. A meta-analysis was not performed due to limited results.
Results
Search results
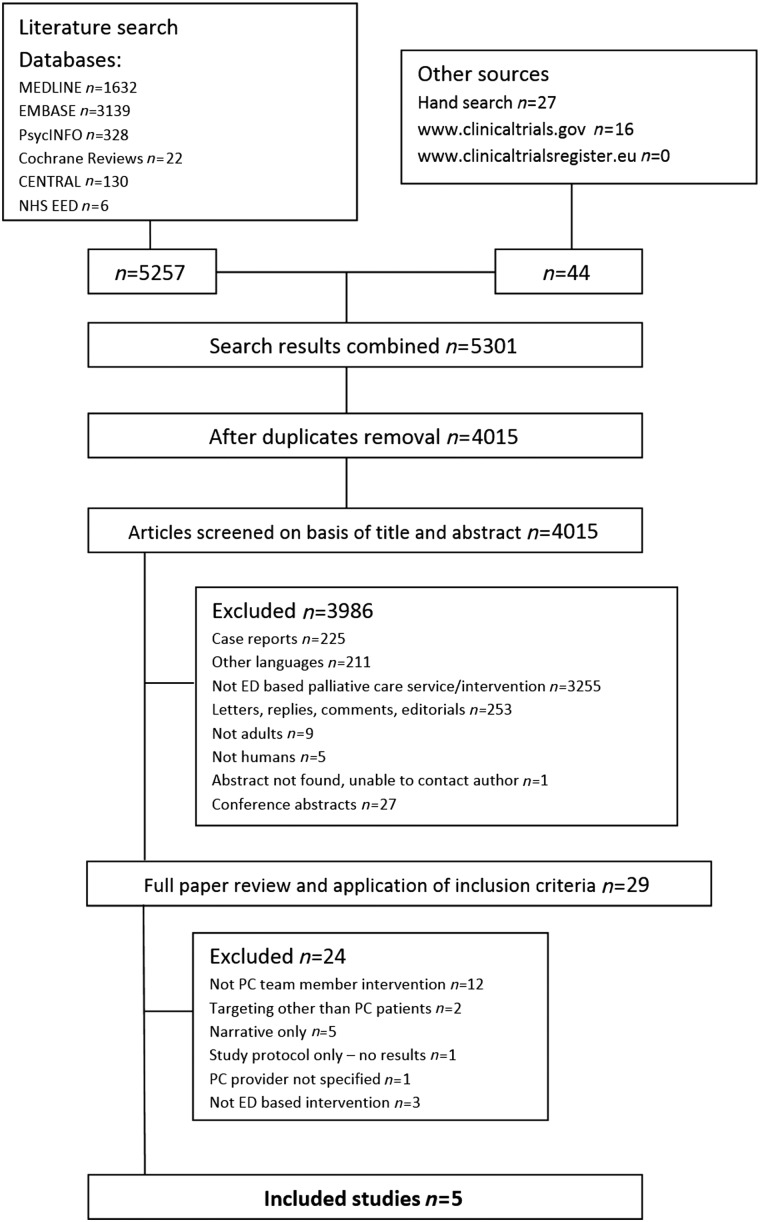
Electronic searches identified 5301 hits, resulting in 4015 references after duplicate removal. Through screening of title and abstract, 3986 references were excluded. We then examined the full text of the 29 remaining references. Five studies were included. Reasons for exclusion are presented in Figure 1.
FIG. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) flowchart.
Design and setting
Three studies are case series and two are cohort studies with intervention and control groups (see Table 1). Four studies originated from the United States (conducted between 2005 and 2010), all from major academic inner city tertiary medical centers. One study was European, including 174 EDs across France and Belgium, conducted in 2004 and 2005.17
Table 1.
Characteristics of the Included Studies
| Study reference | Setting | Methodology | Data collection | Participants | Intervention | Outcomes | Hospital admissions | Length of stay | Impact on symptoms | Impact on HQoL | Use of other health care services | Referrals to palliative care services | NICE SIGN |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glajchen 2011 | Beth Israel Medical Center, New York, U.S. — large academic medical center with 940 beds and 60,000 annual ED visits | Case series; single center | May – December 2007 | n = 139 (51 referred to PC; 88 not referred); n = 56 male; n = 42 cancer; mean age 79.92–80.59 | Two-stage screening protocol (BriefPal) and training | Referrals to specialist PC; symptoms | Not assessed | Not assessed | n = 112 (follow-up) reported symptom reduction in pain, SOB, nausea, anxiety (no comparison) | Not assessed | Not assessed | At the peak of implementation, the BriefPal project accounted for half of all referrals to PC | NICE 4/8 (+) |
| Lamba 2012 | New Jersey University Hospital, U.S. — academic, urban, level-1 trauma ED (100,000 patient visits/year) | Case series; single center; retrospective review of data | March 2008 to June 2009 | n = 89 ED-PC; n = 52 males; subgroup with mean age at death = 45 years | Two advanced NPs and 2 masters-trained bereavement/family support counselors | Survival, LOS, destination postdischarge | Not assessed | Average hospital LOS = 2 days (0.76 on subgroup that died) | Not assessed | Not assessed | 24% returned to ED within 1 month and 59% within 6 months. N = 4 (4%) discharged to nursing facility | N = 11 (12%) discharged to home hospice | NICE 3/8 (+) |
| Mahony 2008 | Montefiore Medical Center, Bronx, N.Y., U.S. — urban community teaching hospital, 80,000 episodes/year | Case series; single center | April 2005 to June 2006 | n = 291 (847 consultations); females 64%; mean age 79 ± 8.4; N = 61 cancer | Two PC NPs working in the ED 11 a.m. – 9 p.m. Monday through Friday | Hospital admission, QoL, destination postdischarge, ED use, survival | 90% of patients enrolled were admitted to the medical center | Not assessed | Not assessed | MVQoL scale (n = 20); 14 = satisfaction with control of their physical symptoms; 13 = “loss of ability to do many of the things that I like” | N = 131 (45%) visited ED in the 12 months postdischarge; N = 110 (41.9% from all admissions) discharged to skilled nursing facilities | n = 83 received homecare and N = 91 hospice; correlations between referral to ED PC and enrollment on hospice (r = 0.49, p < 0.001) | NICE 3/8 (+) |
| Van Tricht 2012 | 174 EDs, both urban and rural (171 in France and 3 in Belgium); mainly university hospitals; all with PC mobile units | Retrospective cohort; multicenter; compared patients who received ED PC vs standard care | November –December 2004 and April – May 2005 | n = 2420; mean age 73 ± 15.1; 1196 male; n = 1373 received PC (exposed); n = 1047 standard care (unexposed) | ED-PC services: analgesia, sedation, hydration, mouth care, reposition, emotional support | Survival, LOS, withhold/withdraw life support, time from admission to death | Not assessed | ED-based PC associated with interval between admission and death longer than 9 hours OR (95% CI) 2.75 (2.21–3.41) p < 0.001 | Not assessed | Not assessed | Not assessed | Not assessed | SIGN 4/13 (0) |
| Wu 2013 | California Pacific Medical Center, U.S.; 2 centers (18,000 and 28,000 annual ED visits) | Retrospective cohort with control group; multicenter | January 2006 to December 2010 | n = 1435 (215 Davies and 1220 Pacific) control; n = 1385; intervention n = 50; mean age 75.6; N = 768 female | ED-based PC service consisting of 2 physicians and an NP | LOS, average time to PC consultation | 90-day readmission; 11/50 patients (22%) ED-based PC vs 179/1385 (13%) that received standard care | Mean LOS intervention (n = 50) = 4.32 days (SE 0.68, p < 0.01); control (n = 1385) = 8.29 days (SE 0.36, p < 0.01) | Not assessed | Not assessed | Not assessed | Not assessed | SIGN 10/13 (++) (downgraded to +; see text) |
ED, emergency department; HQoL, health quality of life; LOS, length of stay; NICE, National Institute for Health and Care Excellence; MVQoL, Missoula Vitas Quality of Life; NP, nurse practitioner; OR, odds ratio; PC, palliative care; QOL, quality of life; SE, standard error; SIGN, Scottish Intercollegiate Guidelines Network; SOB, shortness of breath.
Participants
In total, 4374 participants were included, with a mean of 875 participants per study (ranging from 89 to 2420 participants). Four studies included patients with cancer—16.5%17 to 51%18—and noncancer conditions—44.6%19 to 83.5%.17 The latter predominantly included advanced dementia, HF, COPD,18 renal and liver diseases.19–21 Median/mean age ranged from 66 to 90 years. Gender distribution ranged from 36%21 to 58%22 for males and 42%22 to 64%21 for females.
Types of interventions
We found three main types of ED-based PC interventions, described according to the level of cooperation between ED and PC services:
• ED screening tool used by PC members: Glajchen et al.;19 this is a rapid two-stage screening protocol (BriefPal) that was developed to improve referral of frail older people in ED to PC or hospice care
• Traditional PC consultations in the ED: Van Tricht et al.,20 Mahony et al.,21 and Lamba et al.22 In these interventions, there is typically no specific collaborative relationship to help integrate PC principles into the fabric of ED care23
• Integrated ED PC services: Wu et al.;18 these programs are characterized by a more formal working relationship between the ED and PC program to define partnership goals and objectives23
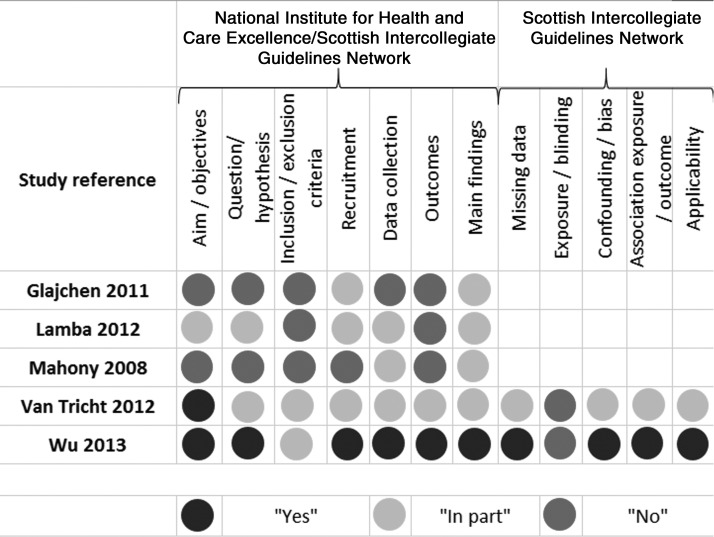
Results from quality assessment are discussed below and are shown in Figure 2 with dots to aid interpretation.
FIG. 2.
Quality assessment of the included studies.
Case series
Using the NICE quality assessment tool for case series,13 the quality of the three case series was considered moderate (see Fig. 2). All case series were single-center and the data were collected retrospectively. Other methodological weaknesses were related to lack of clarity of the hypothesis, aim, and objectives,17,18 inclusion/exclusion criteria,19 data collection,18 and outcomes measured.17–19
Cohort studies
Using the SIGN methodology checklist for cohort studies,14 the quality of the study by Van Tricht et al.20 was considered low (0) while the quality of the study by Wu et al.21 was considered acceptable (+). The two studies reported clear aims, and the intervention and control groups were selected from similar and comparable source populations. However, Van Tricht et al.20 do not clearly state their hypothesis, there is a lack of information on missing data, and the outcomes are not clearly stated. Methodological strengths of the study by Wu et al.21 include having a clear aim and hypothesis; and information on missing data, outcomes, limitations, and results. However, since both studies are retrospective cohort studies and the outcome assessment was not blinded to the exposure status (ED-based PC consultation or standard care), according to SIGN the rating of these two studies cannot be rated higher than “+” (acceptable quality).14 Effects of the interventions are discussed below and summarized in Table 2.
Table 2.
Effects of ED-Based PC Interventions
| Outcomes | Number of participants (studies) | Data | Comments |
|---|---|---|---|
| Hospital admissions | Two studies, n = 1726: Mahony 2008, case series, U.S., n = 291; Wu 2013, retrospective cohort with control group, U.S., n = 1435 | Mahony 2008: 262 patients (90%) that were attended by MMC ED-based PC team ended up admitted to the medical center during the 14-month time data was collected | No comparison (before and after or with a control group) |
| Wu 2013: 90-day readmission rates — 11/50 patients (22%) from intervention group were readmitted versus 179/1385 (13%) from control group | No statistically significant differences | ||
| LOS | Two studies, n = 1524: Lamba 2012, case series, U.S., n = 89; Wu 2013, retrospective cohort with control group, U.S., n = 1435 | Lamba 2012: “Average” (as stated by the authors) hospital LOS of patients to whom an ED-based PC consultation was initiated was 2 days | No comparison (before and after or with a control group); unclear if “average” represents the mean or median, and SD or IQR were not provided |
| Wu 2013: Mean LOS for the intervention group (patients who received ED-based PC consultations; n = 50) was 4.32 days (SE 0.68) compared to 8.29 days (SE 0.36) in the control group (without ED-based PC consultations; n = 1385); in multivariate analysis (controlling for covariates and propensity scores), the initiation of PC at the ED (vs after) | |||
| Symptoms | One study, Glajchen 2011, case series, U.S., n = 139 | Glajchen 2011: Group referred to BriefPal reported “symptom reduction in pain, shortness of breath, nausea, and anxiety” | No comparison; follow-up made to 112 patients via telephone; no information is given about time points, percentages, means/medians, SDs/IQRs, or P values |
| QoL | One study, Mahony 2008, case series, U.S., n = 291 | Mahony 2008: MVQoLI post-ED discharge (20/291 patients, 6.9% response rate): 14 expressed satisfaction with control of their physical symptoms and 13 expressed improved ability to communicate with people close to them | No information was given on the exact time point when the tool was administered after discharge; very low RR |
| Use of other health care services (includes ED readmission) | Two studies, n = 380: Lamba 2012, case series, n = 89; Mahony 2008, case series, n = 291 | Lamba 2012: Subsequent use of ED of patients who received ED-based PC: 24% returned within 1 month and 59% within 6 months; 4/34 patients that received ED-based PC and survived were discharged to skilled nursing facilities (the remaining 30 had gone home (18/34) / home hospice (11/34) / unknown place (1/34) | No comparison (before and after or with a control group) |
| Mahony 2008: ED-based PC services group (n = 291): 45% (n = 131) visited ED in the 12 months subsequent to the index visit; from subgroup admitted (n = 262), 41.9% were subsequently discharged to skilled nursing facilities, 24.2% to home with homecare, and 19.1% were discharged without homecare | No comparison (before and after or with a control group) | ||
| Referrals to PC or hospice care services | Three studies, n = 519: Glajchen 2011, case series, n = 139; Lamba 2012, case series, n = 89; Mahony 2008, case series, n = 291 | Glajchen 2011: At the peak of implementation, the BriefPal project accounted for half of all referrals to in-hospital PC services | No comparison (before and after or with a control group); no information on the usual referral rate |
| Lamba 2012: 11/89 patients (12%) who received ED-based PC consultations were discharged to home hospice | No comparison (before and after or with a control group) | ||
| Mahony 2008: From n = 550 patients referred by the PC team on discharge (total 894 consultations), 83 received homecare after discharge and 91 received hospice services | No comparison (before and after or with a control group) | ||
| Survival | Three studies, n = 3944: Lamba 2012, case series, n = 89; Van Tricht 2012, retrospective cohort, n = 2420; Wu 2013, retrospective cohort, n = 1435 | Lamba 2012: Rates of in-hospital deaths; ED-initiated PC group (n = 89) death rate = 62% (n = 55), compared to 16% ward-initiated PC group (91/583) and 50% ICU-initiated PC group (288/578) | Significance of differences not reported |
| Van Tricht 2012: ED-based PC associated with an interval between ED admission and death longer than 9 hours (adjusted OR 2.75, 95% CI 2.21–3.41, p < 0.0001) | Data adjusted for other variables not reported | ||
| Wu 2013: APRDRG risk of mortality: Control group 2.3% for minor risk (32/2385), 22.5% for moderate risk (311/1385), 50% for major risk (693/1385), 31.8% for extreme risk (441/1385). Intervention: 4% for minor risk (2/50), 24% for moderate risk (12/50), 62% for major risk (31/50), 10% for extreme risk (5/50) (p < 0.01) | All APRDRG ROM and SOI |
APRDRG, All Patient Refined Diagnostic Related Group; ED, emergency department; IQR, interquartile range; LOS, length of stay; MMC, Montefiore Medical Centre; MVQoLI, Missoula Vitas Quality of Life Index; OR, odds ratio; PC, palliative care; QoL, quality of life; ROM, risk of mortality; SD, standard deviation; SE, standard error; SOI, severity of illness.
Hospital admissions
In two studies the authors reported information on hospital admissions (1726 patients). Wu et al.18 found no statistically significant differences in 90-day readmission rates. The authors stated that 11/50 patients (22.0%) that initiated PC at the ED (integrated ED PC) were readmitted, compared to 179/1385 (12.9%) of those that initiated PC after hospital admission. Mahony et al.21 reported on hospital admissions but without comparison with a control group: 90% of the 291 patients that were seen by the ED-based PC team (traditional PC consultations) were admitted to the medical center during the 14-month timeframe of the study.
Length of stay
In two studies the authors reported information on LOS (1524 patients). Wu et al.18 reported the mean LOS for the intervention group (patients who received ED-based PC; n = 50) was 4.32 days (SE 0.68) compared to 8.29 days (SE 0.36) in the control group (without ED-based PC; n = 1385). In multivariate analysis (controlling for covariates and propensity scores), the initiation of PC at the ED (versus after hospital admission) was still associated with shorter LOS, with a mean decrease of 3.63 days (p < 0.01). Lamba et al.22 described the “average” hospital LOS of patients for whom an ED-based PC consultation was initiated was two days (without comparison with a control group).
Symptoms
In one study the authors reported information on symptoms (Glajchen et al.19). Patients in the intervention group (patients screened by BriefPal) reported symptom reduction in pain, shortness of breath, nausea, and anxiety. These outcomes were assessed through follow-up of 112 patients via telephone contact. However, no information was given about time points; and no information was given about descriptive or analytic statistics comparing patients who were screened to those who were not.
Quality of life
In one study the authors reported information on QoL (Mahony et al.24). The patient's QoL was self-reported using the Missoula Vitas Quality of Life Index (MVQoLI; scores range from −20 to +20; higher scores mean higher QoL). This assessment was done post-ED discharge, although no information was given on the specific point in time after the discharge. By then, very few patients completed the MVQoLI (20/291, 6.9% response rate). The authors reported that of those 20 patients, 14 expressed satisfaction with the control of their physical symptoms, and 13 expressed improved ability to communicate with people close to them. There was no information on total scores, results comparison pre-post the intervention (consultations by two PC nurse practitioners), or with a control group.
Use of other health care services
Two studies reported information on the use of other health care services. Lamba et al.22 reported subsequent use of ED by patients who received ED-based PC, but without a comparison group. Twenty-four percent of patients (21/89) returned within one month and 59% (52/89) within six months of their initial contact with PC nurse practitioners. Four out of the 34 patients that received the ED-based PC and survived were discharged to skilled nursing facilities. Other destinations on discharge were home (18/34), home hospice (11/34), and unknown place (1/34). Mahony et al.21 reported that of the 291 patients who received the ED-based PC service, 45% visited the ED in the 12 months subsequent to the index visit (compared to 59% in the 12 months before the index visit). Of those admitted to acute care (90.0%, n = 262), 41.9% were discharged to skilled nursing facilities, 24.2% to home with homecare, and 19.1% were discharged without homecare.
Referral to palliative care or hospice care services
In two studies the authors examined referrals to other PC or hospice care services, but neither provided analytical results. Glajchen et al.19 reported that at the peak of implementation, the BriefPal screening project accounted for half of all referrals to the in-hospital PC service. Lamba et al.22 described that 12% of patients who initiated PC at ED were discharged to home hospice (compared to 4% in ICU and 13% in ward-initiated PC). The statistical significance of these differences was not reported.
Survival
There were conflicting results on survival. Van Tricht et al.20 reported that the provision of traditional ED-based PC was associated with greater odds of experiencing an interval between ED admission and death longer than nine hours (adjusted OR 2.75, 95% CI 2.21 to 3.41). Wu 201318 reported the risk of mortality for 1435 patients according to the All Patient Refined Diagnostic Related Group (APRDRG) risk of mortality (ROM) and severity of illness (SOI).25 The APRDRG is a severity coding methodology that allows assignment of an SOI and ROM score in four categories (minor, moderate, major, and extreme risk). This system is used to evaluate resource utilization and predict inpatient mortality. The authors reported statistically significant differences between the intervention and control groups (p < 0.01). In the latter (standard care; n = 1385), the ROM was 2.3% for minor risk (32/1385), 22.5% for moderate risk (311/1385), 42.4% for major risk (587/1385), and 32.9% for extreme risk (455/1385). In the intervention group (n = 50), the ROM was 4.0% for minor risk (2/50), 24.0% for moderate risk (12/50), 62.0% for major risk (31/50), and 10.0% for extreme risk (5/50). The direction of these differences suggests higher mortality in the intervention group. Finally, Lamba et al.22 reported in-hospital death rates. In the ED-initiated PC group (n = 89), the in-hospital death rate was 62% (n = 55), compared to 16% in the ward-initiated PC group (91/583) and 50% in the ICU-initiated PC group (288/578). However, the authors did not report the statistical significance of these differences. They viewed the in-hospital death rate in the ED-initiated PC group as “very high,” justified due to most patients dying early and at the ED, “signifying a trend for ED clinicians to request PC consults in those who are imminently dying.”22
Discussion
We found no evidence that ED-based PC affects patient outcomes except for indication from one study of no association with 90-day hospital readmission but a possible reduction in LOS (by 3.53 days) if integrated PC is introduced early at the ED rather than after hospital admission. These findings must be interpreted with care, as they derive from a retrospective cohort study with a small intervention group (n = 50). However, if confirmed in future studies, this could suggest that ED-based PC may not avoid hospital readmission (possibly inevitable due to the complexity of patients with PC needs who present at the ED) but may help to plan and provide a faster discharge. It is important to discuss what a reduction in hospital LOS means at the EOL. Extensive evidence shows that well over 50% of people prefer to be cared for and to die at home.12,26 Most patients and caregivers facing advanced illness also prefer this.12,27,28 Therefore, if the result holds true in future trials, an early initiation of PC at the ED might contribute to decrease trends towards hospitalized dying, by helping patients who wish to remain at home spend less time in hospital and go home quicker.
Interestingly, we found conflicting findings about the association of ED-based PC interventions with patient's survival. Lamba et al. reported different in-hospital death rates, depending on where PC interventions commenced; and although the statistical significance of these differences was not reported and the ED-PC group was relatively small (n = 89), the findings indicated there could be more (in-hospital) death in this group (62%) compared to patients who initiated PC at a ward (16%). The difference is not so large when compared to patients who initiated PC at the ICU (50%), which suggests that the high rates may reflect the acute state of patients at the ED and ICU, which makes PC interventions more time restricted and closer to death.
On the contrary, Wu et al. found a lower proportion of people in extreme mortality risk in the group who initiated PC at the ED (10.0%) compared to those who initiated after admission (32.9%). But the overall direction of differences taking into account the other categories (major, moderate, and minor risk) indicates higher mortality risk in the intervention group—the main difference being in a major risk group, which represents 62.0% of all intervention patients and 42.4% of controls. Contrarily, Van Tricht et al. reported that ED-based PC was associated with two times greater odds of an interval between ED admission and death longer than nine hours. The latter results suggest that ED-based PC interventions might prolong survival, similar to what has been found in other recent interventional studies of PC.29,30
Limitations
Only one review author (DS) conducted the initial screening. Only 27 potentially eligible studies were found through hand searches. The studies included were all conducted in high-income countries. Only one example of ED screening tools used by PC members31 and only one example of integrated ED PC services18 were found.
Since we conducted the review searches (August 2014), a systematic review of PC screening/referral projects at the ED was published.32 This did not exclude studies that used non-PC personnel to screen patients. From the seven studies identified, one is relevant to our review but does not change our findings. This was a randomized control trial with 134 patients, comparing early ED-based PC referral to usual care (where PC was provided only if requested by the admitting physician).33 The authors found that early referral increased the likelihood of receiving a PC consultation, which was low as part of usual care within the ED (18% in the usual care group). Finally, the body of evidence we reviewed has strong methodological limitations, mainly related to the retrospective nature of studies.
Conclusions
In this systematic review we found that there is yet insufficient evidence of the effect of ED-based PC interventions, except for indication from one study of no association with 90-day hospital readmission but a possible reduction in LOS if integrated PC is introduced early at the ED rather than after hospital admission. Evidence is very scarce of impact on symptom control, QoL, and referrals to specialist PC services and use of other health care services. Finally, we found conflicting data on survival that require investigation. There is an urgent need for powered and well-conducted randomized controlled trials to examine any potential benefits of these interventions.
Acknowledgments
This study would not have been possible without the important contribution of the Calouste Gulbenkian Foundation, Dr. Jacinta Fernandes and the Unidade Domiciliária de Cuidados Paliativos—Planalto Mirandês, who generously funded and supported it. The study was conducted as Duarte Soares' research project for the MSc in Palliative Care at King's College London, integrated in the DINAMO Project, which aims at enhancing advanced training and research to optimize home palliative care in Portugal (Principal Investigator – Barbara Gomes, Scientific Director – Irene J. Higginson, other members – Pedro L. Ferreira, Hélder Aguiar, Ana F. Lacerda, Vera P. Sarmento, Duarte Soares, Rita Canário, Maja de Brito, Catarina Ribeiro, Diogo M. Branco).
The authors also wish to thank the library services from King's College London; the authors of the included studies, who provided the information asked for; colleagues in the Cicely Saunders Institute and the MSc course, mainly Rita Canário, Vera Sarmento, Ana Lacerda, and Bárbara Antunes, for supporting this project since its inception.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.UN Department of Economic and Social Affairs, Population Division: World Population Ageing 2013. UN, 2013 [Google Scholar]
- 2.Aminzadeh F, Dalziel WB: Older adults in the emergency department: A systematic review of patterns of use, adverse outcomes, and effectiveness of interventions. Ann Emerg Med 2002;39:238–247 [DOI] [PubMed] [Google Scholar]
- 3.Kardamanidis K, Lim K, Da Cunha C, et al. : Hospital costs of older people in New South Wales in the last year of life. Med J Aust 2007;187:383–386 [DOI] [PubMed] [Google Scholar]
- 4.Gomes B, Calanzani N, Curiale V, et al. : Effectiveness and cost-effectiveness of home palliative care services for adults with advanced illness and their caregivers. Cochrane Database Syst Rev 2013;6:CD007760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grudzen CR, Stone SC, Morrison RS: The palliative care model for emergency department patients with advanced illness. J Palliat Med 2011;14:945–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Addington-Hall J, Altmann D, McCarthy M: Variations by age in symptoms and dependency levels experienced by people in the last year of life, as reported by surviving family, friends and officials. Age Ageing 1998;27:129–136 [DOI] [PubMed] [Google Scholar]
- 7.Stone SC, Mohanty S, Grudzen CR, et al. : Emergency medicine physicians' perspectives of providing palliative care in an emergency department. J Palliat Med 2011;14:1333–1338 [DOI] [PubMed] [Google Scholar]
- 8.Smith AK, Fisher J, Schonberg MA, et al. : Am I doing the right thing? Provider perspectives on improving palliative care in the emergency department. Ann Emerg Med 2009;54:86–93.e1 [DOI] [PubMed] [Google Scholar]
- 9.O'Connor AE, Winch S, Lukin W, Parker M: Emergency medicine and futile care: Taking the road less travelled. Emerg Med Australas 2011;23:640–643 [DOI] [PubMed] [Google Scholar]
- 10.Iglesias ML, Lafuente A: [Care for the dying patient in emergency departments.] Asistencia al paciente agonico que va a fallecer en urgencias. An Sist Sanit Navar 2010;33:173–191 [PubMed] [Google Scholar]
- 11.Meier DE, Beresford L: Fast response is key to partnering with the emergency department. J Palliat Med 2007;10:641–645 [DOI] [PubMed] [Google Scholar]
- 12.Gomes B, Calanzani N, Gysels M, et al. : Heterogeneity and changes in preferences for dying at home: A systematic review. BMC Palliat Care 2013;12:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG: Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009;339:332–336 [PMC free article] [PubMed] [Google Scholar]
- 14.Beynon T, Gomes B, Murtagh FE, et al. : How common are palliative care needs among older people who die in the emergency department? Emerg Med J 2011;28:491–495 [DOI] [PubMed] [Google Scholar]
- 15.National Institute for Health and Care Excellence: Quality Assessment Tool for Case Series. National Institute for Health and Care Excellence, 2014. www.nice.org.uk/guidance/cg3/resources/appendix-4-quality-of-case-series-form2 (last accessed December17, 2014)
- 16.Scottish Intercollegiate Guidelines Network: Methodology Checklist: Cohort Studies. Scottish Intercollegiate Guidelines Network, 2014. www.sign.ac.uk/methodology/checklists.html (last accessed December12, 2014)
- 17.Le Conte P, Riochet D, Batard E, et al. : Death in emergency departments: A multicenter cross-sectional survey with analysis of withholding and withdrawing life support. Intensive Care Med 2010;36:765–772 [DOI] [PubMed] [Google Scholar]
- 18.Wu FM, Newman J, Lasher A, Brody A: Effects of initiating palliative care consultation in the emergency department on inpatient length of stay. Palliat Med 2013;16:1362–1367 [DOI] [PubMed] [Google Scholar]
- 19.Glajchen M, Lawson R, Homel P, et al. : A rapid two-stage screening protocol for palliative care in the emergency department: A quality improvement initiative. J Pain Symptom Manage 2011;42:657–662 [DOI] [PubMed] [Google Scholar]
- 20.Van Tricht M, Riochet D, Batard E, et al. : Palliative care for patients who died in emergency departments: Analysis of a multicentre cross-sectional survey. Emerg Med J 2012;29:795–797 [DOI] [PubMed] [Google Scholar]
- 21.Mahony SO, Blank A, Simpson J, et al. : Preliminary report of a palliative care and case management project in an emergency department for chronically ill elderly patients. J Urban Health 2008;85:443–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamba S, Nagurka R, Walther S, Murphy P: Emergency-department-initiated palliative care consults: A descriptive analysis. J Palliat Med 2012;15:633–636 [DOI] [PubMed] [Google Scholar]
- 23.Quest T, Herr S, Lamba S, et al. : Demonstrations of clinical initiatives to improve palliative care in the emergency department: A report from the IPAL-EM Initiative. Ann Emerg Med 2013;61:661–667. [Erratum appears in Ann Emerg Med 2013;61:24.] [DOI] [PubMed] [Google Scholar]
- 24.Byock IR, Merriman MP: Measuring quality of life for patients with terminal illness: The Missoula-VITAS quality of life index. Palliat Med 1998;12:231–244 [DOI] [PubMed] [Google Scholar]
- 25.Lagman RL, Walsh D, Davis MP, Young B: All patient refined-diagnostic related group and case mix index in acute care palliative medicine. J Support Oncol 2007;5:145–149 [PubMed] [Google Scholar]
- 26.Gomes B, Higginson IJ, Calanzani N, et al. : Preferences for place of death if faced with advanced cancer: A population survey in England, Flanders, Germany, Italy, the Netherlands, Portugal and Spain. Ann Oncol 2012;23:2006–2015 [DOI] [PubMed] [Google Scholar]
- 27.Bell CL, Somogyi-Zalud E, Masaki KH: Methodological review: Measured and reported congruence between preferred and actual place of death. Palliat Med 2009;23:482–490 [DOI] [PubMed] [Google Scholar]
- 28.Higginson IJ, Sen-Gupta GJA: Place of care in advanced cancer: A qualitative systematic literature review of patient preferences. J Palliat Med 2000;3:287–300 [DOI] [PubMed] [Google Scholar]
- 29.Bakitas MA, Tosteson TD, Li Z, et al. : Early versus delayed initiation of concurrent palliative oncology care: Patient outcomes in the ENABLE III randomized controlled trial. J Clin Oncol 2015;33:1438–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Temel JS, Greer JA, Muzikansky A, et al. : Early palliative care for patients with metastatic non–small-cell lung cancer. N Engl J Med 2010;363:733–742 [DOI] [PubMed] [Google Scholar]
- 31.Glajchen M, Lawson L, Homel P, et al. :. Palliative Care Model and Brief Intervention (PC-SBI) “BriefPal” Palliative Care Screening Tool. New York: Department of Pain Medicine and Palliative Care, Department of Emergency Medicine, Beth Israel Medical Center, 2011. www.stoppain.org/for_professionals/pdfs/BriefPal_Screening_Instrument.pdf (last accessed December18, 2014) [Google Scholar]
- 32.George N, Phillips E, Zaurova M, et al. : Palliative care screening and assessment in the emergency department: A systematic review. J Pain Symptom Manage 2016;51:108–119 [DOI] [PubMed] [Google Scholar]
- 33.Kistler EA, Morrison R, Richardson LD, et al. : Emergency department-triggered palliative care in advanced cancer: Proof of concept. Acad Emerg Med 2015;22:237–239 [DOI] [PubMed] [Google Scholar]