Abstract
Intraocular pressure (IOP) is still the main treatment target for glaucoma. Outflow resistance mainly exists at the trabecular meshwork (TM) outflow pathway, which is responsible for IOP regulation. Changes of TM cellularity and TM extracellular matrix turnover may play important roles in IOP regulation. In this article, we review basic anatomy and physiology of the outflow pathway and TM stem cell characteristics regarding the location, isolation, identification and function. TM stem cells are localized at the insert region of the TM and are label-retaining in vivo. They can be isolated by side-population cell sorting, cloning culture, or sphere culture. TM stem cells are multipotent with the ability to home to the TM region and differentiate into TM cells in vivo. Other stem cell types, such as adipose-derived stem cells, mesenchymal stem cells and induced pluripotent stem cells have been discovered for TM cell differentiation and TM regeneration. We also review glaucomatous animal models, which are suitable to study stem cell-based therapies for TM regeneration.
Introduction
The conventional pathway, also called the trabecular meshwork (TM) pathway, of aqueous humor outflow consists of the TM, Schlemm's canal (SC), and the aqueous collector channels. The latest studies have shown that the conventional pathway accounts for 40%–96% of the total aqueous humor drainage in humans. This variation is because the conventional pathway compensates the decreased outflow of uveoscleral pathway with aging.1,2 The conventional outflow pathway is a pressure-driven system,3,4 that provides resistance to aqueous humor and allows bulk flow of aqueous humor to pass through it driven by the pressure gradient, thus keeps intraocular pressure (IOP) in a steady state. Outflow resistance in the conventional outflow pathway increases with age5,6 and is higher in primary open-angle glaucomatous eyes.7,8
All current antiglaucoma treatment strategies for reducing IOP can be classified into 3 categories as follows: (1) increase outflow through the alternative routes; (2) reduce aqueous humor production; or (3) shunt aqueous humor away from the diseased conventional outflow pathway, which also shunts nutrients away from the eye. None of these therapies target the conventional route, although the majority of aqueous humor exits via this route, which makes it an attractive prospect for development of new therapies.
It has been suggested that age- and disease-related decrease of TM cells, accelerated apoptosis and senescence,9–15 abnormal extracellular matrix (ECM) accumulation, the presence of cross-linked actin networks in TM cells16–19 and the trabecular beam fusion resulting from adhesions between denuded portions of adjacent trabecular beams20 are associated with an increased aqueous outflow resistance and concomitant increase of IOP. A myocilin mutant mouse glaucoma model21,22 demonstrating TM cell death and IOP elevation emphasizes the TM cell function in the aqueous outflow. TM cells are phagocytic23 with the ability to remove potential obstructive debris in the outflow track and are responsible for ECM degradation and replacement biosynthesis in the outflow pathway.24 It is reasonable to predict that reduction of TM cellularity will affect ECM turnover and cause debris accumulation, hence increase outflow resistance in open-angle glaucoma eyes. Theoretically, repopulation of the TM cells by stem cells may compensate the decreased cellularity in glaucomatous eyes and modify the abnormal ECM and promote the ECM turnover, thus reducing IOP. As juxtacanalicular cells are not depleted in late stages of glaucoma, replacement of corneoscleral TM cells might suffice for IOP regulation.9 This is a new treatment strategy, by which IOP could be reduced via improving the conventional outflow pathway.
TM Anatomy and Physiology
Location and shape
The TM is a wedge-shaped lamellar tissue stretched between the periphery of Descemet's membrane of the cornea anteriorly and the scleral spur posteriorly.25 It is formed by collective tissue beams or lamellae that have a core of collagenous and elastic fibers and are covered by flat cells, which rest on a basal lamina. The beams attach to one another in several layers and form a porous filter-like structure.
TM components and their morphological functions to regulate IOP
The TM consists of 3 regions that differ in structure as follows: the inner uveal meshwork, the deeper corneoscleral meshwork, and the juxtacanalicular tissue (JCT) or cribriform region that is localized directly adjacent to the inner wall of SC endothelium (Fig. 1). From the inner to the outermost layer, there is a decrease in porosity,25,26 thus the lamellae of the uveal TM are a filter. The trabecular outflow resistance resides within the deepest 1/4–1/3 of the TM27 and most of the resistance appears to reside within the JCT and/or SC endothelium.28,29 The cells lining the lamellae of the TM play 2 primary roles: secretion of specific enzymes and ECM, and phagocytosis of debris in the aqueous humor.23 Both functions help maintain aqueous outflow flowing through open spaces between organized trabecular lamellae.30
FIG. 1.

Illustration of the TM structure. Insert region (green, nonfilter region) is between the TM and the corneal endothelium. The TM consists of uveal meshwork (blue), corneoscleral meshwork (red), and JCT (yellow). JCT, juxtacanalicular tissue; TM, trabecular meshwork.
Stem Cells in the TM
In addition to the 3 components of the TM in the outflow facility, there is proof of a fourth region called the insert zone. The insert zone resides at the Schwalbe's line and does not filter aqueous humor into the SC. Several studies have explored the characteristics of the cell population in the insert zone. In 1982, Raviola31 identified an unusual cell population termed Schwalbe's line cells with distinct ultrastructural features different from TM cells. In 1989, Acott et al.32 found out that there was an increase in TM cell division after treating organ cultured human anterior segments with laser trabeculoplasty, and over 60% of the cell division took place in the nonfiltering proportion of TM. More direct evidence for the existence of stem cells in the insert zone of the TM came from immunostaining studies by Whikehart and McGowan,33,34 demonstrating presence of stem cell markers in the TM and in the transition zone between the TM and the corneal endothelial periphery.
It is thus hypothesized that the insert zone is a niche for cells with adult stem cell-/progenitor cell-like properties that serve as a reservoir to repopulate cells into the filtering TM region when injury takes place.35 In the following session, we will be discussing in more detail about the growing evidence and techniques of this hypothesis.
Isolation, Cultivation, and Identification of Trabecular Meshwork Stem Cells
Isolation and cultivation
To isolate and enrich trabecular meshwork stem cells (TMSCs), several techniques can be applied based on the properties of stem cells. For example, formation of colonies is one of the primary characteristics of stem cells. Also, stem cells have the ability to remove DNA-binding fluorescent dyes because stem cells express special ATP-binding cassette-containing pumps.36 Thus stem cells are able to efflux the dyes and remain unstained or weakly stained forming a side population (SP) when analyzed by flow cytometry.36,37 Based on these principles, there have been different methods to isolate and culture stem cells from TM region.
We isolated human TMSCs by sorting side-population (SP) cells using fluorescence-activated cell sorting (FACS) technique. Using SP cell sorting to purify stem cells was discovered in 199638 and has been used to isolate many kinds of adult stem cells. Since there are not enough cells from human TM tissue for direct SP cell sorting, we39,40 first cultured and passaged cells from human TM in stem cell growth medium (SCGM) containing multipurpose reduced-serum media (Opti-MEM) supplemented with 5% fetal bovine serum (FBS), 10 ng/mL epidermal growth factor (EGF), 100 μg/mL bovine pituitary extract, 20 μg/mL ascorbic acid, 200 μg/mL calcium chloride, 0.08% chondroitin sulfate, 100 IU/mL penicillin, 100 μg/mL streptomycin, and 50 μg/mL gentamicin. After 2 to 3 passages, 5 × 105–2 × 106 cells were incubated at 1 × 106 cells/mL in prewarmed DMEM with 2% FBS and either 5 μg/mL Hoechst 3334241 or 10 μM DyeCycle Violet (DCV)42 for 100 min at 37°C. A negative control was performed by preincubation with 50 μg/mL verapamil or 25 μg/mL fumitremorgin C for 20 min before Hoechst or DCV incubation to inhibit Hoechst or DCV dye efflux. After staining, cells were washed twice in Hank's balanced salt solution with 2% FBS and stored on ice and then 2 μg/mL propidium iodide was added to identify nonviable cells immediately before sorting. Cells were analyzed on a flow cytometer high-speed cell sorter, using 350-nm (for Hoechst dye) or 405-nm (for DCV dye) excitation. Designated SP cells showed reduced fluorescence at both blue and red channels (Fig. 2).
FIG. 2.
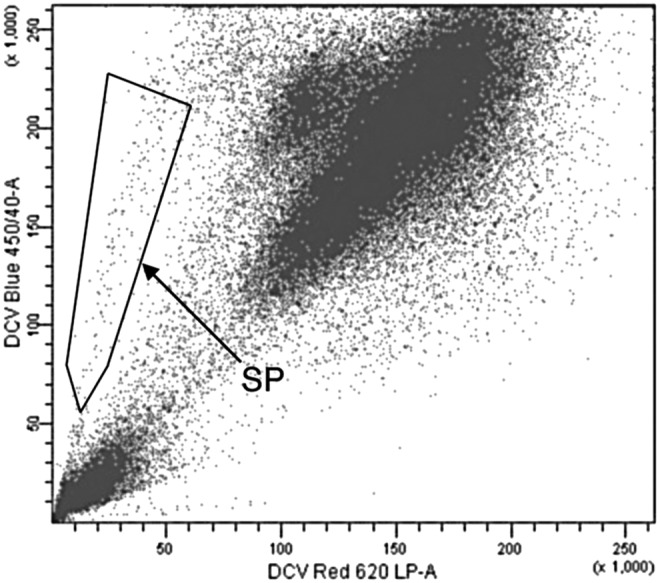
Isolation of TMSCs as SP cells. SP cells were isolated by FACS from passage 3 human TM cells (using DyeCycle Violet Dye; Invitrogen). Cells showing reduction of both blue (450 nm) and red (>620 nm) are the SP cells in the frame. Reproduced from Du et al.39 with permission of the Association for Research in Vision and Ophthalmology. FACS, fluorescence-activated cell sorting; LP, long pass; SP, side population; TMSCs, trabecular meshwork stem cells.
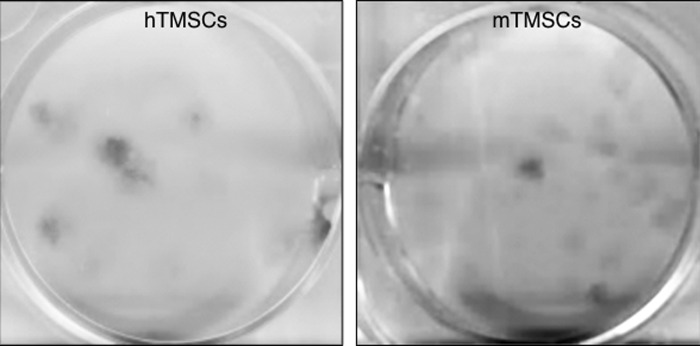
We were also able to isolate the TMSCs by clonal culture. We cultured either human and mouse TMSCs in SCGM at 100 cells per well of 6-well plates precoated with FNC Coating Mix (containing fibronectin, collagen and albumin, AthenaES). Twelve days later, we stained the cells with 0.5% crystal violet solution in 25% methanol and scanned the plates. Figure 3 shows that both human and mouse TMSCs have the ability to form colonies.
FIG. 3.
Both hTMSCs- and mTMSCs-forming colonies. Both hTMSCs and mTMSCs were cultured in SCGM at 100 cells/well for 12 days. Crystal violet stains cell colonies. hTMSCs, human TMSCs; mTMSCs, mouse TMSCs; SCGM, stem cell growth medium.
Gonzalez43 isolated free-floating spheres from human TM cell primary cultures. Primary TM cells were isolated as described by Stamer30 and cultured in low glucose Dulbecco's modified Eagle's medium (DMEM) with l-glutamine and 110 mg/L sodium pyruvate containing 10% fetal bovine serum (FBS), 100 μM nonessential amino acids, and antibiotics at 37°C in a humidified atmosphere of 5% CO2. Free-floating spheres were maintained in StemSpan™ Serum-Free Expansion Medium and could be expanded in vitro for 3 months. Their proliferative potential was diminished after culturing for longer periods of time and cryopreservation.
Tay44 isolated TM cells following the method described by Tripathi45 and digested the TM tissue with 2 mg/mL type I collagenase in DMEM containing 10% FBS. Cells were cultured and passaged in low-glucose DMEM containing 10% FBS, 4 mM L-GlutaMAX™, 1 mM sodium pyruvate, 1% nonessential amino acids, and antibiotics. They found that cells seeded at low densities generated colonies after 14 days, indicating the presence of proliferative cells within the population. They named the cells as TM-derived mesenchymal stem cells (TM-MSC). They observed that 0.15% of seeded TM-MSC were able to form adherent colonies.
Nadri46 cultured the cells in low glucose DMEM supplemented with 20% serum and 200 ng/mL basic-FGF. They indicated that about 57%–76% of cells at different passages were able to form colonies.
Cell markers
Several groups have been exploring specific markers for TM stem cells, or the absence of specific markers for differentiated TM cells as to determine the stem cell properties of TMSCs (Table 1).
Table 1.
Markers of Trabecular Meshwork Stem Cells
| Studies | Detecting methods | Presence/high expression of cell markers | Absence/low expression of cell markers |
|---|---|---|---|
| Tay44 | Flow cytometry, immunostaining | CD73, CD90, CD105, CD145 | CD11b, CD34, CD45, CD79a |
| Du et al.39 | RT-PCR Immunofluorescent staining | ABCG2, Notch1, Muc1, AnkG, CD73, CD90, CD166, Bmi1 | AQP1, MGP, CHI3L1, Myocilin |
| Kelley et al.35 | Immunohistochemistry | HIMFG-1 | CHI3L1 |
| Gonzalez et al.43 | Microarray | Nestin, leukemia inhibitory factor, CHI3L1, matrix Gla protein | |
| McGowan et al.34 | Immunostaining | Oct-3/4, Wnt-1, Pax-6, Sox2 |
In 2006, Gonzalez43 performed microarray studies of neurospheres isolated from human TM tissue, identified high expression of two TM markers, MGP (matrix Gla protein), and CHI3L1(chitinase-3-like-1, also known as YKL-40, cartilage glycoprotein-39, HC-gp39), which indicated that these free-floating spheres originated from HTM cells. In addition, nestin, a marker for neural precursor cells, and leukemia inhibitory factor (LIF), a gene involved in maintenance of undifferentiated progenitor cells, were detected in high levels in the spheres, suggesting that these cells possess certain level of stemness.
Later on, other groups demonstrated that stem cells from the TM express stem cell markers, but lack markers of differentiated TM cells, Schlemm's canal endothelial (SCE) cells, fibroblasts, or hematopoietic lineage cells. In 2012, we identified that human TMSCs expressed stem cell markers ABCG2, Notch1, OCT-3/4, ankyrin G, and mucin 1, but not TM cell markers AQP1, MGP, CHI3L1, or TIMP3. Furthermore, passaged TMSCs were a homogeneous population with more than 95% of the cells positive to CD73, CD90, CD166, or Bmi1.39 Tay et al.44 identified that stem cells derived from the TM expressing markers of CD73, CD90, and CD105 are typically associated with mesenchymal stem cells. Thus, they named the cells as “TM-MSC.” Furthermore, these TM-MSC were identified as exhibiting low expression of CD11b (leukocyte marker), CD34 (hematopoietic stem cell marker), CD45 (pan-hematopoietic marker), and CD79a (B cell marker).
McGowan et al.34 compared unwounded with wounded corneas, which were the corneal rims, with the central corneal part removed by trephination for corneal transplant. They found that stem cell markers nestin, alkaline phosphatase, and telomerase were present in the TM and in the TM insert region of both unwounded and wounded corneas. Additional stem cell markers, Oct-3/4 and Wnt-1, were found in the same regions of wounded corneas. This study suggests the possibility that endogenous TM stem cells can exit the quiescent state to repopulate TM cells.
Label-retaining assays
Label-retaining techniques allow in vivo histological detection of stem cell-enriched cell population such as locating stem cell niches and identifying stem cell status of proliferation or quiescence. Usually a radiolabeled nucleoside analog such as bromodeoxyuridine (BrdU) is administered to animals for a certain time (pulse period) and then taken away for a prolonged period (chase period) before the tissues are examined. BrdU can be incorporated into newly synthesized DNA of replicating cells. The nuclear label is diluted with each cell division. Fast-cycling cells are constantly dividing. Consequently, the amount of original label steadily decreases to the point when the label is no longer detectable. Conversely, stem cells are slow-cycling in vivo and divide less frequently. After the chase period, they retain significant amount of the label, and are therefore identified as label-retaining cells (LCRs).47–49
Acott et al. used [3H]-thymidine pulse-chase protocol to examine cell proliferation in the TM after laser trabeculoplasty in human corneoscleral explant organ cultures.32 There was a 4-fold increase in cell division in laser-treated explant and nearly 60% of this cell division was localized to the anterior nonfiltering region of the TM where it inserted into the cornea beneath Schwalbe's line. Furthermore, 60% of these labeled cells moved to the burn sites by 14 days after laser treatment. This study suggested that the labeled cells served as a source for TM cell renewal and might be stem cells.
Braunger et al.50 identified stem cells in the anterior chamber angle of the monkey eyes by detecting BrdU long-term retention. They treated 4 monkeys with BrdU for 4 weeks and found that the number of BrdU-positive cells was higher at Schwalbe's line covering the peripheral end of Descemet's membrane than in regions of JCT, TM, and scleral spur. This study characterized in detail the specific in situ localization of the stem cell niche in TM region.
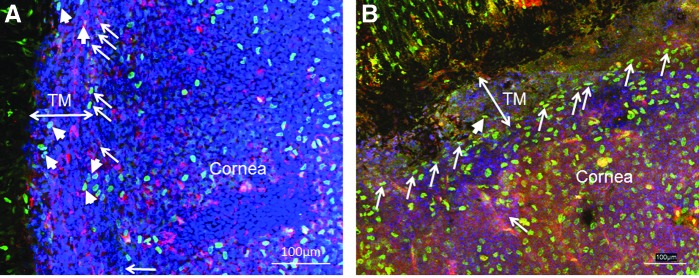
We peritoneally injected BrdU at 50 μg/g body weight into wild-type C57BL/6 newborn mice twice a day for 3 days and traced up to 8 weeks after the last injection. We observed that BrdU-positive cells were at both the TM and the insert region 12 days after injection, whereas BrdU label-retaining cells were limited at the insert region 8 weeks after injection (Fig 4). It confirms that there are label-retaining stem cells in the TM tissue and the insert region is the niche of TM stem cells in mouse eyes. This result confirms the finding by Acott et al32.
FIG. 4.
BrdU-label retaining cells in mouse TM. Newborn mice were given BrdU labeling by intraperitoneal injection. Twelve days after the last BrdU injection (A), BrdU+ (Green) cells are at both the TM region (arrowheads) and the insert region (arrows). Eight weeks after the last BrdU injection (B), BrdU+ (green) cells are limited at the insert region (arrows), and very few are at the TM region (arrowhead). AQP1 stains TM cells and corneal endothelial cells as red. DAPI stains nuclei as blue. Scale bars, 100 μm.
Multipotency
Multipotency is one of the characteristics of adult stem cells. Stem cells are capable of multilineage differentiation and functional reconstruction of damaged tissues in vivo.51 It has been proven that stem cells from the TM are multipotent with the ability to differentiate into various cell types. We have successfully induced TMSCs to differentiate to a variety of cell types that either express neural markers neurofilament, β-tubulin III, GFAP; or keratocyte-specific markers keratan sulfate and keratocan; or express adipocyte markers aP2 and leptin.39
Tay44 proved that stem cells derived from the TM, with gene expression patterns similar to mesenchymal stem cells derived from other tissues, are capable of differentiation into adipocytes, osteocytes and chondrocytes. Natri et al46 isolated a stem cell population from the TM region which are able to differentiate toward mesenchymal and photoreceptor lineages at different induction conditions. All these studies have confirmed that there are stem cells in the TM region and the TM stem cells are multipotent.
Other stem cell sources for TM regeneration
To utilize stem cells to maintain tissue homeostasis, tissue specific functional cells are needed. So far, several groups have been exploring the ability of TMSCs, adipose-derived stem cells (ADSCs), mesenchymal stem cells (MSCs) and induced pluripotent stem cells (iPS) to differentiate into functional TM cells.
TMSCs are multipotent with the potential to differentiate into phagocytic TM cells39 when cultured in the presence of aqueous humor or in the presence of relatively high concentration of serum. Bovine aqueous humor (AH) was collected from enucleated bovine eyes. TM cell differentiation was induced by culturing TMSCs in three different conditions: 50% AH in SCGM, 100% AH, or DMEM/F12 plus 10% FBS. After 10 days, TMSCs readily differentiated into TM cells with phagocytic function and expression of TM markers AQP1, CHI3L1, and TIMP3. This indicates that TMSCs show the most potential for TM regeneration and IOP reduction in glaucomatous eyes.
ADSCs can be readily isolated from human adipose tissue and have multilineage potential to be induced to differentiate into fat, bone, cartilage, and muscle under specific culture conditions.52 Two groups showed their results on successfully inducing ADSCs into TM cells at the ARVO meeting in 2015.53,54
Bone marrow-derived mesenchymal stem cells are multipotent and have been explored for TM regeneration.55 In the study by Manuguerra-Gagne et al,55 MSCs were injected into the anterior chamber of rats with laser-induced glaucoma. MSC injection induced a rapid return to normal IOP levels in experimental glaucomatous rats. Injected MSCs migrated to laser-treated region and restored TM structure one month after injection. Furthermore, injection of MSC-conditioned medium generated in low-oxygen condition also dramatically reduced IOP. This study indicates that both MSCs and MSC-conditioned medium are effective in TM regeneration. Further studies are needed for exploring regenerative mechanisms.
iPS cells are reprogrammed differentiated cells with characteristics similar to embryonic stem cells.56,57 iPS cells can be derived from patients' own dermal fibroblasts that are easily accessible, making iPS an ideal candidate for autologous cell transplant. iPS cells are able to differentiate into TM cells after culture on TM cell-derived extracellular matrix (ECM)58 or co-culture with TM cells.59 The induced TM cells from iPS fully restored intraocular pressure homeostatic function in an ex vivo perfused human organ culture model.58 After co-culturing with human TM cells for up to 21 days, mouse iPS cells became TM-like cells (iPSC-TM) resembling cultured human TM cells morphologically. They also began to express many markers of TM cells while ceasing to express pluripotency markers such as Nanog, Oct4, and Sox2. Functionally, these cells developed the ability to phagocytose particles. Finally, exposure to dexamethasone or phorbol 12-myristate acetate caused a distinct increase in the production and secretion of myocilin and matrix metalloproteinase-3 by this cell population, which is the classic behavior characteristic of TM cells.59
These studies indicate the feasibility of inducing other stem cell types, especially for autologous purpose, into TM cells for TM regeneration in glaucomatous eyes.
Animal Models for TMSC Transplantation Study
To test if stem cell transplantation could increase both cellularity and function of the TM in glaucomatous eyes, we need an appropriate animal model. The model should have the characteristics of (1) decreased TM cellularity and relatively normal skeletal structure of TM and SC, which can provide a scaffold for stem cells to attach to; (2) open anterior chamber angle, which will allow transplanted cells to reach the TM tissue; and (3) increased IOP that lasts for a relatively long period.
One extensively used experimental strategy to make glaucoma-like animal model is to increase IOP to a level that preferentially damage retinal ganglion cells. A translimbal laser photocoagulation model for glaucoma in rats was invented by Levkovitch-Verbin et al.,60 in which IOP was consistently elevated for 21 days. Since then, laser photocoagulation on the anterior segment of the eye has become one of the most used methods for IOP elevation in mice or rats.61–68 Almost all of those experiments resulted in IOP elevation by inducing episcleral vein cauterization, which can also cause nonvascular glaucoma, and severe peripheral anterior synechia, which is one of the main characteristics of primary angle-closure glaucoma, as keeping the normal morphology of anterior segment was not essential in those studies.
We69 recently developed a mouse glaucoma model induced by laser photocoagulation with low energy settings. We showed that IOP elevation lasted for 6 months with TM cellularity and ECM changes, open anterior chamber angle, loss of retinal ganglion cell axons, and decreased photopic negative response, which mirrored almost all the characteristics of human open-angle glaucoma. We also elucidated the pathological changes responsible for the change of outflow and the elevation of IOP. Previously, we discovered that adult corneal stromal stem cells have an ability to remodel tissue matrix in vivo70 and TM stem cells can selectively home to TM region in vivo.71 We hypothesize that homed TM stem cells, like corneal stromal stem cells, can remodel tissue matrix in vivo to promote TM extracellular matrix turnover and maintain TM homeostasis. We believe that this mouse glaucoma model could be applied to studies on stem cell-based therapies for glaucoma to explore if TMSCs can repopulate the TM cellularity and remodel the ECM structure of the conventional outflow pathway. It could also be applied to studies of regeneration of retinal ganglion cells and optic nerves in glaucomatous eyes.
Tg-MYOCY437H mouse model might also be appropriate for testing stem cell-based therapies. It is a transgenic mouse model of open-angle glaucoma induced by Y437H MYOC mutation.22,72 Tg-MYOCY437H mouse demonstrates significant elevation of IOP starting at 3 months of age with open iridocorneal angle and normal morphology of anterior chamber structure. No abnormalities in the iris, cornea, and lens were revealed in Tg-MYOCY437H mouse. Tg-MYOCY437H mouse also presents retinal ganglion cell death and axonal degeneration, which closely resembles the phenotypes seen in patients with primary open-angle glaucoma (POAG) caused by the Y437H MYOC mutation. Significant loss of TM cells was demonstrated in 12-month-old Tg-MYOCY437H mice, which is associated with endoplasmic reticulum stress-induced apoptotic pathway.
Active transforming growth factor-β2 (TGF-β2) is marketly increased in the aqueous humor of eyes with high IOP in POAG patients.73 Connective tissue growth factor (CTGF) is a TGF-β2 target gene with high constitutive TM expression.74 Junglas and the team74 induced a mouse glaucoma model with IOP elevation by either adenoviral-mediated or transgenic CTGF overexpression in the mouse eyes. There is increase of actin stress fibers in the TM cells and increase of TM fibronectin and α-SMA in the TM of the CTGF-overexpressing glaucoma mice. This might be another appropriate animal model for studying stem cell-based therapies.
Stem Cell Transplantation into TM Region
To verify if TMSCs have the ability to home to TM and survive after transplantation, we labeled human TMSCs and fibroblasts with fluorescent membrane dye DiO and injected them into normal mouse anterior chambers.71 We found that injected human TMSCs localized primarily in the TM, remaining viable in the tissue at least 4 months. Within 1 week, some of the injected TMSCs began to express TM cell marker CHI3L1. Meanwhile, fibroblasts injected into mouse anterior chamber distributed in the corneal endothelium, lens epithelium, iris, and TM without expression of CHI3L1. Little apoptosis was detected in injected TM tissue and IOP was not elevated during the experiment. We did not detect any CD45-positive cells after TMSC transplantation. Together with our previous study, we proved that (1) TMSC can be isolated from TM and expanded in vitro, (2) TMSC can home to TM and differentiate into TM cells in vivo, and (3) TMSC anterior chamber transplantation does not cause inflammation. All of these are the essential requirements for using TMSC transplantation to repopulate TM cells and restore TM function in glaucomatous eyes. Transplanting of TMSCs into the abovementioned animal glaucoma models is an essential step for verifying the feasibility of stem cell-based therapy for glaucoma, which is ongoing.
Summary
POAG is one of the most prevalent types of glaucoma and presents as elevated IOP, retinal ganglion cell death, and axonal degeneration. Decreased cellularity in TM, which is demonstrated in glaucomatous eyes, is thought to be associated with IOP elevation, and thus, loss of retinal ganglion cells. TMSCs have been identified in vivo, isolated in vitro, and characterized as capable of regenerating TM cells and homing to the TM region after anterior chamber transplantation. Appropriate animal models, which mirror the main phenotypes of POAG, are currently available. Now, it is possible to verify if stem cell-based therapy is an applicable strategy for treating glaucoma by evaluating the ability of TMSCs and other types of stem cells such as ADSCs and iPS cells to repopulate TM cells and restore TM function in these animal models.
Acknowledgments
This work was supported by NIH grants EY025643 (Y.D.) and P30-EY008098; BrightFocus Foundation (Y.D.); Eye and Ear Foundation (Pittsburgh, PA); Research to Prevent Blindness; and an anonymous philanthropic donation (Y.D.), University of Pittsburgh Summer Premedical Academic Enrichment Program (SPAEP) (A.W.).
Author Disclosure Statement
All authors declare that they have no conflicts of interest.
References
- 1.Fautsch M.P., and Johnson D.H. Aqueous humor outflow: what do we know? Where will it lead us? Invest. Ophthalmol. Vis. Sci. 47:4181–4187, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinreb R.N., Toris C.B., Gabelt B.T., Lindsey J.D., and Kaufman P.L. Effects of prostaglandins on the aqueous humor outflow pathways. Sur. Ophthalmol. 47 Suppl 1:S53–S64, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Roy Chowdhury U., Hann C.R., Stamer W.D., and Fautsch M.P. Aqueous humor outflow: dynamics and disease. Invest. Ophthalmol. Vis. Sci. 56:2993–3003, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goel M., Picciani R.G., Lee R.K., and Bhattacharya S.K. Aqueous humor dynamics: a review. Open Ophthalmol. J. 4:52–59, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Epstein D.L., and Rohen J.W. Morphology of the trabecular meshwork and inner-wall endothelium after cationized ferritin perfusion in the monkey eye. Invest. Ophthalmol. Vis. Sci. 32:160–171, 1991 [PubMed] [Google Scholar]
- 6.Sit A.J., Coloma F.M., Ethier C.R., and Johnson M. Factors affecting the pores of the inner wall endothelium of Schlemm's canal. Invest. Ophthalmol. Vis. Sci. 38:1517–1525, 1997 [PubMed] [Google Scholar]
- 7.Grant W.M. Clinical measurements of aqueous outflow. Am. J. Ophthalmol. 34:1603–1605, 1951 [PubMed] [Google Scholar]
- 8.Johnson M., Chan D., Read A.T., Christensen C., Sit A., and Ethier C.R. The pore density in the inner wall endothelium of Schlemm's canal of glaucomatous eyes. Invest. Ophthalmol. Vis. Sci. 43:2950–2955, 2002 [PubMed] [Google Scholar]
- 9.Alvarado J., Murphy C., and Juster R. Trabecular meshwork cellularity in primary open-angle glaucoma and nonglaucomatous normals. Ophthalmology. 91:564–579, 1984 [DOI] [PubMed] [Google Scholar]
- 10.Alvarado J., Murphy C., Polansky J., and Juster R. Age-related changes in trabecular meshwork cellularity. Invest. Ophthalmol. Vis. Sci. 21:714–727, 1981 [PubMed] [Google Scholar]
- 11.Tripathi R.C. Pathologic anatomy in the outflow pathway of aqueous humour in chronic simple glaucoma. Exp. Eye Res. 25 Suppl:403–407, 1977 [DOI] [PubMed] [Google Scholar]
- 12.Lutjen-Drecoll E. Morphological changes in glaucomatous eyes and the role of TGFbeta2 for the pathogenesis of the disease. Exp. Eye Res. 81:1–4, 2005 [DOI] [PubMed] [Google Scholar]
- 13.He Y., Leung K.W., Zhang Y.H., et al. . Mitochondrial complex I defect induces ROS release and degeneration in trabecular meshwork cells of POAG patients: protection by antioxidants. Invest. Ophthalmol. Vis. Sci. 49:1447–1458, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Liton P.B., Challa P., Stinnett S., Luna C., Epstein D.L., and Gonzalez P. Cellular senescence in the glaucomatous outflow pathway. Exp. Gerontol. 40:745–748, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baleriola J., Garcia-Feijoo J., Martinez-de-la-Casa J.M., Fernandez-Cruz A., de la Rosa E.J., and Fernandez-Durango R. Apoptosis in the trabecular meshwork of glaucomatous patients. Mol. Vis. 14:1513–1516, 2008 [PMC free article] [PubMed] [Google Scholar]
- 16.Chatterhee A., Jin D.J., Kang M.H., Haddadin R., Villarreal G., Toteberg-Harms M., Swaminathan S.S., Rhee D.J. The role of SPARC in trabecular meshwork extracellular matrix turnover and IOP regulation. GLaucoma Today. 12–16, 2012 [Google Scholar]
- 17.Clark A.F., Brotchie D., Read A.T., et al. . Dexamethasone alters F-actin architecture and promotes cross-linked actin network formation in human trabecular meshwork tissue. Cell Motil. Cytoskeleton. 60:83–95, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Read A.T., Chan D.W., and Ethier C.R. Actin structure in the outflow tract of normal and glaucomatous eyes. Exp. Eye Res. 82:974–985, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Hoare M.J., Grierson I., Brotchie D., Pollock N., Cracknell K., and Clark A.F. Cross-linked actin networks (CLANs) in the trabecular meshwork of the normal and glaucomatous human eye in situ. Invest. Ophthalmol. Vis. Sci. 50:1255–1263, 2009 [DOI] [PubMed] [Google Scholar]
- 20.H Gong S.D. The histopathological changes in the trabecular outflow pathway and their possible effects on aqueous outflow in eyes with primary open-angle glaucoma. In: KPaS J. (ed), Glaucoma Research and Clinical Advances 2016–2018. Amsterdam, The Netherlands: Kugler; 2016:17–40 [Google Scholar]
- 21.Shepard A.R., Jacobson N., Millar J.C., et al. . Glaucoma-causing myocilin mutants require the Peroxisomal targeting signal-1 receptor (PTS1R) to elevate intraocular pressure. Hum. Mol. Genet. 16:609–617, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Zode G.S., Kuehn M.H., Nishimura D.Y., et al. . Reduction of ER stress via a chemical chaperone prevents disease phenotypes in a mouse model of primary open angle glaucoma. J. Clin. Invest. 121:3542–3553, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buller C., Johnson D.H., and Tschumper R.C. Human trabecular meshwork phagocytosis. Observations in an organ culture system. Investigative ophthalmology & visual science 1990;31:2156–2163 [PubMed] [Google Scholar]
- 24.Keller K.E., Aga M., Bradley J.M., Kelley M.J., and Acott T.S. Extracellular matrix turnover and outflow resistance. Experimental eye research 2009;88:676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Buskirk E.M. The anatomy of the limbus. Eye. 3:101–108, 1989 [DOI] [PubMed] [Google Scholar]
- 26.Tamm E.R. The trabecular meshwork outflow pathways: structural and functional aspects. Exp. Eye Res. 88:648–655, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Acott T.S., and Kelley M.J. Extracellular matrix in the trabecular meshwork. Exp. Eye Res. 86:543–561, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ethier C.R. The inner wall of Schlemm's canal. Exp. Eye Res. 74:161–172, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Johnson M. What controls aqueous humour outflow resistance?'. Exp. Eye Res. 82:545–557, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stamer W.D., Seftor R.E., Snyder R.W., and Regan J.W. Cultured human trabecular meshwork cells express aquaporin-1 water channels. Curr. Eye Res. 14:1095–1100, 1995 [DOI] [PubMed] [Google Scholar]
- 31.Raviola G. Schwalbe line's cells: a new cell type in the trabecular meshwork of Macaca mulatta. Invest. Ophthalmol. Vis. Sci. 22:45–56, 1982 [PubMed] [Google Scholar]
- 32.Acott T.S., Samples J.R., Bradley J.M., Bacon D.R., Bylsma S.S., and Van Buskirk E.M. Trabecular repopulation by anterior trabecular meshwork cells after laser trabeculoplasty. Am. J. Ophthalmol. 107:1–6, 1989 [DOI] [PubMed] [Google Scholar]
- 33.Whikehart D.R., Parikh C.H., Vaughn A.V., Mishler K., and Edelhauser H.F. Evidence suggesting the existence of stem cells for the human corneal endothelium. Mol. Vis. 11:816–824, 2005 [PubMed] [Google Scholar]
- 34.McGowan S.L., Edelhauser H.F., Pfister R.R., and Whikehart D.R. Stem cell markers in the human posterior limbus and corneal endothelium of unwounded and wounded corneas. Mol. Vis. 13:1984–2000, 2007 [PubMed] [Google Scholar]
- 35.Kelley M.J., Rose A.Y., Keller K.E., Hessle H., Samples J.R., and Acott T.S. Stem cells in the trabecular meshwork: present and future promises. Exp. Eye Res. 88:747–751, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nemeth K., and Karpati S. Identifying the stem cell. J. Invest. Dermatol. 134:e26, 2014 [DOI] [PubMed] [Google Scholar]
- 37.Terunuma A.J.K., and Kapoor V. Side population keratinocytes resembling bone marrow side population stem cells are distinct from label-retaining keratinocyte stem cells. J. Invest. Dermatol. 121:1095–1103, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Goodell M.A., Brose K., Paradis G., Conner A.S., and Mulligan R.C. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J. Exp. Med. 183:1797–1806, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Du Y., Roh D.S., Mann M.M., Funderburgh M.L., Funderburgh J.L., and Schuman J.S. Multipotent stem cells from trabecular meshwork become phagocytic TM cells. Invest. Ophthalmol. Vis. Sci. 53:1566–1575, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yun H., Schuman J., and Du Y. Trabecular meshwork stem cells. In: Pebay A., ed. Regenerative Biology of the Eye. New York: Springer; 2014; p. 203–214 [Google Scholar]
- 41.Du Y., Funderburgh M.L., Mann M.M., SundarRaj N., and Funderburgh J.L. Multipotent stem cells in human corneal stroma. Stem Cells. 23:1266–1275, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Telford W.G., Bradford J., Godfrey W., Robey R.W., and Bates S.E. Side population analysis using a violet-excited cell-permeable DNA binding dye. Stem Cells. 25:1029–1036, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Gonzalez P., Epstein D.L., Luna C., and Liton P.B. Characterization of free-floating spheres from human trabecular meshwork (HTM) cell culture in vitro. Exp. Eye Res. 82:959–967, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tay C.Y., Sathiyanathan P., Chu S.W., Stanton L.W., and Wong T.T. Identification and characterization of mesenchymal stem cells derived from the trabecular meshwork of the human eye. Stem Cells Dev. 21:1381–1390, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Tripathi R.C., and Tripathi B.J. Human trabecular endothelium, corneal endothelium, keratocytes, and scleral fibroblasts in primary cell culture. A comparative study of growth characteristics, morphology, and phagocytic activity by light and scanning electron microscopy. Exp. Eye Res. 35:611–624, 1982 [DOI] [PubMed] [Google Scholar]
- 46.Nadri S., Yazdani S., Arefian E., et al. . Mesenchymal stem cells from trabecular meshwork become photoreceptor-like cells on amniotic membrane. Neurosci. Lett. 541:43–48, 2013 [DOI] [PubMed] [Google Scholar]
- 47.Krisztian Nemeth S.K. Identifying the stem cell. J. Invest. Dermatol. 134:e26, 2014 [DOI] [PubMed] [Google Scholar]
- 48.Cotsarelis G., Cheng S.Z., Dong G., Sun T.T., and Lavker R.M. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 57:201–209, 1989 [DOI] [PubMed] [Google Scholar]
- 49.Hsu Y.C., and Fuchs E. A family business: stem cell progeny join the niche to regulate homeostasis. Nature reviews Molecular cell biology 2012;13:103–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Braunger B.M., Ademoglu B., Koschade S.E., et al. . Identification of adult stem cells in Schwalbe's line region of the primate eye. Invest. Ophthalmol. Vis. Sci. 55:7499–7507, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Verfaillie C.M. Adult stem cells: assessing the case for pluripotency. Trends Cell Biol. 12:502–508, 2002 [DOI] [PubMed] [Google Scholar]
- 52.Tholpady S.S., Llull R., Ogle R.C., Rubin J.P., Futrell J.W., and Katz A.J. Adipose tissue: stem cells and beyond. Clinics in plastic surgery 2006;33:55-62, vi [DOI] [PubMed] [Google Scholar]
- 53.Snider E., Pride C., Patil A., Stamer W.D., and Ethier C.R. Characterization of Mesenchymal Stem Cells vs. Trabecular Meshwork Cells. Invest Ophthalmol Vis Sci 2015;4825 [Google Scholar]
- 54.Zhou Y., Yun H., Yang E., Schuman J.S., and Du Y. Induction of Adipose-derived Stem Cells to Trabecular Meshwork Cells for Glaucoma. Invest Ophthalmol Vis Sci 2015;327926024110 [Google Scholar]
- 55.Manuguerra-Gagne R., Boulos P.R., Ammar A., et al. . Transplantation of mesenchymal stem cells promotes tissue regeneration in a glaucoma model through laser-induced paracrine factor secretion and progenitor cell recruitment. Stem cells 2013;31:1136–1148 [DOI] [PubMed] [Google Scholar]
- 56.Takahashi K., and Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663–676 [DOI] [PubMed] [Google Scholar]
- 57.Yu. J., Vodyanik M.A., Smuga-Otto K., et al. . Induced pluripotent stem cell lines derived from human somatic cells. Science 2007;318:1917–1920 [DOI] [PubMed] [Google Scholar]
- 58.Abu-Hassan D.W., Li X., Ryan E.I., Acott T.S., and Kelley M.J. Induced pluripotent stem cells restore function in a human cell loss model of open-angle glaucoma. Stem cells 2015;33:751–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ding Q.J., Zhu W., Cook A.C., Anfinson K.R., Tucker B.A., and Kuehn M.H. Induction of trabecular meshwork cells from induced pluripotent stem cells. Investigative ophthalmology & visual science 2014;55:7065–7072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Levkovitch-Verbin H., Quigley H.A., Martin K.R., Valenta D., Baumrind L.A., and Pease M.E. Translimbal laser photocoagulation to the trabecular meshwork as a model of glaucoma in rats. Invest. Ophthalmol. Vis. Sci. 43:402–410, 2002 [PubMed] [Google Scholar]
- 61.Aihara M., Lindsey J.D., and Weinreb R.N. Experimental mouse ocular hypertension: establishment of the model. Invest. Ophthalmol. Vis. Sci. 44:4314–4320, 2003 [DOI] [PubMed] [Google Scholar]
- 62.Biermann J., van Oterendorp C., Stoykow C., et al. . Evaluation of intraocular pressure elevation in a modified laser-induced glaucoma rat model. Exp. Eye Res. 104:7–14, 2012 [DOI] [PubMed] [Google Scholar]
- 63.Fu C.T., and Sretavan D. Laser-induced ocular hypertension in albino CD-1 mice. Invest. Ophthalmol. Vis. Sci. 51:980–990, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grozdanic S.D., Betts D.M., Sakaguchi D.S., Allbaugh R.A., Kwon Y.H., and Kardon R.H. Laser-induced mouse model of chronic ocular hypertension. Invest. Ophthalmol. Vis. Sci. 44:4337–4346, 2003 [DOI] [PubMed] [Google Scholar]
- 65.Soto I., Oglesby E., Buckingham B.P., et al. . Retinal ganglion cells downregulate gene expression and lose their axons within the optic nerve head in a mouse glaucoma model. J. Neurosci. 28:548–561, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kwong J.M., Vo N., Quan A., et al. . The dark phase intraocular pressure elevation and retinal ganglion cell degeneration in a rat model of experimental glaucoma. Exp. Eye Res. 112:21–28, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mimura T., and Joyce N.C. Replication competence and senescence in central and peripheral human corneal endothelium. Invest. Ophthalmol. Vis. Sci. 47:1387–1396, 2006 [DOI] [PubMed] [Google Scholar]
- 68.Tsuruga H., Murata H., Araie M., and Aihara M. A model for the easy assessment of pressure-dependent damage to retinal ganglion cells using cyan fluorescent protein-expressing transgenic mice. Mol. Vis. 18:2468–2478, 2012 [PMC free article] [PubMed] [Google Scholar]
- 69.Yun H., Lathrop K.L., Yang E., et al. . A laser-induced mouse model with long-term intraocular pressure elevation. PloS One. 9:e107446, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Du Y., Carlson E.C., Funderburgh M.L., et al. . Stem cell therapy restores transparency to defective murine corneas. Stem Cells. 27:1635–1642, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Du Y., Yun H., Yang E., and Schuman J.S. Stem cells from trabecular meshwork home to TM tissue in vivo. Invest. Ophthalmol. Vis. Sci. 54:1450–1459, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zode G.S., Bugge K.E., Mohan K., et al. . Topical ocular sodium 4-phenylbutyrate rescues glaucoma in a myocilin mouse model of primary open-angle glaucoma. Invest. Ophthalmol. Vis. Sci. 53:1557–1565, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fuchshofer R., and Tamm E.R. The role of TGF-beta in the pathogenesis of primary open-angle glaucoma. Cell Tissue Res. 347:279–290, 2012 [DOI] [PubMed] [Google Scholar]
- 74.Junglas B., Kuespert S., Seleem A.A., et al. . Connective tissue growth factor causes glaucoma by modifying the actin cytoskeleton of the trabecular meshwork. Am. J. Pathol. 180:2386–2403, 2012 [DOI] [PubMed] [Google Scholar]