Abstract
Cerebral stroke is the leading cause of death and permanent disability in elderly persons. The impaired glucose and oxygen transport to the brain during ischemia causes bioenergetic failure, leading to oxidative stress, inflammation, blood-brain barrier dysfunction, and eventually cell death. However, the development of effective therapies against stroke has been hampered by insufficient oral absorption of pharmaceuticals and subsequent delivery to the brain. Nanotechnology has emerged as a new method of treating cerebral diseases, with the potential to fundamentally change currently available therapeutic approaches using compounds with low bioavailability. This perspective review provides an overview of the therapeutic potential of oral nanomedicines for stroke, focusing on novel natural product-loaded delivery system with potent antioxidant and anti-inflammatory effects.
Key Words: : antioxidant activity, bioflavonoids, cerebral ischemia, dietary herbal supplement, nanomedicine, natural product, neuroprotection, stroke
Introduction
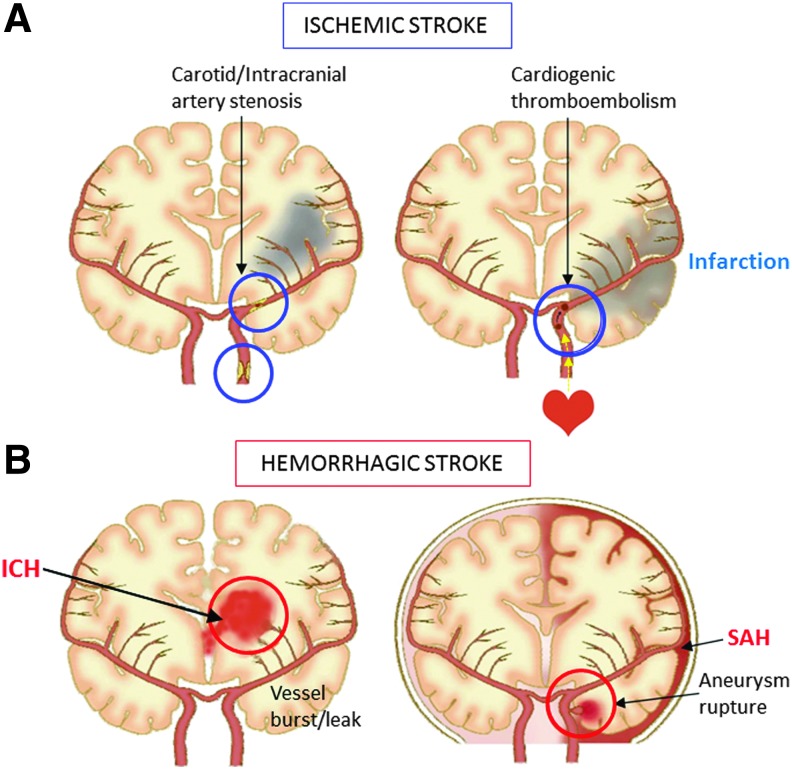
Stroke, also known as “brain attack,” is the leading cause of death and permanent disability in elderly persons.1 Stroke is caused by the interruption of blood supply to the brain, either by clots or vessel rupture, leading to the loss of brain functions. There are two main types of stroke: ischemic (infarction) and hemorrhagic (intracerebral/parenchymal or subarachnoid hemorrhage), which affect the brain in different ways and can have different causes (Fig. 1). Cerebral infarction accounts for nearly 80% of stroke patient cases.2 The most effective treatment of cerebral infarction is to restore the blood supply by recanalization of the occluded arteries, either by thrombolytic agents or endovascular therapy, immediately after arterial occlusion.3 However, recanalization therapy can also aggravate brain damage, referred to as ischemia-reperfusion injury, resulting in poor clinical outcomes due to fatal edema (brain herniation) or intracranial hemorrhage after thrombolysis.4 Although oral anticoagulant/antiplatelet drugs are currently available treatment options to prevent stroke recurrence, protective and preventive therapies to combat ischemia-reperfusion-induced neuronal damage have yet to be discovered.
FIG. 1.
Illustration of stroke subtypes. (A) Ischemic stroke occurs as a result of an obstruction within a blood vessel supplying blood to an area of the brain; usually by a clot from a small vessel lesion, intracranial/carotid stenosis or cardioembolic origin. (B) Hemorrhagic stroke occurs when an artery in the brain leaks or bursts (ICH), or an aneurysm ruptures (SAH). ICH, intracerebral hemorrhage; SAH, subarachnoid hemorrhage. Color images available online at www.liebertpub.com/jmf
Under ischemic conditions, reduced glucose and oxygen transport to the brain cause bioenergetic failure, leading to oxidative stress, inflammation, blood-brain barrier (BBB) dysfunction, and eventually cell death.5 Free radicals, of which reactive oxygen species (ROS) and reactive nitrogen species are two major classes, are important cytotoxic molecules that play a role in these processes. Ischemia and reperfusion insults induce the accumulation of excessive ROS, resulting in oxidative damage to brain tissue6 and edema formation.7 Nitric oxide (NO) is an important regulatory molecule in host defense that plays a vital role in the central nervous system (CNS). Studies have suggested that large amounts of NO produced by inducible nitric oxide synthase (iNOs) are toxic to the injured brain and contribute to the late stage of cerebral ischemia.8 Oxidative injury is further worsened when blood flow is restored during reperfusion.9
Taken together, the research indicates that it is necessary to employ exogenous antioxidant drugs and free radical scavengers to counter cerebral ischemia and reperfusion-induced oxidative stress. At present, there are few effective neuroprotective agents that may be used for treating ischemic stroke. Antioxidant therapies such as edaravone, a low molecular weight ROS scavenger, have been proposed to prevent oxidative stress in acute ischemic stroke patients, with the potential to improve clinical outcomes.10 Edaravone is the first intravenous neuroprotective agent approved for clinical use in Japan; however, its systemic application has been limited by its low tissue accessibility, rapid renal clearance, and acute renal toxity in elderly patients or those with renal insufficiency,11,12 resulting in low accumulation of the ROS scavenger to the target brain lesion.
Therefore, it has become necessary to develop a system capable of providing an elevated pool of such antioxidants to the brain to completely protect neuronal cells against oxidative attack. Nanotechnology has emerged as a new means of treating cerebral diseases, with the potential to fundamentally change currently available therapeutic approaches.13,14 The use of nanotechnology with safe natural products is a rapidly developing field. Currently, nanotechnology brings multiple advantages to the delivery of natural compounds in the treatment of CNS diseases.15 In this review, we will address key aspects of emerging natural product-based oral nanomedicines for stroke prevention and treatment.
Natural Compounds for Disease Prevention Strategy
Natural compounds, also called natural products, are complex chemical molecules found in plants and microorganisms. It is reported that such natural product-centered antioxidant supplements may be an excellent prevention strategy for various diseases such as cancer, infection, and stroke.15–17 Research on the mechanisms of actions of natural products containing minerals, flavonoids, and polyphenols has focused on their antioxidant activities. This section will focus on natural compounds with antioxidant and anti-inflammatory effects that have been studied in relation to ischemic stroke treatment.
Validity of Oral Administration to Stroke Patients
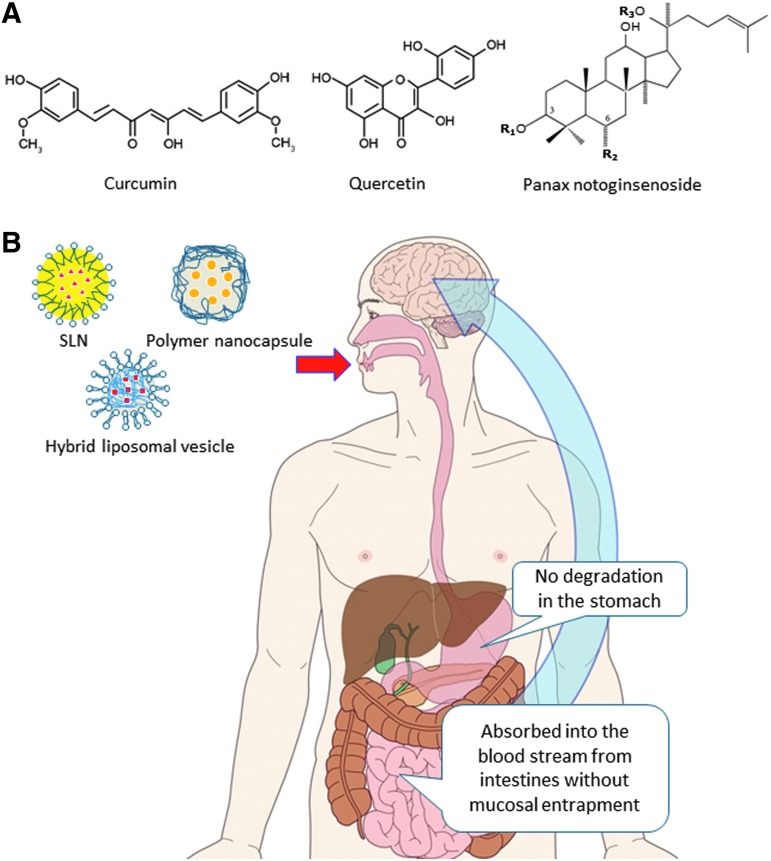
Optimal duration (i.e., therapeutic window) and route of administration for a given drug depends on the individual pharmacokinetic properties of the neuroprotective compounds, on any adverse effects of the drugs, and on the nature of the insult that gave rise to the stroke.18 In clinical practice, it is common to withhold food, drink, and oral medications until speech and swallowing have been adequately assessed by a trained speech therapist to prevent complications such as aspiration pneumonia. It would therefore be ideal if the treatment could be administered initially in an intravenous preparation, and later changed to an oral formulation as soon as the patient has adequate motor control. In fact, there are subgroups of patients who can be identified as being at higher risk of further ischemic events and who potentially could benefit from longer neuroprotection.18 For prolonged treatment to be practical, it would be ideal to have an orally active form with good bioavailability characteristics (Fig. 2) and have an acceptable side effect profile.19
FIG. 2.
Concept of oral nanotherapy using natural compounds for stroke treatment. (A) Chemical structures of curcumin, quercetin, and Panax notoginsenoside. (B) Orally administered nano-units containing natural products show lower degradation in the stomach, are absorbed in the bloodstream from the intestine, and are more efficiently delivered to the brain while preserving the active drug form. SLN, solid lipid nanoparticle. Color images available online at www.liebertpub.com/jmf
Natural Products as Therapeutic Candidates for Stroke
Animal experiments suggest that the biological functions of many herbal formulations derived from natural products are due to their protective effects against oxidation, which is proposed as one of the therapeutic mechanisms of ischemic stroke.20 Furthermore, recent reports indicate that dietary components not only evoke genetic, but also epigenetic components to compensate stroke and related pathologies.17
Among currently available natural products, curcumin, quercetin, and Panax notoginseng are promising therapeutic candidates for stroke prevention (Table 1). The beneficial effects of these drugs are mainly based on their antioxidant and other pleiotropic properties.20
Table 1.
Summary of Oral Nanomedicines for Their Therapeutic Potential in Stroke Prevention
| Natural compound | Partition coefficient (log P) | Topological polar surface area (Å2) | Nanodevice | Route | Dose | Advantage | References |
|---|---|---|---|---|---|---|---|
| Curcumin | 4.12 | 93.06 | Solid lipid nanoparticle | Oral | 50 mg/kg | Improves brain bioavailability and functional recovery | Kakkar et al.39 |
| Quercetin | 2.16 | 127.45 | PLGA nanoparticle | Oral | 2.7 mg/kg | Prevents hippocampal neuronal damage | Ghosh et al.41 |
| Panax notoginsenide | R1, 0.03 | 296 | PEG-PLGA-based core-shell hybrid liposomal vesicles | Oral | 30 mg/kg | Improves brain bioavailability and reduces cerebral edema and infarct volume | Zhang et al.36 |
| Rg1, 0.80 | 239 | ||||||
| Rb1, −0.56 | 377 | ||||||
| N/A | MSC-derived exosomes | IV | 100 μg in 0.5 mL PBS | Improves functional recovery and enhances neurite remodeling, neurogenesis, and angiogenesis | Xin et al.46 |
Log P and surface area values are obtained from sources: http://chemicalize.org and http://pubchem.ncbi.nlm.nih.gov/compound.
IV, intravenous; MSC, mesenchymal stromal cell; N/A, not applicable; PBS, phosphate-buffered saline; PEG, polyethylene glycol;
PLGA, polylactide-co-glycolide acid.
Curcumin is derived from the roots of turmeric (Curcuma longa). Curcumin treatment provides vascular protective effects in persons at risk for stroke.21 The stroke preventive properties of curcumin can be attributed to the following: (1) neuroprotection via free radical scavenging activity, and inhibition of nitric oxide synthase and lipid peroxidation; (2) anti-inflammatory property by inhibiting interleukin (IL)-1, IL-8, and tumor necrosis factor-α production; (3) anti-lipidemic property by lowering cholesterol and boosting high-density lipoproteins; and (4) antiaggregation property by inhibiting platelet aggregation.22,23
Quercetin is a bioflavonoid polyphenolic antioxidant found in white fruit and vegetables such as apples and pears.24 Quercetin is proposed to prevent the oxidation of low-density lipoproteins by scavenging free radicals and chelating transition metal ions. These antioxidant properties of quercetin might be beneficial in preventing stroke.16
Research on the mechanisms of action of natural products from traditional Chinese medicine has focused on their antioxidant activities.25 P. notoginseng (San Qi) is derived from the root of the traditional Chinese herb Tienchi Ginseng, which exhibits various activities, including modulating vascular tone, prevention of hemostasis, and alleviation of pain.26 Panax notoginsenoside (PNS) is the major active component of P. notoginseng, and includes ginsenoside Rg1 (20.5%), ginsenoside Rb1 (29.9%), ginsenoside Rd (8.0%), and notoginsenoside R1 (2.7%).27 In addition to its antioxidant properties,28 pleiotropic physiological effects of PNS including anti-inflammatory action, vasodilator effect, and protective effect on microcirculatory disturbance have also been reported,29,30 which indicates its pharmacological utility.
Nanotechnology for Drug Delivery to the Brain
The major problem with the use of natural products in stroke treatment is their low bioavailability, which has limited their effectiveness in clinical trials.15 Although the natural compounds described above are known as pleiotropic antioxidants for their multiple medicinal benefits, they cannot cross the BBB when administered orally due to water insolubility and low bioavailability, a major stumbling block in CNS therapeutics, and are limited by their instability at physiological pH, rapid metabolism, and rapid systemic elimination.31 Therefore, this has necessitated the development of an effective delivery system to provide an elevated pool of such antioxidants in the brain to protect neuronal cells against oxidative attack.
Colloidal particles ranging in size from 10 to 1000 nm are known as nanoparticles.32 They are manufactured from synthetic or natural polymers and are ideally suited to optimize drug delivery and reduce toxicity. However, it should be noted that most nanomedicines are not used as orally administered drugs for cerebral diseases because nanoparticles between the sizes of 10 and 100 nm are not absorbed via the gastrointestinal tract.33 The development of an orally administered drug with nanomedicine-like characteristics that is absorbed in the blood would be an ideal oral medication for treating stroke. The successful implementation of nanoparticles for drug delivery depends on several factors: the ability to penetrate through several anatomical barriers including the BBB, sustained release of contents, and stability in the nanometer size. This review introduces emerging strategies of neuroregenerative nanoparticle-mediated delivery of natural products, which could strengthen the therapeutic utility for stroke therapy.
Nano-Carriers for CNS Drug Delivery
Polymeric nanoparticles in CNS-targeted drug delivery provide better penetration of therapeutic agents and have a reduced risk in comparison to existing therapies.34 Coating the nanoparticle surface with a hydrophilic polymer, such as polyethylene glycol (PEG) and/or polylactide-co-glycolide acid (PLGA), has been shown to prolong circulation half-life when the polymers are covalently bound on the nanoparticle surface rather than adsorbed.15 Both PEG and PLGA are accepted as effective drug carriers, and current technological advantages of nanoparticles include a long shelf-life, increased carrier capacity and feasibility via various routes of administration including the oral route.35
Liposomes have long been considered as useful carriers for the delivery of therapeutic agents because of their unique properties such as biocompatibility, biodegradability, variable particle size, surface charge, and membrane fluidity. Because liposomes can be unstable, leading to leakage of the entrapped drug after administration, modifications of the liposomal surface with a single layer of hydrophilic polymers or polyelectrolytes, or varying the size of liposomes have been tested in animal experiments. Zhang et al.36 recently introduced a novel liposomal system encapsulating methyl ether PEG-PLGA-based nanoparticles. These so-called core-shell hybrid liposomal vesicles (HLV), increase entrapment efficiency and retard destruction of drug components; the resulting vesicles showed suitable architecture and properties for oral application.
Solid lipid nanoparticles (SLNs) are lipophilic colloidal carriers developed as an alternative system to the existing traditional carriers (emulsions, liposomes, and polymeric nanoparticles).15,37 They are a new generation of submicron-sized lipid emulsions where the liquid lipid (oil) has been substituted by a solid lipid with an ability to penetrate the BBB and a therapeutic potential for CNS disorders.32 As a drug carrier, SLNs offer unique properties such as small size, large surface area, and high loading properties, and are attractive for their potential to improve the performance of oral pharmaceuticals against stroke.38
Curcumin-Loaded SLNs
Free curcumin undergoes significant metabolic transformation, resulting in poor oral bioavailability of <1%. Curcumin embedded within a solid lipid matrix of SLNs is not only protected against enzymatic degradation during absorption, but also attains a long circulation time and reduced clearance in vivo following absorption.39 Furthermore, passive uptake of SLNs by pino- and endocytosis, and active uptake by brain endothelial cells, has also been purported.40
In a recent report, Kakkar et al.39 proposed the use of curcumin (25–50 mg/kg)-loaded solid lipid nanoparticles (C-SLNs; average particle size 134.6 ± 15.4 nm) with an ability to penetrate the BBB and a potential for treating CNS disorders. They showed the neuroprotective potential of oral C-SLNs (treatment for 5 days before and 3 days subsequent to bilateral common carotid artery occlusion) in global cerebral ischemia in rats by achieving improved systemic and cerebral bioavailability of curcumin. Notably, the oral administration of C-SLNs improved blood and brain bioavailability, analyzed with gamma centigram, 4 h after drug application by about 8 and 16 times compared to that of solubilized curcumin (without nano-carriers). Furthermore, animals treated with oral C-SLNs showed improved cognitive function (90%) and neurological motor scores (79%) following ischemia-reperfusion injury as compared to rats treated with solubilized curcumin. Levels of superoxide dismutase, catalase, glutathione, and mitochondrial complex enzyme activities were increased, whereas lipid peroxidation, nitrite, and acetylcholinesterase levels decreased after C-SLNs administration, all of which were restored to levels equivalent to sham control values. These data support the efficacy of C-SLNs against cerebral ischemic insult achieved by packaging curcumin into a suitable carrier system through the oral route.
PLGA-Encapsulated Quercetin
Ghosh et al.41 evaluated the therapeutic efficacy of nanoencapsulated quercetin in combating ischemia-reperfusion-induced neuronal damage in rats by occlusion of the common carotid arteries. PLGA-encapsulated quercetin (2.7 mg/kg; 20–50 nm) was orally administered to rats 2 h before ischemic insult and until post-treatment day 3. In that study, nanoparticulated quercetin treatment before ischemic insult imparted absolute protection of enzymatic antioxidant status and controlled the osmolality in different brain regions. Quercetin also exerted neuroprotective properties of nano-encapsulated quercetin by downregulating iNOS and caspase-3 activities and improved counts of pyramidal neurons in the hippocampal CA1 and CA3 subfields were observed even 3 days after ischemia-reperfusion.41 More interestingly, no animals died in this treatment group after 24 h of reperfusion, as compared to 50% mortality in the control group.
PNS-Loaded Core-Shell HLV
PNS is poorly absorbed when administrated orally.42 Minimal amounts of major PNS components (ginsenoside R1 and Rb1) can be absorbed from the digestive tract by oral administration to rats. It is also reported that the amount of Rg1 (20.46%) absorbed via oral administration was within 1.9–20.0%.43
Zhang et al.36 developed a novel PNS-loaded core-shell HLV (PNS-HLV; 337.8 ± 40.2 nm) to resolve the restricted bioavailability and retard the release of the major components of PNS to a similar degree to enhance its protective effects on global cerebral ischemia/reperfusion in a rat model in vivo. The formation of the HLV and its preparation method, the application of water-in-oil-in-water double emulsion solvent evaporation method and thin-film hydration method, are designed according to the polarities of the main components of PNS.42,44 The encapsulated drugs in HLV are increased synchronously compared with other PNS preparations and a high concentration of drugs could be delivered into the circulation.
It was shown that treatment with oral PNS-HLV (30 mg/kg) for 10 days before the induction of ischemia attenuated the ischemia/reperfusion-induced brain infarction in comparison with PNS-solution, mPEG-PLGA nanoparticle-loaded with PNS, and PNS-loaded liposome-treated groups.36 As a result, orally administered PNS-HLV exhibited greater effectiveness for attenuation of brain damage and bioactivities and achieved higher potency than the free drug, conventional nanoparticles, and liposomes in vivo.
Other Drug Delivery Options
The route of delivery greatly affects a drug's bioavailability in the brain. Oral delivery is beneficial because it has high patient compliance; however, one of the main drawbacks with this approach is the extremely low drug bioavailability. Another route that has been incorporated using nanotechnology is intravenous or intranasal (topical) administration.15
Exosomes (<200 nm) released from diverse cell types in the extracellular fluid are nonimmunogenic and possess the ability to cross the BBB.45 Since exosomes carry biological information from their cells of origin, encapsulating or priming cells with potential therapeutic agents has been shown to improve cellular delivery through exosomes. Xin et al.46 suggested that intravenous administration of exosomes derived from mesenchymal stromal cells to a rat stroke model might improve functional recovery and enhance neurite remodeling, neurogenesis, and angiogenesis. With regard to nanoparticle-mediated delivery of natural products, curcumin-primed exosomes derived from brain endothelial cells have the potential to restore junction proteins and permeability of endothelial cells.46 The efficacy of curcumin-encapsulated exosomes in treating inflammatory cerebral diseases via an intranasal administration has also been demonstrated.47 These novel therapeutic options are applicable to treating acute stroke pathology where the oral route is unavailable.
Safety Issues
Toxicity, nonspecific uptake, and unwanted immune response to nanoparticles after systemic administration might hinder their progress in vivo.48 Although numerous investigators have claimed that nanoparticles composed of biocompatible polymers (PLGA and PEG), phospholipids (liposomes and micelles), and other materials are safe and show no toxicity to healthy intact cells, further studies are required.15 Targeted delivery may be a solution to reduce the side effects and limit potential toxicity. Another promising approach to increase the efficiency of nanotherapy is to develop a system for controlled release (e.g., “on–off” regulation),49 whereby the nanoparticle is dormant in a non-target tissue and is activated in the target area. The ultimate goal for a targeted drug delivery system is to create a specific drug-containing nano-carrier that enhances the delivery or uptake in a specific area of the brain, and thus improves treatment outcomes and reduces related adverse effects.
Concluding Remarks
Recent animal studies have suggested that novel natural product-loaded nanoparticle delivery systems can enhance drug bioavailability in the brain. One of the problems currently facing the continued development of oral nanomedicine delivery systems for stroke prevention and treatment is the transition from bench to bedside. Unfortunately, natural products processed as nanoparticles that have controlled release or targeted delivery properties have not reached clinical trials involving stroke patients. This is in part due to insufficient mechanistic information regarding the interaction between the nanosurface and biosurface (i.e., BBB), and insufficient knowledge about the fate of the nanoparticles once they enter the tissues and cells. Recently, epigenetic mechanisms of natural products (e.g., curcumin) to regulate the functional gene environment by regulating gene expression without altering the gene sequence or structure have been suggested,17 and might be useful for stroke protection and therapeutics by reversing erroneous epigenetic alterations or controlling normal epigenetic mechanisms. To solve the low drug bioavailability issue, the development or discovery of novel nanomedicines could lead to advances in the safe and effective treatment of cerebral stroke.
Acknowledgment
This work was partly supported by the Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science and Life Science Foundation of Japan.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Heron M: Deaths: Leading causes for 2011. Natl Vital Stat Rep 2015;64:1–96 [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, et al. : Heart disease and stroke statistics—2015 Update: A report from the American Heart Association. Circulation 2015;131:e29–e322 [DOI] [PubMed] [Google Scholar]
- 3.Grotta JC, Hacke W: Stroke neurologist's perspective on the new endovascular trials. Stroke 2015;46:1447–1452 [DOI] [PubMed] [Google Scholar]
- 4.Mullen MT, Pisapia JM, Tilwa S, et al. : Systematic review of outcome after ischemic stroke due to anterior circulation occlusion treated with intravenous, intra-arterial, or combined intravenous+intra-arterial thrombolysis. Stroke 2012;43:2350–2355 [DOI] [PubMed] [Google Scholar]
- 5.Crack PJ, Taylor JM: Reactive oxygen species and the modulation of stroke. Free Radic Biol Med 2005;38:1433–1444 [DOI] [PubMed] [Google Scholar]
- 6.Chan PH: Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab 2001;21:2–14 [DOI] [PubMed] [Google Scholar]
- 7.Heo JH, Han SW, Lee SK: Free radicals as triggers of brain edema formation after stroke. Free Radic Biol Med 2005;39:51–70 [DOI] [PubMed] [Google Scholar]
- 8.Nogawa S, Forster C, Zhang F, et al. : Interaction between inducible nitric oxide synthase and cyclooxygenase-2 after cerebral ischemia. Proc Natl Acad Sci U S A 1998;95:10966–10971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chrissobolis S, Faraci FM: The role of oxidative stress and NADPH oxidase in cerebrovascular disease. Trends Mol Med 2008;14:495–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakase T, Yoshioka S, Suzuki A: Free radical scavenger, edaravone, reduces the lesion size of lacunar infarction in human brain ischemic stroke. BMC Neurol 2011;11:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hishida A: Clinical analysis of 207 patients who developed renal disorders during or after treatment with edaravone reported during post-marketing surveillance. Clin Exp Nephrol 2007;11:292–296 [DOI] [PubMed] [Google Scholar]
- 12.Kamouchi M, Sakai H, Kiyohara Y, et al. : Acute kidney injury and edaravone in acute ischemic stroke: The Fukuoka stroke registry. J Stroke Cerebrovasc Dis 2013;22:e470–476 [DOI] [PubMed] [Google Scholar]
- 13.De Jong WH, Borm PJ: Drug delivery and nanoparticles: Applications and hazards. Int J Nanomedicine 2008;3:133–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srikanth M, Kessler JA: Nanotechnology-novel therapeutics for CNS disorders. Nat Rev Neurol 2012;8:307–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watkins R, Wu L, Zhang C, et al. : Natural product-based nanomedicine: Recent advances and issues. Int J Nanomedicine 2015;10:6055–6074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knekt P, Isotupa S, Rissanen H, et al. : Quercetin intake and the incidence of cerebrovascular disease. Eur J Clin Nutr 2000;54:415–417 [DOI] [PubMed] [Google Scholar]
- 17.Kalani A, Kamat PK, Kalani K, et al. : Epigenetic impact of curcumin on stroke prevention. Metab Brain Dis 2015;30:427–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dyker AG, Lees KR: Duration of neuroprotective treatment for ischemic stroke. Stroke 1998;29:535–542 [DOI] [PubMed] [Google Scholar]
- 19.Alavijeh MS, Chishty M, Qaiser MZ, et al. : Drug metabolism and pharmacokinetics, the blood-brain barrier, and central nervous system drug discovery. NeuroRx 2005;2:554–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J, Li Y, Chen X, et al. : Systems pharmacology dissection of multi-scale mechanisms of action for herbal medicines in stroke treatment and prevention. PLoS One 2014;9:e102506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ovbiagele B: Potential role of curcumin in stroke prevention. Expert Rev Neurother 2008;8:1175–1176 [DOI] [PubMed] [Google Scholar]
- 22.Strimpakos AS, Sharma RA: Curcumin: Preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid Redox Signal 2008;10:511–545 [DOI] [PubMed] [Google Scholar]
- 23.Soni KB, Kuttan R: Effect of oral curcumin administration on serum peroxides and cholesterol levels in human volunteers. Indian J Physiol Pharmacol 1992;36:273–275 [PubMed] [Google Scholar]
- 24.Oude Griep LM, Verschuren WM, Kromhout D, et al. : Colors of fruit and vegetables and 10-year incidence of stroke. Stroke 2011;42:3190–3195 [DOI] [PubMed] [Google Scholar]
- 25.Sun K, Fan J, Han J: Ameliorating effects of traditional Chinese medicine preparation, Chinese materia medica and active compounds on ischemia/reperfusion-induced cerebral microcirculatory disturbances and neuron damage. Acta Pharm Sin B 2015;5:8–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lei XL, Chiou GC: Cardiovascular pharmacology of Panax notoginseng (Burk) F.H. Chen and Salvia miltiorrhiza. Am J Chin Med 1986;14:145–152 [DOI] [PubMed] [Google Scholar]
- 27.Chen ZH, Li J, Liu J, et al. : Saponins isolated from the root of Panax notoginseng showed significant anti-diabetic effects in KK-Ay mice. Am J Chin Med 2008;36:939–951 [DOI] [PubMed] [Google Scholar]
- 28.He NW, Zhao Y, Guo L, et al. : Antioxidant, antiproliferative, and pro-apoptotic activities of a saponin extract derived from the roots of Panax notoginseng (Burk.) F.H. Chen. J Med Food 2012;15:350–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo FC, Wang SD, Qi L, et al. : Protective effect of panaxatriol saponins extracted from Panax notoginseng against MPTP-induced neurotoxicity in vivo. J Ethnopharmacol 2011;133:448–453 [DOI] [PubMed] [Google Scholar]
- 30.Sun K, Wang CS, Guo J, et al. : Protective effects of ginsenoside Rb1, ginsenoside Rg1, and notoginsenoside R1 on lipopolysaccharide-induced microcirculatory disturbance in rat mesentery. Life Sci 2007;81:509–518 [DOI] [PubMed] [Google Scholar]
- 31.Egleton RD, Davis TP: Development of neuropeptide drugs that cross the blood-brain barrier. NeuroRx 2005;2:44–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mukherjee S, Ray S, Thakur RS: Solid lipid nanoparticles: A modern formulation approach in drug delivery system. Indian J Pharm Sci 2009;71:349–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jani P, Halbert GW, Langridge J, et al. : Nanoparticle uptake by the rat gastrointestinal mucosa: Quantitation and particle size dependency. J Pharm Pharmacol 1990;42:821–826 [DOI] [PubMed] [Google Scholar]
- 34.Misra A, Ganesh S, Shahiwala A, et al. : Drug delivery to the central nervous system: A review. J Pharm Pharm Sci 2003;6:252–273 [PubMed] [Google Scholar]
- 35.Garinot M, Fievez V, Pourcelle V, et al. : PEGylated PLGA-based nanoparticles targeting m cells for oral vaccination. J Control Release 2007;120:195–204 [DOI] [PubMed] [Google Scholar]
- 36.Zhang J, Han X, Li X, et al. : Core-shell hybrid liposomal vesicles loaded with Panax notoginsenoside: Preparation, characterization and protective effects on global cerebral ischemia/reperfusion injury and acute myocardial ischemia in rats. Int J Nanomedicine 2012;7:4299–4310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hajare AA, Mali SS, Ahir AA, et al. : Lipid nanoparticles: A modern formulation approach in topical drug delivery systems. JADD 2014;1:30–37 [Google Scholar]
- 38.Wong HL, Wu XY, Bendayan R: Nanotechnological advances for the delivery of CNS therapeutics. Adv Drug Deliv Rev 2012;64:686–700 [DOI] [PubMed] [Google Scholar]
- 39.Kakkar V, Muppu SK, Chopra K, et al. : Curcumin loaded solid lipid nanoparticles: An efficient formulation approach for cerebral ischemic reperfusion injury in rats. Eur J Pharm Biopharm 2013;85:339–345 [DOI] [PubMed] [Google Scholar]
- 40.Panariti A, Miserocchi G, Rivolta I: The effect of nanoparticle uptake on cellular behavior: Disrupting or enabling functions? Nanotechnol Sci Appl 2012;5:87–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghosh A, Sarkar S, Mandal AK, et al. : Neuroprotective role of nanoencapsulated quercetin in combating ischemia-reperfusion induced neuronal damage in young and aged rats. PLoS One 2013;8:e57735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Z, Zhang Q, Ding L, et al. : Preparation procedure and pharmacokinetic study of water-in-oil nanoemulsion of Panax notoginseng saponins for improving the oral bioavailability. Curr Drug Deliv 2016;13:600–610 [DOI] [PubMed] [Google Scholar]
- 43.Odani T, Tanizawa H, Takino Y: Studies on the absorption, distribution, excretion and metabolism of ginseng saponins. II. The absorption, distribution and excretion of ginsenoside Rg1 in the rat. Chem Pharm Bull (Tokyo) 1983;31:292–298 [DOI] [PubMed] [Google Scholar]
- 44.Zhang X, Chen G, Wen L, et al. : Novel multiple agents loaded PLGA nanoparticles for brain delivery via inner ear administration: In vitro and in vivo evaluation. Eur J Pharm Sci 2013;48:595–603 [DOI] [PubMed] [Google Scholar]
- 45.Kalani A, Kamat PK, Chaturvedi P, et al. : Curcumin-primed exosomes mitigate endothelial cell dysfunction during hyperhomocysteinemia. Life Sci 2014;107:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xin H, Li Y, Cui Y, et al. : Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab 2013;33:1711–1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhuang X, Xiang X, Grizzle W, et al. : Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther 2011;19:1769–1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dumont CM, Park J, Shea LD: Controlled release strategies for modulating immune responses to promote tissue regeneration. J Control Release 2015;219:155–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yositomi T, Vong LB, Nagasaki Y: Nanomedicine for the treatment of oxidative stress injuries. In: Post-Genomic Approaches in Cancer and Vaccine Development (Kishore R, Sakharkar KR, Sakharkar MK, Chandra R, eds.). River Publishers, Delft, Netherlands, 2015, pp. 199–218 [Google Scholar]