Abstract
Background and Aims Although plant distribution patterns are well documented, our understanding of the ecophysiological mechanisms that control the geographical ranges of plant species remains poor. We used a largely ignored method, the performance of the male gametophyte in vitro, to assess whether the thermal range of pollen germination and tube growth controls species distribution ranges, in this case along an elevational gradient.
Methods Using in vitro pollen germination experiments, we obtained cardinal temperatures (minimal, optimal and maximal) of pollen germination and pollen tube growth for 25 herbaceous species along a mean annual temperature gradient of about 5 °C. These temperatures were correlated with temperatures of the sites where the species were collected. The presence of a phylogenetic signal in the data set as well as an effect of species flowering phenology were also estimated.
Key Results and Conclusions We found a strong positive relationship between temperature conditions at our collection sites and the minimum temperature for both pollen germination and pollen tube growth. In addition, a significant correlation between maximum temperature of pollen tube growth and temperature of flowering month was apparent. We conclude that the restriction of pollen germination and growth by low temperatures is an important contributor to the climatic restriction of plant species distributions. Improved knowledge of this thermal precursor to seed production could, from a functional perspective, enhance our understanding of species distributions along climatic gradients and our ability to predict how anthropogenic climate change might affect plant community composition.
Keywords: pollen germination, pollen tube growth, plant distribution, elevational gradient, biogeography, temperature, climate change
INTRODUCTION
Temperature has been long recognized as a major factor controlling plant distribution along latitudinal and elevational gradients (Salisbury, 1926; Woodward and Jones, 1984; Archibold, 1995). The identification of plant–environment relationships has catalysed the generation of many vegetation–climate models to describe plant distribution patterns at different geographical scales (Meusel et al., 1965; Woodward, 1987; Prentice et al., 1992). Although these models are highly informative, the exact ecophysiological mechanisms that shape the distribution of species with respect to temperature range remain unclear.
One suggested mechanism regulating climatic restriction of plant species relates to the sensitivity of the shoot. From studies on plant persistence at low temperatures it has been proposed that the level of frost resistance of vegetative organs, such as buds, leaves and stems, can predict the extent to which species are distributed in regions with harsh climates (Sakai and Larcher, 1987; Körner, 1999; Larcher, 2000; Taschler and Neuner, 2004). However, differences in distributional ranges are not always clear-cut; plant species from different biomes often overlap in their frost tolerance (Sakai and Larcher, 1987; Körner, 1999; Larcher, 2003). Thus, this ecophysiological mechanism provides only a partial explanation of the large-scale discontinuities in plant distributions. This ‘frost-sensitivity’ explanation also fails to consider the fact that the geographical limits of vegetative survival may be determined at stages of the plant life cycle other than that of the mature plant, and by very different environmental conditions (see Woodward, 1987, 1997; García et al., 2000). Despite its fundamental importance in shaping the distribution of species and vegetation types globally, and the uncertainties surrounding future impacts of climate warming (Parmesan and Hanley, 2015), an accurate mechanistic appreciation of how temperature shapes the distribution of a plant species remains elusive. The identification of the rule base that explains how climate restricts plant distribution remains one of the major challenges of modern ecology (Bykova et al., 2012).
In contrast to the ambiguous relationships between climate and vegetative growth, reproduction processes are intimately related to ambient temperatures (Zinn et al., 2010; Bykova et al., 2012). They may therefore be a primary determinant of the geographical boundaries of species (e.g. Pigott and Huntley, 1981; Pigott, 1992; García et al., 2000; Jump and Woodward, 2003). For a given species, ‘environmental favourability’ declines from the core to the periphery of its distributional area, a fact that may negatively affect the reproduction performance of the plant. Indeed, seed set progressively decreases from a maximal capacity at the centre of this area to conditions where the quality and quantity of the produced seeds are below that necessary for successful and long-term regeneration at the distributional edge (García et al., 2000; Jump and Woodward, 2003). Consequently, the inability to reproduce, or poor reproduction, beyond its ecological optimum limits the further geographical expansion of a species (Grubb, 1977; Woodward and Jones, 1984; Pigott, 1992; McKee and Richards, 1996). For example, seed production of lowland species decreases drastically with increasing elevation (Hofgaard, 1993; Kullman, 1993), while alpine species are able to complete reproduction even under the extreme climatic conditions of high elevations (Ladinig and Wagner, 2005; Wagner et al., 2010). However, although the climatic control of species ranges may be mediated through seed production (Grubb, 1977; Woodward, 1987), we remain uncertain as to the key stage of the reproduction process: the stage that is critical in ecophysiological terms and has therefore strong predictive value in defining the distributional range of a species.
Variation in seed crop production in relation to climate may stem from a specific physiological limitation of seed development (Pigott, 1992; Zinn et al., 2010). Although various stages of seed development are temperature-dependent (e.g. Henttonen et al., 1986; Peet et al., 1997), numerous experimental studies have demonstrated that for the completion of successful fertilization, both pollen germination (PG) and pollen tube growth (PTG) are also highly temperature-dependent (Weinbaum et al., 1984; Elgersma et al., 1989; Kakani et al., 2005; Boavida and McCormick, 2007; Steinacher and Wagner, 2012). Despite dependence on habitat temperature for the success of both the PG and the PTG components of the progamic phase of fertilization, however, few studies have related temperature requirements of PG and PTG to the geographical range of the investigated species. Nonetheless, Pigott and Huntley (1981) demonstrated that the temperature sensitivity of PTG and the short period of stigmoid and stylar receptivity in Tilia cordata may account for its northern distributional limit in the British Isles (see also Pigott and Warr, 1989; Pigott, 1992).
Here, we attempt to take the ground-breaking but still unrecognized work of ecologists such as Pigott and Huntley (1981) to the next level. We explore the extent to which temperature requirements of PG and pollen tube elongation of contrasted distributional ranges may be predicted from the temperatures associated with their natural habitat. More specifically, we test the hypothesis that the specific temperature requirements of PG and PTG in vitro predict positively species occurrence along a gradient of mean annual temperature (MAT). The distribution of a species with a high temperature requirement for these two processes is expected to be limited to the higher part of the temperature gradient, due to increasing negative temperature stress.
To test our hypothesis, we selected an elevational gradient as a study system, because it is one of the most powerful ‘natural experiments’ for testing ecological and evolutionary responses of biota to geophysical influences, such as temperature (Körner, 2007a, b). The strong negative correlation between MAT and elevation (0·6 K per 100 m; Sakai and Larcher, 1987; Körner, 1999, 2007a, b) offers an ideal opportunity to explore macroecological mechanisms of plant–climate interactions over short spatial distances and offers additional insight into the likely impact of anthropogenic climate change on plant ecophysiology and distribution (Dunne et al., 2003).
MATERIALS AND METHODS
Study system: study area and species selection
Fieldwork was carried out in the Berchtesgaden National Park located in the Bavarian Alps (south-east Germany). The National Park is approx. 200 km2 in area and characterized as typically alpine topography, with steep mountain peaks composed of Triassic limestone and dolomite (Marke et al., 2013). The climate is typically montane with large altitudinal decrease in mean annual air temperatures from +7 to – 2 °C [from 603 to 2713 m above sea level (a.s.l.), respectively]. Mean annual precipitation in the region varies, ranging from approx. 1500 to 2600 mm (Marke et al., 2013).
For the purpose of this study, 25 herbaceous species with different elevational distributions (and therefore with different distributional ranges along the MAT gradient) within southern Germany were selected (see Table 1; Supplementary Data, Table S1). All chosen species occur in a single vegetation type (calcareous grasslands) and share similar habitat preferences with regard to light, water, soil characteristics and chemistry (Ellenberg et al., 1991; Oberdorfer, 2001); thus, temperature is likely to be the main explanatory variable for their distributional ranges. Nomenclature follows Oberdorfer (2001).
Table 1.
Cardinal temperatures of pollen germination (PG) and pollen tube growth (PTG) for the investigated species
| Species | Species distribution characteristics |
Flowering phenology |
Pollen germination |
Pollen tube growth |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Range of MAT (°C) | MAT of collection site (°C) | Flowering month | TFM (°C) | Tmin | Topt | Tmax | Tmin | Topt | Tmax | |
| Aconitum napellus L. | 2·4–4·4 | 3·8 | VIII | 11·3 | – | – | – | 2·4 | 21·2 | 37·5 |
| Anemone nemorosa L. | 3·9–9·0 | 6·1 | IV | 5·0 | 7·5 | 26·3 | 36·0 | 5·0 | 31·1 | 34·0 |
| Anemone pulsatilla L. | 6·2–9·0 | 8·0 | IV | 8·1 | 9·0 | 17·3 | 37·2 | 9·0 | 32·2 | 34·0 |
| Caltha palustris L. | 4·9–9·0 | 6·8 | IV | 6·0 | 9·0 | 23·2 | 34·0 | 8·9 | 28·4 | 34·0 |
| Campanula alpina Jacq. | 1·9–3·6 | 2·7 | VI | 8·6 | 0·0 | 16·4 | 34·0 | 0·0 | 17·8 | 34·0 |
| Campanula scheuchzeri Vill. | 2·2–6·2 | 3·5 | VII | 10·6 | 0·0 | 17·3 | 35·9 | 0·0 | 16·8 | 39·0 |
| Carex caryophyllea Latourr. | 4·9–9·0 | 6·3 | IV | 15·7 | 0·0 | 15·9 | 40·0 | 0·0 | 34·3 | 40·0 |
| Carex firma Host | 1·8–6·7 | 6·3 | V | 10·5 | 0·2 | 15·8 | 35·0 | 0·0 | 23·9 | 35·7 |
| Carex flacca Schreb. | 4·9–9·0 | 6·3 | V | 14·1 | – | – | – | 4·0 | 30·0 | 34·0 |
| Erica carnea L. | 3·1–7·0 | 6·3 | IV | 15·7 | 0·0 | 26·0 | 34·8 | 0·0 | 28·8 | 40·0 |
| Gentiana asclepiadea L. | 3·4–7·5 | 5·4 | VIII | 9·3 | – | – | – | 8·0 | 19·2 | 35·6 |
| Gentiana pannonica Scop. | 2·6–5·6 | 5·1 | VII | 12·8 | 0·9 | 28·5 | 35·0 | 2·0 | 19·6 | 36·0 |
| Gentianella aspera (Hegetschw. & Heer) Dostál ex Skalický, Chrtek & Gill | 1·9–3·6 | 3·7 | VIII | 11·3 | 2·5 | 26·1 | 34·8 | 1·0 | 16·4 | 40·0 |
| Globularia cordifolia L. | 4·9–9·0 | 8·0 | IV | 8·1 | 9·0 | 30·4 | 40·0 | 9·0 | 29·6 | 40·0 |
| Helleborus niger L. | 4·9–7·5 | 6·3 | III | 5·5 | 0·0 | 14·5 | 33·4 | 5·0 | 23·5 | 34·4 |
| Parnassia palustris L. | 1·8–7·4 | 2·8 | VIII | 10·2 | 6·5 | 31·8 | 34·0 | 0·0 | 31·0 | 34·0 |
| Phyteuma orbiculare L. | 2·3–4·9 | 3·5 | VI | 10·6 | 0·0 | 21·6 | 33·9 | 0·0 | 16·3 | 30·5 |
| Primula minima L. | 1·9–3·6 | 2·7 | IV | 5·8 | 0·0 | 16·1 | 34·0 | 0·0 | 26·9 | 34·0 |
| Plantago lanceolata L. | 4·4–7·4 | 6·1 | VII | 12·9 | 5·0 | 20·6 | 36·8 | 4·7 | 25·8 | 38·2 |
| Primula veris L. | 6·2–9·0 | 8·0 | IV | 8·1 | 9·0 | 22·7 | 33·9 | 9·0 | 24·8 | 34·7 |
| Ranunculus acris L. | 5·9–9·0 | 6·3 | VI | 13·4 | 12·2 | 31·0 | 35·0 | 12·0 | 26·2 | 35·9 |
| Ranunculus serpens subsp.polyanthemophyllus (W.Koch & H.E.Hess) Kerguélen | 5·9–9·0 | 6·7 | V | 11·0 | 5·0 | 21·7 | 35·0 | 5·0 | 22·8 | 35·0 |
| Silene flos-cuculi (L.) Greuter & Burdet | 3·9–9·0 | 6·1 | VI | 12·9 | 9·0 | 17·8 | 34·0 | 9·0 | 25·3 | 34·0 |
| Soldanella alpina L. | 1·9–6·2 | 4·4 | IV | 8·0 | 2·0 | 18·6 | 33·6 | 2·5 | 23·3 | 33·9 |
| Trollius europaeus L. | 2·6–5·6 | 5·4 | VI | 12·1 | – | – | – | 6·3 | 30·7 | 34·0 |
Species distribution characteristics are based on a vegetation survey carried out at 40 sites along a gradient from 650 to 2570 m a.s.l. in the Berchtesgaden National Park. The cardinal temperatures were estimated by fitting the generalized plant growth model to PG rate and PTG length, which were obtained in in vitro germination experiments along a temperature gradient from 5 to 35 °C (for details see Material and Methods section). Tmin, lowest temperature; Topt, optimum temperature; Tmax, maximum temperature of PG or PTG; MAT, mean annual temperature.
We surveyed vegetation at 40 sites along an altitudinal gradient from 650 to 2570 m a.s.l. to determine distributional (and therefore climatic) ranges of our target species. Ten random 1-m2 plots were set up at each site and in each plot we recorded the cover of each vascular plant species. The relative abundance of a species at a site was calculated as the mean value (% cover) of its abundance in all plots. The species’ relative abundances among sites were compared, and the site with the highest value for that species (i.e. with putative optimal ecological conditions) was identified. Pollen grains of the selected species were subsequently collected from this ‘optimal’ site. Pollen samples were mainly collected in the Berchtesgaden National Park from between 800 and 2050 m a.s.l., although pollen grains from Anemone pulsatilla L., Globularia cordifolia L. and Primula veris L. were collected from calcareous grassland located at 450 m a.s.l. (Garchinger Heide, southern Bavaria, Germany). For every collection site, data on MAT as well as mean monthly temperature from March to September (see below) were obtained from the closest weather station (difference in elevation less than 50 m and none more than 2 km from the site). Data are presented as mean values for the last 10 years of records.
MAT as a proxy for habitat temperature environment
Using temperature of the flowering period as a main factor influencing cardinal temperatures of PT and PTG would be the optimal approach in the current study, as this better reflects the conditions the pollen actually experiences. However, we were compelled to use MAT as a proxy for species habitat temperature conditions, for the following reasons. Flowering time, especially in mountain plants, is a highly flexible trait, strongly dependent on both habitat characteristics (e.g. altitude, slope aspect and inclination, shadow from surrounding trees or rocks) and particular weather conditions (thickness and distribution of snow, inter-annual temperature variation, etc.). Due to these two factors, individual plants may flower over a prolonged period: for example, in 2010, Sesleria albicans, one of the most common species in the study region, flowering began on 1 May at 800 m a.s.l. and ended on 10 July at 2430 m a.s.l. Furthermore, ground-level temperatures, where flowers are actually located, differ from meteorological records measured to international standards at 2 m above the ground (Geiger et al., 2009). Thus, to obtain reliable temperature data for the flowering period for any given species, it was necessary to observe flowering phenology within entire distribution ranges over a long period, as well as conduct direct measurements of ambient temperatures at flower height. Due to logistical limitations (phenological observations and fresh pollen collections were simultaneously carried out by one person only in a remote area at sites located from between 800 and 2050 m a.s.l.), phenological observations for a given species were confined to one year (either 2010 or 2011) and only in a site with putative optimal ecological conditions. For similar reasons, we were unable to obtain near-ground temperatures during species flowering (a pollen collection site was visited for several hours only). Under these circumstances and despite the possible confounding effect of coldest month, MAT was therefore the only feasible proxy for temperature conditions during flowering and seed set for sites located within a relatively small geographically compact region.
Flowering phenology
As temperature conditions during flowering may also be correlated with the temperature requirements of PG and PTG (e.g. early-flowering species show lower temperature thresholds for these two processes; Weinbaum et al., 1984; Luza et al., 1987), the flowering phenology of the studied species was also considered. To that end, phenological observations of flower development were conducted two to three times a month from March to September in either 2010 or 2011 (Table S1) in every ‘optimal’ site (see above) following the Biologische Bundesanstalt, Bundessortenamt and Chemical Industry (BBCH) code. This code provides a detailed growth stage key that includes intermediate stages as well as stages marking the end of phenophases and thus allows observation of the entire development cycle of all mono- and dicotyledonous plants using a decimal coding system (Meier, 2001). Using this key also obviates the necessity of being present at the exact start of each phenological stage and the frequency distribution of phenophases within the population can instead be assessed at each sampling date (Cornelius et al., 2013). We focused here on three key phenological events: the onset of flowering (non-graminoids: first flowers open; graminoids: first anthers visible), full flowering (non-graminoids: 50 % of flowers open; graminoids: 50 % of anthers mature) and the cessation of flowering (non-graminoids: petals dehydrated or fallen; graminoids: all spikelets/panicles have completed flowering but some dehydrated anthers may remain). On each sampling date, the phenological stage of the majority of individuals of a species was recorded. The timing of the onset of phenophases was defined as the sampling date when the stage was first observed. When the majority of individuals of the target species were in flower, this was adjudged to be in phenological terms the flowering date and flower buds were collected for the PG experiments.
Pollen collection
To minimize the impact of intraspecific variation on PG measurements (Kakani et al., 2005), fresh flower buds (1–3 d before opening) were randomly collected in the field from at least 30 individuals separated by a distance of 2–5 m from each other. After collection, buds were taken immediately to the laboratory and disinfected by spraying with 96 % ethanol. The anthers were then removed manually and left to dry for 2–3 d at room temperature in a desiccator filled with silica gel (relative humidity approx. 30 %). To extract the pollen grains, the dried anthers were subsequently crushed into small pieces and passed through a 200 -µm sieve. Prior to the experiments, the pollen grains were stored at 5 °C for not more than 7 d.
Pollen germination experiments
The media used for PG contained nutrient salts (0·01 % H3BO4, 1 mm Ca(NO3)2, 1 mm CaCl2 and 1 mm MgSO4) and different concentrations of sucrose, from 10 to 30 % (Brewbaker and Kwack, 1963). To reduce the risk of microbial and fungal contamination, the glassware and stock solutions of the salts were autoclaved before use; the PG media were sterilized by filtration. Fresh germination media were prepared and stored at 5 °C for no longer than 3 d prior to use.
Preliminary tests were required to determine the species-specific pollen sensitivity to the sugar concentration of the germination media (Bajaj, 1987). To this end, hydrated pollen (see below) was mixed with germination media that varied in sucrose content (from 10 to 30 % with a 2 % step) and allowed to germinate at room temperature (22 °C) for 18 h. The medium with the highest germination rates and longest pollen tubes was considered as optimal and subsequently used in the germination experiments.
To avoid the pollen grains bursting, a hydration procedure was performed over a saturated KCl solution for 6 h at 5 °C (Connor and Towill, 1993). After hydration, the pollen was mixed with the appropriate PG media, and 150 µL of the mixture was pipetted into a germination chamber produced by cutting a 96-well PCR plate into 24 pieces (with four wells each). The pre-processed pollen samples were maintained at nine different temperatures (5, 9, 12, 16, 20, 23, 27, 31 and 34 °C) on a thermogradient table (RUMED 5990, Rubarth Apparate GmbH, Laatzen, Germany). There were six replicates at each temperature except for Globularia cordifolia for which, due to a shortage of pollen, the number of replicates was reduced to four. After 18 h, PG was terminated by pipetting approx. 100 µL of formalin acetic alcohol (Pigott and Huntley, 1981) into each germination chamber, then stored at 5 °C before measurement.
PG was estimated by examining 300 pollen grains in wells (inverted microscope; approx. 10–15 microscopic fields of view) for each replicate. Germination was defined as having occurred when the length of the pollen tube was at least double grain diameter (Kakani et al., 2005). PG was determined by dividing the number of germinated pollen grains per field of view by the total number of pollen grains per field of view, expressed as a percentage.
To estimate the effect of temperature on PTG, images of 25 randomly selected pollen tubes from each replicate were captured with Axiovision version 4.3. software using an AxioCamMR camera (Carl Zeiss, Oberkochen, Germany). The pollen tube lengths were measured manually using ImageJ software (Abramoff et al., 2004).
Statistical analysis
All statistical calculations were performed in R software version 3.2.0 (R Core Development Team, 2012).
Cardinal temperatures
To quantify minimum (Tmin), optimum (Topt) and maximum (Tmax) temperatures of PG and PTG, the generalized plant growth model (eqn 1; Yin and Kropff, 1996) was fitted to PG rate and PTG length versus test temperatures. This flexible model, which is directly specified from the cardinal temperatures, is found to be the best predictive model for the cardinal temperatures of plant growth processes [e.g. seed germination (Derakhshan et al., 2014) or flowering time (Yin et al., 1995)]. An iterative optimization method was applied to estimate the model parameters based on the port algorithm in library nls implemented in R software version 3.2.0 (R Development Core Team, 2012). For this, residual sums of squares were used to detect the best estimates of parameters.
| (1) |
where Tmin, Topt and Tmax are the minimum, optimum and maximum temperatures for PG rate or pollen tube length (R), T is the temperature at which germination and tube growth were studied, Rmax is a maximum value of R at Topt and a is coefficient defining the curvature of the relationship.
Phylogenetic signal in the cardinal temperatures of PG and PTG
As related species are likely to share similar attributes (Harvey and Pagel, 1991), prior to estimating the relationship between the cardinal temperatures of PG and PTG and MAT, we calculated an estimated value of K, a Brownian motion-based metric of the strength of phylogenetic signal (Blomberg et al., 2003), using the phylosignal function in the ‘picante’ library (Kembel et al., 2010). K = 1 indicates that closely related species have trait values that are similar to those expected given Brownian motion; K < 1 indicates that closely related species have trait values that are less similar than expected given a Brownian model of evolution.
Relationship between PG/PTG and MAT
To assess the relationship between the temperature experienced by the plant in its natural habitat and the temperature requirements of the progamic phase of fertilization, cardinal temperatures (response variables) were correlated with collection site MATs (predictor) using phylogenetic generalized least squares (PGLS) regression models implemented in the cape library (Orme et al., 2013). We preferred to use PGLS for our regression models, because closely related species are likely to have similar trait values (Harvey and Pagel, 1991) and therefore violate assumptions of independence for traditional statistical analysis (Kraft et al., 2015). Whereas components of the error term in an ordinary least squares regression are assumed to follow a normal distribution around a mean of zero and variance σ2, PGLS corrects for the effect of non-independence in observations by incorporating an expected model of evolution and phylogeny into the variance–covariance matrix specified for the error term of a linear model (Kraft et al., 2015). Our analysis assumed a simple continuous evolutionary model of Brownian motion subject to the scaling factor, λ (Pagel, 1999). λ, estimated in each model using a maximum-likelihood (ML) approach, is a constant that allows one to assess the strength of a phylogenetic signal in the residuals of a regression model (Kraft et al., 2015), which ranges between 0 and 1. More specifically, λ = 0 indicates phylogenetic independence among observations (i.e. the variation of a trait is modelled as a function of an independent evolution along the branches leading to the tips) and thus the resulting PGLS is equivalent to ordinary least squares (Kraft et al., 2015). When λ = 1, the trait shows variation expected under the Brownian motion model of evolution (Freckleton et al., 2003). Intermediate values of λ indicate varying degrees of phylogenetic dependence in the data. The phylogenetic tree used in the analysis was based a documented phylogeny of a large European flora (Durka and Michalski, 2012). In all the models, non-linear terms were also considered (Tcardinal ∼ MAT + MAT2).
Effect of flowering phenology on PG and PTG variations
To account for any possible effect of flowering phenology on PG and PTG variations among the studied species, we also included in all PGLS models temperature of the flowering month (TFM) both as an independent variable and as an interaction with MAT (TFM:MAT) as predictors of a cardinal temperature. The PGLS models with all variables included were reduced via backward selection of the least significant variables until we achieved the minimal adequate model (Crawley, 2007). Following model-fitting, the model requirements (a normal distribution and homogeneous variances in the residuals) were checked. All explanatory variables were considered to be not collinear, because they were not correlated to each other.
RESULTS
Distributional ranges and flowering phenology
Distributional ranges along the MAT gradient differed considerably among the study species from typical lowland species, such as Anemone pulsatilla or Primula veris (MAT range 6·2–9·0 °C for both), to species with main distribution in the alpine belt, e.g. Campanula alpina and Primula minima (MAT range 1·9–3·6 °C for both species; Table 1).
Flowering phenology also varied, from early-flowering species, such as Helleborus niger or Soldanella alpina (March and April, respectively) to late-flowering species, e.g. Aconitum napellus to Parnassia palustris (both flower in August). Correspondingly, TFM varied from 5 °C (Anemone nemorosa) to 15·7 °C (Erica carnea and Carex caryophyllea) with an average of 10·3 °C.
Cardinal temperatures of PG
Cardinal temperatures for PG differed greatly; Tmin ranged from 0 °C (e.g. Campanula alpina) to 12·2 °C (Ranunculus acris), with a mean of 4·1 °C. The optimum temperature (Topt) ranged from 14·5 °C (Helleborus niger) to 31·8 °C (Parnassia palustris), with a mean of 21·9 °C. The Tmax values varied from 33·4 °C for Helleborus niger to 40·0 °C for Carex caryophyllea and Globularia cordifolia, with a mean of 35·3 °C (Table 1).
Due to the presence of anther debris obscuring pollen grains, PG rates could not be measured exactly for Aconitum napellus, Carex flacca, Gentiana asclepiadea or Trollius europaeus.
Cardinal temperatures of PTG
We found considerable variation in pollen tube length at the minimum, optimum and maximum cardinal temperatures (Table 1). The values of Tmin ranged from 0 °C (e.g. Erica carnea and Phyteuma orbiculare) to 12 °C (Ranunculus acris). The magnitude of Topt ranged from 16·3 °C (Phyteuma orbiculare) to 34·3 °C (Carex caryophyllea). The Tmax values ranged from 30·5 °C for Phyteuma orbiculare to 40 °C for, for example, Erica carnea and Gentianella aspera. The mean values for Tmin, Topt and Tmax were 4·1, 25·0 and 35·7 °C, respectively.
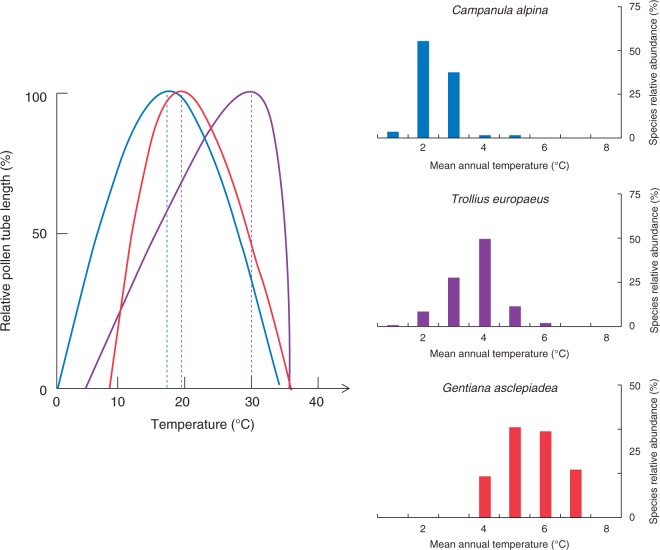
PTG responses to the temperature treatments of three species (Campanula alpina, Trollius europaeus and Gentiana asclepiadea) from climatically contrasted habitats showing reduction in Tmin as MAT decreases are illustrated in Fig. 1. Estimated cardinal temperatures for PG and PTG are presented in Table 1.
Fig. 1.
Differences in the temperature requirements for PTG of three species (Campanula alpina, Trollius europaeus and Gentiana asclepiadea) occurring in climatically contrasting habitats. The cardinal temperatures were estimated by fitting the generalized plant growth model to the experimental data for pollen tube length (see Materials and Methods section). The species relative abundances along the gradient of mean annual temperatures relate to the vegetation survey in the study area (see Materials and Methods section).
Phylogenetic signal
A phylogenetic signal was not detected in PG, irrespective of the cardinal temperature [Tmin (K = 0·10, P = 0·63); Topt (K = 0·10, P = 0·67); Tmax (K = 0·36, P = 0·15)]. Among cardinal temperatures for PTG, a moderate phylogenetic signal was only detected in Topt (K = 0·43, P = 0·03). The low and non-significant K values (K = 0·11, P = 0·72 and K = 0·23, P = 0·37, respectively) indicate that Tmin and Tmax of PTG are not phylogenetically constrained. Measures of the phylogenetic signal (measured as Bloomberg’s K) are presented in Table 2.
Table 2.
Phylogenetic conservatism in cardinal temperatures of PG and PTG rate according to Bloomberg’s K-statistics (for details see Materials and Methods section)
| Trait | Pollen germination |
Pollen tube growth |
||
|---|---|---|---|---|
| K | P | K | P | |
| Tmin | 0·10 | 0·63 | 0·11 | 0·72 |
| Topt | 0·10 | 0·67 | 0·43 | 0·03 |
| Tmax | 0·36 | 0·15 | 0·23 | 0·37 |
K = 1 indicates that closely related species have trait values that are similar to those expected given Brownian motion. K < 1 indicates that closely related species have trait values that are less similar than expected given a Brownian model of evolution.
Correlations between cardinal temperatures and habitat temperatures
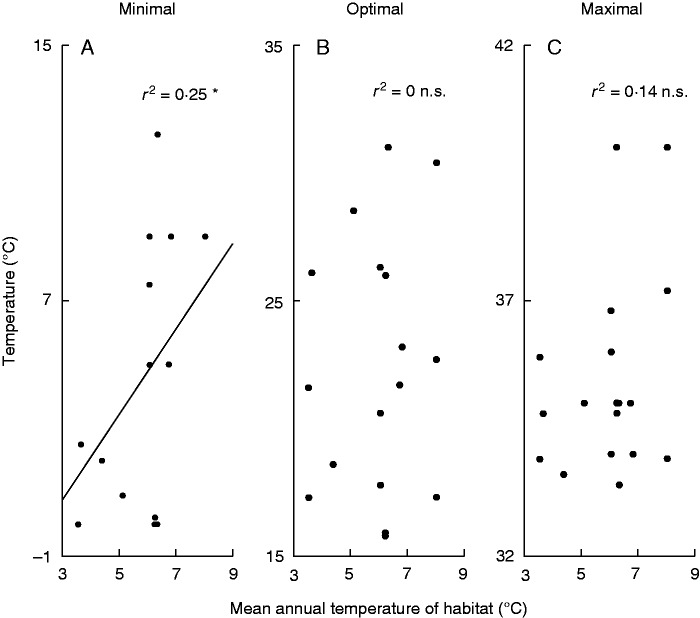
Among studied cardinal temperatures of PG, only Tmin was positively linearly correlated with MAT (Table 3; Fig. 2A, r2 = 0·25, P = 0·01; test accounted for phylogeny via PGLS).
Table 3.
The relationship between PG and PTG, habitat mean annual temperature (MAT) and mean temperature of flowering month (TFM) estimated by phylogenetic least squares analysis (see Material and Methods section for details)
| Adj. R2 | P | λ | |
|---|---|---|---|
| Pollen germination | |||
| Tmin ∼ MAT | 0·25 | 0·01 | 0·118 |
| Topt ∼ MAT | –0·02 | 0·45 | 0 |
| Tmax ∼ MAT | 0·14 | 0·06 | 0·113 |
| Pollen tube growth | |||
| Tmin ∼ MAT | 0·46 | <0·001 | 0·281 |
| Topt ∼ MAT | 0·07 | 0·11 | 0·69 |
| Tmax ∼ TFM | 0·19 | 0·03 | 0 |
The minimum adequate models with corresponding coefficient of determination (Adj. R2), significance of model terms (P) and measure of phylogenetical signal included as parameter in the models (λ; shown as here as a maximum-likelihood estimate) are shown.
Fig. 2.
Relationships between the temperature requirements of pollen germination (PG) and mean annual temperature of habitats where species occur: (A) minimum temperature of PG; (B) optimum temperature of PG; (C) maximum temperature of PG. Statistical significances: *0·01 < P < 0·05, n.s. (not significant) P > 0·05.
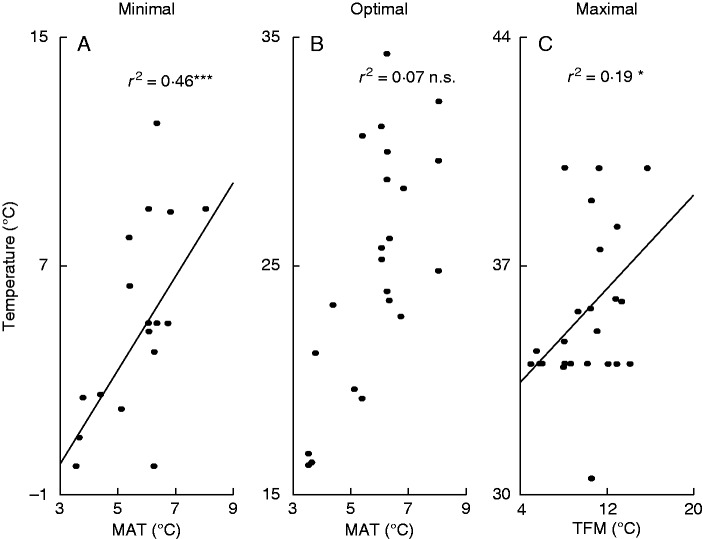
An analysis of the PTG data showed that minimum temperature was also a significant predictor of the species occurrence along the MAT gradient (Table 3; Fig. 3A, r2 = 0·46, P < 0·001; test accounted for phylogeny via PGLS). In contrast, the optimum and maximum temperatures of PTG were not correlated with MAT.
Fig. 3.
Relationships between the temperature requirements of pollen tube growth (PTG) and mean annual temperature (MAT) of habitats where species occur (A and B are minimum and optimum temperature of PTG, respectively). (C) Relationship between the maximum temperature of PTG and temperature of flowering month (TFM) when pollen was collected. Statistical significances: ***P < 0·001, *0·01 < P < 0·05, n.s. (not significant) P > 0·05.
Effect of flowering phenology on PG and PTG variations
A significant correlation between TFM and temperature requirements of the progamic phase of fertilization was found only for Tmax of PTG (Table 3; Fig. 3C; r2 = 0·19, P = 0·03; tests accounted for phylogeny via PGLS), suggesting that PTG of species which flower under relatively high ambient temperatures is suppressed by higher temperatures.
DISCUSSION
It has been suggested that because of their association with more favourable climatic conditions, pollen grains of species from habitats with a higher MAT are adapted to germinate and grow under relatively high temperatures (Weinbaum et al., 1984; Jakobsen and Martens, 1994; Pasonen et al., 2000; Kremer and Jemrić, 2006). Our results are consistent with this hypothesis: the pollen of the majority of ‘low-altitude’ species began to germinate and grow at relatively high temperatures. For example, species collected in the warmest habitat in our study system (MAT 8 °C; Table 1), namely Anemone pulsatilla, Globularia cordifolia and Primula veris, had the highest temperature requirements (except Ranunculus acris) for both PG and PTG (in all cases 9 °C).
A decreasing MAT along a climatic gradient is coupled with an increasing probability of negative temperature stress: this may take the form of freeze–thawing cycles in spring or autumn or as freezing episodes during the growing season (Sakai and Larcher, 1987; Körner, 1999). In species of cold habitats, fertilization can be adapted to this stress by plants reducing their temperature requirements for PG and pollen tube elongation (Zamir et al., 1981; Steinacher and Wagner, 2012). Our results are again consistent with previous findings: the minimal temperature of PG and PTG was on average 4·3 °C less for species from relatively cold habitats (MAT < 5·4 °C; Table 1). Thus, the minimal temperatures for both PG and PTG are strongly negatively correlated with the mean annual habitat temperature (Figs 2A and 3A). Moreover, these specific temperature requirements were good predictors of species occurrence along a MAT gradient (for example PTG; Fig. 1).
In addition to habitat temperature conditions, flowering phenology was also correlated with the temperature requirements of PG and PTG in vitro. It is known from crop plants that pollen of species and cultivars that flower under relatively high temperatures germinates and grows tubes at relatively high temperatures (e.g. Luza et al., 1987; Kakani et al., 2005). Our results partially confirm this pattern; the positive correlation between TFM and cardinal temperatures of PG and PTG was only found in the linear model for Tmax of PTG (Table 3; Fig. 3C). This finding suggests that regardless of MAT of a habitat, species flowering at different times of year differ in their upper temperature thresholds for PTG. As an example from our data set, Carex caryophyllea, which flowers at below 15·7 °C, shows a difference of PTG Tmin (0 °C) of 6 °C to Anemone nemorosa (TFM 5 °C). The increased tolerance of PTG of species flowering under relatively warmer environmental conditions to high temperatures could be explained by higher temperature regimes within their flowers. However, in our dataset the overall effect of flowering phenology on the expression of maximal temperature of PTG is minor: TFM explained only 12 % of the trait variation (Table 3).
The strong correlation between habitat temperature and the two studied components of the progamic phase was consistent with the temperature requirements of PG and PTG impacting upon species distribution. The PG and PTG of species characteristic of warm habitats both require relatively high temperatures to induce the progamic phase of fertilization successfully after pollen adhesion on stigmas. When moving along the temperature gradient, a decreased MAT may result in increasingly suboptimal temperatures for PG and PTG and negatively affect seed production. Potentially, this reduction in reproductive output will in turn affect a species distribution, limiting its capacity to expand its geographical range or even to maintain existing populations (Grubb, 1977; Pigott and Huntley, 1981; Turnbull et al., 2000). In contrast, species whose pollen can germinate and grow at lower temperatures can successfully complete the progamic phase of fertilization, even in regions with a low MAT. This allows range expansions poleward or into higher elevations. Notwithstanding this, the influence of traits such as slow growth rate and short stature, which lead to low competitive ability (Woodward, 1975; Körner, 1999), may limit expansion into higher MAT regions.
Our results suggest that the inclusion of a ‘pollen dimension’ enables improved understanding of existing patterns of plant biogeography and probably plant distributional responses to anthropogenic climate change. For example, the strong correlation between PG and PTG minimal temperature and habitat temperature found here suggests that upward range shifts and changes in plant abundances in alpine vegetation (Gottfried et al., 2012; Pauli et al., 2012; Rosbakh et al., 2014; Mondoni et al., 2015) could be partly due to changes in seed set. We speculate that the temperature rise and associated phenological shifts observed in the Alps in recent decades have alleviated restrictions of harsh high-altitude environments (both extreme weather events, such as frosts, and generally low average temperature) on sexual reproduction for lowland species. These species, which require relatively high temperatures to initiate PG and PTG, could benefit from the relaxation of the low-temperature filter by increasing seed production and contribute to range extension into alpine vegetation. Furthermore, in the last decade, a strong emphasis has been placed on modelling of vegetation–climate interactions (Parmesan and Hanley, 2015). However, these models still suffer from an essential lack of temperature-specific ecological data and a mechanistic understanding of how environmental factors shape plant ecophysiology and current species distributions (Mondoni et al., 2015; Parmesan and Hanley, 2015). We suggest that the temperature requirements of PG and PTG in vitro could easily be integrated into environmental niche models as they can quantitatively estimate the success of reproduction under specific climatic conditions and are technically more easily measured than those relating to many other stages of the plant life cycle.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of Table S1: Collection sites of the studies species.
ACKNOWLEDGMENTS
We thank Susanne Weissflog, Isabel Seoane Berger, Stefanie Meier, Wolfgang Gass and Darya Barteneva for help with the PG experiments and data processing and Irina Kempel for methodological support. Accommodation and access to the collection sites was provided by the Berchtesgaden National Park. The research was funded by FORKAST project (TP 12 Poschlod). We thank John Hodgson, Kingsley Dixon, Widmar Tanner, Mick Hanley and three anonymous referees for comments on earlier versions of the manuscript.
LITERATURE CITED
- Abramoff MD, Magalhães PJ, Ram SJ. 2004. Image processing with ImageJ. Biophotonics international 11: 36–42. [Google Scholar]
- Archibold OW. 1995. Ecology of world vegetation. London: Chapman & Hall. [Google Scholar]
- Bajaj YPS. 1987. Cryopreservation of pollen and pollen embryos, and the establishment of pollen banks. International Review Of Cytology 107: 397–420. [Google Scholar]
- Blomberg SP, Garland T, Ives AR. 2003. Testing for phylogenetical signal in comparative data: behavorial traits are more labile. Evolution 57: 717–745. [DOI] [PubMed] [Google Scholar]
- Boavida L, McCormick S. 2007. Temperature as a determinant factor for increased and reproducible in vitro pollen germination in Arabidopsis thaliana. The Plant Journal 52: 570–582. [DOI] [PubMed] [Google Scholar]
- Brewbaker JL, Kwack BH. 1963. The essential role of calcium ion in pollen germination and pollen tube growth. American Journal of Botany 50: 859–865. [Google Scholar]
- Bykova O, Chuine I, Morin X, Higgins SI, Linder P. 2012. Temperature dependence of the reproduction niche and its relevance for plant species distributions. Journal of Biogeography 39: 2191–2200. [Google Scholar]
- Connor KF, Towill LE. 1993. Pollen-handling protocol and hydration/dehydration characteristics of pollen for application to long-term storage. Euphytica 68: 77–84. [Google Scholar]
- Cornelius C, Estrella N, Franz H, Menzel A. 2013. Linking altitudinal gradients and temperature responses of plant phenology in the Bavarian Alps. Plant Biology 15: 57–69. [DOI] [PubMed] [Google Scholar]
- Crawley MJ. 2007. The R book. Chichester: John Wiley & Sons. [Google Scholar]
- Derakhshan A, Gherekhloo J, Vidal RA, Prado RD. 2014. Quantitative description of the germination of littleseed canarygrass (Phalaris minor) in response to temperature. Weed Science 62: 250–257. [Google Scholar]
- Dunne JA, Harte J, Taylor KJ. 2003. Subalpine meadow flowering phenology responses to climate change: integrating experimental and gradient methods. Ecological Monographs 73: 69–86. [Google Scholar]
- Durka W, Michalski SG. 2012. Daphne: a dated phylogeny of a large European flora for phylogenetically informed ecological analyses. Ecology 93: 2297. [Google Scholar]
- Elgersma A, Stephenson AG, Nijs APM. 1989. Effects of genotype and temperature on pollen tube growth in perennial ryegrass (Lolium perenne L.). Sexual Plant Reproduction 2: 225–230. [Google Scholar]
- Ellenberg H, Weber H, Düll R, Wirth V, Werner W, Paulissen D. 1991. Zeigerwerte von Pflanzen in Mitteleuropa. Scripta Geobotanica 18: 9–160. [Google Scholar]
- Freckleton RP, Harvey PH, Pagel M. 2003. Bergmann’s rule and body size in mammals. American Naturalist 161: 821–825. [DOI] [PubMed] [Google Scholar]
- García D, Zamora R, Gómez JM, Jordano P, Hódar JA. 2000. Geographical variation in seed production, predation and abortion in Juniperus communis throughout its range in Europe. Journal of Ecology 88: 435–446. [Google Scholar]
- Geiger R, Aron RH, Todhunter P. 2009. The climate near the ground. London: Rowman & Littlefield. [Google Scholar]
- Gottfried M, Pauli H, Futschik A, et al. 2012. Continent-wide response of mountain vegetation to climate change. Nature Climate Change 2: 111–115. [Google Scholar]
- Grubb PJ. 1977. The maintenance of species-richness in plant communities: the importance of the regeneration niche. Biological Reviews 52: 107–145. [Google Scholar]
- Harvey PH, Pagel MD. 1991. The comparative method in evolutionary biology. Oxford: Oxford University Press. [Google Scholar]
- Henttonen H, Kanninen M, Nygren M, Ojansuu R. 1986. The maturation of Pinus sylvestris seeds in relation to temperature climate in Northern Finland. Scandinavian Journal of Forest Research 1: 243–249. [Google Scholar]
- Hofgaard A. 1993. Seed rain quantity and quality, 1984–1992, in a high altitude old-growth spruce forest, northern Sweden. New Phytologist 125: 635–640. [DOI] [PubMed] [Google Scholar]
- Jakobsen HB, Martens H. 1994. Influence of temperature and aging of ovules and pollen on reproductive success in Trifolium repens L. Annals of Botany 74: 493–501. [Google Scholar]
- Jump AS, Woodward FI. 2003. Seed production and population density decline approaching the range-edge of Cirsium species. New Phytologist 160: 349–358. [DOI] [PubMed] [Google Scholar]
- Kakani VG, Reddy KR, Koti S, et al. 2005. Differences in in vitro pollen germination and pollen tube growth of cotton cultivars in response to high temperature. Annals of Botany 96: 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kembel SW, Cowan PD, Helmus MR, et al. 2010. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26: 1463–1464. [DOI] [PubMed] [Google Scholar]
- Körner C. 1999. Alpine plant life, functional plant ecology of high mountain ecosystems. Berlin: Springer. [Google Scholar]
- Körner C. 2007a. Climatic treelines: conventions, global patterns, causes. Erdkunde 61: 316–324. [Google Scholar]
- Körner C. 2007b. The use of ‘altitude’ in ecological research. Trends in Ecology & Evolution 22: 569–574. [DOI] [PubMed] [Google Scholar]
- Kraft TS, Wright SJ, Turner I, et al. 2015. Seed size and the evolution of leaf defences. Journal of Ecology 103: 1057–1068. [Google Scholar]
- Kremer D, Jemrić T. 2006. Pollen germination and pollen tube growth in Fraxinus pennsylvanica. Biologia 61: 79–83. [Google Scholar]
- Kullman L. 1993. Tree limit dynamics of Betula pubescens ssp. tortuosa in relation to climate variability: evidence from central Sweden. Journal of Vegetation Science 4: 765–772. [Google Scholar]
- Ladinig U, Wagner J. 2005. Sexual reproduction of the high mountain plant Saxifraga moschata Wulfen at varying lengths of the growing season. Flora - Morphology, Distribution, Functional Ecology of Plants 200: 502–515. [Google Scholar]
- Larcher W. 2000. Temperature stress and survival ability of Mediterranean sclerophyllous plants. Plant Biosystems 134: 279–295. [Google Scholar]
- Larcher W. 2003. Physiological plant ecology: ecophysiology and stress physiology of functional groups. Berlin: Springer. [Google Scholar]
- Luza JG, Polito VS, Weinbaum SA. 1987. Staminate bloom date and temperature responses of pollen germination and tube growth in two walnut (Juglans) species. American Journal of Botany 74: 1898–1903. [Google Scholar]
- Marke T, Strasser U, Kraller G, et al. 2013. The Berchtesgaden National Park (Bavaria, Germany): a platform for interdisciplinary catchment research. Environmental Earth Sciences 69: 679–694. [Google Scholar]
- McKee J, Richards AJ. 1996. Variation in seed production and germinability in common reed (Phragmites australis) in Britain and France with respect to climate. New Phytologist 133: 233–243. [DOI] [PubMed] [Google Scholar]
- Meier U. 2001. Growth stages of plants . BBCH Monograph. Berlin: Blackwell Wissenschafts-Verlag Berlin. [Google Scholar]
- Meusel H, Jäger EJ, Weinert E. 1965. Vergleichende Chorologie der zentraleuropäischen Flora. Jena: VEB Gustav Fischer Verlag. [Google Scholar]
- Mondoni A, Pedrini S, Bernareggi G, et al. 2015. Climate warming could increase recruitment success in glacier foreland plants. Annals of Botany 116: 907–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdorfer E. 2001. Pflanzensoziologische Exkursionsflora. Stuttgart: Eugen Ulmer. [Google Scholar]
- Orme D, Freckleton R, Thomas G, et al. 2013. Caper: Comparative analysis of phylogenetics and evolution in R. http://CRAN.R-project.org/package=caper. [Google Scholar]
- Pagel M. 1999. Inferring the historical patterns of biological evolution. Nature 401: 877–884. [DOI] [PubMed] [Google Scholar]
- Parmesan C, Hanley ME. 2015. Plants and climate change: complexities and surprises. Annals of Botany 116: 849–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasonen HL, Käpylä M, Pulkkinen P. 2000. Effects of temperature and pollination site on pollen performance in Betula pendula Roth – evidence for genotype–environment interactions. Theoretical and Applied Genetics 100: 1108–1112. [Google Scholar]
- Pauli H, Gottfried M, Dullinger S, et al. 2012. Recent plant diversity changes on Europe’s mountain summits. Science 336: 353–355. [DOI] [PubMed] [Google Scholar]
- Peet MM, Willits DH, Gardner R. 1997. Response of ovule development and post-pollen production processes in male-sterile tomatoes to chronic, sub-acute high temperature stress. Journal of Experimental Botany 48: 101–111. [Google Scholar]
- Pigott CD. 1992. Are the distribution of species determined by failure to set seed? In: Marshall C, Grace J, eds. Fruit and seed production: aspects of development, environmental physiology and ecology. Cambridge: Cambridge University Press, 203–216. [Google Scholar]
- Pigott CD, Huntley JP. 1981. Factors controlling the distribution of Tilia cordata at the northern limits of its geographical range. III Nature and causes of seed sterility. New Phytologist 87: 817–839. [Google Scholar]
- Pigott CD, Warr SJ. 1989. Pollination, fertilization and fruit development in sycamore (Acer pseudoplatanus L.). New Phytologist 111: 99–103. [Google Scholar]
- Prentice IC, Cramer W, Harrison SP, et al. 1992. A global biome model based on plant physiology and dominance, soil properties and climate. Journal of Biogeography 19: 117–134. [Google Scholar]
- R Core Development Team. 2012. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Rosbakh S, Bernhardt-Römermann M, Poschlod P. 2014. Elevation matters: contrasting effects of climate change on the vegetation development at different elevations in the Bavarian Alps. Alpine Botany 124: 143–154. [Google Scholar]
- Sakai A, Larcher W. 1987. Frost survival of plants. Responses and adaptation to freezing stress. Berlin: Springer. [Google Scholar]
- Salisbury EJ. 1926. The geographical distribution of plants in relation to climatic factors. The Geographical Journal 67: 312–335. [Google Scholar]
- Steinacher G, Wagner J. 2012. Effect of temperature on the progamic phase in high-mountain plants. Plant Biology 14: 295–305. [DOI] [PubMed] [Google Scholar]
- Taschler D, Neuner G. 2004. Summer frost resistance and freezing patterns measured in situ in leaves of major alpine plant growth forms in relation to their upper distribution boundary. Plant, Cell & Environment 27: 737–746. [Google Scholar]
- Turnbull LA, Crawley MJ, Rees M. 2000. Are plant populations seed-limited? A review of seed sowing experiments. Oikos 88: 225–238. [Google Scholar]
- Wagner J, Steinacher G, Ladinig U. 2010. Ranunculus glacialis L.: successful reproduction at the altitudinal limits of higher plant life. Protoplasma 243: 117–128. [DOI] [PubMed] [Google Scholar]
- Weinbaum SA, Parfitt DE, Polito VS. 1984. Differential cold sensitivity of pollen grain germination in two Prunus species. Euphytica 33: 419–426. [Google Scholar]
- Woodward FI. 1975. The climatic control of the altitudinal distribution of Sedum rosea (L.) Scop. and S. telephium L. II. The analysis of plant growth in controlled environments. New Phytologist 74: 335–348. [Google Scholar]
- Woodward FI. 1987. Climate and plant distribution. Cambridge: Cambridge University Press. [Google Scholar]
- Woodward FI. 1997. Life at the edge: a 14-year study of a Verbena officinalis population’s interactions with climate. Journal of Ecology 85: 899–906. [Google Scholar]
- Woodward FI, Jones N. 1984. Growth studies of selected plant species with well-defined European distributions: I. Field observations and computer simulations on plant life cycles at two altitudes. Journal of Ecology 72: 1019–1030. [Google Scholar]
- Yin X, Kropff MJ. 1996. The effect of temperature on leaf appearance in rice. Annals of Botany 77: 215–221. [Google Scholar]
- Yin XY, Kropff MJ, Mclaren G, Visperas RM. 1995. A nonlinear model for crop development as a function of temperature. Agricultural and Forest Meteorology 77: 1–16. [Google Scholar]
- Zamir D, Tanksley SD, Jones RA. 1981. Low temperature effect on selective fertilization by pollen mixtures of wild and cultivated tomato species. Theoretical and Applied Genetics 59: 235–238. [DOI] [PubMed] [Google Scholar]
- Zinn KE, Tunc-Ozdemir M, Harper JF. 2010. Temperature stress and plant sexual reproduction: uncovering the weakest links. Journal of Experimental Botany 61: 1959–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.