Abstract
Background and aims Angiosperms display remarkable diversity in flower colour, implying that transitions between pigmentation phenotypes must have been common. Despite progress in understanding transitions between anthocyanin (blue, purple, pink or red) and unpigmented (white) flowers, little is known about the evolutionary patterns of flower-colour transitions in lineages with both yellow and anthocyanin-pigmented flowers. This study investigates the relative rates of evolutionary transitions between different combinations of yellow- and anthocyanin-pigmentation phenotypes in the tribe Antirrhineae.
Methods We surveyed taxonomic literature for data on anthocyanin and yellow floral pigmentation for 369 species across the tribe. We then reconstructed the phylogeny of 169 taxa and used phylogenetic comparative methods to estimate transition rates among pigmentation phenotypes across the phylogeny.
Key Results In contrast to previous studies we found a bias towards transitions involving a gain in pigmentation, although transitions to phenotypes with both anthocyanin and yellow taxa are nevertheless extremely rare. Despite the dominance of yellow and anthocyanin-pigmented taxa, transitions between these phenotypes are constrained to move through a white intermediate stage, whereas transitions to double-pigmentation are very rare. The most abundant transitions are between anthocyanin-pigmented and unpigmented flowers, and similarly the most abundant polymorphic taxa were those with anthocyanin-pigmented and unpigmented flowers.
Conclusions Our findings show that pigment evolution is limited by the presence of other floral pigments. This interaction between anthocyanin and yellow pigments constrains the breadth of potential floral diversity observed in nature. In particular, they suggest that selection has repeatedly acted to promote the spread of single-pigmented phenotypes across the Antirrhineae phylogeny. Furthermore, the correlation between transition rates and polymorphism suggests that the forces causing and maintaining variance in the short term reflect evolutionary processes on longer time scales.
Keywords: flower colour, anthocyanin, aurone, yellow flowers, comparative analysis, snapdragon, evolutionary transition, Antirrhineae, Antirrhinum, Linaria
INTRODUCTION
Colours in nature are important cues for organisms to signal warnings or rewards. In angiosperms, flower pigmentation is important for pollinator attraction and is linked to a suite of non-pollinator-related traits (Faegri and Van der Pijl, 1966; Strauss and Whittall, 2006). Transitions in flower colour across a phylogeny are common in many plant lineages, which allows us to draw conclusions about their mechanism and consequences from many replicated evolutionary events (Rausher, 2008; Streisfeld and Rausher, 2011; Wessinger and Rausher, 2012). Moreover, mutations causing flower colour changes are abundant and conspicuous, and the underlying synthetic pathways are well understood (Grotewold, 2006). Flower colour therefore provides an excellent model for the investigation of evolutionary change because we can link molecular genetic changes to the ecology and demography of the organism.
Floral pigments fall into only a handful of molecular families conserved across the angiosperms (Rausher, 2006). To date, most of what we know about flower colour transitions has focused on the gain or loss of one or more anthocyanin pigments, which confer red, blue, pink and purple colours (Grotewold, 2006; Rausher, 2008; Wessinger and Rausher, 2012). In the wild, mutations at a single transcription factor regulating structural anthocyanin synthesis enzymes have been shown to be sufficient to effect a gain or loss of anthocyanin pigmentation in Petunia (Quattrocchio et al., 1999), Antirrhinum (Schwinn et al., 2006), Aquilegia (Whittall et al., 2006) and Mimulus (Cooley et al., 2011; Streisfeld et al., 2013), although additional mutations may occur later (Zufall and Rausher, 2004). However, many plant lineages also include species with yellow flowers, with pigments derived from the carotenoid, aurone or betalainin pathways (Grotewold, 2006). Transitions involving the gain or loss of yellow pigments have been less well studied, but studies on carotenoid gain in Brassica and Chrysanthenum point to loss-of-function mutations at loci involved in the downstream degradation of pigments to colourless compounds (Ohmiya et al., 2006; Zhang et al., 2015). A pattern common to all floral colour transitions examined so far is that a mutation at a single locus is sufficient to cause a shift in flower colour.
We currently know little about the evolution of flower colour when both anthocyanin and yellow pigments are present. Different combinations of yellow and anthocyanin pigments give rise to four colour phenotypes (Fig. 1). A floral tissue may produce (1) neither pigment (white), (2) anthocyanin pigment only, (3) yellow pigment only or (4) anthocyanin and yellow pigments simultaneously, conferring an orange or red phenotype (Hackbarth et al., 1942; Stanton, 1987; Cooley et al., 2011; Streisfeld et al., 2013). The addition of a second pigment pathway makes transitions between yellow and anthocyanin pigmentation fundamentally different from those previously studied because they imply changes at a minimum of two loci; one pathway must be turned off and the other turned on. Alternatively, apparent ‘dual’ transitions might occur if a single mutation influences both pigment classes at once (qwd and qdw, Fig. 1) or if one pigment is masked by the other but becomes visible once that pigment is removed (qay and qya, Fig. 1). Groups of plants with both pigments provide considerable scope for interactions (e.g. epistasis and pleiotropy) that may constrain evolutionary transitions.
Fig. 1.
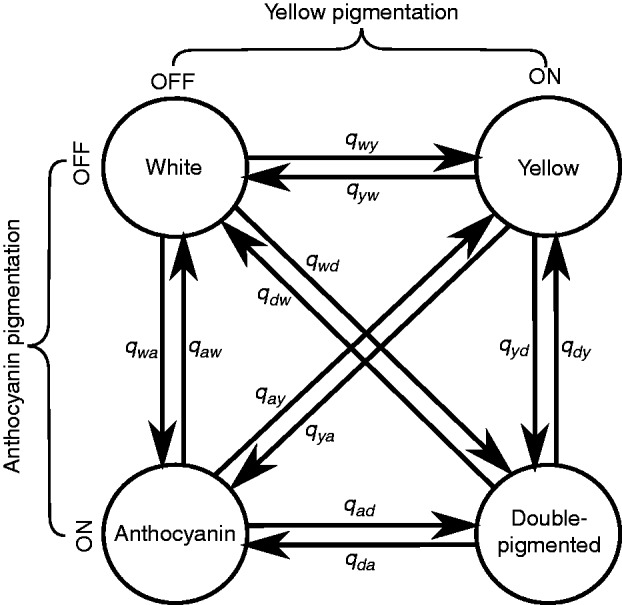
Model for the pigmentation state space and possible transitions. Combinations of alleles controlling anthocyanin and yellow pigments can give rise to four phenotypes. The transition rate from phenotype x to phenotype y across the phylogeny between two phenotypes is labelled qxy.
In this study we examine the history of transitions in flower colour in the tribe Antirrhineae. This group comprises approx. 370 mostly short-lived perennial herbs distributed throughout Eurasia and North America (Sutton, 1988). Floral pigmentation is present in 90 % of species, and pigment morphs are well distributed among genera (Sutton, 1988; Fig. 2). The genetic basis of pigmentation in the model snapdragon Antirrhinum majus has been studied for over a century, and is known to follow the two-dimensional model described above and in Fig. 1 (Wheldale, 1907; Baur, 1924; Whibley et al., 2006). Flower colour in A. majus is derived primarily from the magenta anthocyanin cyanidin and yellow aurone pigments (Geissman et al., 1954; Schwinn et al., 2006; Ono et al., 2006), and crosses between species show that homologous loci are present throughout the Old World Antirrhinum (Hackbarth et al., 1942). Studies beyond Antirrhinum have been limited, but work on Linaria, the largest genus in the tribe, have recovered similar pigments and genetic architectures (Tjebbes, 1929; Harborne, 1966; Valdés, 1970). Note that both aurones and anthocyanins are derived from chalcone pigments, whereas carotenoids and betalainins are not. There is therefore greater potential for pleiotropy and substrate competition between these pathways (Ono et al., 2006), which might not be the case for systems with carotenoid and betalainin pigmentation. Nevertheless, the abundant variation in pigmentation phenotypes in the Antirrhineae makes this tribe a promising testing ground for investigations of transitions between yellow and anthocyanin-pigmented floral phenotypes. Only one study has examined flower colour transitions in a phylogenetic context for the Antirrhineae. This study revealed a bias towards gain of anthocyanins (Smith and Goldberg, 2015). However, they considered only a section of the tribe, and did not consider yellow pigmentation.
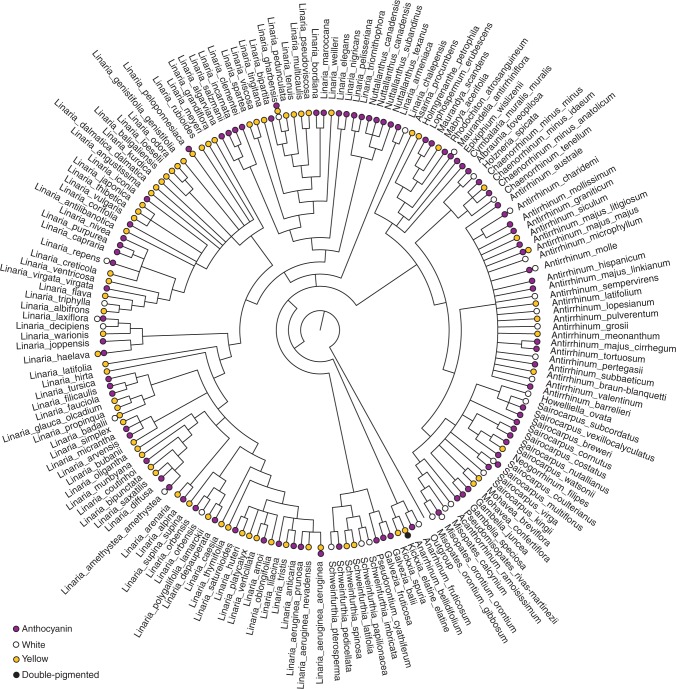
Fig. 2.
Majority-rule consensus tree of Antirrhineae taxa for which molecular data are available. Circles indicate floral colour phenotype. Polymorphic taxa have more than one circle.
Here we present the first attempt to examine patterns of flower colour transitions when two floral pigments underlie colour variation. We use phylogenetic comparative analyses of 169 species from the Antirrhineae to investigate the evolutionary processes acting on anthocyanin and yellow pigmentation. We reconstruct phylogenetic relationships and then estimate transition rates between colour phenotypes. Our results suggest that long-term evolutionary transitions occur at one pathway at a time, especially between anthocyanin-pigmented and unpigmented taxa. These results provide novel insights into the role of interactions between pigments in constraining the possible evolutionary pathways between flower colour phenotypes.
MATERIALS AND METHODS
Flower colour
We collected data on flower colour from classical taxonomic literature (Chavannes, 1833; Munz, 1926; Pennell, 1947; Rothmaler, 1956; Speta, 1980; Elisens, 1985; Fernandéz Cases, 1988; Sutton, 1988; Thompson, 1988; Güemes, 1994; Whibley et al., 2006) on all species given by Sutton (1988). The corollas of Antirrhinneae typically have a ‘major’ colour, but often have some secondary pigmentation, such as purple veins or a yellow palate. We recorded the primary colour throughout the face of the flower described by the authors as either white, yellow, anthocyanin-pigmented or double-pigmented. As it was not possible to differentiate red, blue, pink or purple clearly from many descriptions, we grouped these together as anthocyanin-pigmented. We classified ambiguous descriptions of pale flowers such as ‘whitish-pink’ or ‘whitish-yellow’ as anthocyanin-pigmented or yellow, respectively, as these cases represent low levels of pigmentation. In some cases, such as species of Mimulus, double-pigmented flowers appear red rather than orange (Cooley et al., 2011; Streisfeld et al., 2013). We could not distinguish single- and double-pigmented red flowers from taxonomic descriptions, and scored these as anthocyanin-pigmented phenotypes. However, because only 6·7 % of the taxa included in the phylogeny (below) are described as red, any true double-pigmented red morphs are unlikely to have a large effect on our results.
Colour descriptions in the taxonomic literature are often subjective and brief, but it is not feasible to collect spectral reflectance data for such a large, widely dispersed tribe. We took several measures to identify and exclude taxa with ambiguous records. Where possible (162 taxa) we cross-referenced flower-colour descriptions from multiple authors, and excluded two taxa with contradictory sources. We excluded two taxa with ambiguous descriptions among pigment types (Linaria pedunculata and Linaria albifrons), as well as three taxa with no information on flower colour (Linaria paradoxa, Misopates salvagense and Sairocarpus elmeri). We present colour descriptions of each author verbatim in the Supporting Information.
For discrete traits, the presence of polymorphic taxa presents an ongoing challenge for phylogenetic comparative analyses. Despite the presence of discrete polymorphisms within taxa in many phylogenies, there are presently no methods to explicitly account for this in a comparative framework. In the case of flower colour, polymorphism within taxa can occur within or among populations (e.g. Schemske and Bierzychudek, 2001; Hopkins and Rausher, 2012), and in some instances may represent an incipient transition. Alternatively, this may reflect a variant that has risen to sufficient frequency to be noted by taxonomists and may be transient with little effect on long-term evolutionary trajectories. Moreover, polymorphic taxa could introduce a spurious correlation between diversification and transition rates if a particular phenotype appears often in a polymorphism. In our dataset, we found 24 taxa described as polymorphic. However, there is no information available on the frequency or population distribution of alternative phenotypes in each species. Therefore, to account for this in the comparative analysis, we entered a separate entry for each colour. This effectively splits each species into two on the basis of the colour states present. We then ran all subsequent analyses on two separate datasets: one including all available taxa (polymorphic dataset), and another excluding the 24 polymorphic taxa (monomorphic dataset).
Phylogenetic reconstruction
We reconstructed the phylogenetic relationships for 169 Antirrhineae species using internal transcribed spacer sequences retrieved from GenBank (Benson, 2000). Details of sequencing and accession numbers are described in Fernández-Mazuecos et al. (2013). We added duplicate sequences for each colour morph for polymorphic taxa, meaning each morph is represented as a bifurcation at the tip of the tree with branch lengths of zero. For the monomorphic dataset, we pruned polymorphic taxa entirely. We aligned these sequences in MAFFT (Katoh and Standley, 2013) and estimated the phylogeny in MrBayes 3.2.2 (Ronquist and Huelsenbeck, 2003) using the GTR substitution model with gamma-distributed rate variation across sites. After a burn-in of 10 000 generations, we ran the Markov chain for 10 000 000 generations. We generated a sample of 1000 trees from the posterior distribution by sampling every 10 000 generations to avoid autocorrelation between trees. Including polymorphic taxa, flower colour data were available for 186 species, representing approximately half of the tribe.
Joint estimation of transition and diversification rates
We used the R package diversitree to estimate transition rates between states whilst accounting for possible differences in diversification rates among states (R Core Development Team, 2005; Maddison et al., 2007; FitzJohn et al., 2009; FitzJohn, 2012). We denote the transition rate from phenotype x to phenotype y as qxy, and label white, yellow, anthocyanin-pigmented and double-pigmented phenotypes w, y, a and d, respectively (Fig. 1). We compared two models of trait evolution. In the ‘constrained’ model we constrained dual transitions between white and double pigmentation (qwd, qdw) and between anthocyanin and yellow pigmentation (qay, qya) to zero. To account for the possibility of dual transitions being very rapid, or for one pigment masking another with low expression levels, we also ran a ‘full’ model with all 12 possible transitions between phenotype allowed. We ran each model for both polymorphic and monomorphic datasets.
Transition rate estimates can be biased if differences in speciation (λ) and extinction (μ) rate among states are not accounted for. However, it has recently been shown that these estimates are frequently subject to alarming biases due to correlations with unmeasured characters (Rabosky and Goldberg, 2015). We therefore fitted models which allow for different λ and μ among states so that transition rate estimates are not constrained by these, but confine our interpretation of results to flower-colour transition rates.
We found this dataset to be sensitive to the hill-climbing algorithm implemented in diversitree, which gave radically different results depending on the starting point. We therefore estimated transition rates among floral phenotypes using the MultiMusse Markoc chain Monte Carlo (MCMC) command in diversitree using an exponential prior with a mean of 0·1, and adjusting for taxon sampling. To account for phylogenetic uncertainty we ran the chain for 100 generations on a single tree, and then swapped to a new tree drawn at random from the sample of trees from MrBayes. We first allowed the chain to explore 100 trees as burn-in, and then stored the final generation of chains for each of 1000 subsequent trees. We estimated the marginal likelihood of the full and constrained models as the harmonic mean of the likelihood of each generation in the Markov chain (Newton and Raftery, 1994). This approach has been criticised because it is insensitive to changes in the prior (Friel and Wyse, 2012), but because we used a non-hierarchical model with a fixed prior, the estimator is appropriate. To test the significance of differences in support we applied a χ2-test with four degrees of freedom to the ratio of harmonic mean likelihoods for both models, corresponding to the difference in the number of parameters to be estimated under each model.
Asymmetry in rate estimates
Hypothesis testing for differences in rate parameters, such as asymmetry in the direction of transitions, is often approached by comparing the degree of support for two models using likelihood ratio tests (Pagel, 1994). However, an asymmetry can itself bias the test (Goldberg and Igić, 2008) and requires extensive model exploration, which is impractical for this dataset because of the need to use an MCMC. Instead we assess asymmetry in transition rates by comparing the vectors of parameter estimates from the posterior distributions of Markov chain outputs. The posterior probability (pp) that parameter x is greater than parameter y is simply the frequency of generations in which xi > yi (Kruschke, 2010).
RESULTS
We collected floral trait data on 343 monomorphic taxa, of which 27 were white, 144 were anthocyanin-pigmented, 170 were yellow and two were double pigmented. Of these taxa, 19, 70, 63 and one, respectively, could be included in phylogenetic analyses. We found descriptions of polymorphism in a further 23 taxa, including all four combinations of white, anthocyanin-pigmented and yellow morphs, as well as one yellow/double-pigmented polymorphism (Table 1, see Supplementary Data Table S1 for colour descriptions). Of these, white/anthocyanin polymorphisms account for more than all other combinations combined. A further three taxa were listed by Sutton (1988), but no colour information was given by any author. Yellow and anthocyanin-pigmented taxa are well distributed across the phylogeny (Fig. 2) and frequently occur as sister species. In this sample of taxa, white species are distributed sparsely across the tree and occur very rarely as closely related species.
Table 1.
Frequencies of colour phenotype combinations in polymorphic taxa in the whole tribe (total) and the taxa for which molecular data are available (phylogeny)
| Total | Phylogeny | |
|---|---|---|
| White/anthocyanin | 12 | 8 |
| White/yellow | 3 | 1 |
| Anthocyanin/yellow | 6 | 4 |
| Yellow/double pigmented | 1 | 1 |
| White/anthocyanin/yellow | 2 | 1 |
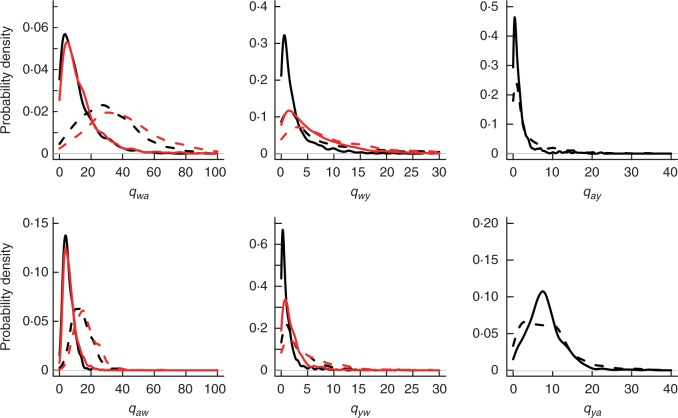
When only monomorphic taxa were included we found better point estimates for parameters, with narrower, more peaked posterior distributions (Fig. 3, Supplementary Data Fig. S1). This suggests that the phylogenetic signal was stronger when polymorphic taxa were excluded. Bayes factor comparisons for models of trait evolution revealed strong support for the constrained model that excludes dual transitions (diagonal transitions in Fig. 1), when only monomorphic taxa are considered (d.f. = 4, P < 0·0001). When polymorphic taxa are included in the dataset there was no difference in support for either the full or the constrained model (d.f. = 4, P = 0·315).
Fig. 3.
Posterior probability distribution of transition rate estimates between white, anthocyanin and yellow taxa. Black lines show the distribution under the full model, and red lines show distributions under the constrained model. Solid lines show distributions for monomorphic taxa only, whereas dashed lines are for datasets including polymorphic taxa.
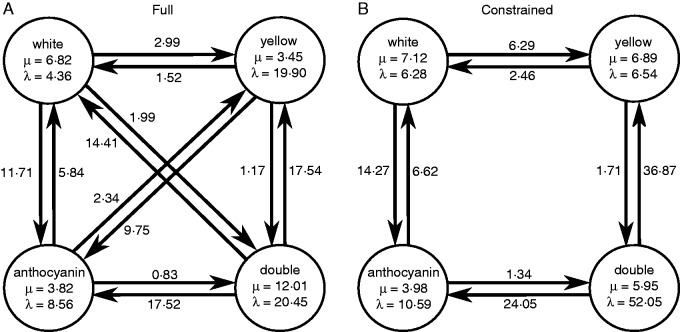
We found evidence for asymmetries in transition rates between taxa (Fig. 4, Table 2). The overall flux between white and anthocyanin pigmentation in both directions was two- to four-fold greater than those between white and yellow pigmentation across models and datasets. Transition rates away from white to anthocyanin pigmentation were twice those towards white, and this pattern had particularly strong support when polymorphic taxa were included. Similarly, transitions from white to yellow were twice as common as from yellow to white (Figs 3 and 4), but statistical support for this observation was equivocal (Table 2). Transitions from yellow to anthocyanin-pigmented phenotypes were around two- to four-fold higher than those towards yellow, but this asymmetry was much weaker when polymorphic taxa were included. Under all models and datasets, transitions away from double pigmentation to all other phenotypes were much greater than those towards double pigmentation (pp ≥ 0·936). Estimates of speciation and extinction varied, and showed no overall pattern of association with particular states (Supplementary Data Figs S2 and S3).
Fig. 4.
Parameter rate point estimates for monomorphic taxa under (A) the full and (B) constrained models. States and transitions correspond to those given in Fig. 1, and point estimates are the means of the posterior distributions.
Table 2.
Posterior probabilities of rate asymmetries for monomorphic and polymorphic datasets under the full and constrained models
| Hypothesis | Monomorphic |
Polymorphic |
||
|---|---|---|---|---|
| Full | Constrained | Full | Constrained | |
| qwa > qaw | 0·705 | 0·745 | 0·871 | 0·910 |
| qwy > qyw | 0·685 | 0·756 | 0·628 | 0·689 |
| qya > qay | 0·893 | – | 0·759 | – |
| qdw > qwd | 0·854 | – | 0·877 | – |
| qda > qad | 0·972 | 0·999 | 0·973 | 0·993 |
| qdy > qyd | 0·936 | 0·974 | 0·962 | 0·986 |
| qwa + qaw >qwy + qyw | 0·905 | 0·845 | 0·919 | 0·932 |
DISCUSSION
Transitions between flower colour phenotypes have been frequent in the Antirrhineae, and yet the distribution of possible phenotypes is strongly biased towards yellow and anthocyanin-pigmented species. In this study we have used phylogenetic comparative methods and accounted for phylogenetic uncertainty to investigate the patterns in floral colour transitions that have contributed to this heterogeneity. Interestingly, when only monomorphic taxa were considered we found greater support for a model that allowed substitutions at one pigment pathway at a time. However, when polymorphic taxa were included there was no difference in support for this model or one allowing apparent dual transitions causing apparent substitutions at two pathways simultaneously. This suggests that many of the inferred dual transitions are due to the effect of polymorphism, representing potentially transient events at the tips of the tree. Transitions among monomorphic taxa, by contrast, are more likely to represent completed transitions deeper in the tree, and better reflect long-term evolutionary patterns. This is confirmed by the markedly greater phylogenetic signal for the monomorphic dataset. Greater support for the constrained model implies that transitions in flower colour phenotypes occur primarily via stepwise substitutions at each pigment pathway, with a detectable waiting time in each state. This is consistent with the observation that while mutations arise just as frequently in structural and regulatory loci, transitions in flower colour on evolutionary timescales are typically due to mutations in genes regulating specific pigment pathways (Streisfeld and Rausher, 2011; Wessinger and Rausher, 2012). By examining the finer patterns of transitions we can use these data to elucidate the possible mechanisms that generate differences in transition rates.
Low overall transition rates will hinder the distribution of phenotypes from reaching equilibrium. Our results suggest that the frequency of transitions is not a limiting factor in shifts between unpigmented and single-pigmented flowers, consistent with previous observations in Ipomoea (Smith et al., 2010). Yellow and anthocyanin-pigmented taxa frequently occur as sister taxa across the phylogeny, and transition rate estimates indicate frequent transitions between these colours via white. In contrast, the paucity of double-pigmented taxa and the very low estimates of qwd, qad and qyd suggest that transitions to double pigmentation are very rare. It is nevertheless possible that double-pigmented taxa do indeed arise but rapidly go extinct, or that double pigmentation appears only fleetingly before one of the two pigments is lost. We found five species with yellow/anthocyanin polymorphisms, where recombination between pigmentation genes could lead to double-pigmented morphs, but this appears not to be common. Double-pigmented flowers do arise in the wild via hybridization between yellow and magenta populations of Antirrhinum majus, but are confined to narrow hybrid zones, suggesting that some selective mechanism prevents their spread (Whibley et al., 2006). It may be that there is a higher cost associated with producing multiple pigments, or that pollinators discriminate against double-pigmented taxa, as has been shown in Raphanus sativus (Stanton, 1987). Thus, although direct evidence is lacking, it is likely that some form of natural selection prevents the spread of double-pigmented colour morphs in the wild, and prevents transitions to double pigmentation across the phylogeny.
We found evidence for asymmetries in the direction of flower colour transitions leading to the gain of pigmentation. Transitions away from white to yellow and especially anthocyanin pigmentation were greater than transitions to unpigmented flowers. Although statistical support was weak, this observation contrasts with other systems that have found a bias in transitions towards white from anthocyanin pigmentation, which are often irreversible (Rausher, 2006; Whittall et al., 2006; Smith et al., 2010). Nevertheless, Cooley et al. (2011) demonstrated parallel gains of anthocyanin pigmentation in two species of the luteus group of Mimulus. This result also mirrors the findings of Smith and Goldberg (2015), who also found an asymmetry in gains of anthocyanins in a subset of the Antirrhineae, and showed that this asymmetry was stronger than in three other tribes examined. Our data show that this pattern is general across the whole tribe, and holds for yellow pigmentation as well. This asymmetry, coupled with the overall paucity of white taxa, suggests that some kind of selective mechanism acts to promote the spread of pigmented morphs in white species.
Investigations into the maintenance of flower colour polymorphisms have revealed that the agents and mechanisms of selection are diverse and may depend on local environmental and demographic conditions. Some studies have shown that white phenotypes are visited less often by pollinators (e.g. Waser and Price, 1981), more sensitive to drought stress (Warren and Mackenzie, 2001) and associated with greater inbreeding depression under stressful conditions (Burdon et al., 1983). In contrast, others have found that white morphs are preferred by pollinators (Stanton, 1987) or show higher growth rates than anthocyanin-pigmented morphs under well-watered conditions (Schemske and Bierzychudek, 2001; Warren and Mackenzie, 2001). The phenotypes observed on the tips of a phylogeny today are the cumulative result of processes acting over long-term evolutionary time scales, providing ample scope for fluctuating selection to favour different morphs through time. Fluctuating selection that disfavours white morphs on average will tend to increase extinction rates among white species, increasing the rate of transitions to pigmented flowers and may ensure the persistence of white taxa at low frequency.
In our study, the greater support for the constrained model is consistent with a model of repeated substitutions at single loci influencing the anthocyanin and aurone pathways independently. Flower colour polymorphisms often have a simple genetic basis, with molecular studies identifying a strong tendency for transitions to be driven by changes at a single regulatory gene (reviewed by Wessinger and Rausher, 2012). Indeed, anthocyanin pigmentation in Antirrhinum majus is controlled primarily by a single MYB-transcription factor (Schwinn et al., 2006). Alleles at flower colour loci throughout the genus Antirrhinum segregate as Mendelian traits, explaining the majority of pigment variation across the flower (Wheldale, 1907; Baur, 1924; Hackbarth et al., 1942). Support for the constrained model is consistent with a model of repeated substitutions at single loci influencing the anthocyanin and aurone pathways independently. This stepwise model of pigment evolution also suggests that although anthocyanins and aurones are both derived from chalcones (Ono et al., 2006), mutations tend to be specific to a single pathway with little competition for substrates.
For Mendelian traits in a monomorphic population (i.e. with no standing variation), a major constraint on adaptation is the waiting time for a new mutation to arise. As there are many more ways for mutation to cause a loss-of-function than gain-of-function mutation, one would expect a bias towards pigment losses. The apparent abundance of gain-of-function mutations observed in the Antirrhineae may be due to repeated introgression of adaptive alleles via rare hybridization events with closely related taxa, especially because homologous loci seem to control pigmentation across multiple species (Hackbarth et al., 1942). A recent study showed that the repeated evolution of red flowers among subspecies of Mimulus aurantiacus was due to adaptive introgression of an allele of the transcription factor MaMyb2 (Stankowski and Streisfeld, 2015). If a similar process occurs in Antirrhineae, adaptive introgression would accelerate transition rates between phenotypic states, and allow for more rapid adaptive shifts to changing environments.
Our survey of the Antirrhineae revealed a marked heterogeneity in the abundances of different polymorphism types. More than half of the species reported to be polymorphic for flower colour have white and anthocyanin morphs, while only three taxa have white and yellow morphs (Table 1). This relative abundance of anthocyanin polymorphisms may be due to the much longer length of the anthocyanin pathway compared with the aurone pathway, and hence the greater mutational target size (Richards, 1997; Nakayama et al., 2000). A survey of the British flora revealed a similar pattern (Warren and Mackenzie, 2001), and our results suggest that this trend is general throughout Eurasia and North America for this group. Interestingly, this abundance of anthocyanin/white polymorphism is mirrored by consistently higher estimates of transitions between these phenotypes across models and datasets (Table 2). The abundance of allelic variants in the anthocyanin pathway may be a major driver of the high rates of transition between anthocyanin and white flowers. This suggests that the processes causing and maintaining variation in the short term may reflect evolutionary processes on longer time scales.
These findings highlight three areas which future efforts might focus on to improve our understanding of the evolution of floral pigmentation. Firstly, increased attention is needed to quantify the relative costs and benefits of yellow and anthocyanin pigments. This would elucidate mechanisms that might promote the spread of single-pigmented phenotypes, and whether there is some cost to double pigmentation that prevents its establishment. Secondly, we need a better understanding of the genetic architecture of flower colour transitions. Genomic approaches should provide the opportunity to sequence flower-colour genes for many species and populations. This will enable us to determine whether multiple independent alleles at these loci segregate within species or genera, and whether these alleles are able to introgress and cause multiple shifts in flower colour. Finally, the correlation between polymorphism and transition rates reflects the gradual rather than binary nature of evolutionary transitions, and highlights the need for phylogenetic comparative methods to explicitly incorporate information on phenotype or allele frequencies. Novel insight into flower-colour evolution is likely to come from considering pigmentation biology at the molecular, organismal and population levels.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Figure S1: posterior probability distributions for transition rate estimates. Figure S2: posterior probability distributions for extinction rates. Figure S3: posterior probability distributions for speciation rates. Table S1: taxon colour descriptions.
ACKNOWLEDGEMENTS
We thank Melinda Pickup, Spencer Barrett, Nick Barton and four anonymous reviewers for helpful discussions on previous versions of this manuscript. We also thank Jana Porsche for her efforts in tracking down the more obscure references.
LITERATURE CITED
- Baur E. 1924. Untersuchungen über das Wesen, Entstehung und die Vererbung von Rassenunterschieden bei Antirrhinum majus. Leipzig: Gebrüder Bornträger. [Google Scholar]
- Benson DA, Karsch-Mizrachi I, Lipman DJ, Ostell J, Rapp BA, Wheeler DL. 2000. GenBank. Nucleic Acids Research 28: 15–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdon JJ, Marshall DR, Brown AHD. 1983. Demographic and genetic changes in populations of Echium plantagineum. Journal of Ecology 71: 667–679. [Google Scholar]
- Chavannes EL. 1833. Monographie des Antirrhinées. Paris: Treuttel et Würtz. [Google Scholar]
- Cooley AM, Modliszewski JL, Rommel ML, Willis JH. 2011. Gene duplication in Mimulus underlies parallel floral evolution via independent trans-regulatory changes. Current Biology 21: 700–704. [DOI] [PubMed] [Google Scholar]
- Elisens WJ. 1985. Monograph of the Maurandyinae (Scrophulariaceae-Antirrhineae). Systematic Botany Monographs 5: 1–97. [Google Scholar]
- Faegri K, Van der Pijl L. 1966. The principles of pollination ecology. Oxford: Pergamon Press. [Google Scholar]
- Fernandéz Cases J. 1988. Asientos para una flora occidental. Fontqueria 20: 63. [Google Scholar]
- Fernández-Mazuecos M, Blanco-Pastor JL, Vargas P. 2013. A phylogeny of toadflaxes (Linaria Mill.) based on nuclear internal transcribed spacer sequences: systematic and evolutionary consequences. International Journal of Plant Science 174: 234–249. [Google Scholar]
- FitzJohn RG. 2012. Diversitree: comparative phylogenetic analyses of diversification in R. Methods in Ecology and Evolution 3: 1084–1092. [Google Scholar]
- FitzJohn RG, Maddison WP, Otto SP. 2009. Estimating trait-dependent speciation and extinction rates from incompletely resolved phylogenies. Systematic Biology 58: 595–611. [DOI] [PubMed] [Google Scholar]
- Friel N, Wyse J. 2012. Estimating the evidence: a review. Statistica Neerlandica 66: 288–308. [Google Scholar]
- Geissman T, Jorgensen EC, Johnson BL. 1954. The chemistry of flower pigmentation in Antirrhinum majus color genotypes. I. The flavonoid components of the homozygous P, M, Y color types. Archives of Biochemistry and Biophysics 49: 368–388. [DOI] [PubMed] [Google Scholar]
- Goldberg EE, Igić B. 2008. On phylogenetic tests of irreversible evolution. Evolution 62: 2727–2741. [DOI] [PubMed] [Google Scholar]
- Grotewold E. 2006. The genetics and biochemistry of floral pigments. Annual Review of Plant Biology 57: 761–780. [DOI] [PubMed] [Google Scholar]
- Güemes J. 1994. Antirrhinum subbaeticum Güemes, Mateu & Sánchez-Gómez (Scrophulariaceae), especie nueva de la península Ibérica. Anales del Jardin Botanico de Madrid 51: 237–247. [Google Scholar]
- Hackbarth J, Michaelis P, Scheller G. 1942. Untersuchungen an dem Antirrhinum-Wildsippensortiment von E. Baur. Zeitschrift für induktive Abstammung und Vererbungslehre 80: 1–102. [Google Scholar]
- Harborne J. 1966. Comparative biochemistry of flavonoids I.: Distribution of chalcone and aurone pigments in plants. Phytochemistry 5: 111–115. [Google Scholar]
- Hopkins R, Rausher MD. 2012. Pollinator-mediated selection on flower color allele drives reinforcement. Science 335: 1090–1092 [DOI] [PubMed] [Google Scholar]
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30: 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruschke J. 2010. Doing Bayesian data analysis: a tutorial introduction with R. Oxford: Academic Press. [Google Scholar]
- Maddison WP, Midford PE, Otto SP. 2007. Estimating a binary character’s effect on speciation and extinction. Systematic Biology 56: 701–710. [DOI] [PubMed] [Google Scholar]
- Munz PA. 1926. The Antirrhinoideae–Antirrhineae of the New World. Proceedings of the Californian Academy of Science 15: 323–397. [Google Scholar]
- Nakayama T, Yonekura-Sakakibara K, Sato T. et al. 2000. Aureusidin synthase: a polyphenol oxidase homolog responsible for flower coloration. Science 290: 1163–1166. [DOI] [PubMed] [Google Scholar]
- Newton MA, Raftery AE. 1994. Approximate Bayesian inference with the weighted likelihood bootstrap. Journal of the Royal Statistical Society. Series B (Methodological) 56: 3–48. [Google Scholar]
- Ohmiya A, Kishimoto S, Aida R, Yoshioka S, Sumitomo K. 2006. Carotenoid cleavage dioxygenase (CmCCD4a) contributes to white color formation in Chrysanthemum petals. Plant Physiology 142: 1193–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono E, Fukuchi-Mizutani M, Nakamura N. et al. 2006. Yellow flowers generated by expression of the aurone biosynthetic pathway. Proceedings of the National Academy of Sciences 103: 11075–11080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagel M. 1994. Detecting correlated evolution on phylogenies: a general method for the comparative analysis of discrete characters. Proceedings of the Royal Society of London B 255: 37–45. [Google Scholar]
- Pennell FW. 1947. Some hitherto undescribed Scrophulariaceae of the Pacific states. Proceedings of the Academy of Natural Sciences of Philadelphia 99: 155–199. [Google Scholar]
- Quattrocchio F, Wing J, van der Woude K. et al. 1999. Molecular analysis of the anthocyanin2 gene of Petunia and its role in the evolution of flower color. The Plant Cell Online 11: 1433–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Development Team. 2005. R: A language and environment for statistical computing Vienna: R Foundation for Statistical Computing. http://www.R-project.org.
- Rabosky DL, Goldberg EE. 2015. Model inadequacy and mistaken inferences of trait-dependent speciation. Systematic Biology 64: 340–355. [DOI] [PubMed] [Google Scholar]
- Rausher MD. 2006. The evolution of flavonoids and their genes In Grotewald E, ed. The science of flavonoids. London: Springer, 175–211. [Google Scholar]
- Rausher MD. 2008. Evolutionary transitions in floral color. International Journal of Plant Science 169: 7–21. [Google Scholar]
- Richards AJ. 1997. Plant breeding systems. London: Chapman & Hall. [Google Scholar]
- Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. [DOI] [PubMed] [Google Scholar]
- Rothmaler W. 1956. Taxonomische Monographie der Gattung Antirrhinum. Berlin: Akademie Verlag. [Google Scholar]
- Schemske DW, Bierzychudek P. 2001. Evolution of flower color in the desert annual Linanthus parryae: Wright revisited. Evolution 55: 1269–1282. [DOI] [PubMed] [Google Scholar]
- Schwinn K, Venail J, Shang Y. et al. 2006. A small family of MYB-regulatory genes controls floral pigmentation intensity and patterning in the genus Antirrhinum. The Plant Cell 18: 831–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SD, Goldberg EE. 2015. Tempo and mode of flower color evolution. American Journal of Botany 102: 1014–1025. [DOI] [PubMed] [Google Scholar]
- Smith SD, Miller RE, Otto SP, FitzJohn RG, Rausher MD. 2010. The effects of flower color transitions on diversification rates in morning glories (Ipomoea subg. Quamoclit, Convolvulaceae). In: Long M, Gu H, Zhou Z, eds. Darwin’s heritage today. Beijing: Higher Education Press, 202–226. [Google Scholar]
- Speta F. 1980. Die Gattungen Chaenorhinum (DC.) REICHENB. und Micorrhinum (ENDL.) FOURR. im östlichen Teil ihrer Areale (Balkan bis Indien). Stapfia 7: 1–72. [Google Scholar]
- Stankowski S, Streisfeld MA. 2015. Introgressive hybridization facilitates adaptive divergence in a recent radiation of monkeyflowers. Proceedings of the Royal Society of London B 282: 20151666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton ML. 1987. Reproductive biology of petal color variants in wild populations of Raphanus sativus: I. Pollinator response to color morphs. American Journal of Botany 7: 178–187. [Google Scholar]
- Strauss SY, Whittall JB. 2006. Non-pollinator agents of selection on floral traits. Oxford: Oxford University Press. [Google Scholar]
- Streisfeld MA, Rausher MD. 2011. Population genetics, pleiotropy, and the preferential fixation of mutations during adaptive evolution. Evolution 65: 629–642. [DOI] [PubMed] [Google Scholar]
- Streisfeld MA, Young WA, Sobel JM. 2013. Divergent selection drives genetic differentiation in an R2R3-MYB transcription factor that contributes to incipient speciation in Mimulus aurantiacus. PLOS Genetics 9: e1003385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton DA. 1988. A revision of the tribe Antirrhineae. London: British Museum (Natural History; ). [Google Scholar]
- Thompson D. 1988. Systematics of Antirrhinum (Scrophulariaceae) in the New World. Systematic Botany Monographs 22: 1–142. [Google Scholar]
- Tjebbes K. 1929. Species crosses in the genus Linaria. Beretning on det 18. skandinav. Naturforskermøde 529–534. [Google Scholar]
- Valdés B. 1970. Flavonoid pigments in flower and leaf of the genus Linaria. Phytochemistry 9: 1253–1260. [Google Scholar]
- Warren J, Mackenzie S. 2001. Why are all colour combinations not equally represented as flower-colour polymorphisms? New Phytologist 151: 237–241. [DOI] [PubMed] [Google Scholar]
- Waser NM, Price MV. 1981. Pollinator choice and stabilizing selection for flower color in Delphinium nelsonii. Evolution 35: 376–390. [DOI] [PubMed] [Google Scholar]
- Wessinger CA, Rausher MD. 2012. Lessons from flower colour evolution on targets of selection. Journal of Experimental Botany 63: 5741–5749. [DOI] [PubMed] [Google Scholar]
- Wheldale M. 1907. The inheritance of flower colour in Antirrhinum majus. Proceedings of the Royal Society of London B 79: 288–305. [Google Scholar]
- Whibley AC, Langlade NB, Andalo C. et al. 2006. Evolutionary paths underlying flower color variation in Antirrhinum. Science 313: 963–966. [DOI] [PubMed] [Google Scholar]
- Whittall JB, Voelckel C, Kliebenstein DJ, Hodges SA. 2006. Convergence, constraint and the role of gene expression during adaptive radiation: floral anthocyanins in Aquilegia. Molecular Ecology 15: 4645–4657. [DOI] [PubMed] [Google Scholar]
- Zhang B, Liu C, Wang Y. et al. 2015. Disruption of a CAROTENOID CLEAVAGE DIOXYGENASE 4 gene converts flower colour from white to yellow in Brassica species. New Phytologist 206:1513–1526. [DOI] [PubMed] [Google Scholar]
- Zufall RA, Rausher MD. 2004. Genetic changes associated with floral adaptation restrict future evolutionary potential. Nature 429: 847–850. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.