Abstract
Background and Aims Nickel (Ni)-hyperaccumulating species produce high-Ni litters and may potentially influence important ecosystem processes such as decomposition. Although litters resembling the natural community conditions are essential in order to predict decomposition dynamics, decomposition of mixed-species litters containing hyperaccumulated Ni has never been studied. This study aims to test the effect of different litter mixtures containing hyperaccumulated Ni on decomposition and Ni release across serpentine and non-serpentine soils.
Methods Three different litter mixtures were prepared based on the relative abundance of the dominant species in three serpentine soils in the island of Lesbos, Greece where the Ni-hyperaccumulator Alyssum lesbiacum is present. Each litter mixture decomposed on its original serpentine habitat and on an adjacent non-serpentine habitat, in order to investigate whether the decomposition rates differ across the contrasted soils. In order to make comparisons across litter mixtures and to investigate whether additive or non-additive patterns of mass loss occur, a control non-serpentine site was used. Mass loss and Ni release were measured after 90, 180 and 270 d of field exposure.
Key Results The decomposition rates and Ni release had higher values on serpentine soils after all periods of field exposure. The recorded rapid release of hyperaccumulated Ni is positively related to the initial litter Ni concentration. No differences were found in the decomposition of the three different litter mixtures at the control non-serpentine site, while their patterns of mass loss were additive.
Conclusions Our results: (1) demonstrate the rapid decomposition of litters containing hyperaccumulated Ni on serpentine soils, indicating the presence of metal-tolerant decomposers; and (2) imply the selective decomposition of low-Ni parts of litters by the decomposers on non-serpentine soils. This study provides support for the elemental allelopathy hypothesis of hyperaccumulation, presenting the potential selective advantages acquired by metal-hyperaccumulating plants through litter decomposition on serpentine soils.
Keywords: Litter mixtures, Ni release, hyperaccumulation, additive interactions, Alyssum lesbiacum, ultramafic soils
INTRODUCTION
Serpentine (ultramafic) soils are stressful environments for plant growth due to multiple limitations posed by their physical characteristics (e.g. low soil moisture-holding capacity) and especially by their chemical composition (low Ca/Mg molar quotients, low concentrations of macronutrients, elevated concentrations of heavy metals such as Ni, Cr and Co; Kazakou et al., 2008). Abiotic stress seems to drive low productivity levels of serpentine relative to non-serpentine habitats, especially in ecosystems where water is not the major determinant of plant productivity (Alexander et al., 2007; Adamidis et al., 2014a). However, less is known about the effect of abiotic stress on other important ecosystem processes, such as litter decomposition, determining carbon (C) and nutrient recycling in ecosystems and controlling C fluxes between the soil and the atmosphere.
Stressful conditions of serpentine soils may influence litter decomposition directly by affecting decomposer communities and indirectly by affecting litter quality (due to the high heavy metal concentrations of serpentine plants) (Kazakou et al., 2008). Heavy metal addition in soils in general has a negative effect on soil microbial communities and thus slows down litter decomposition (reviewed by Giller et al., 1998). Azarbad et al. (2013) recorded negative effects on the structure and functioning of microbial communities, across two metal pollution gradients. However, it has been hypothesized that serpentine soils may host Ni-tolerant fungal and bacterial communities (Amir and Pineau, 1998a, b; Boyd and Martens, 1998; Boyd, 2007) that may facilitate the decomposition of litter containing hyperaccumulated Ni. In this context, a higher proportion of Ni-resistant bacteria has been documented close to the Ni-hyperaccumulators Sebertia acuminate (Schlegel et al., 1991) and Alyssum bertolonii (Mengoni et al., 2001) than in free soil.
Although metal-hyperaccumulating plants (with metal concentrations in their dry mass up to 100 times higher than in normal plants) produce litter with a high metal concentration (Reeves and Baker, 2000), the decomposition of litter with hyperaccumulated metals has rarely been studied in the field (but see Boyd et al., 2008). The hypothesis of interference with neighbouring plants, also called elemental allelopathy (Boyd and Martens 1998), refers to the ability of a plant to prevent or impair the growth of another species, by releasing high levels of metals through litter decomposition (Boyd and Martens 1992). Thus, metal-hyperaccumulating plants acquire a selective advantage through the important process of litter decomposition. According to this hypothesis, the restriction of the majority of metal-hyperaccumulating plants in metaliferous soils and/or the exclusive expression of the hyperaccumulation phenotype on serpentine soils for facultative hyperaccumulators may be dependent on the litter decomposition process. In other words, if litter containing hyperaccumulated Ni decomposes inadequately on non-serpentine soils and thus Ni release is slow in relation to serpentine soils, the hyperaccumulating plants may lack the selective advantage described by the elemental allelopathy hypothesis of hyperaccumulation. So far little research on the hypothesis of elemental allelopathy has been conducted and the few studies that have tested this hypothesis experimentally did not use systems resembling the natural litter decomposition process (e.g. insufficient time for decomposition, grinding and sieving leaf biomass, mixing biomass and soil to homogeneity) (e.g. Zhang et al., 2005, 2007).
In natural communities, plant litter consists of more than one species, and hence decomposition of plant litter mixtures has been documented to be more frequently accelerated or slowed down when compared with that expected from single-species estimates, i.e. non-additive effects (Gartner and Gardon, 2004). Litter diversity is expected to generate synergistic effects on mass loss (observed values higher than predicted) through two major mechanisms: (1) transfer of nutrients from leaves with higher to leaves with lower concentrations of nutrients may enhance the decomposition rate of the latter (Wardle et al., 1997); and (2) high rates of moisture retention by some litter materials may benefit associated materials (Wardle et al., 2003). In contrast, compounds such as tannins and polyphenols may have an antimicrobial effect (McArthur et al., 1994; Nilsson et al., 1998; Schimel et al., 1998) and/or inhibit colonization by fungi (Harrison, 1971), thus generating antagonistic interactions. Oil vesicles in Eucalyptus globulus leaf are also found to inhibit fungal growth and may have an effect on the prolonged decomposition of eucalypt leaves on streams (Canhoto et al., 2002). Hyperaccumulated Ni is also expected to generate antagonistic effects on mass loss (observed values lower than predicted) because of the toxic effect of Ni on decomposers (Hoiland, 1995; Oorts et al., 2007). Boyd et al. (2008) recorded the highest Ni release during the decomposition of the leaf litter with the highest Ni concentration, indicating that the intensity of Ni release is positively related to the level of hyperaccumulated Ni. Thus, it is expected that litter with high Ni concentrations will have a stronger inhibitory effect on decomposer communities which will finally result in higher undecomposed litter mass.
In the present study, we focus on the decomposition dynamics and Ni release during decomposition of mixed-species litters containing hyperaccumulated Ni across serpentine and non-serpentine sites. In addition, we aim to determine whether additive or non-additive interactions occur on mixed-species litters. To our knowledge, this study is the first to report on the decomposition dynamics of mixed-species litters containing hyperaccumulated Ni under contrasted conditions. More precisely, this study focuses on the serpentine endemic Alyssum lesbiacum which has been established as an Ni hyperaccumulator (Brooks et al., 1979; Reeves et al., 1997; Kazakou et al., 2010). It has been demonstrated that A. lesbiacum presents an intra-specific variation of Ni hyperaccumulation across the different populations found on serpentine soils of the island of Lesbos (eastern Mediterranean) (Kazakou et al., 2010; Adamidis et al., 2014b). This significant intra-specific variation in leaf Ni concentrations of A. lesbiacum is particularly useful for studying the effects of hyperaccumulated Ni on decomposition of mixed-species litters, because the different populations not only present different relative abundances for this species but also support different leaf hyperaccumulated Ni concentrations. The hypotheses tested were that (1) the decomposition of mixed-species litters containing hyperaccumulated Ni will be accelerated on serpentine sites compared with non-serpentine sites and thus litter mass remaining on serpentine soils will be lower in relation to non-serpentine soils; (2) the Ni release will be higher on serpentine soils compared with non-serpentine soils and will depend on the initial litter hyperaccumulated Ni; (3) litter mixtures with higher concentrations of hyperaccumulated Ni will slow down litter decomposition and will thus have a higher litter mass remaining on a control non-serpentine site; and (4) non-additive patterns of mass loss, with antagonistic effects emerging on litter mixtures containing high concentrations of hyperaccumulated Ni, will be revealed.
MATERIALS AND METHODS
Study sites and material collection
This study was conducted at three sites (Ampeliko, AM; Olympos, OL; Loutra, LO) located in the central and south-eastern part of the island of Lesbos (Greece). In each of the three sites, a serpentine and an adjacent non-serpentine locality (six localities in total) were chosen for comparisons. An additional non-serpentine site (Xenia) was also selected and used as the control site, in order to make comparisons between three different mixed-species litters and to investigate whether additive or non-additive patterns of mass loss occur. The serpentine and non-serpentine character of the selected localities has been confirmed by soil analysis (Kazakou et al., 2010). The serpentine localities present higher values of soil metals (Kazakou et al., 2010) and lower soil pH values (Adamidis et al., 2014a) than non-serpentine soils. The selection of serpentine localities was based on the presence of large populations of the serpentine-endemic and Ni-hyperaccumulating species A. lesbiacum and was designed to cover a wide elevational range of serpentine environments (10–760 m above sea level) across the island and to include an accessible adjacent (0·6–7 km) non-serpentine area with similar disturbance history and climatic conditions. Despite the lack of A. lesbiacum on non-serpentine sites, the vegetation physiognomy of all sites was similar. A detailed description of the sites is given in Adamidis et al. (2014a) and in Kazakou et al. (2010).
Leaf litter was collected from the dominant species (sensu Garnier et al., 2004) occurring on localities within serpentine environments where A. lesbiacum was abundant (according to Kazakou et al., 2010). More precisely, at the AM serpentine locality, we collected three dominant species (A. lesbiacum, Plantago lagopus and Hordeum bulbosum), at the LO serpentine locality we collected four dominant species (A. lesbiacum, P. lagopus, Aegilops biuncialis and Crepis commutata), and at the OL serpentine locality we collected two dominant species (A. lesbiacum and C. commutata). The contribution of each dominant species was also confirmed by the approximate composition of freshly produced litter samples (10 × 10 cm) in serpentine plots where A. lesbiacum was very abundant. For A. lesbiacum that sheds its leaves once they senesce, plants were gently shaken and the dead leaves that dropped were collected. In species that retain dead leaves on the plant (A. biuncialis, C. commutata. H. bulbosum and P. lagopus), dead leaves were cut off from the standing plant. After collection of the plant material, in order to deter decay processes, leaves were immediately cleaned, air-dried at 40 °C until constant weight was reached and then stored at room temperature.
Litterbag preparation and experimental design
We prepared three different mixed-species litters based on the relative contribution of each dominant species to the total biomass of the dominant species in each serpentine community (species loading ratios are given in Table 1). Our goal was to generate litters resembling the natural community conditions (Gartner and Cardon, 2004; Bonanomi et al., 2010) and not to produce litters with artificial species loading ratios that could create gradients of litter hyperaccumulated Ni. Approximately 1 g (± 0·015 g) of dried plant material was placed in every litterbag (10 × 10 cm), recording the exact mass of plant material used. Litterbags were made from a plastic mesh with 0·5 mm holes, allowing access to decomposers but excluding large invertebrates. For each different mixed-species litter, 90 replicate litterbags were prepared and allowed to decompose at their original serpentine site (30 replicates), at an adjacent non-serpentine site (30 replicates) and at the Xenia site (30 replicates). Single-species litters were also prepared for each dominant species of each serpentine locality in order to test the non-additive hypothesis. For each single-species litter, 12 replicate litterbags were prepared and placed at the Xenia site. Ten replicate samples for each mixed-species litterbag and four replicate samples for each single-species litterbag were recovered from each site after 90, 180 and 270 d of exposure in the field. All samples were air-dried and re-weighed in order to determine the remaining mass and the Ni concentration of the litters.
Table 1.
Composition, species loading ratios and initial Ni concentration of the three litter mixtures
| Litter mixture | Initial Ni concentration (mg kg–1) | Species loading ratios (%) |
|---|---|---|
| AM | 11 937 | Al, Pl, Hb (88:9:3) |
| OL | 10 927 | Al, Cc (99:1) |
| LO | 2863 | Al, Pl, Ab, Cc (47:25:10:18) |
Species abbreviations are Al, Alyssum lesbiacum (Candargy) Rech. f.; Pl, Plantago lagopus L.; Hb, Hordeum bulbosum L.; Ab, Aegilops biuncialis Vis.; Cc, Crepis commutata (Spreng.) Greuter.
AM, Ampeliko; OL, Olympos; LO, Loutra.
Ni determination in litter
The Ni concentration was determined in all litter samples before burial in the ground and after 90, 180 and 270 d of exposure. The harvested litterbags were not rinsed with water in order to avoid Ni leaching; however, leaves from each litterbag were gently cleaned with a soft brush in order to remove adhering debris and soil particles. After pulverization using a laboratory mixer-mill, the samples were digested with concentrated HNO3 in a closed vessel microwave digestion system (CEM Mars Xpress), according to USEPA’s method 3051A (US Environmental Protection Agency, 2007). Ni was measured in the diluted digests by flame atomic absorption spectrometry (Perkin-Elmer, 5100ZL). Ni concentrations in litter tissues were calculated on a dry weight basis. All reagents used in the analysis were appropriate for trace metal determination (Merck, Suprapur), and all water used was of Type 1 ultrapure quality (18·2 MΩ cm resistivity), produced by a Milli-Q purification system (Millipore). Sample handling in the laboratory was carried out in a Class 100 laminar flow hood (NUAIRE, NU 154-524E) to avoid contamination. The quality control and the performance of the analytical procedure are described in detail in Adamidis et al. (2014b).
Data analysis
The percentage of the litter mass remaining is denoted as LMR. Three-way analyses of variance (ANOVAs) were used to examine the main effects of ‘litter mixture’ (AM, OL, and LO), ‘soil type’ (serpentine–non serpentine), ‘time’ (90, 180 and 270 d of field exposure) and their interactions on LMR and litter Ni concentration. Bonferroni multiple comparisons were used to test for pairwise differences between both soil types and harvest time periods. All dependent variables were tested for normality using the Kolmogorov–Smirnov test at a significance level of 0·05.
To assess whether there is a significant differentiation between the decomposition patterns of the three mixed-species litters placed in the control non-serpentine site, we conducted a two-way ANOVA, using LMR as the response variable and ‘litter mixture’ along with ‘time’ as predictors.
For the mixed-species litters, the expected values of litter mass remaining were calculated as follows (e.g. Bonanomi et al., 2010; Dimitrakopoulos, 2010; Lecerf et al., 2007):
| (1) |
where ELMR is the expected litter mass remaining (%), OLMR is the observed mass remaining (%) in single-species litter of species i, and pi is the initial proportion of species i in the mixed-species litter. Finally, the LMR in mixed-species litters (observed) was compared with the average mass remaining of the component species in single-species litters (expected) using the following calculation: [(observed – expected)/expected] × 100 (Loreau, 1998). The product of this calculation was then plotted against duration of field exposure. For each litter mixture × time combination, 95 % confidence intervals (CIs) were calculated. The points with CIs crossing the y = 0 were considered additive (Ball et al., 2008). In the case of non-additive interactions (deviation between observed and expected values), negative values indicated that the LMR in mixtures was underestimated by the predicted values from single-species litters (synergistic effects), and positive values indicated overestimated LMR values in mixtures in relation to what was expected based on single-species litters (antagonistic effects). All the statistical analyses were carried out using the R statistical platform (R Development Core Team, 2009).
RESULTS
Differences in decomposition rates across serpentine and non-serpentine environments
The three-way ANOVA revealed significant effects of ‘litter mixture’ (F2,164 = 80·88; P < 0·001), ‘soil type’ (F1,164 = 17·19; P < 0·001) and ‘time’ (F2,164 = 98·17; P < 0·001) on LMR. Significant ‘soil type × litter mixture’ (F2,164 = 22·52; P < 0·001) and ‘soil type × time’ (F2,164 = 3·49; P = 0·033) interactions were also revealed, indicating that differences among litter mixtures and time of harvest affected the response of the LMR to soil type. In contrast, all litter mixtures followed the same pattern of litter mass loss across time as indicated by the non-significant ‘litter mixture × time’ interaction (P > 0·05). The LMR of litter mixture AM, after 90 d of field exposure, was found to be significantly higher on serpentine soils of the AM site relative to non-serpentine soils (Fig. 1A). However, after 180 d, the LMR did not differ significantly across different soil types and no further decline was documented after 270 d in the field (Fig. 1A). No significant differentiation was documented on the LMR of the litter mixture OL across the different soil types of site OM, after either 90 d or 180 d of field exposure (Fig. 1B). However, after 270 d, the litter mixture OL presented significantly lower LMR values on serpentine soils than on non-serpentine soils (Fig. 1B). The litter mixture LO presented significantly lower LMR values on LO serpentine soils when compared with non-serpentine soils after all harvest time periods (90, 180 and 270 d; Fig. 1C).
Fig. 1.
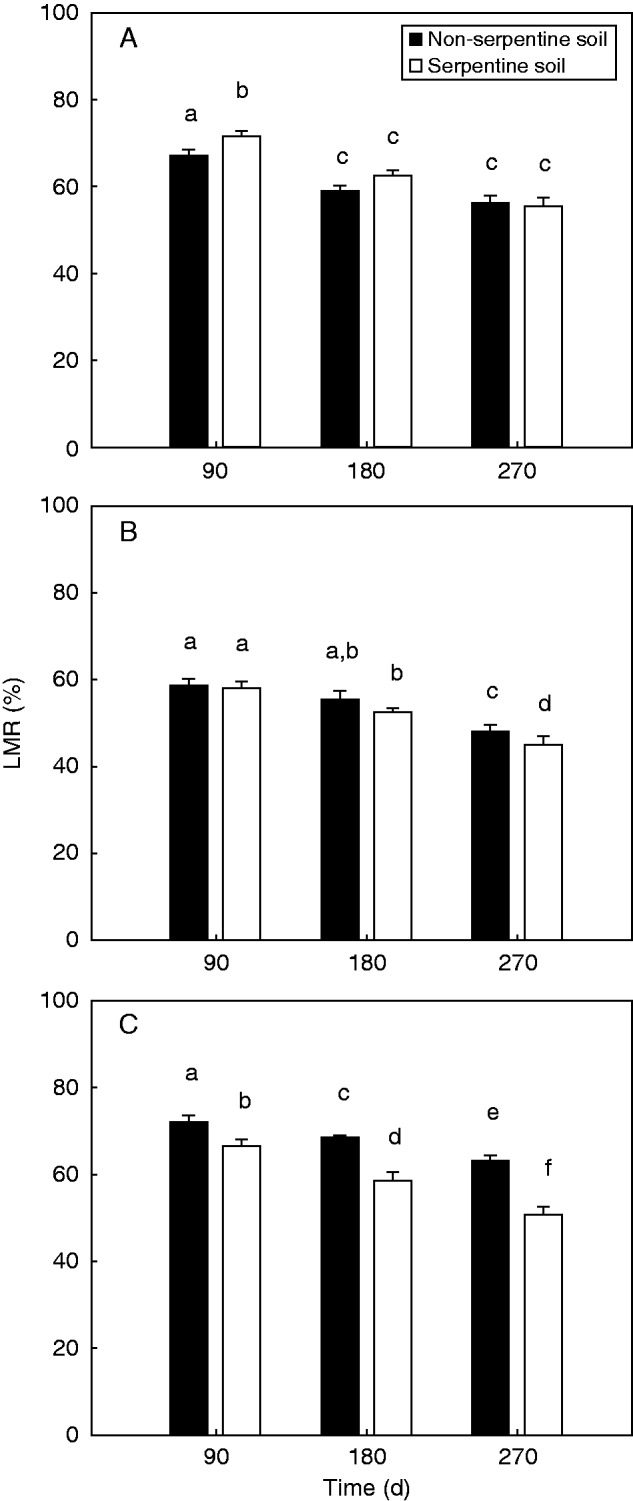
Percentage of litter mass remaining (LMR ± s.e.) after 90, 180 and 270 d of exposure on non-serpentine and serpentine soils for: (A) Ampeliko (AM); (B) Olympos (OL); and (C) Loutra (LO) litter mixtures. Different lower case letters indicate significant differences (at P < 0·05) among harvest time and/or soil types, by Bonferroni multiple comparison test.
Differences in Ni release between serpentine and non-serpentine environments
Significant effects of ‘litter mixture’ (F2,219 = 9097·71; P < 0·001), ‘soil type’ (F1,219= 346·46; P < 0·001) and ‘time’ (F3,219 = 3369·70; P < 0·001) were revealed on litter Ni concentration. In addition, significant ‘soil type × litter mixture’ (F2,219 = 182·87; P < 0·001) and ‘soil type × time’ (F3,219 = 112·95; P < 0·001) interactions were revealed, indicating that differences among litter mixtures and time of exposure affected the response of litter Ni concentration to soil type. The Ni concentration of the AM litter mixture (holding the highest initial Ni concentration; see Table 1), was significantly lower on serpentine soils of the AM site compared with non-serpentine soils (Fig 2A). Its litter Ni concentration decreased 82 % after 90 d of exposure in the serpentine soils of AM (Fig. 2A), remained stable until 180 d and finally showed an additional decrease of 4 % after 270 d (Fig. 2A). In non-serpentine soils of AM, the Ni concentration of the AM litter mixture decreased 70 % after 90 d of exposure and afterwards no further decline was noted (Fig. 2A). The OL litter mixture significantly decreased its Ni concentration after 90 d of exposure on both serpentine and non-serpentine soils (49 and 47 % decrease, respectively) of the OL site, and no further decline was documented afterwards (Fig. 2B). The litter Ni concentration of the OL litter mixture was significantly lower on serpentine soils compared with non-serpentine soils after all harvest times (Fig. 2B). The LO litter mixture (holding the lowest initial Ni concentration; see Table 1) significantly decreased its Ni concentration (34 %) after 90 d on the non-serpentine soils of the LO site, and no further decline developed afterwards (Fig. 2C). The Ni concentration of the same litter mixture decreased 25 % only after 180 d of exposure on the serpentine soils of the LO site and remained stable afterwards (Fig. 2C).
Fig. 2.
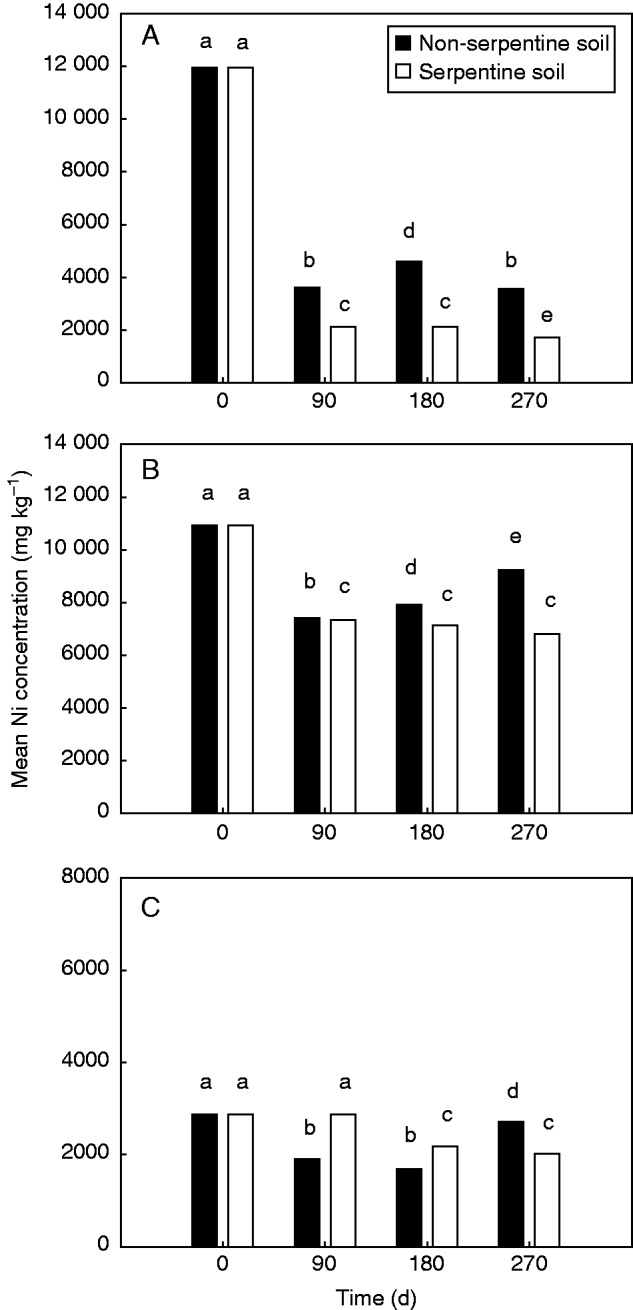
Litter Ni concentration (mg kg–1), at 0 d and after 90, 180 and 270 d of exposure on non-serpentine and serpentine soils for: (A) Ampeliko (AM); (B) Olympos (OL); and (C) Loutra (LO) litter mixtures. Different lower case letters indicate significant differences (at P < 0·05) among harvest time and/or soil types, by Bonferroni multiple comparison test.
Differences in decomposition dynamics of different litter mixtures on a control non-serpentine environment
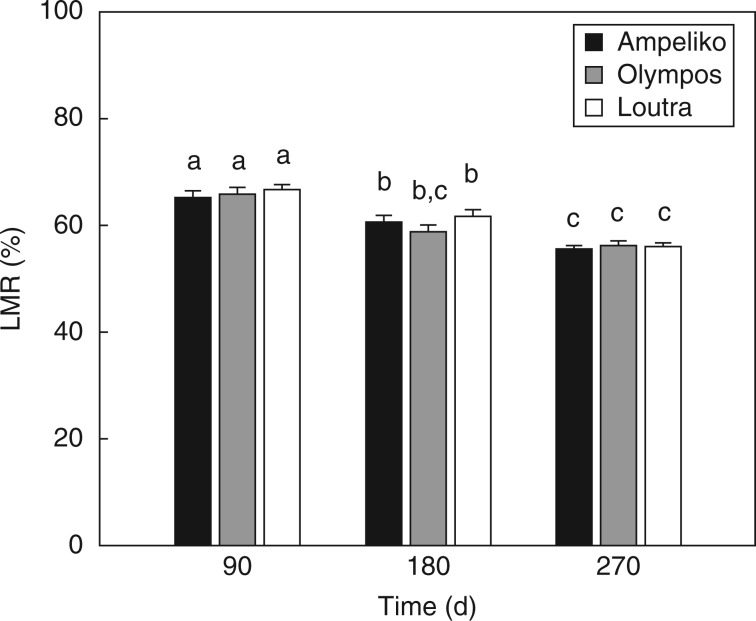
The ANOVA comparing the decomposition dynamics of the three different litter mixtures on the control non-serpentine site revealed no significant effect of ‘litter mixture’ or ‘litter mixture × time’ interaction (P > 0·05 in all cases) on LMR (Fig. 3). However, a significant effect of ‘time’ emerged on LMR (F2,90 = 59·31; P < 0·001).
Fig. 3.
Percentage of litter mass remaining (LMR ± s.e.) after 90, 180 and 270 d of exposure on the control non-serpentine site (Xenia) for Ampeliko (AM), Olympos (OL) and Loutra (LO) litter mixtures. Different lower case letters indicate significant differences (at P < 0·05) among harvest time and/or litter mixtures, by Bonferroni multiple comparison test.
Considering the LMR, additive interactions were found in 89 % of all tested mixed-species litter mixtures (Fig. 4). A non-additive interaction was revealed on OL litter mixture after 180 d of field exposure and indicated a synergistic effect on mass loss (Fig. 4).
Fig. 4.
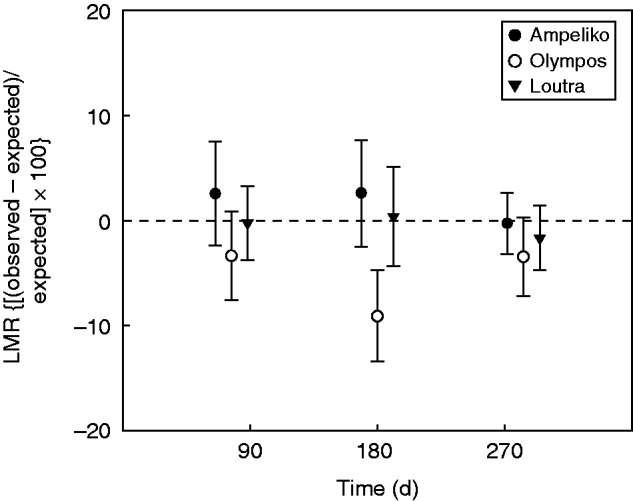
Litter mass remaining (LMR) in the mixed-species litterbags relative to the expected values calculated from the corresponding single-species litterbags. Values are plotted against the days of field exposure. Points for which the 95 % CIs cross y = 0 represent significant additive interactions; points with positive values for which the 95 % CIs do not cross y = 0 represent significant non-additive antagonistic interactions; and points with negative values for which the 95 % CIs do not cross y = 0 represent significant non-additive synergistic interactions.
DISCUSSION
Decomposition across serpentine and non-serpentine environments
The first hypothesis tested in this study was that the LMR of mixed-species litters containing hyperaccumulated Ni would be lower on serpentine soils compared with non-serpentine soils. Our results revealed that the LMR of mixed-species litters demonstrated, on average, higher values on non-serpentine soils than on serpentine soils, confirming our first hypothesis. Boyd et al. (2008) found no significant differentiation of the mass loss of leaf material containing hyperaccumulated Ni across serpentine sites that hosted hyperaccumulator and non-hyperaccumulator populations of S. coronatus. Our results are consistent with the study of Quinn et al. (2011) that found higher rates of decomposition on litters containing high concentrations of Se in seleniferous environments, relative to low-Se litters. Although Quinn et al. (2011) did not include non-seleniferous habitats in their study, they provided strong evidence for the presence of Se-tolerant decomposers in the studied seleniferous habitats. In our study, the higher decomposition rates of litters containing hyperaccumulated Ni on serpentine environments may also indicate the presence of decomposers adapted to high soil Ni concentrations. However, the LMR differentiations between contrasting environments appeared more pronounced as the initial hyperaccumulated Ni concentration of the litter decreased (Fig. 1). In particular, the LMR differentiations were very notable in the LO litter mixture (Fig. 1C), having the lowest initial concentration of hyperaccumulated Ni, were weaker in the case of the OL litter mixture (Fig. 1B) and finally were absent in the case of the AM litter mixture (Fig. 1A) having the highest initial Ni concentration. These results suggest that litters with extremely high Ni concentrations (in our case the AM litter with 11 937 mg Ni kg–1) may inhibit decomposer activity not only on non-serpentine soils but also on serpentine soils. Based on our results, we can assume that serpentine communities may support a higher percentage of Ni-resistant bacteria. However, although decomposition of litter containing Ni is accelerated on serpentine communities, bacterial activity tends to slow down with increasing litter Ni concentrations.
Ni release across serpentine and non-serpentine environments
Our second hypothesis stating that the Ni release will be higher on serpentine soils compared with non-serpentine soils and will depend on the initial litter hyperaccumulated Ni was confirmed. In particular, the AM and OL litter mixtures, having high initial Ni concentrations (11 937 and 10 927 mg kg–1, respectively), indicated lower litter Ni concentrations on serpentine soils after all the different periods of field exposure (Fig. 2A, B). The LO litter mixture, having the lowest initial Ni concentration (2863 mg Ni kg–1), presented significantly lower litter Ni concentrations on serpentine soils compared with non-serpentine soils only after 270 d of field exposure (Fig. 2C). At this point, our analysis has revealed that litter Ni concentrations increase during litter decomposition on non-serpentine soils. This finding, opposite to the expected pattern, is quite obvious in the cases of OL and LO litter mixtures (Fig. 2B, C). Our novel experimental design successfully captured for the first time this intriguing process, possibly implying the selective decomposition of low-Ni parts of litter by the decomposer communities on non-serpentine soils. In particular, the selective decomposition on low-Ni parts by the non-Ni-resistant decomposers of non-serpentine soils may have resulted in an increase in Ni litter concentration. Although selective decomposition of recalcitrant litter compounds and lignin has been previously reported (Gelbrich et al., 2008; Osono et al., 2011), the selective decomposition of low-metal litter parts has never, to our knowledge, been reported, and thus further investigation of this process is required.
For AM and OL litter mixtures, the hyperaccumulated Ni was released rapidly after 90 d of field exposure, on both serpentine (82 and 49 % decrease, respectively) and non-serpentine (70 and 47 % decrease, respectively) soils (Fig. 2A, B). The most extreme release of hyperaccumulated Ni was documented from the AM litter mixture having the highest initial Ni concentration on the serpentine soil (Fig. 2A). Similarly, Boyd et al. (2008) recorded the most extreme Ni release (72–91 % decrease of Ni content) from the leaves with the highest Ni concentration after 1 month of exposure on the site hosting hypraccumulator populations of S. coronatus. In contrast, the Ni concentration of the LO litter mixture decreased by just 25 % after 180 d of exposure on the serpentine soils and by 34 % after 90 d of exposure on the non-serpentine soils, and no significant decline was documented afterwards. Our results, in accordance with those by Boyd et al. (2008), seem to indicate that the rapidity of Ni release is positively related to the initial Ni litter concentration, even if a different experimental design is required in order to determine the mathematical model behind this relationship. A rapid release of Ni has also been documented for the Ni-hyperaccumulator Alyssum murale (Zhang et al., 2005, 2007), while other studies have reported the rapid release of Zn (Boucher et al., 2005) and Se (Quinn et al., 2011) through litter decomposition. The rapidity of release of the hyperaccumulated Ni that has been reported so far does not provide strong support for the hypothesis that Ni-hyperaccumulating species may lower soil Ni concentrations through the hyperaccumulated Ni being bound to undecomposed litter (Ernst, 1972; Baker, 1981). On the contrary, the accelerated decomposition of mixed-species litters containing hyperaccumulated Ni along with the rapid litter Ni release during decomposition in serpentine soils (where the Ni hyperaccumulation process takes place) demonstrated by our study provide support for the elemental allelopathy hypothesis of hyperaccumulation. However, in order to investigate the validity of the elemental allelopathy hypothesis (Boyd and Jaffré, 2001), further studies are necessary to test the fate of the released litter hyperaccumulated Ni (but see El Mehdawi et al., 2011 for elemental allelopathy through Se hyperaccumulation).
Decomposition across different litter mixtures on a control non-serpentine environment
The Xenia non-serpentine site was used in order to investigate our third hypothesis stating that litter mixtures with higher concentrations of hyperaccumulated Ni will present a higher LMR on a control non-serpentine site. The selection of a non-serpentine site as a control site was deliberate, in order to examine further which level of litter Ni concentration is able to slow down litter decomposition in a non-serpentine environment. Surprisingly, the LMR did not significantly differ either across the three different litter mixtures (Fig. 3) or after all time periods, hence offering no substantial support for our third hypothesis. According to our results, initial litter Ni concentrations up to approx. 11 000–12 000 mg kg–1 could not induce significant delay on mixed-species litter decomposition compared with litters with 2863 mg Ni kg–1 on a non-serpentine site. Boyd et al. (2008) also did not record a significantly slower decomposition of leaves containing 9200 mg Ni kg–1 relative to leaves with low Ni concentrations (16 and 130 mg kg–1), and they proposed a litter Ni concentration threshold of > 10 000 mg kg–1 in order to induce delay in the decomposition process. Although our experimental design has overlapped this threshold without observing any significant Ni effect on the decomposition rates on the control non-serpentine site, our lower litter Ni concentrations (2863 mg kg–1) may have been high enough to slow down decomposition rates.
Additive patterns of mass loss emerged for the majority of the different litter mixture × time combinations (Fig. 4), providing no support for our fourth hypothesis stating that non-additive patterns of mass loss will be present, with antagonistic effects emerging on litter mixtures containing high concentrations of hyperaccumulated Ni. Although additive patterns of mass loss have been reported by several studies (e.g. Ball et al., 2008; Bonanomi et al., 2010; Pakeman et al., 2011), these patterns seem to be the exception rather than the rule (Gartner and Cardon, 2004; Hättenschwiller et al., 2005). In opposition to our fourth hypothesis, no antagonistic effects were documented on litter mixtures containing high concentrations of hyperaccumulating Ni. A non-additive interaction was revealed on the OL litter mixture, presenting synergistic effects on mass loss after 180 d of field exposure (Fig. 4). Although Bonanomi et al. (2010) have reported a transition from additive to antagonistic interactions at the later stages of a microcosm experiment, in our case this may be an isolated result which is difficult to interpret. Our additivity vs. non-additivity results are consistent with several other studies reporting additive patterns of mass loss derived by the cancelling out of opposite (synergistic and antagonistic) interactions (Tardif and Shipley, 2013; Tardif et al., 2014; Jewel et al., 2015). The observed patterns captured in our study are the result of the effects of both different litter mixtures and different litter Ni concentration. Hence, their comprehensive interpretation requires experimental designs encompassing the variation of diversity (number of species), species abundance and litter chemistry in the litter mixtures.
Conclusions and future research
Our results provide support for studies providing evidence of the presence of specialist decomposers in serpentine habitats, although further studies investigating the decomposer communities across the different environments are necessary. In addition, our experimental design has captured for the first time the interesting phenomenon of the increase of litter Ni concentration during the decomposition process on non-serpentine soils. We have hypothesized that this pattern may be due to the selective decomposition of low-Ni parts of litters by the decomposer communities on non-serpentine soils. This interesting and novel hypothesis needs to be investigated further in future studies. The decomposition of the three different litter mixtures did not differ significantly on a control non-serpentine site; however, experimental designs including broader ranges of hyperaccumulated Ni concentrations may highlight a potential Ni effect on litter decomposition rates. Finally, the decomposition dynamics of the studied litter mixtures were found to be well predicted by the monoculture litters of the component species; however, future research through related in situ experimental designs is needed in order to extend our knowledge about the mechanisms behind the variable processes of additive and non-additive patterns on both mass loss and Ni release. In general, our study, using an experimental design resembling natural decomposition: (1) indicates the presence of Ni-resistant decomposers in serpentine habitat that may have contributed to the accelerated decomposition of high-Ni litter; (2) demonstrates the increase of litter Ni concentration during decomposition on non-serpentine soils, thus implying the selective decomposition of low-Ni parts of litters by the decomposer communities on non-serpentine soils; and, finally, (3) lends support to the elemental allelopathy hypothesis of hyperaccumulation, presenting the potential selective advantages acquired by the metal-hyperaccumulating plants through the important process of litter decomposition on serpentine soils.
ACKNOWLEDGEMENTS
We would like to thank Dimitra Syrou for editorial assistance, and the Editor Bill Shipley and two anonymous reviewers for their constructive comments on an earlier version of the manuscript. This research has been co-financed by the European Union [European Social Fund (ESF)] and Greek national funds through the Operational Program ‘Education and Lifelong Learning’ of the National Strategic Reference Framework (NSRF) – Research Funding Program: Heracleitus II. Investing in knowledge society through the European Social Fund.
LITERATURE CITED
- Adamidis GC, Kazakou E, Baker AJM, Reeves RD, Dimitrakopoulos PG. 2014a.. The effect of harsh abiotic conditions on the diversity of serpentine plant communities on Lesbos, an eastern Mediterranean island. Plant Ecology and Diversity 7: 433–444. [Google Scholar]
- Adamidis GC, Aloupi M, Kazakou E, Dimitrakopulos PG. 2014b.. Intra-specific variation in Ni tolerance, accumulation and translocation patterns in the Ni-hyperaccumulator Alyssum lesbiacum. Chemosphere 95: 496–502. [DOI] [PubMed] [Google Scholar]
- Alexander EB, Coleman RG, Keeler-Wolf T, Harrison S. 2007.. Serpentine geoecology of western North America. New York: Oxford University Press. [Google Scholar]
- Amir H, Pineau R. 1998a.. Effects of metals on the germination and growth of fungal isolates from New Caledonian ultramafic soils. Soil Biology and Biochemistry 30: 2043–2054. [Google Scholar]
- Amir H, Pineau R. 1998b.. Influence of plants and cropping on microbiological characteristics of New Caledonian ultramafic soils. Australian Journal of Soil Research 36: 457–471. [Google Scholar]
- Azarbad H, Niklińska M, Van Gestel CAM, Van Straalen NM, Röling WFM, Laskowski R. 2013.. Microbial community structure and functioning along metal pollution gradients. Environmental Toxicology and Chemistry 32: 1992–2002. [DOI] [PubMed] [Google Scholar]
- Baker AJM. 1981.. Accumulators and excluders – strategies in the response of plants to heavy metals. Journal of Plant Nutrition 3: 643–654. [Google Scholar]
- Ball BA, Hunter MD, Kominoski JS, Swan CM, Bradford MA. 2008.. Consequences of non-random species loss for decomposition dynamics: experimental evidence for additive and non-additive effects. Journal of Ecology 96: 303–313. [Google Scholar]
- Bonanomi G, Incerti G, Antignani V, Capodilupo M, Mazzoleni S. 2010.. Decomposition and nutrient dynamics in mixed litter of Mediterranean species. Plant and Soil 331: 481–496. [Google Scholar]
- Boucher U, Balabane M, Lamy I, Cambier P. 2005.. Decomposition in soil microcosms of leaves of the metallophyte Arabidopsis halleri: effect of leaf-associated heavy metals on biodegradation. Environmental Pollution 135: 187–194. [DOI] [PubMed] [Google Scholar]
- Boyd RS. 2007.. The defense hypothesis of elemental hyperaccumulation: status, challenges and new directions. Plant and Soil 293: 153–176. [Google Scholar]
- Boyd RS, Jaffré T. 2001.. Phytoenrichment of soil Ni concentration by Sebertia acuminata in New Caledonia and the concept of elemental allelopathy. South African Journal of Science 97: 535–538. [Google Scholar]
- Boyd RS, Martens SN. 1992.. The raison d’etre for metal hyperaccumulation by plants In: AJM Baker, J Proctor, RD Reeves, eds. The vegetation of ultramafic (serpentine) soils. Andover, UK: Intercept, 279–289. [Google Scholar]
- Boyd RS, Martens SN. 1998.. The significance of metal hyperaccumulation for biotic interactions. Chemoecology 8: 1–7. [Google Scholar]
- Boyd RS, Davis MA, Balkwill K. 2008.. Does hyperaccumulated nickel affect leaf decomposition? A field test using Senecio coronatus (Asteraceae) in South Africa. Chemoecology 18: 1–9. [Google Scholar]
- Brooks RR, Morrison RS, Reeves RD, Dudley TR, Akmans Y. 1979.. Hyperaccumulation of nickel by Alyssum Linnaeus (Cruciferae). Proceedings of the Royal Society B: Biological Sciences 203: 387–403. [DOI] [PubMed] [Google Scholar]
- Canhoto C, Barlocher F, Graca MAS. 2002.. The effects of Eucalyptus globulus oils on fungal enzymatic activity. Archiv für Hydrobiologie 154: 121–132. [Google Scholar]
- Dimitrakopoulos PG. 2010.. Influence of evenness on the litter-species richness–decomposition relationship in Mediterranean grasslands. Journal of Plant Ecology 3: 71–78. [Google Scholar]
- El Mehdawi AF, Quinn CF, Pilon-Smits EAH. 2011.. Effects of selenium hyperaccumulation on plant–plant interactions: evidence for elemental allelopathy. New Phytologist 191: 120–131. [DOI] [PubMed] [Google Scholar]
- Ernst WHO. 1972.. Ecophysiological studies on heavy metal plants in South Central Africa. Kirkia 8: 125–145. [Google Scholar]
- Garnier E, Cortez J, Billes G, Navas ML, et al. 2004.. Plant functional markers capture ecosystem properties during secondary succession. Ecology 85: 263–2637. [Google Scholar]
- Gartner TB, Cardon ZG. 2004.. Decomposition dynamics in mixed-species leaf litter. Oikos 104: 230–246. [Google Scholar]
- Gelbrich J, Mai C, Militz H. 2008.. Chemical changes in wood degraded by bacteria. International Biodeterioration and Biodegradation 61: 24–32. [Google Scholar]
- Giller KE, Witter E, McGrath SP. 1998.. Toxicity of heavy metals to microorganisms and microbial processes in agricultural soils: a review. Soil Biology and Biochemistry 30: 1389–1414. [Google Scholar]
- Harrison AF. 1971.. The inhibitory effect of oak leaf litter tannins on the growth of fungi, in relation to litter decomposition. Soil Biology and Biochemistry 3: 167–172. [Google Scholar]
- Hättenschwiller S, Tiunov AV, Scheu S. 2005.. Biodiversity and litter decomposition in terrestrial ecosystems. Annual Review of Ecology Evolution and Systematics 36: 191–218. [Google Scholar]
- Hoiland K. 1995.. Reaction of some decomposer basidiomycetes to toxic elements. Nordic Journal of Botany 15: 305–318. [Google Scholar]
- Jewell MD, Shipley B, Paquette A, Messier C, Reich PB. 2015.. A traits-based test of the home-field advantage in mixed-species tree litter decomposition. Annals of Botany 116: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazakou E, Dimitrakopoulos PG, Baker AJM, Reeves RD, Troumbis AY. 2008.. Hypotheses, mechanisms and trade-offs of tolerance and adaptation to serpentine soils: from species to ecosystem level. Biological Reviews 83: 495–508. [DOI] [PubMed] [Google Scholar]
- Kazakou E, Adamidis GC, Baker AJM, Reeves RD, Godino M, Dimitrakopoulos PG. 2010.. Species adaptation in serpentine soils in Lesbos Island (Greece): metal hyperaccumulation and tolerance. Plant and Soil 332: 369–385. [Google Scholar]
- Lecerf A, Risnoveanu G, Popescu C, Gessner MO, Chauvet E. 2007.. Decomposition of diverse litter mixtures in streams. Ecology 88: 219–227. [DOI] [PubMed] [Google Scholar]
- Loreau M. 1998.. Separating sampling and other effects in biodiversity experiments. Oikos 82: 600–602. [Google Scholar]
- McArthur JV, Aho JM, Rader RB, Mills GL. 1994.. Interspecific leaf interactions during decomposition in aquatic and flood-plain ecosystems. Journal of the North American Benthological Society 13: 57–67. [Google Scholar]
- Mengoni A, Barzanti R, Gonnelli C, Gabbrielli R, Bazzicalupo M. 2001.. Characterization of nickel-resistant bacteria isolated from serpentine soil. Environmental Microbiology 3: 691–698. [DOI] [PubMed] [Google Scholar]
- Nilsson MC, Gallet C, Wallstedt A. 1998.. Temporal variability of phenolics and batatasin-III in Empetrum hermaphroditum leaves over an eight-year period: interpretations of ecological function. Oikos 81: 6–16. [Google Scholar]
- Oorts K, Ghesquiere U, Smolders E. 2007.. Leaching and aging decrease nickel toxicity to soil microbial processes in soils freshly spiked with nickel chloride. Environmental Toxicology and Chemistry 26: 1130–1138. [DOI] [PubMed] [Google Scholar]
- Osono T, Hobara S, Hishinuma T, Azuma JI. 2011.. Selective lignin decomposition and nitrogen mineralization in forest litter colonized by Clitocybe sp. European Journal of Soil Biology 47: 114–121. [Google Scholar]
- Pakeman RJ, Eastwood A, Scobie A. 2011.. Leaf dry matter content as a predictor of grassland litter decomposition: a test of the ‘mass ratio hypothesis’. Plant and Soil 342: 49–57. [Google Scholar]
- Quinn CF, Wyant K, Wangeline AL, et al. 2011.. Enhanced decomposition of selenium hyperaccumulator litter in a seleniferous habitat – evidence for specialist decomposers. Plant and Soil 341: 51–61. [Google Scholar]
- R Core Team. 2009.. R: a language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: http://www.R-project.org/. [Google Scholar]
- Reeves RD, Baker AJM, Kelepertsis A. 1997.. The distribution and biogeochemistry of some serpentine plants of Greece In: T Jaffré, RD Reeves, T Becquer, eds. Ecologie des milieux sur roches ultramafiques et sur sols metallifères. ORSTOM: Nouméa, 205–207. [Google Scholar]
- Reeves RD, Baker AJM. 2000.. Metal-accumulating plants In: I Raskin, BD Ensley, eds. Phytoremediation of toxic metals. New York: John Wiley & Sons, 193–229. [Google Scholar]
- Schimel JP, Cates RG, Ruess R. 1998.. The role of balsam poplar secondary chemicals in controlling soil nutrient dynamics through succession in the Alaskan taiga. Biogeochemistry 42: 221–34. [Google Scholar]
- Schlegel HG, Cosson JP, Baker AJM. 1991.. Nickel hyperaccumulating plants provide a niche for nickel-resistant bacteria. Botanica Acta 104: 18–25. [Google Scholar]
- Tardif A, Shipley B. 2013.. Using the biomass-ratio and idiosyncratic hypotheses to predict mixed-species litter decomposition. Annals of Botany 111: 135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif A, Shipley B, Bloor JM, Soussana JF. 2014.. Can the biomass-ratio hypothesis predict mixed-species litter decomposition along a climatic gradient? Annals of Botany 113: 843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Environmental Protection Agency. 2007.. Method 3051A, microwave assisted acid digestion of sediments, sludges, soils, and oils, revision 1. Test methods for evaluating solid waste. Washington DC: USEPA. [Google Scholar]
- Wardle DA, Bonner KI, Nicholson KS. 1997.. Biodiversity and plant litter: experimental evidence which does not support the view that enhanced species richness improves ecosystem function. Oikos 79: 247–258. [Google Scholar]
- Wardle DA, Nilsson M-C, Zackrisson O, Gallet C. 2003.. Determinants of litter mixing effects in a Swedish boreal forest. Soil Biology and Biochemistry 35: 827–835. [Google Scholar]
- Zhang L, Angle JS, Delorme T, Chaney RL. 2005.. Degradation of Alyssum murale biomass in soil. International Journal of Phytoremediation 7: 169–176. [DOI] [PubMed] [Google Scholar]
- Zhang L, Angle JS, Chaney RL. 2007.. Do high-nickel leaves shed by the nickel hyperaccumulator Alyssum murale inhibit seed germination of competing plants? New Phytologist 173: 509–516. [DOI] [PubMed] [Google Scholar]