Abstract
Background and Aims Some plant groups, especially on islands, have been shaped by strong ancestral bottlenecks and rapid, recent radiation of phenotypic characters. Single molecular markers are often not informative enough for phylogenetic reconstruction in such plant groups. Whole plastid genomes and nuclear ribosomal DNA (nrDNA) are viewed by many researchers as sources of information for phylogenetic reconstruction of groups in which expected levels of divergence in standard markers are low. Here we evaluate the usefulness of these data types to resolve phylogenetic relationships among closely related Diospyros species.
Methods Twenty-two closely related Diospyros species from New Caledonia were investigated using whole plastid genomes and nrDNA data from low-coverage next-generation sequencing (NGS). Phylogenetic trees were inferred using maximum parsimony, maximum likelihood and Bayesian inference on separate plastid and nrDNA and combined matrices.
Key Results The plastid and nrDNA sequences were, singly and together, unable to provide well supported phylogenetic relationships among the closely related New Caledonian Diospyros species. In the nrDNA, a 6-fold greater percentage of parsimony-informative characters compared with plastid DNA was found, but the total number of informative sites was greater for the much larger plastid DNA genomes. Combining the plastid and nuclear data improved resolution. Plastid results showed a trend towards geographical clustering of accessions rather than following taxonomic species.
Conclusions In plant groups in which multiple plastid markers are not sufficiently informative, an investigation at the level of the entire plastid genome may also not be sufficient for detailed phylogenetic reconstruction. Sequencing of complete plastid genomes and nrDNA repeats seems to clarify some relationships among the New Caledonian Diospyros species, but the higher percentage of parsimony-informative characters in nrDNA compared with plastid DNA did not help to resolve the phylogenetic tree because the total number of variable sites was much lower than in the entire plastid genome. The geographical clustering of the individuals against a background of overall low sequence divergence could indicate transfer of plastid genomes due to hybridization and introgression following secondary contact.
Keywords: Diospyros, genome skimming, island floras, New Caledonia, next-generation sequencing, nuclear ribosomal DNA, rapid radiation, complete plastid genomes
INTRODUCTION
New Caledonia comprises an archipelago in the southern Pacific known for its characteristic, rich endemic flora (Lowry, 1998; Morat et al., 2012). Due to its complex geological history, New Caledonia features a mosaic of soil types (Pelletier, 2006; Maurizot and Vendé-Leclerc, 2009), which, in combination with its elevational and climatic heterogeneity, results in many different habitats. One of the genera that has adapted to a wide range of these habitats is Diospyros (Ebenaceae).
Diospyros is a large genus of woody dioecious plants found worldwide in the tropics and subtropics, including 31 species in New Caledonia. Previous studies based on plastid markers (Duangjai et al., 2009) showed that Diospyros colonized New Caledonia at least four times via long-distance dispersal. Two of the successful dispersal events each resulted in a single species that still persists; an additional dispersal event led to a small clade comprising five species; and yet another event gave rise to a putatively rapidly radiating group of 24 endemic species. These 24 species have been shown to be highly similar genetically using low-copy nuclear and plastid markers (Duangjai et al., 2009; Turner et al., 2013a). Most of these closely related species are morphologically and ecologically clearly differentiated, and current species delimitations (White, 1993) have been generally confirmed by analyses of amplified fragment length polymorphisms (AFLPs; Turner et al., 2013b) and restriction site-associated DNA sequencing (RADseq; Paun et al., 2016).
On New Caledonia, Diospyros species are found in many habitats, but they often grow in proximity to each other. At some localities, several species are microsympatric, which allows interspecific gene flow if reproductive isolation is still incomplete. Dating analysis based on four plastid and two low-copy nuclear DNA regions showed that the ancestors of this group of New Caledonian Diospyros species arrived in New Caledonia around 9 million years ago (Turner et al., 2013a). Given that Diospyros includes long-lived perennial plants, it becomes obvious that they have evolved relatively recently. Resolving the phylogenetic relationships in such a young group of rapidly radiating and potentially hybridizing taxa poses significant challenges (Glor, 2010). We test here the usefulness of next-generation sequencing (NGS)-based genome skimming to obtain phylogenetic data, in particular by sequencing whole plastomes and the full-length nuclear ribosomal DNA region (i.e. nrDNA).
The plastid genome has proved useful for molecular phylogenetic investigations of plants at different taxonomic levels. In the past two decades, sequences from the plastid genome have been extensively used to infer phylogenetic relationships among plants (e.g. Chase et al., 1993; Barfuss et al., 2005; Duangjai et al., 2009; Russell et al., 2010). Uniparental inheritance, low mutation rates and high copy number are well-known features of plastid genomes and the basis for their standard usage in plant systematics. Due to the slow rate of evolution of the plastid genome, the level of variation is often low compared with nuclear DNA in general and mitochondrial markers in animals (Schaal et al., 1998). The high level of conservation has prevented the development of a universally applicable single barcoding region in plants (CBOL Plant Working Group, 2009). Recently, whole-plastid genome sequencing has become affordable, and this has been employed to generate more highly resolved phylogenetic trees (e.g. Ku et al., 2013; Yang et al., 2013; Barrett et al., 2014; Malé et al., 2014).
Genes coding for nuclear ribosomal RNA are found in the genome in multiple copies arranged in tandem repeats, and therefore it is feasible to obtain full-length sequences from low-coverage NGS approaches. Because of concerted evolution, these thousands of copies mostly behave as single-copy genes, and potential evidence of hybridization is normally eliminated within a few generations (Chase et al., 2003). Each nrDNA repeat consists of coding and non-coding elements. In plants, the four rRNA genes are arranged in two clusters. One of these clusters comprises three genes (18S, 5·8S and 26S) separated by two internal transcribed spacers (ITSs), the external transcribed spacer (ETS) and the non-transcribed spacer (NTS). The second cluster includes the coding region of one rRNA (5S) and a spacer between the repeats. The nrDNA genes are relatively conserved but contain enough variation that they have been used for phylogenetic reconstructions at higher taxonomic levels (Maia et al., 2014). The non-coding spacers, ITS and ETS, between the genes are much more variable and represent a useful source of phylogenetic information among closely related species (e.g. Devos et al., 2006; Sanz et al., 2008; Akhani et al., 2013; Nürk et al., 2013; Zhu et al., 2013).
Here we aimed to use whole plastid genomes as well as complete sequences of the nrDNA for phylogenetic analyses for species of the New Caledonian Diospyros to investigate whether this approach would produce an improved estimate of phylogenetic relationships in this putatively rapidly radiating group.
MATERIALS AND METHODS
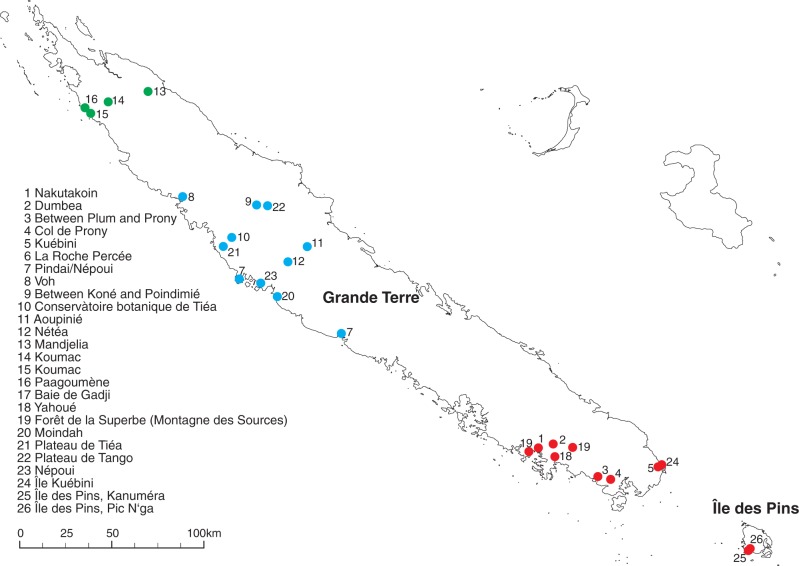
Leaf material from New Caledonian Diospyros species was collected on the main island, Grande Terre, and on one smaller island in the south, Île des Pins (Fig. 1). For the widespread species, at least two representative individuals were sequenced (Table 1). In total we included here 36 individuals of New Caledonian Diospyros species (corresponding to 21 identified and one unidentified species) as well as one individual of Diospyros olen (from New Caledonia, but not closely related to the other New Caledonian Diospyros species) and one individual of Diospyros ferrea from Thailand. Diospyros olen and D. ferrea were used as outgroups. Wherever possible, we used the exact same individuals for which Sanger sequence data are available from previous studies (Turner et al., 2013a). However, because of poor DNA quality (unsuitable for NGS) or unavailability for some of the accessions, we had to include in this study another accession from the same population or locality.
Fig. 1.
Map of New Caledonia indicating the 26 sampling localities for this study. Numbered dots indicate sampling sites (see also Table 1). Dots are coloured according to sampling region (north, green; middle, blue; south, red).
Table 1.
Table of accessions including all individuals used in this study. The identification numbers of sampling localities are given in Fig. 1. Voucher codes: JMXXXX: collection number J. Munzinger; Tree No. XXXXX: Tree of New Caledonian Plant Inventory and Permanent Plot Network (NC-PIPPN, Ibanez et al., 2014); KUFF, Herbarium of the Faculty of Forestry Kasertsat University Bangkok; MPU, Herbarium of the University of Montpellier; NOU, Herbarium of IRD Nouméa; P, Herbarium of the Natural History Museum Paris; WU, Herbarium of the University of Vienna
| Taxon | Accession number | Sampling location | Voucher |
|---|---|---|---|
| D. calciphila F.White | BT313 | 25 | MPU026746 |
| D. cherrieri F.White | BT293 | 23 | NOU079547 |
| D. erudita F.White | BT280 | 21 | WU062858 |
| D. ferrea (Wild.) Bakh. | Eb045 | Duangjai 106 (KUFF) | |
| D. flavocarpa (Vieill. ex P.Parm.) F.White | BT130 | 9 | MPU026741 |
| BT156 | 11 | MPU026737 | |
| D. glans F.White | BT093 | 5 | Turner et al. 093 (MPU) |
| D. impolita F.White | BT102 | 6 | NOU019538 |
| D. inexplorata F.White | BT308 | 24 | NOU005818 |
| D. labillardierei F.White | BT122 | 9 | NOU052188 |
| D. minimifolia F.White | BT135 | 10 | NOU019556 |
| BT233 | 17 | NOU019554 | |
| BT263 | 20 | NOU079549, WU062872 | |
| D. olen Hiern | BT001 | 1 | NOU052191 |
| D. pancheri Kosterm. | BT028 | 3 | MPU026742 |
| BT031 | 3 | MPU026742 | |
| D. parviflora (Schltr.) Bakh. | BT041 | 4 | Turner et al. s.n. (NOU) |
| BT090 | 5 | NOU2519 | |
| BT147 | 10 | NOU052175 | |
| BT187 | 13 | NOU031409 | |
| BT250 | 19 | Tree no. 23109 | |
| BT290 | 22 | NOU079550 | |
| D. perplexa F.White | BT004 | 1 | MPU026738 |
| D. pustulata F.White | BT111 | 7 | NOU019572 |
| BT140 | 10 | NOU052177 | |
| BT261 | 20 | NOU079544 | |
| D. revolutissima F.White | BT120 | 8 | NOU023189 |
| BT221 | 16 | NOU084762 | |
| D. tridentata F.White | BT207 | 14 | NOU052179 |
| D. trisulca F.White | BT185 | 13 | NOU031344 |
| D. umbrosa F.White | BT176 | 12 | JM6635 (NOU) |
| BT247 | 19 | NOU023234 | |
| D. veillonii F.White | BT227 | 17 | NOU019582 |
| D. vieillardii (Hiern) Kosterm. | BT025 | 2 | Turner et al. s.n. (NOU) |
| BT100 | 5 | NOU006676 | |
| BT215 | 15 | NOU023242 | |
| D. yahouensis (Schltr.) Kosterm. | BT238 | 18 | P00057340 |
| D. sp. Pic N’ga | BT318 | 26 | NOU054315 |
DNA was extracted from silica gel-dried leaf material using a modified sorbitol/high-salt cetyltrimethylammonium bromide (CTAB) method (Tel-Zur et al., 1999). Extracts were purified using the NucleoSpin gDNA Clean-up kit (Marcherey-Nagel, Germany), according to the manufacturer’s protocol.
From each sample, 300 ng of DNA was sheared (in two cycles of 45 s with 30 s break between the two shearing runs) using an ultrasonicator (Bioruptor Pico, Diagenode, Belgium), targeting a mean fragment size of 400 bp. Library preparation was performed using the NEBNext Ultra DNA Library Prep Kit for Illumina (New England Biolabs, USA) according to the manufacturer’s protocol. All individuals were barcoded and pooled to reach an equal representation of each individual in the final libraries. In total, two libraries (containing 14 and 24 samples per library, respectively) were prepared for the 38 samples used. The two libraries were sequenced on an Illumina HiSeq as 100-bp paired-end reads at the VBCF (Vienna Biocenter Core Facilities, Vienna, Austria; http://www.vbcf.ac.at/facilities/next-generation-sequencing/). Demultiplexing of the raw data was performed, allowing for a maximum of one mismatch using the Picard BamIndexDecoder (included in the Picard Illumina2bam package; available from https://github.com/wtsi-npg/illumina2bam). The number and quality of raw reads obtained from each individual were evaluated with FastQC (Andrews, 2010).
Assembling and annotating plastid genomes
Reads originating from the plastid genome (pt DNA) were filtered using a multistep and iterative in-house established pipeline. First, the individual raw files were imported into the CLC GENOMIC WORKBENCH v. 6.5 (Qiagen) and trimmed by quality at P <0·05, retaining reads of at least 30 bp. Then the reads of D. ferrea were mapped on the complete plastid genome of Camellia sinensis (Theaceae, Ericales, GenBank: KC143082.1). For this initial mapping of both coding and non-coding regions (the latter comprising introns and intergenic spacers), a mismatch cost of 2 and insertion and deletion cost of 3 were used, requiring at least 80 % of a read to be at least 90 % similar to the target for each successful mapping. With these settings, 403 980 reads of D. ferrea mapped to the Camellia plastome. We re-extracted the initial paired-end read data corresponding to these reads using FastQ.filter.pl (Rodriguez-R and Konstantinidis, 2016, available at https://github.com/lmrodriguezr/enveomics/) and have assembled them de novo in the CLC GENOMIC WORKBENCH, with automatic optimization of the word and bubble sizes and updating the contigs after mapping back the reads. We obtained three contigs, which have been concatenated by aligning them to the C. sinensis reference sequence manually in the program BioEdit v. 7.2.5 (Hall, 1999). From the coverage information and by comparison with the C. sinensis reference we were able to identify both inverted repeats and confirm their presence in the plastid genome of Diospyros. Both inverted repeats were reconstructed together and duplicated to represent a complete plastid genome. The base composition of the assembled contigs was extracted from the alignments using BioEdit v. 7.2.5 (Hall, 1999).
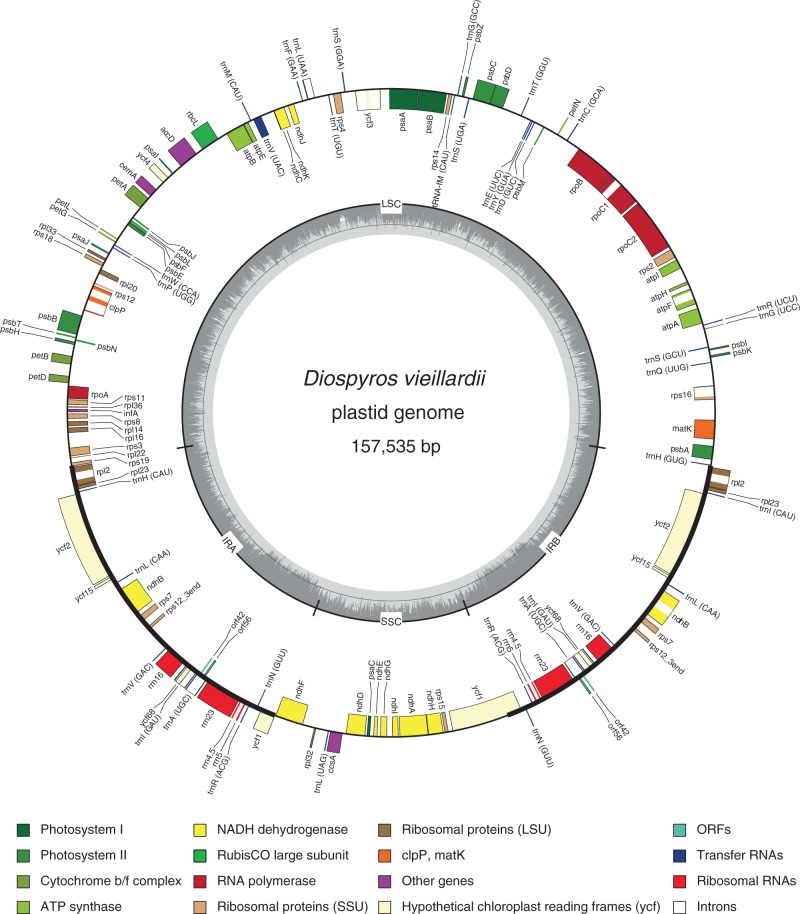
The plastid genomes of the rest of the Diospyros species were obtained in a similar way, but the initial mapping step was performed on the assembled D. ferrea genome. Finally, annotation of coding regions was performed using DOGMA (Wyman et al., 2004) using only the D. vieillardii BT025 plastid genome. The circular plastid genome map (Fig. 2) was visualized with OGDRAW (Lohse et al., 2007).
Fig. 2.
Graphic representation of the annotated plastid genome of Diospyros vieillardii.
Due to the difficulties with assembling the mitochondrial genome of plants as well as the low level of phylogenetic information in plant mtDNA (Malé et al., 2014), we did not attempt to assemble the mitochondrial genome of Diospyros.
Assembly of nrDNA repeats
Reads containing sequences from the nrDNA region were collected and assembled using the program MITObim v. 1.7 (Hahn et al., 2013). As initial seed, we used previously generated Sanger sequences from the ITS region of Diospyros vieillardii BT025 (B. Turner, unpubl. res.). Characteristics of assemblies such as the number of reads assembled in each contig and coverage were inspected from the MITObim output files. Assembled sequences were aligned using Muscle v. 3.8 (Edgar, 2004). The alignment was manually inspected using the program BioEdit v. 7.2.5 (Hall, 1999). The beginning and end of each coding region were estimated by comparing the Diospyros alignment with annotated sequences of Solanum lycopersicum (GenBank: AY366529, AY552528). We also extracted the 5S nrDNA region using the procedure described above. As initial seed we used the 120-bp coding fragment of 5S nrDNA sequences of Actinidia chinensis (GenBank: AF394578).
Phylogenetic analyses
For the phylogenetic analyses of the plastid sequence data, only one copy of the inverted repeat was included in the final alignment. For analyses of the nrDNA sequences, only the transcribed regions were used.
Parsimony analyses including bootstrapping were performed using PAUP* v. 4b10 (Swofford, 2003). They were run using a heuristic search with stepwise addition, random sequence addition (1000 replicates) and tree bisection–reconnection. Gaps were treated as missing data. To estimate clade support, bootstrapping with 1000 replicates was performed using the same settings as above (including 1000 random replicates per bootstrap replicate). Estimation of consistency index (CI) and retention index (RI) was done with PAUP. Likelihood analysis including bootstrapping was performed using RAxML v. 8.1.3 (Stamatakis, 2014). We used the Broyden–Fletcher–Goldfarb–Shanno (BFGS) method to optimize generalized time reversible (GTR) rate parameters, the gamma model of rate heterogeneity and 1000 rapid bootstrap inferences with a subsequent thorough maximum likelihood (ML) search. The results were visualized with FigTree 1·4 (available from http://tree.bio.ed.ac.uk/software/figtree/). We rooted the trees obtained with D. olen according to earlier results (Turner et al., 2013a).
To reduce the file size and speed up analyses in the combined analyses, D. olen and constant positions were removed using Mesquite v. 3.01 (Maddison and Maddison, 2014). In trees conducted from the combined data set D. ferrea was used as outgroup. Parsimony and likelihood analyses were performed as described for the individual data sets. In addition, we conducted a Bayesian analysis for the combined data set using BEAST v. 1.8.1 (Drummond et al., 2012). The best evolutionary models for the two subsets (ptDNA and nrDNA) were evaluated using jModeltest v. 2.1.6 (Guindon and Gascuel, 2003; Darriba et al., 2012). For the plastid partition, the transversional model with equal frequencies (TVMef; Posada, 2003) showed the best fit to the data, and for the nrDNA partition Tamura and Nei’s model (TrN; Tamura and Nei, 1993) with among-site rate variation modelled with a gamma distribution (TrN+Γ) showed the best fit. Base frequencies (uniform), substitution rates among bases (gamma shape 10) and alpha (gamma shape 10) were inferred by jModeltest for each data set. For flexibility, we used a relaxed uncorrelated log-normal clock model (Drummond et al., 2006). As a speciation model, we used a Yule model because the investigated group is so young that we expected a low proportion of lineage extinction (Yule, 1925; Gernhard, 2008). For further details regarding the parameters used see Supplementary Data Fig. S1. Two independent Metropolis-coupled Markov chain Monte Carlo (MCMC) analyses, each with 20 million generations, were run, sampling each 1000th generation. The initial 10 % of trees obtained from each MCMC run were removed as burn-in; the remaining trees of both runs were used to calculate the maximum clade credibility tree.
For directly comparing the results of the present study with previous ones (Turner et al., 2013a) based on four plastid markers (atpB, rbcL, trnK-matK, trnS-trnG; 5979 bp) and two low-copy nuclear markers (ncpGS, 717 bp; PHYA, 1189 bp), a subset of those data corresponding to the individuals included in this study was analysed in the same way as described above for the Bayesian analysis. In most cases, to represent each species we used either the same accession or individuals from the same population. In the few cases where no data from individuals of the same population were available, then the geographically closest individual assigned to the same species was used. Due to these differences, results of the previous studies are not strictly comparable to those produced here, but we have compared them nonetheless due to their overall similarity.
A general pattern of geographical clustering was visually observed in the resulting trees, in particular in the plastid data. Based on the geographical coordinates of samples and the distance matrix of pairwise uncorrected P-values (calculated with SplitsTree), we tested the significance of geographical clustering of the samples in the trees using a Mantel test. We estimated the correlation of geographical and genetic discontinuities in the data by performing this analysis on the plastid and the combined data sets with Isolation by Distance web service (IBDWS) (Jensen et al., 2005).
To investigate the relationships between populations we constructed a neighbour network based on the plastid markers, using SplitsTree4 v. 4.13.1 (Hudson and Bryant, 2006) and uncorrected P distance estimates. Based on the results from the Mantel test [Table 2 and RADseq results (Paun et al., 2016)], we excluded samples of D. olen, D. ferrea, D. vieillardii, D. umbrosa, D. flavocarpa, D. cherrieri and D. veillonii from this analysis to get a clearer picture of the relationships within the recently and rapidly radiated species group.
Table 2.
The extent of geographical clustering in the data, as evidenced with Mantel tests performed on IBDWS
| Dataset | Mantel’s r | Significance |
|---|---|---|
| Plastid data | ||
| Including all individuals, except the outgroups D. olen and D. ferrea | 0·103 | P = 0·09 |
| Excluding D. olen, D. ferrea and all D. vieillardii | 0·242 | P < 0·001 |
| Excluding D. olen, D. ferrea, D. vieillardii, D. umbrosa and D. flavocarpa | 0·383 | P < 0·001 |
| Excluding D. olen, D. ferrea, D. vieillardii, D. umbrosa, D. flavocarpa, D. cherrieri and D. veillonii | 0·427 | P < 0·001 |
| Combined | ||
| Including all individuals except the outgroups D. olen and D. ferrea | 0·079 | P = 0·10 |
| Excluding D. olen, D. ferrea and all D. vieillardii | 0·121 | P < 0·05 |
| Excluding D. olen, D. ferrea, D. vieillardii, D. umbrosa and D. flavocarpa | 0·302 | P < 0·001 |
| Excluding D. olen, D. ferrea, D. vieillardii, D. umbrosa, D. flavocarpa, D. cherrieri and D. veillonii | 0·374 | P < 0·001 |
RESULTS
After the demultiplexing step, the number of raw Illumina sequences ranged from 10 to 29 million paired-end reads per individual. Details of sample characteristics are given in Table 3.
Table 3.
Details of samples used here for sequencing of whole plastid genomes and nrDNA
| Raw reads | Plastid genome |
Nuclear ribosomal DNA |
|||||
|---|---|---|---|---|---|---|---|
| Percentage of reads mapping | Coverage (×) | GC (%) | Percentage of reads mapping | Coverage (×) | GC (%) | ||
| D. calciphila BT313 | 19549968 | 1·34 | 162 | 37·37 | 0·17 | 444 | 59·24 |
| D. cherrieri BT293 | 15164658 | 3·30 | 309 | 37·36 | 0·11 | 229 | 58·52 |
| D. erudita BT280 | 12612118 | 3·03 | 236 | 37·36 | 0·15 | 246 | 59·36 |
| D. ferrea Eb045 | 9943646 | 4·81 | 300 | 37·34 | 0·51 | 559 | 58·46 |
| D. flavocarpa BT130 | 10129514 | 0·99 | 62 | 37·36 | 0·29 | 331 | 57·07 |
| D. flavocarpa BT156 | 15656792 | 0·53 | 51 | 37·37 | 0·10 | 219 | 58·53 |
| D. glans BT093 | 14436316 | 0·91 | 81 | 37·36 | 0·19 | 386 | 58·48 |
| D. impolita BT102 | 18636398 | 1·14 | 131 | 37·36 | 0·08 | 209 | 59·26 |
| D. inexplorata BT308 | 16201056 | 1·06 | 106 | 37·36 | 0·24 | 511 | 59·65 |
| D. labillardierei BT122 | 26574012 | 0·95 | 156 | 37·36 | 0·15 | 538 | 58·6 |
| D. minimifolia BT135 | 26685314 | 1·19 | 199 | 37·36 | 0·09 | 314 | 58·82 |
| D. minimifolia BT233 | 25526086 | 0·84 | 134 | 37·35 | 0·13 | 384 | 58·24 |
| D. minimifolia BT263 | 16154630 | 1·95 | 198 | 37·35 | 0·29 | 616 | 59·14 |
| D. olen BT001 | 24966688 | 1·51 | 236 | 37·44 | 0·38 | 1084 | 58·32 |
| D. pancheri BT028 | 27590124 | 0·80 | 136 | 37·35 | 0·09 | 316 | 59·38 |
| D. pancheri BT031 | 29453086 | 0·71 | 130 | 37·36 | 0·09 | 361 | 58·98 |
| D. parviflora BT041 | 27178316 | 0·83 | 140 | 37·36 | 0·09 | 356 | 58·54 |
| D. parviflora BT090 | 25432978 | 0·40 | 63 | 37·37 | 0·19 | 656 | 59·34 |
| D. parviflora BT147 | 17887304 | 0·65 | 71 | 37·36 | 0·28 | 690 | 58·69 |
| D. parviflora BT187 | 26648984 | 0·45 | 74 | 37·36 | 0·21 | 690 | 56·6 |
| D. parviflora BT250 | 17588828 | 0·43 | 47 | 37·36 | 0·37 | 877 | 59·02 |
| D. parviflora BT290 | 19187356 | 0·39 | 47 | 37·36 | 0·28 | 737 | 59·08 |
| D. perplexa BT004 | 18085506 | 0·74 | 82 | 37·36 | 0·30 | 595 | 57·43 |
| D. pustulata BT111 | 15958418 | 2·46 | 242 | 37·36 | 0·28 | 467 | 57·69 |
| D. pustulata BT140 | 17029506 | 1·80 | 188 | 37·36 | 0·26 | 580 | 59·52 |
| D. pustulata BT261 | 15585090 | 1·19 | 114 | 37·36 | 0·37 | 745 | 59·94 |
| D. revolutissima BT221 | 20867576 | 1·50 | 193 | 37·37 | 0·20 | 551 | 57·98 |
| D. revolutissima BT120 | 17111770 | 1·46 | 154 | 37·35 | 0·21 | 466 | 59·16 |
| D. tridentata BT207 | 14190292 | 0·89 | 77 | 37·35 | 0·21 | 356 | 57·79 |
| D. trisulca BT185 | 17297816 | 0·75 | 80 | 37·36 | 0·29 | 710 | 58·67 |
| D. umbrosa BT176 | 18856642 | 0·85 | 98 | 37·38 | 0·48 | 632 | 55·92 |
| D. umbrosa BT247 | 28845756 | 0·59 | 104 | 37·36 | 0·10 | 385 | 59·19 |
| D. veillonii BT227 | 26594158 | 1·80 | 297 | 37·36 | 0·30 | 1056 | 58·65 |
| D. vieillardii BT025 | 26595776 | 0·91 | 152 | 37·34 | 0·38 | 1160 | 58·29 |
| D. vieillardii BT100 | 22649344 | 2·10 | 297 | 37·33 | 0·11 | 327 | 58·52 |
| D. vieillardii BT215 | 27710030 | 0·80 | 138 | 37·32 | 0·14 | 501 | 58·94 |
| D. yahouensis BT238 | 29242716 | 0·80 | 144 | 37·36 | 0·15 | 578 | 59·68 |
| D. sp. Pic N’ga BT318 | 18515350 | 4·63 | 531 | 37·36 | 0·21 | 500 | 59·37 |
Plastid genomes
We obtained between 75 262 (47× average coverage, for D. parviflora BT250) and 857 039 (531× coverage, for D. sp. Pic N’ga BT318) pairs of reads per individual that mapped to the plastid genome (for details see Table 3). The GC content of the plastid genomes of Diospyros (∼37 %) is similar to those of many other angiosperms [average ∼37 %; e.g. Ardisia (Ku et al., 2013); Camellia (Yang et al., 2013); Potentilla (Ferrarini et al., 2013); Musa (Martin et al., 2013); Zingiberales (Barrett et al., 2014)].
The size (∼ 157 kb) and gene order of the plastid genome of D. vieillardii (Fig. 2) is similar to that of C. sinensis (GenBank: KC143082.1). This plastid genome is the first fully sequenced plastid genome of Ebenaceae reported in the literature.
The plastid matrix of Diospyros used for phylogenetic analyses (including only one of the inverted repeats) included 133 210 characters, of which 1295 variable positions were parsimony-uninformative and 384 (0·3 %) were potentially parsimony-informative. Phylogenetic reconstruction using parsimony produced 1127 equally parsimonious trees (results not shown) with a CI of 0·93 and RI of 0·87. Phylogenetic relationships between the species were in many cases not well supported, and in several cases individuals of the same species failed to form well-supported clusters. Similar results were obtained using maximum likelihood (Supplementary Data Fig. S2).
Nuclear ribosomal DNA
Between 0·08 % (D. impolita BT102) and 0·51 % (D. ferrea Eb045) of the total reads pertained to the nrDNA region (for details see Table 3). The contigs obtained had average coverages ranging from 209× (for D. impolita BT102) to 1159× (for D. vieillardii BT025). The NTS of the intergenic spacer was difficult to align and therefore excluded from further analyses. The aligned nrDNA matrix of Diospyros included 7233 characters, of which 368 variable positions were parsimony-uninformative and 141 (1·9 %) were potentially parsimony-informative. The parsimony phylogenetic reconstruction with only the nrDNA sequences produced 84 equally parsimonious trees (results not shown) with a CI of 0·66 and RI of 0·53. Phylogenetic relationships generally disagreed with results obtained from other markers, but these incongruent relationships are all weakly supported (results not shown). Several species failed to form unique groups. Similar results were obtained using maximum likelihood (Supplementary Data Fig. S3).
The assembled 5S nrDNA region containing coding and non-coding parts was short (less than 500 bp) and therefore did not contain many informative characters. Phylogenetic trees based on this fragment were poorly resolved, and therefore the trees are not presented or discussed further. The 5S nrDNA region was also not included in the combined analysis.
Analyses of the combined data set
In addition to the individual analyses of the two data sets, we also combined them to determine whether this approach provides better resolution/support.
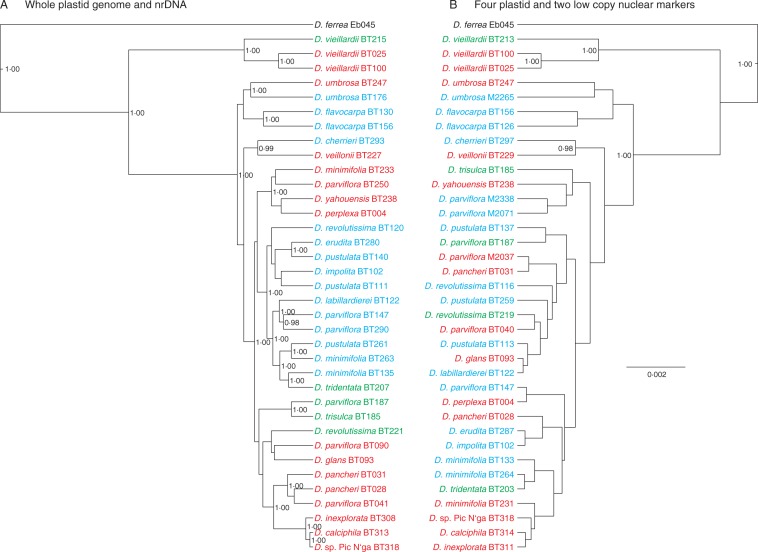
The combined matrix (i.e. reduced to variable positions as explained in Materials and methods) included 1136 characters, of which 437 were potentially parsimony-informative. Phylogenetic reconstruction using parsimony produced a single most parsimonious tree of 1580 steps (Supplementary Data Fig. S4) with a CI of 0·73 and RI of 0·52. Phylogenetic relationships depicted in the combined analysis were better resolved than in the trees obtained from the individual analyses. A comparable pattern was found in the Bayesian analysis of the combined data set (Fig. 3). All three individuals of D. vieillardii clustered together and were sister to the rest of the New Caledonian endemic species group. Individuals from D. flavocarpa and D. umbrosa formed unique groups and were sister to the rest of the remaining accessions, among which D. cherrieri and D. veillonii were sister to the rest. The results from the combined analysis were also in agreement with earlier results based on plastid and nuclear markers (Turner et al., 2013a).
Fig. 3.
Phylogenetic trees resulting from the Bayesian analysis of the (A) combined data set of the present study (variable nucleotide positions in the whole plastid genome and nrDNA) and (B) combined data set of a previous study including four plastid and two low-copy nuclear markers (Turner et al., 2013a). The numbers indicate posterior probabilities >90. Trees are scaled to the same branch length. Samples are coloured according to sampling region (see Fig. 1).
There is a trend of geographical clustering visible in the Bayesian tree (Fig. 3) and in the neighbour network (Supplementary Data Fig. S5). The neighbour network (Fig. S5) clearly shows that individuals and populations from the south and the middle of New Caledonia clustered according to their sampling region. The Mantel test (Table 2) confirmed a significant geographical clustering of the genetic information (Fig. 3), in particular in the plastid data across the crown group.
DISCUSSION
Previous standard approaches to phylogenetic analysis of Diospyros species including samples from New Caledonia used nine (Duangjai et al., 2009) and four (Turner et al., 2013a) plastid markers (alignment length >8 and 6·4 kb, respectively). They demonstrated low levels of sequence divergence among these species, indicating a fairly recent and rapid radiation. Inclusion of low-copy nuclear markers, which have been shown to be highly informative and useful for resolving phylogenetic relationships at lower taxonomic levels in some taxa [e.g. Paeonia (Tank and Sang, 2001) and Passiflora (Yockteng and Nadot, 2004)], only partly improved resolution among the New Caledonian Diospyros species (Turner et al., 2013a). Similar results have been observed in two genera of Cunoniaceae from New Caledonia [Spiraeanthemum (Pillon et al., 2009a) and Codia (Pillon et al., 2009b)].
AFLP markers are typically used at low taxonomic level in closely related species and population analyses (e.g. Despré et al., 2003; Tremetsberger et al., 2006; Gaudeul et al., 2012). In the case of the New Caledonian Diospyros species, we used AFLPs to evaluate species limits (Turner et al., 2013b), and in most cases we found congruence with the species concepts of White (1993). However, the AFLP approach was not useful in resolving phylogenetic relationships among these species (Turner et al., 2013b) because there were few of the individual AFLP fragments shared by two or more species. It would appear from the AFLP results that fragmentation of an original widespread population occurred in many regions of New Caledonia more or less simultaneously, resulting in genetically distinct populations that correspond to the morphologically based species delimitations of White (1993) without leaving much evidence for interspecific relationships.
The phylogenetic tree obtained here based on the whole plastid genome (Fig. S2) is similar to the phylogenetic tree previously based on four plastid markers (Turner et al., 2013a). The combined tree of this study (Fig. 3A) is similar both in resolution and structure to the phylogenetic tree based on four plastid and two low-copy nuclear markers (Fig. 3B). Although not always represented by the same individuals, general relationships of species are in agreement.
The nuclear ribosomal region included a higher percentage of parsimony-informative characters (1·9 versus 0·3 %) than the plastid DNA, which is in agreement with findings in other plant groups (Hamby and Zimmer, 1992; Doyle, 1993; Malé et al., 2014). Despite this variability, nrDNA was still too short to contain enough variation (141 versus 384 informative sites) and failed to resolve phylogenetic relationships among the New Caledonian Diospyros species (Fig. S3).
Our results clearly show that for this group of species, in which standard plastid and nuclear markers were not helpful for resolving the phylogenetic relationships, using the whole plastid genome does not greatly increase resolution and support. There are only a few other studies available in which whole plastid genomes have been used to resolve phylogenetic relationships at the intraspecific level among closely related species. Some studies [e.g. in Chrysobalanaceae (Malé et al., 2014) and eucalypts (Myrtaceae) (Bayly et al., 2013)] have revealed that this approach can be useful for resolving phylogenetic relationships among genera, but they failed to resolve phylogenetic relationships among closely related species and to group together individuals of the same taxonomic species. In cases of recently radiating species groups, in particular following an extreme bottleneck associated with a long-distance dispersal event such as the arrival of Diospyros in New Caledonia, plastid genomes appear to be insufficient for inference of phylogenetic relationships. The basis of the rapid radiation of Diospyros in New Caledonia is not yet clear, but it has been speculated that it is an adaptive origin associated with different soil types (Paun et al., 2016), as recently shown for the genus Geissois in Cunoniaceae (Pillon et al., 2014).
The individuals of D. vieillardii, D. umbrosa, D. flavocarpa, D. cherrieri and D. veillonii form a minimally isolated group in the combined analyses (Fig. 3). These species form clusters that are successively sister to the rest of the taxa, which are well supported collectively but form a highly unresolved central cluster. This unresolved central cluster is less than 6 million years old (Paun et al., 2016) and could be the result of two lineages that developed in isolation and then subsequently colonized some of the same habitats. This too could be the result of simultaneous parallel divergence in different parts of New Caledonia combined with effects of local and more recent hybridization (as was indicated in the AFLP study; Turner et al., 2013b). Retention of ancient polymorphisms present in the original colonizing population, which probably also underwent a severe bottleneck, could not produce such a geographically structured pattern.
Comparisons of the phylogenetic tree based on plastid sequences (Fig. S2) with the tree derived from RAD data [Paun et al., 2016 (Supplementary Data Fig. S6)] showed several clusters of individuals (D. trisulca and D. parviflora from L13, D. pustulata and D. minimifolia from L20, D. pancheri and D. parviflora from L3 and 4; Table 1, Fig. 1) that occur with high statistical support in the plastid results, but are not present in the nuclear tree. These clusters consist of individuals found in the same or very nearby locations, which could indicate introgressive hybridization and transfer of plastid genomes as a relevant phenomenon (Naciri and Linder, 2015, and references therein). Geographical rather than taxonomic clustering was observed for all populations of D. minimifolia and D. parviflora that also failed to form unique groups in nuclear results [AFLP (Turner et al., 2013b) RAD (Paun et al., 2016)]. Phenomena like introgressive hybridization and transfer of plastid genomes could also explain the geographical clustering of individuals observed in the plastid data set (Figs S2 and S5; Table 2), whereas in nuclear-based data sets [AFLP (Turner et al., 2013b), RAD (Paun et al., 2016)] no such geographical clustering was observed. Similar geographical clustering for plastid results has previously been reported in other plant groups [e.g. Nothofagus (Acosta and Premoli, 2010)].
Although there are more than 140 kb of DNA sequence included in this study, it effectively corresponds to only two markers (plastid genome and rDNA region). As not all genes evolve in the same mode and at the same tempo, phylogenies based on different genes might show different phylogenetic relationships (Heled and Drummond, 2010). It is therefore important to use many phylogenetic markers for reconstruction of relationships to overcome the limitations of individual genes and produce results as close as possible to the real species tree. Phylogenetic trees based on plastid data should always be viewed as gene trees because of the typical evolutionary pathways of these organelles (Naciri and Linder, 2015). The trees presented here may hence show the evolutionary history of the particular region investigated, and may differ from the species trees. In the case of the New Caledonian Diospyros species, we consider the SNP data derived from RAD (Paun et al., 2016) as the best approximation of the species tree, because it involves nearly 8500 independent markers from the nuclear genome.
Conclusions
Although New Caledonian Diospyros are morphologically and ecologically diverse, they show little genetic divergence. For these rapidly radiating Diospyros species, in which standard plastid and nuclear markers were not helpful for resolving the phylogenetic relationships, using the whole plastid genome does not greatly increase resolution and support. Plastid markers grouped accessions according to geographical provenance, which could result from local transfer of plastid genomes due to hybridization and introgression following secondary contact.
We are now conducting additional nuclear genome studies (both coding and non-coding regions) to determine whether other approaches could help us determine the potential adaptive nature of this radiation, which has thus far defeated our attempts using standard and next-generation methods.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Figure S1: BEAST input file for the Bayesian analysis of the combined data set including all settings and priors of both data sets. Figure S2: maximum likelihood tree based on plastid sequences. Figure S3: maximum likelihood tree based on nrDNA sequences. Figure S4: single most parsimonious tree based on the combined data matrix. Figure S5: neighbour network based on the plastid data set. Individuals are coloured according to their sampling region. Figure S6: maximum likelihood tree based on RAD SNP data.
ACKNOWLEDGEMENTS
We thank Emiliano Trucchi for his help and ideas concerning data analysis and IRD’s Nouméa team for assistance with field work, especially Céline Chambrey. Voucher specimens are deposited in the herbaria of Noumea (NOU), University of Montpellier (MPU), University of Vienna (WU) and the Faculty of Forestry of the Kasertsat University Bangkok (KUFF). This work was supported by the Austrian Science Fund (P 22159-B16 to R. S.).
LITERATURE CITED
- Acosta MC, Premoli AC. 2010.. Evidence of chloroplast capture in South American Nothofagus (subgenus Nothofagus, Notofagaceae). Molecular Phylogenetics and Evolution 54: 235–242. [DOI] [PubMed] [Google Scholar]
- Akhani H, Malekhammadi M, Mahdavi P, Gharibiyan A, Chase MW. 2013.. Phylogenetics of the Irano-Turanian taxa of Limonium (Plumbaginaceae) based on ITS nrDNA sequences and leaf anatomy provides evidence for species delimitation and relationships of lineages. Botanical Journal of the Linnean Society 171: 519–550. [Google Scholar]
- Andrews S. 2010. FastQC: a quality control tool for high throughput sequence data. http://www.bioinformatics.babraham.ac.uk/projects/fastqc/.
- Barfuss MHJ, Samuel R, Till W, Stuessy TF. 2005.. Phylogenetic relationships in subfamily Tillandsioideae (Bromeliaceae) based on DNA sequence data from seven plastid regions. American Journal of Botany 92: 337–351. [DOI] [PubMed] [Google Scholar]
- Barrett CF, Specht CD, Leebens-Mack J, Stevenson DW, Zomlefer WB, David JI. 2014.. Resolving ancient radiations: can complete plastid gene sets elucidate deep relationships among the tropical gingers (Zingiberales)? Annals of Botany 113: 119–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayly MJ, Rigault P, Spokevicius A, et al. 2013.. Chloroplast genome analysis of Australian eucalypts – Eucalyptus, Corymbia, Angophora, Allosyncarpia and Stockwellia (Myrtaceae). Molecular Phylogenetics and Evolution 69: 704–716. [DOI] [PubMed] [Google Scholar]
- CBOL Plant Working Group. 2009.. A DNA barcode for land plants. Proceedings of the National Academy of Sciences of the USA 106: 12794–12797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase MW, Soltis DE, Olmstead RG, et al. 1993.. Phylogenetics of seed plants: an analysis of nucleotide sequences from the plastid gene rbcL. Annals of the Missouri Botanical Garden 80: 528–548, 550–580. [Google Scholar]
- Chase MW, Knapp S, Cox AV, et al. 2003.. Molecular systematics, GISH and the origin of hybrid taxa in Nicotiana (Solanaceae). Annals of Botany 92: 107–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darriba D, Taboada GL, Doallo R, Posada D. 2012.. jModelTest 2: more models, new heuristics and parallel computing. Nature Methods 9: 772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Despré L, Giells L, Redoutet B, Taberlet P. 2003.. Using AFLP to resolve phylogenetic relationships in a morphologically diversified plant species complex when nuclear and chloroplast sequences fail to reveal variability. Molecular Phylogenetics and Evolution 27: 185–196. [DOI] [PubMed] [Google Scholar]
- Devos N, Raspé O, Jacquemart A-L, Tyteca D. 2006.. On the monophyly of Dactylorhiza Necker ex Nevski (Orchidaceae): is Coleoglossum viride (L.) Hartman a Dactylorhiza? Botanical Journal of the Linnean Society 152: 261–269. [Google Scholar]
- Doyle JJ. 1993.. DNA, phylogeny, and the flowering of plant systematics. BioScience 43: 380–389. [Google Scholar]
- Drummond AJ, Ho SYW, Phillips MJ, Rambaut A. 2006.. Relaxed phylogenetics and dating with confidence. PLoS Biology 4: 699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond AJ, Suchard MA, Xie D, Rambaut A. 2012.. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Molecular Biology and Evolution 29: 1969–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duangjai S, Samuel R, Munzinger J, et al. 2009.. A multi-locus plastid phylogenetic analysis of the pantropical genus Diospyros (Ebenaceae), with an emphasis on the radiation and biogeographic origins of the New Caledonian endemic species. Molecular Phylogenetics and Evolution 52: 602–620. [DOI] [PubMed] [Google Scholar]
- Edgar RC. 2004.. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32: 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrarini M, Moretto M, Ward JA, et al. 2013.. Evaluation of the PacBio RS platform for sequencing and de novo assembly of a chloroplast genome. BMC Genomics 14: 670. doi:10.1186/1471-2164-14-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudeul M, Rouhan G, Gardner MF, Hollingsworth PM. 2012.. AFLP markers provide insights into the evolutionary relationships and diversification of New Caledonian Araucaria species (Araucariaceae). American Journal of Botany 99: 68–81. [DOI] [PubMed] [Google Scholar]
- Gernhard T. 2008.. The conditioned reconstructed process. Journal of Theoretical Biology 253: 769–788. [DOI] [PubMed] [Google Scholar]
- Glor RE. 2010.. Phylogenetic insights on adaptive radiation. Annual Review of Ecology, Evolution and Systematics 41: 251–270. [Google Scholar]
- Guindon S, Gascuel O. 2003.. A simple, fast and accurate method to estimate large phylogenies by maximum-likelihood. Systematic Biology 52: 696–704. [DOI] [PubMed] [Google Scholar]
- Hahn C, Bachmann L, Chevereux B. 2013.. Reconstructing mitochondrial genomes directly from genomic next-generation sequencing reads – a baiting and iterative mapping approach. Nucleic Acids Research 41: e129. doi:10.1093/nar/gkt371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall TA. 1999.. BioEdit: a user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symposium Series 41: 95–98. [Google Scholar]
- Hamby RK, Zimmer EA. 1992. Ribosomal RNA as a phylogenetic tool in plant systematics In: PS Soltis, DE Soltis, JJ Doyle, eds. Molecular systematics of plants. New York: Chapman & Hall, 50–91. [Google Scholar]
- Heled J, Drummond AJ. 2010.. Bayesian inference of species trees from multilocus data. Molecular Biology and Evolution 27: 570–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson DH, Bryant D. 2006.. Application of phylogenetic networks in evolutionary studies. Molecular Biology and Evolution 23: 254–267. [DOI] [PubMed] [Google Scholar]
- Ibanez T, Munzinger J, Dagostini G, et al. 2014.. Structural and floristic diversity of mixed tropical rain forest in New Caledonia: new data from the New Caledonian plant inventory and permanent plot network (NC-PIPPN). Applied Vegetation Science 17: 386–397. [Google Scholar]
- Jensen JL, Bohonak AJ, Kelley ST. 2005.. Isolation by distance, web service. BMC Genetics 6: 13. doi:10.1186/1471-2156-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku C, Hu J-M, Kuo C-H. 2013.. Complete plastid genome sequence of the basal asterid Ardisia polysticta Miq. and comparative analyses of asterid plastid genomes. PLoS One 8: e62548. doi:10.1371/journal.pone.0062548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohse M, Drechsel O, Bock R. 2007.. OrganellarGenomeDRAW (OGDRAW): a tool for the easy generation of high-quality custom graphical maps of plastid and mitochondrial genomes. Current Genetics 52: 267–274. [DOI] [PubMed] [Google Scholar]
- Lowry II PP. 1998. Diversity, endemism and extinction in the flora of New Caledonia: a review In: Peng CF, Lowry II PP, eds. Rare, threatened, and endangered floras of Asia and the Pacific rim. Taiwan: Institute of Botany, Taipei, 181–206. [Google Scholar]
- Maddison WP, Maddison DR. 2014. Mesquite: a modular system for evolutionary analysis V. 3.01. http://mesquiteproject.org.
- Maia VH, Gitzendanner MA, Soltis PS, Wong GK-S, Soltis DE. 2014.. Angiosperm phylogeny based on 18S/26S rDNA sequence data: constructing a large data set using next-generation sequence data. International Journal of Plant Sciences 175: 613–650. [Google Scholar]
- Malé P-JG, Bardon L, Besnard G, et al. 2014.. Genome skimming by shotgun sequencing helps resolve the phylogeny of a pantropical tree family. Molecular Ecology Resources 14: 966–975. [DOI] [PubMed] [Google Scholar]
- Martin G, Baurens F-C, Cardi C, Aury J-A, D’Hont A. 2013.. The complete chloroplast genome of banana (Musa acuminata, Zingiberales): insight into plastid monocotyledon evolution. PLoS One 8: e67350. doi:10.1371/journal.pone.0067350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurizot P, Vendé-Leclerc M. 2009. New Caledonia geological map, scale 1/500000. Direction de l’Industrie, des Mines et de l’Energie, Service de la Géologie de Nouvelle-Calédonie, Bureau de Recherches Géologiques et Minières.
- Morat P, Jaffré T, Tronchet F, et al. 2012.. Le référentiel taxonomique florical et les caractéristiques de la flore vasculaire indigène de la Nouvelle-Calédonie. Adansonia 34: 177–219. [Google Scholar]
- Naciri Y, Linder HP. 2015.. Species delimitation and relationships: the dance of the seven veils. Taxon 64: 3–16. [Google Scholar]
- Nürk NM, Mandiñán S, Crine MA, Chase MW, Blattner FR. 2013.. Molecular phylogenetics and morphological evolution of St. John’s wort (Hypericum; Hypericaceae). Molecular Phylogenetics and Evolution 66: 1–16. [DOI] [PubMed] [Google Scholar]
- Paun O, Turner B, Trucchi E, Munzinger J, Chase MW, Samuel R. 2016.. Processes driving the adaptive radiation of a tropical tree (Diospyros, Ebenaceae) in New Caledonia, a biodiversity hotspot. Systematic Biology 65: 212–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier B. 2006. Geology of the New Caledonia region and its implications for the study of the New Caledonian biodiversity In: C Payri, B Richer de Forges, eds. Compendium of marine species from New Caledonia. Documents Scientifiques et Techniques II4. New Caledonia: Institut de Recherche pour le Développement Nouméa, 17–30. [Google Scholar]
- Pillon Y, Hopkins HCF, Rigault F, Jaffré T, Stacy EA. 2014.. Cryptic adaptive radiation in tropical forest trees in New Caledonia. New Phytologist 202: 521–530. [DOI] [PubMed] [Google Scholar]
- Pillon Y, Hopkins HCF, Munzinger J, Amir H, Chase MW. 2009a.. Cryptic species, gene recombination and hybridization in the genus Spiraeanthemum (Cunoniaceae) from New Caledonia. Botanical Journal of the Linnean Society 161: 137–152. [Google Scholar]
- Pillon Y, Munzinger J, Amir H, Hopkins HCF, Chase MW. 2009b.. Reticulate evolution on a mosaic of soils: diversification of the New Caledonian endemic genus Codia (Cunoniaceae). Molecular Ecology 18: 2263–2275. [DOI] [PubMed] [Google Scholar]
- Posada D. 2003.. Using MODELTEST and PAUP* to select a model of nucleotide substitution. Current Protocols in Bioinformatics 6.5: 1–14. doi: 10.1002/0471250953.bi0605s00. [DOI] [PubMed] [Google Scholar]
- Rodriguez-R LM, Konstantinidis KT. 2016.. The enveomics collection: a toolbox for specialized analyses of microbial genomes and metagenomes. PeerJ Preprints. https://doi.org/10.7287/peerj.preprints.1900v1 (last accessed 30 March 2016).
- Russell A, Samuel R, Rupp B, et al. 2010.. Phylogenetics and cytology of a pantropical orchid genus Polystachya (Polystachyinae, Vandeae, Orchidaceae): evidence from plastid DNA sequence data. Taxon 59: 389–404. [Google Scholar]
- Sanz M, Vilatersana R, Hidalgo O, et al. 2008.. Molecular phylogeny and evolution of floral characters of Artemisia and allies (Anthemideae, Asteraceae): evidence from nrDNA ETS and ITS sequences. Taxon 57: 66–78. [Google Scholar]
- Schaal BA, Hayworth DA, Olsen KM, Rauscher JT, Smith WA. 1998.. Phylogeographic studies in plants: problems and prospects. Molecular Ecology 7: 465–474. [Google Scholar]
- Stamatakis A. 2014.. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swofford DL. 2003. PAUP*. Phylogenetic analysis using parsimony (*and other methods). Version 4. Sunderland, MA: Sinauer Associates. [Google Scholar]
- Tamura K, Nei M. 1993.. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Molecular Biology and Evolution 10: 512–526. [DOI] [PubMed] [Google Scholar]
- Tank DC, Sang T. 2001.. Phylogenetic utility of the glycerol-3-phosphate acyltransferase gene: evolution and implications in Paeonia (Paeoniaceae). Molecular Phylogenetics and Evolution 19: 421–429. [DOI] [PubMed] [Google Scholar]
- Tel-Zur N, Abbo S, Myslabodski D, Mizrahi Y. 1999.. Modified CTAB procedure for DNA isolation from epiphytic cacti of genera Hylocereus and Selenicereus (Cactaceae). Plant Molecular Biology Reporter 17: 249–254. [Google Scholar]
- Tremetsberger K, Stuessy TF, Kadlec G, et al. 2006.. AFLP phylogeny of South American species of Hypochaeris (Asteraceae, Lactuceae). Systematic Botany 31: 610–626. [Google Scholar]
- Turner B, Munzinger J, Duangjai S, et al. 2013a.. Molecular phylogenetics of New Caledonian Diospyros (Ebenaceae) using plastid and nuclear markers. Molecular Phylogenetics and Evolution 69: 740–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner B, Paun O, Munzinger J, Duangjai S, Chase MW, Samuel R. 2013b.. Amplified fragment length polymorphism (AFLP) data suggest rapid radiation of Diospyros species (Ebenaceae) endemic to New Caledonia. BMC Evolutionary Biology 13: 269. doi:10.1186/1471–2148–13–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White F. 1993. Flore de la Nouvelle-Calédonie et Dépendances. 19. Ébénacées. Paris: Muséum National d’Histoire Naturelle. [Google Scholar]
- Wyman SK, Jansen RK, Boore JL. 2004.. Automatic annotation of organellar genomes with DOGMA. Bioinformatics 20: 3252–3255. [DOI] [PubMed] [Google Scholar]
- Yang J-B, Yang S-X, Li H-T, Yang J, Li De-Zhu. 2013.. Comparative chloroplast genomes of Camellia species. PLoS ONE 8: e73053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yockteng R, Nadot S. 2004.. Phylogenetic relationships among Passiflora species based on the glutamine synthetase nuclear gene expressed in chloroplast (ncpGS). Molecular Phylogenetics and Evolution 31: 379–396. [DOI] [PubMed] [Google Scholar]
- Yule GU. 1925.. A mathematical theory of evolution, based on the conclusions of Dr. J. C. Willis, F.R.S. Philosophical Transactions of the Royal Society London, Series B 213: 21–87. [Google Scholar]
- Zhu W-D, Nie Z-L, Wen J, Sun H. 2013.. Molecular phylogeny and biogeography of Astilbe (Saxifragaceae) in Asia and eastern North America. Botanical Journal of the Linnean Society 171: 377–394. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.