Abstract
Metallic stents are commonly used to promote revascularization and maintain patency of plaqued or damaged arteries following balloon angioplasty. To mitigate the long-term side effects associated with corrosion-resistant stents (i.e. chronic inflammation and late stage thrombosis), a new generation of so-called “bioabsorbable” stents is currently being developed. The bioabsorbable coronary stents will corrode and be absorbed by the artery after completing their task as vascular scaffolding. Research spanning the last two decades has focused on biodegradable polymeric, iron-based, and magnesium-based stent materials. The inherent mechanical and surface properties of metals make them more attractive stent material candidates than their polymeric counterparts. Unfortunately, iron produces a voluminous, retained oxide product in the arterial wall, whereas magnesium and its alloys corrode too rapidly. A third class of metallic bioabsorbable materials that are based on zinc has been introduced in the last few years. As summarized in this contribution, this new zinc-based class of materials demonstrates the potential for an absorbable metallic stent with the mechanical and biodegradation characteristics required for optimal stent performance. They appear to be free of flaws that limit the application of iron- and magnesium-based alloys, and polymers. This review compares bioabsorbable materials and summarizes progress towards bioabsorbable stents. It emphasizes on current understanding of physiological and biological benefits of zinc and its biocompatibility. Finally, the review provides an outlook on challenges in designing zinc-based stents of optimal mechanical properties and biodegradation rate.
Keywords: cardiovascular disease, endovascular stent, biodegradable stent, zinc
1 Introduction
Coronary artery disease, including phenomena such as atherosclerosis, are detrimental to the continuity of blood flow. Stents are commonly used in conjunction with balloon angioplasty procedures to restore and maintain blood flow in diseased vessels. Bare metal stents provide mechanical support as vascular scaffolding, and present generation polymer-coated drug-eluting stents provide additional molecular therapy. These constructs are needed only for a short amount of time during reendothelialization and arterial wall stabilization [1]. Their short-term benefit can ultimately be overshadowed by long-term complications such as chronic inflammation leading to in-stent restenosis, late stage thrombosis, and vessel size mismatch due to metal caging [2].
Bioabsorbable stents hold potential as alternatives for vascular repair that circumvent many of the current long-term health risks [3]. Ideally, they would retain their mechanical properties for approximately six months before being broken down, metabolized, and excreted by the body, thereby restoring native vasomotion and eliminating sources of inflammation or thrombosis.[4] A near two decades long investigation into bioabsorbable stent materials has included both polymeric and metallic materials. Poly-L-lactic acid (PLLA) has been shown to possess acceptable biocompatibility [5], but a polymeric stent requires a greater strut thickness than most metal stents because of the polymer’s lower ultimate tensile strength [6]. Other limitations seen in polymer stents include the inability to expand completely with balloon dilatation [7] along with restenosis rates similar to those observed for conventional bare metal stents [8].
Metallic stents are superior in comparison to their polymeric counterparts in terms of mechanical strength. Metals that contain elements present in the body are considered biocompatible and therefore suitable materials for constructing bioabsorbable stents. Specifically, magnesium (Mg) and iron (Fe) have been shown to hold promise due to their mechanical properties and purported biocompatibility of elements [9]. Unfortunately, magnesium alloys show a relatively high rate of degradation and associated evolution of hydrogen gas [10] which has raised concerns of cytotoxicity and systemic toxicity [11]. More importantly, present Mg alloys can dissolve within 60 to 90 days from implantation [4], which is premature for vascular stenting applications. On the other hand, Fe corrodes at a reasonable rate for stenting applications (0.1 – 0.2 mm/year), but accumulates a voluminous corrosion product that repels neighboring cells and biological matrix, and does not appear to be excreted or metabolized at an appreciable rate.[12]
A possible breakthrough in the field of biodegradable metallic stents was made in 2013 with the introduction of zinc (Zn) and its alloys, which harmlessly degrade at a rate of ~0.02 mm/year. At present, this biodegradation rate appears to be ideal for stent applications.[13] To realize the full potential of this discovery, it is necessary to achieve the rigorous biocompatibility, degradation, and mechanical properties required for bioabsorbable stent applications. The development of Zn and its alloys to attain these benchmarks is being pursued and is briefly reviewed in this paper.
This review is organized as follows: Coronary artery diseases and their treatments are reviewed in Section 2. A history of coronary artery stenting is briefly reviewed in Section 3. The concept of biodegradable stents is introduced in Section 4. Section 5 reviews and discusses biodegradable stent materials. Zinc as a new bioabsorbable stent material is introduced in Section 6 and its toxicity, biological significance and physiological degradation are discussed in details. Research progress towards development of zinc-based stents is reviewed in Section 7, which includes an overview of the mechanical properties of zinc alloys and biodegradataion rates as compared to other materials. The review concludes with a short summary and future directions on development of first zinc-based grafting stents.
2 Coronary Artery Disease and Common Treatments
Coronary artery disease is the leading cause of death in the United States.[14] This disease is characterized by the progressive, often decades long, occlusion of one or more of the four coronary arteries that deliver blood to the myocardium. The cause of the slow progressive occlusion is the intimal protrusion of plaques. Depending on their pathological stage, plaques contain combinations of smooth muscle cells with synthetic proliferative phenotypes, inflammatory macrophages and foam cells, necrotic cells, calcification, and a concentrated deposition of lipids under a fibrous cap, covered by a dysfunctional endothelium.[15] Progressive occlusion can lead to a state of decreased reserve blood flow, a deficiency that becomes manifest during stressful activities and produces chest pain (angina) and shortness of breath. Because the arterial wall can remodel extensively to increase luminal diameter, disease progression is often asymptomatic until a large plaque size is obtained. As plaque size gradually overwhelms arterial remodeling, luminal narrowing and endothelial dysfunction can eventually promote plaque rupture, followed by acute thrombus formation and the complete sudden occlusion of the coronary artery.[16]
Drug administration, coronary artery bypass grafting and stenting are three common treatments used to combat coronary artery disease.
Medications
The current treatment of coronary artery disease by medication predominantly targets serum lipoproteins with statins. These orally administered drugs act systemically to reduce serum low-density lipoprotein (LDL) levels by inhibiting a critical LDL precursor enzyme known as 3-hydroxy-methylglutaryl coenzyme A (HMG-CoA) reductase. Numerous clinical studies have shown that statins reduce major cardiovascular events in patients with a wide variety of coronary artery disease states[17] and statins are now widely used. However, although clearly effective for inhibiting atheroma progression, statins do not effectively reverse arterial disease.[18] Furthermore, there are several side effects associated with statin use, such as muscle pain and injury,[19] liver damage,[19] increased blood sugar and type 2 diabetes,[20] and an increased risk of hemorrhage.[21]
Coronary Artery Bypass Grafting (CABG)
CABG is the preferred intervention in patients with progressed atherosclerosis in multiple coronary arteries. This intricate surgical approach is highly invasive and requires direct access to the heart, as well as dissections of the saphenous vein, radial artery, or internal mammary artery for use as autologous grafting material. Autologous vessels are then surgically anastamosed to bypass coronary lesions to potentially treat all four coronary arteries. Recent clinical data has demonstrated the superiority of autologous arterial vs. venous conduits.[22] Because CABG requires the use of specific autologous conduits, there is a limited potential for repeat surgeries. Furthermore, its high cost, invasiveness, and long recovery time makes CABG a less desirable treatment option when reasonable alternatives are available.
Stents
Although CABG is a more effective approach than percutaneous coronary intervention (PCI) for treating patients with multivessel disease and complex lesions,[23] PCI is currently preferred for interventional treatment of atherosclerosis in patients presenting with a less complicated state of arterial disease. The present generation drug eluting stents (DES) provide metal scaffolding to enlarge luminal diameter and increase blood flow through diseased arteries while releasing anti-proliferative agents into the arterial wall to inhibit the restenosis response that is common to arterial stents. While DES represent an improvement relative to earlier generation bare metal stents,[24] as further discussed in the next section, they retain limitations inherent to the presence of foreign materials in the artery. These include chronic inflammatory reactions due to the permanent presence of foreign material, inability of the smooth muscle layer to dilate or contract due to the stiff metal scaffolding, and the potential for late stent thrombosis due to impaired arterial healing from the eluting drug and chronic inflammation. Partially degradable stents consisting of a bare metal – based material overlaid fully or partially with a bioabsorbable drug-eluting polymer are being developed to reduce late stage complications associated with a permanent polymer coating. These include risks associated with the long-term dual anti-platelet therapy that is often required to reduce late stage thrombosis events.[25] These devices have met with mixed results,[26] potentially due to the continued presence of the non-native metallic scaffolding, which restricts smooth muscle contraction/dilation responses and promotes chronic inflammation.
3 Development and Advances in Stenting Technology
Development and refinement of the cardiac catheterization, angioplasty and related catheter-based interventions, which have permitted the safe and effective treatment of coronary and peripheral artery disease, are among the greatest achievements of interventional cardiology, and are primarily attributed to accomplishments over the past century (Table 1). Although the concept of interventional cardiology evolved over the past millennia, Andreas Grüntzig performed the first balloon angioplasty in the 1970s,[27] which gave birth to modern interventional cardiology, also known as percutaneous transluminal coronary angioplasty. Unfortunately further investigation identified subsequent problems with (plain) balloon angioplasty including acute vessel closure (due to dissection and elastic recoil), neointimal proliferation, and restenosis.[28] Complications such as acute myocardial infarction triggered by elastic recoil and rebound occlusion of the artery were reported in 5–10% of patients, and typically occurred in minutes or hours after the balloon angioplasty procedure. Injuries, including arterial structure dissection, induced by angioplasty exposed circulating blood cells to the sub-endothelial matrix causing platelet aggregation and thrombosis,[29] as well as provoking medial smooth muscle cell necrosis and proliferation that concluded in restenosis.[30] These post-angioplasty complications lead to the development of a second revolutionary treatment in the 1980s: the coronary stent – used to scaffold the balloon-dilated artery to eliminate late recoil and seal the injuries.
Table 1.
Major historical milestones in interventional cardiology (based on references [31] and the web site: http://ptca.org/history_timeline.html)
| Year/Time | Inventor or Agency/Country | Landmark event |
|---|---|---|
| 3000 B.C. | Egypt | Bladder catheterization performed using metal (bronze, gold, silver) pipes |
| 400 B.C. | Greece | Function of cardiac valves is studied in cadavers using catheters made from hollow reeds and pipes |
| 1711 | Hales | The first cardiac catheterization of a horse using brass pipes, glass tube and goose trachea |
| 1844 | Bernard/France | Catheters are used to record intracardiac pressure in animals; term “cardiac catheterization” is defined |
| 1929 | Forssmann/Germany | First documented human cardiac catheterization |
| 1941 | Cournand and Richards/USA | Cardiac catheter used as a diagnostic tool |
| 1956 | Forssmann, Cournand and Richards/USA | Nobel Prize awarded to pioneers in cardiac catheterization |
| 1964 | Dotter/USA | Introduction of concept of remodeling the artery – transluminal angioplasty |
| 1967 | Favaloro/USA | First saphenous vein graft (bypass) surgery |
| 1967 | Judkins/USA | Introducing the catheter via groin puncture: “Judkins technique” |
| 1974 | Gruentzig/Switzerland | The first experimentation with peripheral human balloon angioplasty |
| 1977 | Gruentzig/Switzerland | The first percutaneous transluminal coronary angioplasty on conscious patient |
| 1979 | Hartzler/USA | Balloon angioplasty was used to treat an acute myocardial infarction |
| 1986 | Jacques Puel and Sigwart/France | The implantation of a stent in a human coronary artery |
| 1987 | Schatz/USA | First FDA-approved stent in USA |
| 1991 | Cannon and Roubin/USA | First treatment of acute myocardial infarction with coronary stenting |
| 1994 | FDA/USA | Approval of the use of stents to treat acute and threatened vessel closure for cases of failed balloon angioplasty |
| 1999 | Sousa | Implantation of the first drug-eluting stent in human coronary artery |
| 2002/2003 | EME and FDA | Approval of the first drug-eluting stent (Cypher, Johnson & Johnson/Cordis) in Europe and USA |
| 2011 | EME | Approval of bioresorbable vascular scaffold in Europe |
FDA = Food and Dug Administration; EME = European Medicines Agency
Stents introduced in the 1980s and 1990s were of the permanent “bare metal” type, consisting of high-strength, corrosion-resistant alloys such as stainless steel and cobalt-chromium (Table 2). Besides providing mechanical support, these stents demonstrate the radio-opacity that enables precise deployment and positioning under X-ray fluoroscopic guidance. Compared to 316 L stainless steel, cobalt chromium alloys exhibited superior radiopacity and radial strength, which together enabled the construction of stents with thinner struts that reduced the restenosis rate.[32]
Table 2.
Examples of stents of different strut dimensions (after O’Brien and Carroll[33])
| Stent name/manufacturer | Material | 0.2% Yield strength [MPa] | Strut thickness [μm] |
|---|---|---|---|
|
| |||
| BX Velocity®/Johnson & Johnson | 316L | 340 | 140 |
| Express®/Boston Sci. | 316L | 340 | 132 |
| Driver®/Medtronic | CoCr (MP35N) | 415 | 91 |
| Vision™/Abbott | CoCr (L605) | 510 | 81 |
| TriMaxx™/Abbott | 316L/Ta/316L | N/A | 74 |
Thinner struts are also thought to reduce stent thrombosis by reducing the surface area of blood contacting foreign material, reducing direct contact related injuries to the arterial endothelium, and reducing the amount of metallic surface area that requires neo-endothelialization. For these reasons, a stent material with high strength is desirable. The rigorous strength is one of the main requirements that polymeric stent materials cannot provide. By providing mechanical support as vessel scaffolding, these first generation metal stents were able to reduce early elastic recoil and restenosis as compared to balloon angioplasty alone [28a].
By providing the required intravascular mechanical support, these earlier generation metallic stents reduced emergency bypass surgery rates to below 0.5%. Furthermore, the restenosis rate—typically 40–30% with plain balloon angioplasty—was reduced to only 20–30%.[32–33] Despite their obvious advantages, an iatrogenic problem emerged in the form of the in-stent neointimal hyperplasia that produced the aforementioned restenosis rates.[34] Additionally, as an “acute” foreign device, a bare metal stent may trigger platelet adhesion and activate the coagulation cascade. Hence, the bare metal stents have thrombogenic potential.
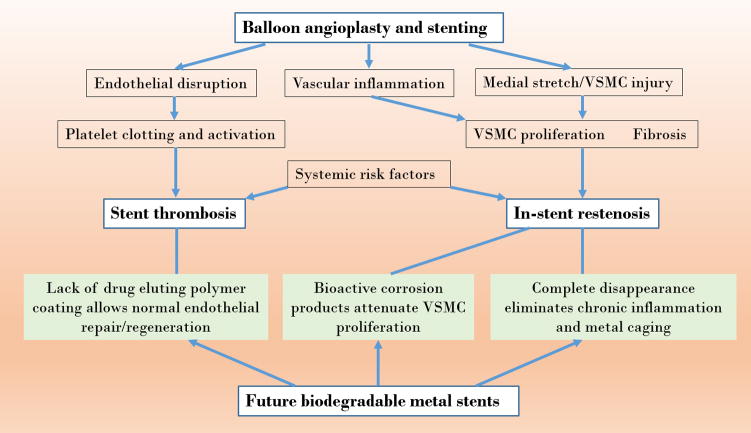
To improve biocompatibility of stent materials, inorganic coatings such as a native oxide layer, titanium-nitride-oxide coating, iridium oxide coating or carbon/diamond coating were tested but failed to provide conclusive and effective performance improvements over the stent lifetime.[33] These coatings did little to deter neointimal proliferation and the resulting restenosis. Unfortunately, the harmful side effects from the permanent presence of corrosion resistant stents remains a significant concern as this contributes to stent thrombosis and in-stent restenosis [31b] (Figure 1). Polymer-coated, DES are commonplace today because they provide additional molecular therapy with the purpose of reducing inflammatory and smooth muscle cell proliferative effects and improving vascular compatibility.[1b, 33, 35] The addition of a drug eluting polymer coating for metallic stents has considerably reduced restenosis rates (by approximately 60 – 80%) mainly by inhibiting excessive vascular smooth muscle cell (VSMC) proliferation. This improved performance has resulted in widespread commercialization of the DES concept, also known as “second generation” stents. However, a side effect of these drugs is an impaired endothelial regeneration that has increased the rate of stent thrombosis. Future fully biodegradable stents (discussed in the next section) are expected to overcome some of the serious limitations that are still seen with second generation DES. For instance, stent disappearance is expected to eliminate chronic inflammation and restore vasomotility, while biodegradable materials that produce bioactive degradation products (such as zinc, whose corrosion products may suppress smooth muscle cell proliferation) may eliminate the drug eluting polymer coatings that inadvertently impair endothelial regeneration and predispose for thrombosis (Figure 1).
Figure 1.
Pathophysiological impact of stents and possible benefits of biodegradable metallic stents (VSMC = vascular smooth muscle cell).
Currently available DES are often a stainless steel or cobalt chromium platform with a permanent polymer overlayer containing a specific drug. For example, CoCr is the platform for the second generation Xience V and Endeavor stents; stainless steel is used for Sirolimus-eluting and Paclitaxel-eluting stents. Early trials by Johnson & Johnson Company et al. have shown that DES are capable of reducing the rate of restenosis to zero within six months.[36] Although the DES provide significantly improved outcomes within one year, the long term outcomes are not statistically different relative to bare metal stents.[37]
4 Concept of Bioabsorbable Stents and Their Current Status
The concept of bioabsorbable stents has probably been around since the introduction of metallic stents,[38] but this different paradigm has only now gained momentum. Because of this, the idea of degradability is perceived as potentially “revolutionary” in stent research (third generation stents). The current belief is that biodegradable stents will drastically reduce, if not eradicate, all of the long-term health risks associated with bare metal stents and DES.[3b, 39] Particularly well designed biodegradable metallic stents could conceivably eliminate all the drawbacks of bare and drug-eluting metallic stents such as stent thrombosis and in-stent restenosis (Figure 1). A series of attempts to bring this concept to reality date back more than a decade, to the beginning of the XXIst century.[40] In the last ~15 years, each major stent manufacturer has shown interest in this new concept, along with dozens of academic laboratories in the Americas, Europe, and Asia. The concept may be achieved by engineering stents that retain mechanical properties for approximately 4 – 6 months before being broken down, metabolized, and harmlessly excreted by the body.[3b, 4, 39] Already, as early as in 2011, the European Medicines Agency approved the use of Absorb BVS (Abbott Vacular) made of a biodegradable poly-L-lactic acid (PLLA) polymer (Table 1).
Biodegradable polymers have been tested for cardiovascular stent applications since the late 1980s. Biodegradable metals, although considered for implants much earlier (in XVII century for Fe[41] and at the turn of the XIX century for Mg[42]), practically attracted interest for cardiovascular applications at the beginning of XXI century. Pure Fe was the first metal tested, although the majority of research was done on Mg and its alloys. All of these materials were implanted in animal arteries (Table 3) and were proven safe and reliable. As of today only Mg – based and PLLA – based stents went beyond animal testing and were tested clinically in humans (Tables 3 and 4).
Table 3.
Examples of pre-clinical and clinical tests on biodegradable polymers and metals.
| Implant | Implant Site and Time | Major Test Results |
|---|---|---|
| PLLA Igaki-Tamai stent (with ST638 or ST494) [48] | Porcine coronary arteries, 21d | Neointimal formation and geometric remodeling were significantly less at the ST638-loaded stent site than at the ST494 site. |
| Igaki-Tamai stent [49] | Human patient artery, 6 months to 10 years | No stent thrombosis and no major cardiac event occurred within 30 days. No major cardiac event, except for repeat angioplasty, developed within 6 months. Long-term (>10 years) clinical outcomes showed acceptable major adverse cardiac events and scaffold thrombosis rates without stent recoil and vessel remodeling. |
| PLLA stent[50] | Porcine Coronary Artery, 16 weeks | Histological examination revealed no inflammation and minimal neointimal hyperplasia |
| Copolymeric polylactide stent[51] | Rabbit aorta, 34 months | No inflammatory reaction observed after 6 months and it was completely degraded by 24 months. |
| Paclitaxel-eluting PDLLA[52] | Porcine coronary arteries, 3 months | The histomorphometric analysis at 3 weeks demonstrated inhibition of neointimal formation by 53% with the paclitaxel-loaded PDLLA when compared to the PDLLA stent, and by 44% when compared to metal stents. |
| Pure Fe stent [40] | Rabbits (descending aorta), 6–18 months | No thrombogenicity, no significant neointimal proliferation and systemic toxicity, faster degradation at junctions of the stent |
| Pure Fe stent [53] | Porcine (descending aorta), 360 d | Degradation product adjacent to the stent struts and within adventitia accompanied by macrophages; no sign of toxicity |
| Pure Fe stent [54] | Pig coronary artery, 28d | Fe stent is very safe |
| Pure Fe wire [12] | Rat (artery lumen or artery matrix), 1–9 months | Fe wire experienced substantial corrosion within artery matrix, whereas it experienced minimal biocorrosion in blood-contacting environment |
| AE21[2] (Mg alloy) | Pig (coronary artery), 56d | Mg alloy is satisfactory; 40% loss of perfused lumen diameter due to neointima formation; degradation time needs to be extended |
| WE43 [55] (Mg alloy) | Minipig (coronary artery), 56d | Mg alloy stents are safe and reliable; the struts are covered by neointima after 6 d; higher minimal lumen diameter on week 4 and 12 than the 316L stent group |
| WE 43 [56] | Domestic (28d) or minipigs Pig (coronary artery, 3 months) | Degradation after 28 d post-surgery; less neointima compare to 316L stent; stenosis increased from 28 d to 3 months; decreased lumen area |
| WE43 [57] | Domestic pig coronary artery,28d | Reduced endothelial proliferation, presence of gas pockets |
| Mg stent [58] | Left pulmonary artery of a preterm baby patient, 5 months | Complete degradation occurred during 5 months; no in-stent obstruction or neointimal hypertrophy was observed; the degradation level was tolerated and the stent secured reperfusion of the previously occluded left pulmonary artery. |
Table 4.
Biodegradable stents developed or under development (as per Iqbal et al.[31b] and Boland et al. [59])
| Stent | Manufacturer | Material | Coating | Drug | Strut thickness [μm] | Absorption time [month] |
|---|---|---|---|---|---|---|
| Polymeric | ||||||
| a Igaki-Tamai | Kyto Medical | PLLA | None | None | 170 | 24 |
| d ABSORB BVS 1.0 | Abbott Vascular | PLLA | PDLLA | Everolimus | 156 | 18–24 |
| a ABSORB BVS 1.1 | Abbott Vascular | PLLA | PDLLA | Everolimus | 156 | 18–24 |
| a ABSORB GT1 | Abbott Vascular | PLLA | None | Everolimus | 156 | 18–24 |
| a DeSolve | Elixir | PLLA | None | Myolimus | 150 | 12–24 |
| d Ideal BTI | Xenogenics | PLLA | Salicylate | Sirolimus | 200 | 6–9 |
| pc Ideal BioStent | Xenogenics | SA/AA | Salicylate | Sirolimus | 175 | 12 |
| d REVA | REVA Medical | PTD-PC | None | None | 200 | 24 |
| c ReZolve | REVA Medical | PTD-PC | None | Sirolimus | 200 | 4–6 |
| c Fantom | REVA Medical | PTD-PC | None | NA | 114–228 | 24 |
| a ART 18AZ | ART | PDLLA | None | None | 170 | 3–6 |
| c Fortitude | Amaranth | PLLA | None | None | 150–200 | 3–6 |
| pc Xinsorb | Huaan Biotech | PLLA | NA | Sirolimus | 160 | NA |
| pc Acute BRS | Orbus Neich | PLLA, PLDA-ECL | NA | EPC, sirolimus | 150 | NA |
| Metallic | ||||||
| d AMS 1.0 | Biotronik | Mg alloy | None | None | 165 | <4 |
| d DREAMS-1 | Biotronik | Mg alloy | None | Paclitaxel | 120 | 9 |
| c DREAMS-2 | Biotronik | Mg alloy | PLLA | Sirolimus | 125 | 9 |
with obtained market approval in Europe,
in clinical trials,
discontinued,
used in pre-clinical trials.
To date, fully bioabsorbable polymeric stent technology has progressed considerably further relative to their more desirable metallic counterparts,[43] as summarized by data in Table 4, with several devices having already obtained market approval in Europe or in clinical trials. This may be due to the pre-existence of numerous well-characterized Food and Drug Administration (FDA) approved polymeric materials from which to manufacture a fully bioabsorbable polymeric stent, with the most frequently used polymer being PLLA [44]. However, the recent ABSORB II trial[45]—which evaluated the performance of a polymeric scaffold relative to a conventional metallic drug-eluting device—found poor post-intervention luminal gains when polymeric devices were employed. This was due, in part, to the reluctance of participating physicians to fully expand the brittle polymeric material.[46] Furthermore, a 10-year follow-up with patients that had received an Igaki-Tamai PLLA coronary stent found poor tissue remodeling through histological analysis.[47] The area previously occupied by PLLA appeared to be filled with proteoglycan, a component of extracellular matrix, but was acellular.
Biodegradable polymeric materials have the developmental advantage of degrading predominantly by a simple hydrolysis reaction, producing predictable degradation products, and degrading through similar mechanisms whether evaluated in vitro or in vivo [60]. In contrast, the development of a suitable metallic material for stenting applications, though promising [2], has been elusive. This may be due to the lack of suitable pre-existing materials, as well as the high cost and complexity of developing new materials. Metallic materials often corrode via complex mechanisms that produce a wide range of products, and the rates and products of corrosion can differ fundamentally between in vitro and in vivo conditions [61]. This has made it difficult to translate success at the bench top into a successful stent. Consequently, the scientific and industrial community has engaged in more than a decade-long focus on Mg and Fe [62] that has failed to realize the promise of acceptable fully bioabsorbable metallic alternative to the emerging fully biodegradable polymeric stents.
Mechanical strength similarity to conventional stents allows clinicians to have reasonable deployment expectations when using a biodegradable metallic stent. The potentially beneficial bioactivity of corrosion products [63] raises the exciting prospect that pathogenic cell responses to stent implantation may be modulated as the stent corrodes. Therefore, the ability to control corrosion rates and behavior by conventional metallurgical and alloying approaches may allow for corrosion product-mediated reprogramming of host responses near the host-implant interface. As of today, only stents made of Mg alloys have been reported to go through clinical trials.[43] The first design of Mg-based stents from Biotronik (AMS-1.0), composed of about 93 wt% of Mg and 7 wt% of rare earth elements, degraded in electrolyte solutions in about 60 days.[31b] Although the degradation rate was too high, pre-clinical studies indicated a rapid endotheliazation.[4] Clinical studies on 63 patients confirmed the stent’s safety, with no cardiac death, myocardial infarction, or thrombosis, although the target lesion revascularization was ~24% and ~27% at 4 and 12 months, respectively.[64] Improved metallic stents from Biotronik (DREAMS-1 and DREAMS-2) utilize a Mg-alloy exhibiting a slower degradation rate and improved radial strength than what was used for AMS-1.0. DREAMS-1 additionally incorporated anti-proliferative drugs to reduce neointimal hyperplasia and prevent restenosis. DREAMS-2 is an improved version of DREAMS-1 that instead of incorporateing drug into a porous structure of metal, is additionally covered with a drug-eluting PLLA thin coating. Immunosuppressive and antiproliferative drug prevents restenosis, whereas PLLA reduces the stent’s degradation rate at the early stage.
5 Search for Ideal Bioabsorbable Stent Material Candidates
Bioabsorbable stents are designed to provide mechanical support for the arterial wall during the remodeling period and to degrade with the progression of tissue regeneration [65]. Ideally, in order to achieve the appropriate scaffolding, the mechanical properties of the candidate materials should be close to those of 316L stainless steel, which has been traditionally considered the gold standard material for stent constructs [66]. The stent material itself and its degradation products should also be non-toxic and compatible in the cardiovascular environment [67]. As briefly reviewed in Section 4, biodegradable polymers and two metals (Fe and Mg) went through a number of animal and clinical studies, proving of being non-toxic and promising materials for cardiovascular applications.
Specific design criteria that have been suggested for biodegradable stent materials in the scientific literature are listed in Table 5. It should be recognized that although a material tensile strength of >300 MPa is recommended, stents made of polymers – with much less favorable mechanical properties – were demonstrated in recent years and even reached clinical trials, as discussed in the previous section.
Table 5.
Summary of material criteria and constraints for a bioabsorbable stent – based on Ref.[68] – with a few additions.
| Criterion | Constraints |
|---|---|
| Bioabsorption | Mechanical integrity for 3–6 months; Full absorption in 12–24 months |
| Biocompatibility | Non-toxic and non-inflammatory; No allergenic potential; No harmful release or retention of particles |
| Mechanical properties | Yield strength > 200 MPa; Ultimate tensile strength > 300 MPa; Yield strength/elastic modulus ratio >0.16; Elongation to failure > 15–18%; Elastic recoil on expansion < 4%; Resistance to cyclic fatigue >10–20 million cycles before failure |
| Microstructure | Homogenous and approximately isotropic; Small grain size (< 30μm) |
| Corrosion rate | Penetration rate < 0.02 mm/year[69] |
The mechanical properties and degradation rates for polymers and metals tested for cardiovascular stent applications are compiled in Tables 6 and 7. The mechanical properties reported in these tables include elastic (Young’s) modulus (YM), yield strength (YS), ultimate tensile strength (UTS) and elongation. These mechanical properties are indicators of stent radial strength, acute and chronic recoil, axial and radial flexibility, deliverability, profile and lifetime integrity.[70] The YM provides a measure of how well the stent material resists deformation. Stents are typically delivered through a balloon catheter and then expanded upon proper positioning in the artery. The stent material needs to sustain deformations without cracking or fragmenting during delivery. The critical value for the YM of materials used for stents is not well defined but it is preferable to have a high value to reduce stent recoil. As shown in Table 6, polymers generally exhibit a one order of magnitude lower YM than metals. Additionally, polymers are typically viscoelastic and can deform permanently over their lifetime when in use under stress. Although rarely mentioned in the stent literature, viscoelasticity is a disadvantage of using polymers for stent applications.
Table 6.
Mechanical and degradation properties of polymers tested for cardiovascular stent applications [49b, 72]. (The values heavily depend on the molecular weight and should be treated as approximate values only.)
Table 7.
Mechanical and degradation properties of Fe and Mg and their alloys tested for cardiovascular stent applications.
| Material | Metallurgy | Grain size [μm] | Young Modulus [GPa] | Yield Strength [MPa] | Ultimate Tensile Strength [MPa] | Elongation [%] | Degradation rate [mm/year] |
|---|---|---|---|---|---|---|---|
| Iron and Iron Alloys | |||||||
| SS316L [74] | annealed | 12–30 | 193 | 190 | 490 | 40 | - |
| Armco Fe [75] | annealed | 40 | 200 | 150 | 200 | 40 | 0.20 |
| Fe-35Mn [76] | annealed | <100 | 230 | 430 | 30 | 0.44 | |
| Fe-10Mn-1Pd [77] | heat-treated | - | 60 | 850–950 | 1450–1550 | 2–8 | - |
| Fe-21Mn-0.7C-1Pd [78] | heat-treated | - | 50–100 | 690–1095 | 1020–1320 | 24–48 | 0.21 |
| Fe [79] | electrocasted annealed at 550°C | 2–8 | 54 | 270 | 290 | 18 | 0.46–1.22 |
| Alloyed Fe with (Mn, Co, Al, W, Sn, B, C and S) [80] | as rolled | 100–400 | - | 390–450 | 520–860 | 5–10 | 0.09–0.19 |
| Nanocrystalline Fe [81] | Equal channel angular processing | 0.08–0.20 | - | - | 250–450 | - | 0.09–0.2 |
| Magnesium and Magnesium Alloys | |||||||
| Pure Mg [82] | as cast | - | 41 | 20 | 86 | 13 | 407 |
| WE43 alloy [83] | extruded T5 | 10 | 44 | 195 | 280 | 2 | 1.35 |
| AM60B-F [82–84] | die cast | 25 | 45 | - | 220 | 6–8 | 8.97 |
| AZ91D [83, 85] | die cast | - | - | 150 | 230 | 3 | 2.80 |
| AZ31 [83, 86] | extruded | - | 45 | 125–135 | 235 | 7 | 1.17* |
| ZW21 [67, 87] | extruded | 4 | - | 200 | 270 | 17 | - |
| WZ21 [67, 87] | extruded | 7 | - | 140 | 250 | 20 | - |
| Mg-Zn [88] | extruded | - | 42 | 170 | 280 | 19 | 0.16 |
| Mg-Zn-Mn [83, 89] | extruded | - | - | 247 | 280 | 22 | 0.92* |
| Mg-Ca [83, 90] | extruded | - | - | 136 | 240 | 11 | 1.71 |
the degradation rate for AZ31 and Mg-Zn-Mn is from in vivo test, others are calculated from potentiodynamic polarization test.
Materials with a high UTS (>300 MPa) and low YS (~200 MPa) value are preferred for the design of stents. A high UTS, combined with high YM, is needed to increase the stent’s radial strength. A low YS is desirable for ease of crimping the stent onto a balloon tipped catheter and then expanding the stent at low balloon pressures during deployment. A YS that is too high can trigger acute recoil during or after balloon deflation. Unfortunately, most of the materials with high UTS also have a high YS. Many polymers do not exhibit a proportional limit in tension and so YS is often not reported. As shown in Tables 6 and 7, the UTS values for metals are superior to those of polymers. Additionally, the YM value should be as high as possible to prevent acute recoil, and elastic recoil of stent on expansion should be below 4%. Poncin and Proft additionally suggest using the YS/YM ratio in characterizing the elastic range of materials, which provides an indication of the expected recoil upon deflation of the balloon.[71] This value is between 0.16 amd 0.32 for stainless steel and cobalt alloys used in manufacturing of permanent stents. Bioabsorbable stent materials should probably have similar YS/YM values.
Both UTS and elongation to failure influence fatigue resistance and fracture toughness of the stents. An elongation of 30% or higher is typically preferred in materials used for stent design, although the acceptable criterion often reported is >15–18%.
The minimum lifetime before failure of permanent stents made of 316L stainless steel is 10 years, which translates to ~400 million cycles. The same criteria do not apply to absorbable stents; at present, there is no standard defined for this new generation of cardiovascular stents. Since a bioabsorbable stent needs to retain its mechanical integrity for 3 to 6 months, the stent material should be acceptable if it is able to sustain ~10 to 20 million cycles before failure occurs.
In Table 7, we also report the grain size of the metals due to the fact that small grains in a metal’s microstructure favor fatigue resistance and homogeneous corrosion, although they elevate the YS – which can be beneficial, but also raises the likelihood of acute recoil.
The last column in Tables 6 and 7 summarizes the available degradation rate data for the listed metals. For polymers, the degradation lifetime is provided instead due to very limited degradation rate data for polymers.
Despite the increased challenges faced in their development, metallic materials hold several important advantages over polymeric materials. Metallic stents are considered to be superior to polymeric devices in terms of mechanical performance (i.e. ultimate tensile strength elastic range[70]) and ease of translation to a clinical environment. Their greater mechanical strength and better elastic properties are more similar to traditional metallic stents and permit a greater flexibility in stent designs and a wider range of expandable diameters during deployment. The reduced radial strength and ductility of polymeric stents have necessitated substantially larger struts (which have the side effect of increasing vascular injury and blood flow disruptions) and the introduction of a locking mechanism to maintain luminal cross sectional area following deployment [3b]. The larger polymer stents require a larger catheter for delivery relative to metal stents, which may exclude pediatric populations [91]. The larger stent struts may also increase susceptibility to early and midterm thrombosis [92]. The locking mechanism further constrains stent design flexibility and the freedom to control the final stent diameter during deployment. It may also be a concern from a device safety standpoint, as this complex feature may increase the risks of device failure. Even in a successful deployment, lower material ductility may also affect the clinician’s willingness to expand a polymer stent sufficiently to completely overcome recoil and achieve full deployment. This effect was hypothesized to have led to significantly lower post-procedure luminal gains with a polymeric stent relative to a metallic stent in the Absorb II clinical trial [93].
As compared to polymers, Fe- and Mg-based metallic absorbable scaffolds:
exhibit similar radial force to stainless steel [82] and cobalt chromium stents [54];
display the superior profile of metallic scaffolds, which makes them more deliverable [94]; and
can bioabsorb at comparable rates with arterial remodeling and wound healing [82].
The first implantation of Fe stents in the descending aorta of New Zealand white rabbits demonstrated no significant inflammatory response, neointimal proliferation or systemic toxicity.[40] Subsequent implantations of Fe stents in minipigs [53] and juvenile domestic pigs [54] confirmed that Fe degrades without excess inflammation, local toxicity or thrombosis. Additionally, the high radial strength of Fe could make its stent struts thinner, while a high ductility makes it easy to deliver via catheter-based systems [67]. However, reports on Fe stents have indicated that it cannot corrode completely during the follow up period.[40] In order to increase the degradation rate for Fe, tremendous work has been focused either on the development of alloys or modification of the microstructure by heat [82], mechanical [81] or solution treatment [95]. Another limitation for Fe stents comes from the large volume of potentially hazardous iron oxide products, which may not degrade easily in the human body [96]. Table 7 lists the mechanical and degradation properties of Fe and Fe alloys tested for cardiovascular stent applications. Stainless steel 316 L (SS 316L) is included as the gold standard metal for clinical stent applications. Alloying with manganese (Mn) [76] or electro casting [79] was shown to increase the strength and degradation rate of pure Fe [76]. Fe-Mn alloys [76–77, 80, 95, 97] exhibited similar mechanical properties to those of SS 316L. The as-formed austenitic phase decreased the magnetic susceptibility which enhanced compatibility with the magnetic resonance imaging (MRI). From biological facts, the presence of Mn is more appropriate than nickel (used for the SS 316L), which is more toxic and carcinogenic [98]. Electron casting and annealing at 550°C produced a fine grain structure with an average grain size of 4 μm, resulting in a superior ductility and ultimate tensile strength (UTS) [79].
Mg has been considered to be another attractive base-metal candidate because of its good biocompatibility and low thrombogenicity [88a, 99]. Table 7 lists the mechanical and degradation properties of Mg and its alloys tested for cardiovascular stent applications. Mg alloys have a large range of UTS and elongation, from 86 to 280 MPa and from 3% to 20%, respectively. However, pure Mg usually corrodes too fast in aggressive chloride environments including body fluid [99c]. This fast degradation could not only make a Mg stent lose mechanical integrity in a short time, but also overload the tissue with degradation products that may lead to neointimal formation [67]. Many attempts have been conducted to improve the performance of Mg by alloying [100] and advanced processing techniques [99c, 101]. For example, it was demonstrated that alloying Mg with Li can change the crystal structure from hexagonal to body-centered cubic (bcc), producing an increase in ductility, but in exchange the UTS dropped to 132 MPa [102], much too low a value for a cardiovascular stent.
Biocompatibility and corrosion facts of degradable alloys and polymers are listed in Table 8. For biodegradable metals, the released metallic ions may induce local and systemic toxicity to host cells. Therefore, the overall amount of the element used to design a final device and the local release rate for each ion during degradation should be carefully examined. The degradation mode for polymers seems less harmful, but its in vivo long term overdose effects should not be neglected. A possible cause for concern for polymers is a recent report that a degraded Igaki-Tamai polymer stent was replaced with proteoglycans.[47] This may indicate poor extracellular matrix regeneration within the footprint of a PLLA stent. Poor matrix regeneration may be a consequence of the mode of polymer degradation vs. that of metals, which proceeds by a bulk degradation that may produce voids inside the material vs. surface corrosion taking place directly at the tissue-metal interface, which allows for an expansion of the tissue front directly into the degrading implant footprint.
Table 8.
Key properties and aspects of potential biodegradable metals and polymers for cardiovascular stent applications [103]
| Fe-alloys | Mg-alloys | Polymers | |
|---|---|---|---|
| Essential trace element | Yes | Yes | No |
| Recommended daily intake | 6–20 mg | 375–500 mg | - |
| Blood serum level | 5.0–17.6 g/l | 0.73–1.06 mM | - |
| In vivo long term overdose effects | Damage of lipid membranes, proteins and DNA; Stimulus for inflammations; Increase of free radicals. |
Excessive Mg leads to nausea; Reduction of the excitability of neuromuscular, smooth muscular and cardiac regions. |
Adverse tissue reactions; Inflammatory tissue reactions, necrosis and aneurysms. |
| Effect on local pH during degradation | Alkalescent | Alkalescent | Acidic |
| Corrosion mode | Localized corrosion | Mostly localized and pitting | Hydrolytic (volume) or enzymatic (surface) |
| Expected gaseous corrosion products | None | Hydrogen | None |
| Expected solid corrosion products | Fe(OH)2, α-FeO(OH), Fe3O4 | Mg(OH)2, MgO, MgCl2, (Ca1-xMgx)10(PO4)6OH2 | Water soluble and non-soluble oligomers |
6 Emergence of Zinc as a Bioabsorbable Metal
Pure Zn exhibits an elongation to failure of 60–80% (exceptional) and a tensile strength of ~120 MPa (unacceptable). However, minor alloying additions to improve ductility, strength, and corrosion uniformity could very well pave the way to a fully bioabsorbable Zn-based stent. This is one of the primary objectives of current research activities in the area of material development. By using Zn and its alloys, many of the core engineering problems associated with Mg and Fe could be avoided. As discussed previously, the native corrosion rate of pure Mg is known to extend into the hundreds of micrometers per year in the arterial environment, while high-purity Zn implants exhibited a native corrosion rate in the tens of micrometers per year.[13] Not only does the lower intrinsic biocorrosion rate of Zn afford greater freedom for metallurgical manipulation vis-à-vis strengthening and enhanced arterial biocompatibility, but slower degradation also allows for the engineering of lower-profile stents due to the improved material longevity. With respect to degradable ferrous biometals, Zn appears to avoid many of the pitfalls associated with nominally non-resorbable Fe oxides that have been observed in the course of arterial biocorrosion at longer times [12]. The full extent of the mechanical range, corrosion behavior, and host response to Zn-based stent materials is only now starting to be realized. Material development efforts are truly in their infancy. A more detailed comparative discussion of Zn-based material strength and corrosion properties is undertaken later in the section entitled “Progress towards Zn Stents.”
6.1 Tolerance and Biological Significance of Zinc
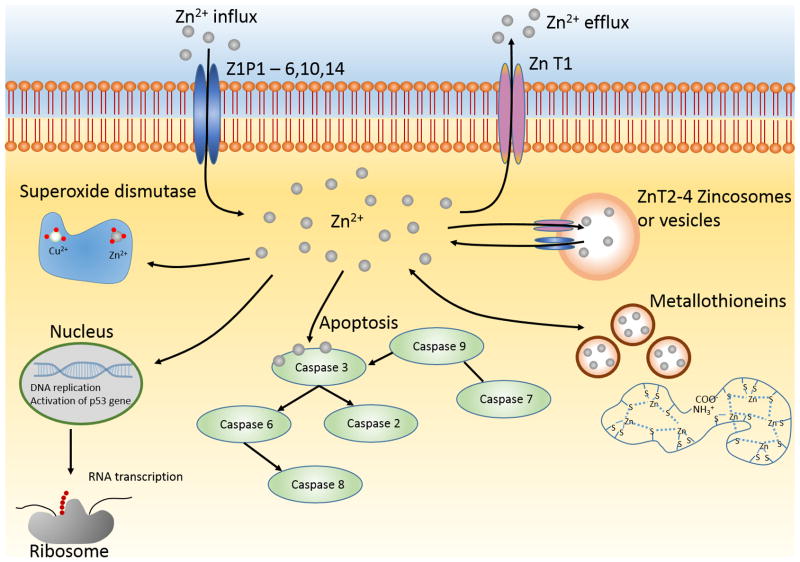
In human physiology, zinc is considered to be an essential trace element and plays an indispensable role in human health [104]. Figure 2 illustrates the major biological roles zinc plays within the cell, and throughout the human body. As seen in the figure, Zn is closely regulated via channels within the cell wall. Once Zn has entered the cytoplasm, it plays many different roles such as regulation of DNA replication, apoptosis coordination, and metal-based enzymes- as described in detail below.
Figure 2.
Biological roles of zinc.
Zn is also relatively nontoxic, especially when compared with the other elements of group IIb of the periodic table- cadmium and mercury which are 10 and 50 times more toxic [105]. The recommended dietary allowance (RDA) for Zn is 11 mg/day for men and 8 mg/day for women [106]. This is much lower than the median lethal dose (LD50) value of 27 g of zinc/day [107]. There are three main modes of Zn exposure in the human body which includes inhalation, dermal contact, or ingestion [108].
Inhalation effects are primarily caused by industrial processes with Zn-containing smoke. The usual effect of inhalation of this smoke is what is called metal fume fever (MFF). This disease is the result of inhaling fresh metal fumes and symptoms include muscle soreness, nausea, fatigue, fever and adverse respiratory problems [109]. When patients develop MFF, it is unknown whether the symptoms are caused by the exposure level of metal fumes and small particles (<1μm) or zinc exposure levels [110]. Acute oral exposure to zinc is not a large concern, due to the emetic dose of 225–400 mg zinc being much lower than the LD50 value of 27 g of zinc/day [111]. When dosages of supplemental zinc are taken over large periods of time, copper deficiency can be the result[112] . This is the result of the competitive binding of zinc and copper to metallothioneins. High dietary zinc levels will upregulate MTs, and these MTs in turn have a higher affinity for copper over zinc [113]. Free copper ions are bound to the MTs present within enterocytes, and this complex is then excreted. The symptoms of copper deficiency include anemia, leukopenia, and increased low-density lipoprotein to high-density lipoprotein (LDL:HDL) cholesterol levels [114]. It has also been shown that the excess zinc/copper deficiency can lead to a neurotoxic syndrome [115]. The exact pathogenesis of nervous system deficiencies is poorly understood, whether the primary factor is the excess zinc or the resultant copper shortage is an open question [116].
Dermal exposure is another route of exposure for zinc, but there are a limited number of studies on this route and the mechanism is not clear. Zinc chloride (ZnCl2) has been shown to be a severe irritant in levels of 10 g/L when dried topically and led to ulceration of the skin, but this does not necessarily demonstrate that zinc per se is producing a toxic effect as the native pH of the ZnCl2 solution is approximately 5.6 [117]. It appears that other forms of Zn compounds, such as zinc oxide and zinc sulfate, produce no irritating effects [118]. Zn is a common supplement for treatment of dermatological conditions and wound healing, which demonstrates dermal Zn exposure should not be a major concern [118b, 119].
Due to zinc’s role as an essential trace element in the human body, Zn deficiency can lead to many more problems as opposed to systemic Zn toxicity. The frequency of Zn deficiency worldwide is higher than 20%, and largely affects people in developing countries and the elderly [120]. Zn deficiency can lead to growth retardation, hypogonadism in males, skin changes, poor appetite, and thymic atrophy [121].
As mentioned above, Zn is an essential trace element in the human body. It plays important roles in many enzymes and proteins, both structurally and regulatory. Zn has even been shown to have a direct signaling function at different cellular signal transduction levels [122]. Zn is a completely intracellular element within the body, with 40% in the nucleus, 50% cytoplasmic, and the remainder is found within the cell membrane [123]. In terms of locations throughout the body, 85% of Zn is located in muscle and bone, 11% in the skin and liver, with the remaining located throughout the rest of the tissues [124].
Zn has three main roles within the cell, including catalytic, structural, and regulatory. Zn is required for the function of over 300 enzymes, and therefore essential to the catalysis and co-catalysis performed by these enzymes, including wound healing, brain development, and membrane stability [125]. Zn is essential to the structural integrity of many proteins, especially metalloproteins and membrane proteins [123]. Zn can also act in a direct regulatory role via both and activator or inhibitor role and regulate the stability of proteins and the activity of enzymes [125]. It is also closely regulated via cellular mechanisms. Because Zn ions are hydrophilic, they cannot cross the cell membrane by passive diffusion [123]. Instead, the metallothionein/thionein pair of proteins can bind and unbind the free Zn ions at pico- to nanomolar levels [126].
There are many specialized roles of Zn throughout the body that have been studied. Zn is linked intimately to gene expression within the cell. Not only is Zn essential to the structure of chromatin, but it plays a role in DNA replication and RNA transcription, as well as DNA repair [127]. Zn also plays an important role as an antioxidant within the cells. There are several ways in which Zn will act indirectly as an antioxidant. Instead of directly interacting with the free radical reaction, Zn will stabilize cell membranes and DNA, maintain metallothionein levels (which are free radical scavengers), and are an essential component of superoxide dismutases [123, 128]. Zn has also been shown to have possible antiatherogenic effects due to its membrane stabilizing abilities. Polyunsaturated lipids and inflammatory cytokines can destabilize vascular endothelial membranes, and Zn can prevent this destabilization while also interfering with pathways involved in apoptosis, leading to higher endothelium integrity and lower levels of atherosclerosis [129].
As mentioned above, Zn can play a role in different pathways linked to cellular apoptosis. When the cells are deprived of Zn, or Zn is chelated, the mode of cell death is commonly apoptosis [122]. Two well-known triggers of apoptosis are DNA damage and activation of the p53 gene [130]. Zn stabilizes the tertiary structure of the DNA-binding domain of p53 and modulation of this binding may be a regulatory mechanism of apoptosis [131]. Zn is also linked to inhibiting apoptosis by keeping caspase-3 inactivated and preventing the initiation of the caspase cascade [132]. When intracellular Zn is in excess it has been shown to be pro-apoptotic, it can activate p38 and potassium channels and inhibit energy metabolism [133]. Overall, it has been shown that Zn plays a very complex role in apoptosis and it is difficult to show consistently if apoptosis is activated or inhibited depending on the Zn concentration [134].
6.2 Zinc Physiological Degradation
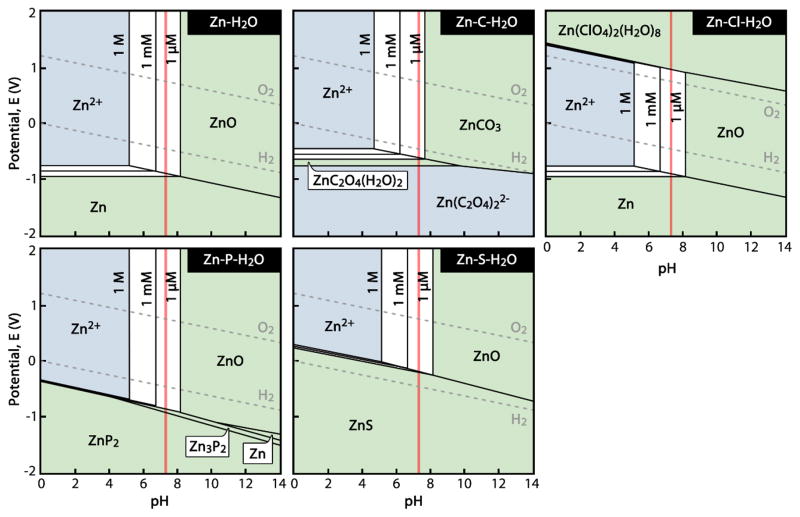
Given the overall good tolerance of most tissues to excess Zn2+ and the tight control over free Zn2+ via metallic ion buffering systems, it seems that low doses of Zn2+ would be well tolerated systemically and locally in many locales in vivo. However, it is not currently known in what quantities Zn2+ or complexed variants thereof are released from the surface of corroding metallic Zn implants. A useful exercise is to consider the equilibrium Pourbaix (E-pH) diagrams for aqueous systems containing physiological ions. Zn-H2O and Zn-X-H2O diagrams are shown for X = {C, Cl, P, and S} and [Zn2+] = {10−6, 10−3, and 1 M} at 37°C in Figure 3. Physiological concentrations of each “X” were adopted from Black [135]. It appears from Figure 3 that the equilibrium behavior of Zn at pH = 7.3 in solutions containing Cl, P, and S will tend to be largely the same, with [Zn2+] reaching stability at concentrations around 100 μM in equilibrium with solid ZnO. In contrast, C (in the form of CO32− and HCO3−) limits the equilibrium concentration of [Zn2+] to a relatively low value on the order of 10 μM. The Zn-C-H2O system also appears to arrive at equilibrium with ZnCO3 as the solid product instead of ZnO. These calculated diagrams may help explain the previous observation of a ZnO-like phase forming alongside a zinc carbonate (or, more generally, a Zn-O-C) phase after 4.5 months’ residence in the murine artery [136]. The relatively low equilibrium [Zn2+] would correspond to a local free Zn2+ release easily mitigated by the previously discussed Zn2+ buffering mechanisms [137]. This may help explain the lack of necrosis observed for Zn implants in vivo [63].
Figure 3.
Zn-H2O and Zn-H2O-X Pourbaix diagrams for physiological concentrations of X = {C, Cl, P, and S} at 37°C. Aqueous species have a light blue background, concentration-dependent regions between [Zn2+] = 1 μM and 1 M are white, and solid species are shown with green backgrounds. Physiological pH = 7.3 is shown by a red line. (Calculated using FactSage software.)
The series of diagrams in Figure 3 is largely consistent with published Pourbaix diagrams for the Zn-H2O system, though some examples suggest that Zn(OH)2 and variants thereof dominate instead of ZnO [138]. This appears to be a result of so-called atmospheric “carbonate-hydroxide” Zn corrosion theories formulated prior to 1970 [139]. Zn(OH)2 is a poorly-investigated phase that is difficult to synthesize outside of high-pH environments (i.e. ammonia or NaOH) [140]. Thus, its long-term existence is dubious in biocorrosion. It is also worth noting that, Figure 3 lacks the equilibrium phase Zn3(PO4)2·2H2O phase at pH ≈ 7.1–8.0 calculated by Zberg et al. for Zn in a simulated body fluid [141]. The slow, uniform corrosion behavior of Mg-Zn-Ca metallic glasses was previously attributed to the selective dissolution of Mg and subsequent formation of a phase similar to Zn3(PO4)2·2H2O. It is also possible that a quasi-apatitic phase containing Zn may have formed on those metallic glasses in a conversion reaction similar to that which has been observed to occur on Mg [142].
The Pourbaix diagrams are interesting in interpreting long-term in vivo results, but one must be cognizant of the fact that they simply display conditions of thermodynamic equilibrium. They can be grossly misleading when employed in making mechanistic or kinetic predictions. With respect to surface interactions, however, some guidance may be found in the modern conventional corrosion literature. An exhaustive review of corrosion literature for zinc corrosion behavior in the presence of Cl−, SO42−, CO32−/HCO3−, HPO42−, and metallic cations (Mg2+, Ca2+, Na+, and K+) is beyond the scope of the present contribution. However, some recent work has revealed new interesting aspects of Zn corrosion and electrochemistry should be mentioned. For instance, Thomas et al. [143] examined the formation and destabilization of passive ZnO surface layers, which may be useful in the future formulation of physiological corrosion mechanisms. Such an example of this is the pH-dependence of Zn corrosion studied by Thomas et al. [144] that was able to connect corrosion kinetics and thermodynamics in chloride solutions. At pH ≈ 7.3, it is shown that the aqueous species ZnCl+ and ZnOH+ participate in the corrosion reaction in addition to the thermodynamically predicted Zn2+. Importantly, the authors outline conditions under which acidification arising from Zn anodic dissolution at pH ≈ 7–10 may disrupt passive surface layers that were previously formed. In a separate study, Thomas et al. [143] also performed electrochemical experiments on Zn in both active and passive states while measuring dissolved O2 content adjacent to the metallic surface. Their results pointed to a strong dependence of corrosion on the dissolved O2 concentration due to the dominance of the cathodic oxygen reduction reaction, which varied greatly depending on the activity or passivity of the metallic Zn. These recent results demonstrate that relatively little is still known about Zn corrosion, let alone its degradation within the arterial environment.
6.3 Observed Microscale Toxicity and Biocompatibility for a Model Stent Material
The success of zinc-based stent materials will depend a great deal upon whether they provoke a biocompatible response from blood borne and tissue specific cells near the implant. Given the anticipated sustained, yet potentially time dependent flux of zinc ions and zinc-bearing corrosion products towards the cells and tissue surrounding a zinc-based implant in combination with the multitude of interactions between zinc corrosion products and numerous proteins and biological processes inside the cell, clarifying specific cellular responses to a corroding zinc based material is likely to be a challenging endeavor. This challenge will be exacerbated in alloyed materials that introduce new corrosion product phases, which can alter the overall biocompatibility of the material. Simplified in vitro cell culture systems that expose cells to zinc corrosion products will be helpful to investigate cell tolerances and specific functional changes and protein modifications that take place within the different vascular cell types. However, the complex interaction of zinc-bearing corrosion products with local vascular cells, inflammatory cell recruitment/activation and the activity of their secreted agents, fibrous capsule formation or its absence, coupled with the challenges in recapitulating physiological corrosion in an in vitro setting will make long term in vivo evaluations absolutely essential.
It should be noted that metallic zinc (Zn), although one of the few physiologically relevant metals, had not received much research attention for application in a bioabsorbable stent prior to 2013. The interest in Zn as a bioabsorbable metal was cultivated by a recent publication[13] in which it was shown that Zn degradation proceeds in rat arteries at a rate of 10–20 μm/year, nearly identical to the 20 μm/year benchmark value for ideal bioabsorbable materials. This first in vivo study with Zn relied upon an established rat-based model wherein a wire sample (0.25mm nominal diameter) is lead into the arterial media layer to simulate the environment of an encapsulated stent, or is lead into the rat artery lumen in contact with the endothelium.[96] The usefulness of the wire model is that it allows for simplified investigations at the interface between the candidate metal and the arterial environment that are more realistic than standard in vitro approaches, yet are without the high cost, time, and materials processing complications associated with a stent-based approach in large animals. In fact, the traditional reliance upon expensive and complex stent-based approaches for preliminary investigations related to materials selection and characterization, in conjunction with in vitro models that do not adequately simulate the physiological environment has hindered the discovery and development of new materials. We introduced and have been using the wire implantation model to characterize and prescreen candidate stent materials prior to proceeding along the more complicated path to stent manufacturing and large animal studies, where questions specific to the performance of actual stents can be addressed.
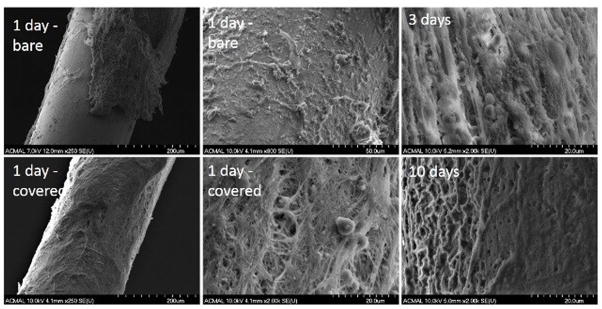
As stents are initially placed in contact with flowing blood, the evaluation of a candidate stent material’s biocompatibility should include whether or not the material provokes a robust platelet or inflammatory cell activation within the first several days to weeks. Such a response is thought to largely depend upon the candidate material’s intrinsic properties rather than its geometrical form (eg. wire vs. stent). Here we show preliminary observations related to zinc hemocompatibilty, assessed by implanting a high purity (99.99%) zinc wire into the rat arterial lumen (Figure 4). The exposed wire surface shown in this image depicts a passivation layer partially covered with healthy red blood cells, without any evidence of excessive thrombosis or a harmful inflammatory response. The same approach can be used to evaluate the evolution of early inflammation and thrombogenesis to the surface, by merely extending the time of wire incubation in the rat artery (Figure 5). A low thrombus thickness and lack of inflammatory cell infiltration is evident from this set of images, depicting the positive biocompatibility of pure zinc at early times. Because each unique alloy composition and set of processing conditions will produce a different surface, it will be necessary to evaluate the hemo and immuno compatibility of each candidate material in a similar manner as was shown here for pure zinc. The simplicity and effectiveness of the wire implant approach demonstrates its value as a prescreening tool.
Figure 4.
SEM image of a pure Zn wire implanted into the arterial lumen of a rat for 1 day, showing crystallization of surface and intact attached red blood cells, at this particular region of the wire.
Figure 5.
SEM images of a pure Zn wire implanted into the arterial lumen of a rat for 1 – 10 days. Shown for the one day sample are wire surfaces with (bottom left) and without full coverage of a thrombus layer (top left) and close-up images of these regions (middle column – showing bare region at top and covered region at bottom). The surfaces of separate wires are shown at 3 (top right) and 10 (bottom right) days.
Although wire implantations of several days to weeks are useful for evaluating the hemo and immuno compatibility of surface layers or coatings, substantially longer implantation durations are necessary for evaluating the biocompatibility of cells to corrosion products. Initial results with our rat-based in vivo model showed that the pure zinc wire did not elicit a negative inflammatory response or smooth muscle cell proliferation for implant durations of up to 6 months.[145] Additionally, the corrosion product experienced a considerable amount of tissue regeneration within the original footprint of the implant, a feature rarely seen after magnesium degradation, and remains unreported in iron implants. As a sample of this work, Figure 6 shows an H&E stained cross section of a pure Zn wire explanted from the abdominal aortic wall after a four month residence.
Figure 6.
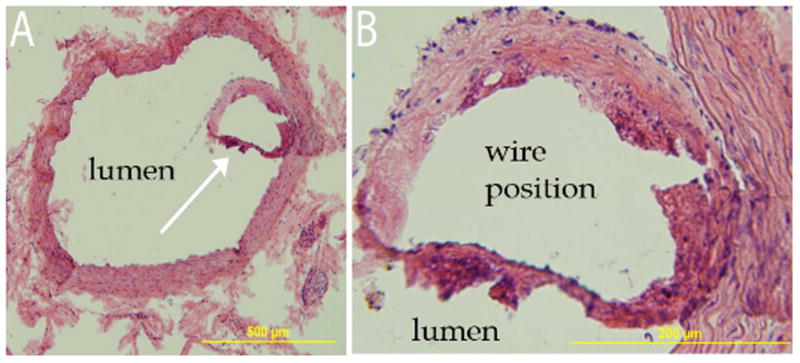
Hematoxylin and Eosin stained images from an excised zinc wire after residence in the arterial lumen for four months. White arrow in A identifies encapsulating tissue surrounding the wire, which is the region that is magnified in B. Please note that the wire cross section was originally present within the encapsulating tissue and detached during staining.
A thin neo-intima surrounding the wire without cellular hyperplasia or harmful inflammation is evident from the images shown in Figure 6. Taken together with our recent report, [145] these images indicate that (i) a continuous neo-endothelium is present around the zinc wire encapsulating tissue; (ii) several layers of smooth muscle cells are present, but not in numbers that constitute hyperplasia; and (iii) microphages occupy the corrosion region up to the metallic interface. Good neointimal tissue compatibility and the vigorous presence of cells in and around the corrosion layer further establish the biocompatibility of the zinc biometal.[146]
Further investigation into metallic zinc is needed to unravel the underlying mechanisms behind the positive biocompatibility response we observed. The current work of assessing compatibility focuses heavily on histomorphometric features of tissue sections, and luminal cross-section area loss. Many studies have failed to perform an in-depth characterization of the cellular response to degrading bio-metals, and the activity of their interfaces. The near-ideal physiological corrosion rate of zinc coupled with nominally positive arterial biocompatibility results suggests zinc as an excellent candidate for alloying and degradable vascular scaffolding devices. The presented approaches for evaluating the properties of pure zinc as an arterial implant material can be readily adopted to evaluate novel zinc alloys and processing conditions prior to proceeding along the more complicated path to stent manufacturing and large animal implantations.
7 Progress towards Zn Stents
7.1 Mechanical Properties Relative to General Requirements
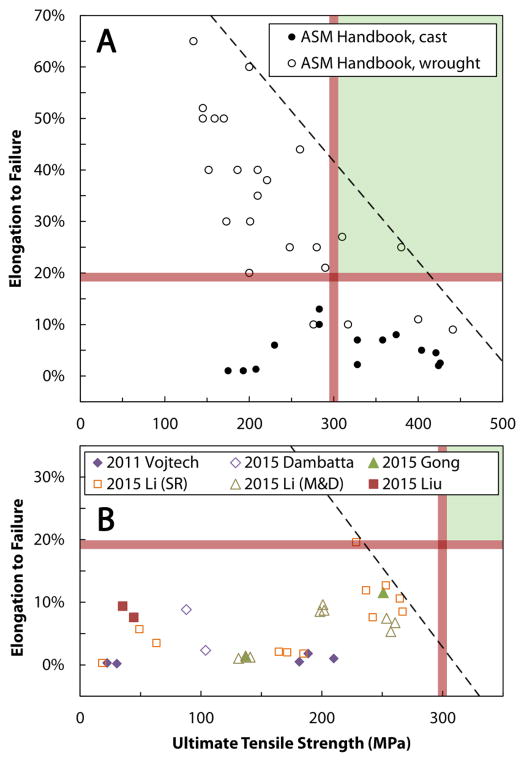
The aforementioned mechanical and corrosion requirements (Table 5) adopted from Werkhoven et al. [147] and other sources [70, 148] are a useful lens through which the state-of-the-art in zinc alloy development may be viewed. It is informative to use an Ashby-style materials selection chart [149] to visualize two of the more basic mechanical requirements—UTS and elongation to failure. Figure 7 shows elongation to failure versus UTS for two families of Zn-based materials: conventional cast and wrought alloys [150] (Figure 7 (A)); and experimental biometals [151] (Figure 7 (B)). A full accounting of mechanical and compositional information may be found in the Supplementary Data.
Figure 7.
Materials selection plots of elongation to failure versus ultimate tensile strength. Data for conventional cast and wrought alloys from the ASM Handbook [150] are shown in (A). A comparable presentation is given in (B) for experimental Zn-based biodegradable metals from the following literature sources: “2011 Vojtech” [151a], “2015 Dambatta” [151b], “2015 Gong” [151c], “2015 Li (SR)” [151d], “2015 Li (M&D)” [151e], and “2015 Liu” [151f]. Red lines denote approximate mechanical benchmarks, the green shaded area denotes a region in which both requirements are satisfied, and the dashed black line shows apparent limits in strength and ductility for each group of materials. Note the difference in ultimate tensile strength (x-axis) scales.
Figure 7 (A) shows that conventional Zn alloys tend to exhibit relatively high elongations to failure in the wrought state (10–65%) and a wide UTS range (130–440 MPa). Unsurprisingly, high ductilities are observed in alloys with lower strengths and vice versa. Cast alloys display a similar UTS range (175–425 MPa), but much lower elongations to failure (< 13%). When the approximate stent material mechanical benchmarks of 18–20% elongation to failure and 300 MPa UTS are overlaid on Figure 8(A), it is seen that a portion of the conventional Zn alloy elongation-UTS property space is included. This suggests that conventional alloy families and processing methods are capable of producing a Zn-based material with the requisite UTS and elongation to failure for stent application.
While the handbook data paint a favorable picture for the applicability of Zn in bioabsorbable stents, experimental alloys have failed to reach the 18–20% elongation/300 MPa UTS benchmarks. Figure 7(B) shows that Zn-based biometals reported to date—including Zn-Mg, Zn-Ca, Zn-Sr, several ternary alloys thereof, a single Zn-Al-Cu alloy, and pure Zn—have by-and-large exhibited poor elongations (< 20%) and only moderate UTS values (20–265 MPa). When compared to benchmark values, it is clear that the entire family of current experimental Zn biometals falls short of the target values. The apparent emphasis on alloying Zn with alkaline earth metal biological trace elements (Mg, Ca, and Sr) may be contributing to the systemic under-performance of these experimental Zn biometals relative to conventional Zn alloys. While advances in material processing may be able to increase the strengths and ductilities of Zn-alkaline earth metal alloys, it seems clear that other alloying routes should be pursued. Perhaps an appropriate starting point would be the more successful conventional alloys that do not contain systemically toxic alloying additions (i.e. Pb and Cd).
As observed from this analysis, a wide array of strength and elongation values have been reported for absorbable Zn-based materials. In contrast, determination of Young’s modulus for experimental zinc alloys has heretofore been unsatisfactory. Precise ultrasonic measurements are required to predict the potential for elastic recoil via the ratio of tensile strength to modulus, as well as to assist in comparative modeling investigations that may help uncover performance differences in the biodegradable metals in silico.
7.2 Biodegradation Rates in Comparison to Other Materials
Given the early stage of research on degradable Zn, broad statements regarding corrosion rates for Zn-based materials are difficult to make. However, the authors have conducted separate studies of 99.9%+ pure Mg and 99.99% Zn using the arterial implant method [12] mentioned before and the same method of analysis. Mg biocorrosion measurements yielded rates in the hundreds of micrometers per year (300–600 μm/year), and the 250 μm diameter implant became fragmented after 32 days in vivo [152]. In contrast, pure Zn exhibited corrosion rates a full order of magnitude lower, ranging from 15 to 50 μm/year over the course of six months in the murine arterial wall [136]. Zn implants (also 250 μm diameter) remained intact after 6 months’ residence in the artery. These directly comparable results indicate that Zn is an intrinsically slower-corroding material than Mg.
The in vitro corrosion susceptibility of experimental Zn alloys is a difficult issue that is distinct from the comparison of in vivo studies. Corrosion rate measurements can now be found in the literature for both electrochemical and weight-loss measurements, primarily in Hank’s solution [151d–f]. This solution selection is justified by referencing ASTM standard G31 72 from 2004, titled “Standard practice for laboratory immersion corrosion testing of metals.” The suitability of ASTM standard corrosion techniques for magnesium-based biomaterials was debunked by Witte et al. in 2006,[61a] was the topic of a focused ASTM-FDA workshop in 2012 [153], and the “correct” corrosion methodology is still a matter of contention. Sufficed to say, invocation of an ASTM standard is not appropriate practice for testing the biocorrosion of Mg- or Zn- based biometals. Some attention was recently called to this issue by Törne et al. [154], who attempted to address the divergence in corrosion rates between conventional in vitro immersion mediums by evaluating the behavior of Zn in PBS, Ringer’s solution, human plasma, and whole blood. Few concrete recommendations were offered by Törne and coauthors, but Ringer’s solution appeared to induce corrosion that was nominally similar to blood and plasma. While corrosion data for Zn in PBS, Hank’s solution, blood plasma, etc. are now available, the direct comparison of in vitro corrosion rates gleaned from disparate methods is fallacious, and will be avoided in this review.
That being said, electrochemical and immersion (in vitro) and long-term ex post (in vivo) corrosion data are absolutely needed to understand the basic corrosion processes underpinning Zn bioabsorption. Due to their importance and the ease with which misleading results may be obtained, such experiments should be approached with great caution. Some of the lessons learned from Mg biocorrosion studies [155] may be applied to the in vitro evaluation of candidate biometals and help investigators avoid fundamental mistakes. Such important considerations include:
the careful selection and consistent formulation of a pseudophysiological solution that successfully replicates in vivo biocorrosion for a particular environment (ionic composition and buffering system should be carefully considered);
use of a large volume-to-surface area ratio used in Zn corrosion studies, particularly due to the lower equilibrium [Zn2+] according to Figure 3; and
proper temperature, atmospheric composition, gas exchange conditions, and buffer equilibration to pH ≈ 7.3 before and during in vitro corrosion.
After the development of an in vitro corrosion experiment, it is also important to compare the results with a published in vivo reference to ensure the same patterns of corrosion initiation and product generation are realized. The authors’ work mentioned previously [136] could provide a useful in vivo touchstone for absorbable stent materials. However, benchmark behavior for Zn in an orthopedic setting is not available. It therefore currently impossible to validate an in vitro test for Zn-based orthopedic implants.
7.3 MRI Compatibility
Magnetic resonance imaging (MRI) is a noninvasive imaging alternative to x-ray, angiography, and computed tomography for diagnosing diseases of the heart and blood vessels. Real-time MRI provides safe and accurate navigation, positioning and deployment of stents during endovascular interventions.[156] As a diagnostic tool in the clinic, it provides a post-procedural anatomic and hemodynamic assessment, capable of early recognition of significant complications after stenting. The permanent stents currently in use, such as 316L stainless steel, Co–Cr alloys and Ni–Ti alloys exhibit an unsatisfactory MRI compatibility. This is due to the presence of constituents with high magnetic susceptibility values, which produce void artifacts during imaging that are several orders of magnitude greater than their geometrical dimensions [ref]. Zinc (and most likely many of its alloys) with magnetic (volume) susceptibility of (− 15.7 × 106) is one of only a few metals with a high MRI compatibility, which is close to that of polymers and ceramics and much lower than that of stainless steel (+(3.5 to 6.7) × 109), iron (+0.2 × 106), and magnesium (+11.7 × 106).[157] The high MRI compatibility of zinc adds an extra advantage to this promising new metallic candidate for biodegradable stents over both iron- and magnesium-based stent materials presently under development.
8 Summary and Future Challenges
In this review, the need for a degradable metal vascular scaffold has been clarified, as have the published corrosion and mechanical benchmarks required of a material considered for this application. The strengths and limitations of existing polymeric and metallic (Fe, Mg) stent material candidates have been reviewed at length. In view of a decade of metallurgical endeavors in magnesium and iron processing that have failed to produce a suitable material, new directions should be considered.
At this stage, it appears that a carefully formulated Zn-based biometal may exhibit mechanical properties, corrosion rate, and bioactivity that are suitable for a bioabsorbable endovascular stent. Corrosion rates in the tens of micrometers per year and elongations to failure in excess of 70% for pure Zn leave ample room for creative alloying and processing routes to be applied to obtain an appropriate mechanical profile. Furthermore, the residence of Zn in the arterial environment appears to mitigate the factors associated with smooth muscle cell hyperplasia and, ultimately, restenosis. Per literature review, it is unlikely that Zn-based stents will contribute to substantial systemic or local toxicity, and indications are good that bespoke Zn alloys will be able to meet mechanical benchmarks.
Moving forward, the challenges facing Zn-based stent development are largely the same as those facing Mg-based stents. Primarily, a material has not yet been reproducibly shown to possess both the mechanical and corrosion properties that will enable stenting with degradable biometals. This is the grand pursuit in the study of degradable biometals for stents, but straightforward “melt-and-corrode” methodologies currently pervasive in the literature are not sufficient to advance the field towards this end goal. Issues that commonly hinder material development and have been discussed in this review include:
cytotoxicity studies performed according to published standards with little critical thought or understanding of relevant biochemical interactions,
the measurement of corrosion rates using inappropriate in vitro methods without an in vivo reference,
a lack of methods detail regarding material processing and pre- and post-corrosion material characterization; and
a relatively poor understanding of the long-term in vivo corrosion mechanism that underpins loss of function and ultimately clears the corrosion products.
Moving forward, we must remain cognizant of these challenges and discourage work that does not address these issues of process reproducibility, cytotoxicity and corrosion methodology. With a regrouped effort on developing Zn-based materials and processes for bioabsorbables, a degradable biometal stent may be realized in the near future.
Supplementary Material
Acknowledgments
U.S. National Institute of Health – National Heart, Lung, and Blood Institute (Grant #1R15HL129199-01) and U.S. National Institute of Health – National Institute of Biomedical Imaging and Bioengineering (Grant #5R21 EB 019118-02) are acknowledged for funding this work. PKB was supported by an American Heart Association predoctoral research fellowship administered by the Midwest Division.
Abbreviations
- ASTM
American Society for Testing and Materials
- CABG
coronary artery bypass graftig
- DES
drug-eluting stent
- DNR
deoxyribonucleic acid
- ECL
e-caprolactone
- EME
European Medicines Agency
- EPC
endothelial progenitor cells
- Fe
iron
- FDA
Food and Dug Administration
- HDL
high-density lipoprotein
- HMG-CoA
3-hydroxy-methylglutaryl coenzyme A
- LD50
median lethal dose
- LDL
low-density lipoprotein
- Mg
magnesium
- PBS
phosphate-buffered saline
- PCI
percutaneous coronary intervention
- PCL
poly-caprolactone
- PDLLA
poly-d,l-lactic acid
- PGA
polyglycolide acid
- PLLA
poly-l-lactic acid
- PTD-PC
poly-tyrosine-derived polycarbonate
- RDA
recommended dietary (daily) allowance
- RNA
ribonucleic acid
- SA/AA
salicylic acid/adipic acid
- TD-PCP
poly-tyrosine derived polycarbonate
- UTS
ultimate tensile strength
- VSMC
vascular smooth muscle cell
- YM
Young modulus
- YS
yield strength
- Zn
zinc
References
- 1.a O’Connell BM, McGloughlin TM, Walsh MT. Biomed Eng Online. 2010:9. doi: 10.1186/1475-925X-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Baber U, Mehran R, Sharma SK, Brar S, Yu J, Suh JW, Kim HS, Park SJ, Kastrati A, de Waha A, Krishnan P, Moreno P, Sweeny J, Kim MC, Suleman J, Pyo R, Wiley J, Kovacic J, Kini AS, Dangas GD. J Am Coll Cardiol. 2011;58:1569–1577. doi: 10.1016/j.jacc.2011.06.049. [DOI] [PubMed] [Google Scholar]
- 2.Heublein B, Rohde R, Kaese V, Niemeyer M, Hartung W, Haverich A. Heart. 2003;89:651–656. doi: 10.1136/heart.89.6.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.a Waksman R. J Invasive Cardiol. 2006;18:70–75. [PubMed] [Google Scholar]; b Onuma Y, Ormiston J, Serruys PW. Circ J. 2011;75:509–520. doi: 10.1253/circj.cj-10-1135. [DOI] [PubMed] [Google Scholar]
- 4.Waksman R, Pakala R, Kuchulakanti PK, Baffour R, Hellinga D, Seabron R, Tio FO, Wittchow E, Hartwig S, Harder C, Rohde R, Heublein B, Andreae A, Waldmann KH, Haverich A. Catheter Cardiovasc Interv. 2006;68:607–617. doi: 10.1002/ccd.20727. [DOI] [PubMed] [Google Scholar]
- 5.Tamai H, Igaki K, Tsuji T, Kyo E, Kosuga K, Kawashima A, Matsui S, Komori H, Motohara S, Uehata H, Takeuchi E. Journal of Interventional Cardiology. 1999;12:443–449. [Google Scholar]
- 6.Bunger CM, Grabow N, Sternberg K, Goosmann M, Schmitz KP, Kreutzer HJ, Ince H, Kische S, Nienaber CA, Martin DP, Williams SF, Klar E, Schareck W. J Endovascular Ther. 2007;14:725–733. doi: 10.1177/152660280701400518. [DOI] [PubMed] [Google Scholar]
- 7.Grabow N, Bunger CM, Schultze C, Schmohl K, Martin DP, Williams SF, Sternberg K, Schmitz KP. Ann Biomed Eng. 2007;35:2031–2038. doi: 10.1007/s10439-007-9376-9. [DOI] [PubMed] [Google Scholar]
- 8.Di Mario C, Griffiths H, Goktekin O, Peeters N, Verbist J, Bosiers M. J Interv Cardiol. 2004;17:391–395. doi: 10.1111/j.1540-8183.2004.04081.x. [DOI] [PubMed] [Google Scholar]
- 9.a Wang H, Estrin Y, Zuberova Z. Materials Letters. 2008;62:2476–2479. [Google Scholar]; b Saris NEL, Mervaala E, Karppanen H, Khawaja JA, Lewenstam A. Clinica Chimica Acta. 2000;294:1–26. doi: 10.1016/s0009-8981(99)00258-2. [DOI] [PubMed] [Google Scholar]; c Moravej M, Mantovani D. International Journal of Molecular Sciences. 2011;12:4250–4270. doi: 10.3390/ijms12074250. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Zhu SF, Huang N, Xu L, Zhang Y, Liu HQ, Sun H, Leng YX. Materials Science & Engineering C-Biomimetic and Supramolecular Systems. 2009;29:1589–1592. [Google Scholar]
- 10.Gu XN, Zheng YF, Cheng Y, Zhong SP, Xi TF. Biomaterials. 2009;30:484–498. doi: 10.1016/j.biomaterials.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 11.Ghali E, Revie RW. Corrosion Resistance of Aluminum and Magnesium Alloys: Understanding, Performance, and Testing. Wiley; Hoboken: 2010. [Google Scholar]
- 12.Pierson D, Edick J, Tauscher A, Pokorney E, Bowen PK, Gelbaugh J, Stinson J, Getty H, Lee CH, Drelich J, Goldman J. J Biomed Mater Res B. 2012;100B:58–67. doi: 10.1002/jbm.b.31922. [DOI] [PubMed] [Google Scholar]
- 13.Bowen PK, Drelich J, Goldman J. Advanced materials. 2013;25:2577–2582. doi: 10.1002/adma.201300226. [DOI] [PubMed] [Google Scholar]
- 14.O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX. J Am Coll Cardiol. 2013;61:E78–E140. doi: 10.1016/j.jacc.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 15.Bentzon JF, Otsuka F, Virmani R, Falk E. CircRes. 2014;114:1852–1866. doi: 10.1161/CIRCRESAHA.114.302721. [DOI] [PubMed] [Google Scholar]
- 16.Ambrose JA, Singh M. F1000Prime Rep. 2015;7(08):5. doi: 10.12703/P7-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.a Pedersen TR, Kjekshus J, Berg K, Haghfelt T, Faergeman O, Thorgeirsson G, Pyorala K, Miettinen T, Wilhelmsen L, Olsson AG, Wedel H, Kristianson K, Thomsen H, Nordero E, Thomsen B, Lyngborg K, Andersen GS, Nielsen F, Talleruphuus U, McNair A, Egstrup K, Simonsen EH, Simonsen I, Vejbychristensen H, Sommer L, Eidner PO, Klarholt E, Henriksen A, Mellemgaard K, Launbjerg J, Freuergaard P, Nielsen L, Madsen EB, Ibsen H, Andersen U, Enemark H, Haarbo J, Martinsen B, Dahlstrom CG, Thyrring L, Thomassen K, Jensen G, Rasmussen SL, Skov N, Hansen KN, Larsen ML, Haastrup B, Hjaere I, Thuroe A, Leth A, Munch M, Worck R, Nielsen B, Thorn AG, Pedersenbjergaard O, Fournaise B, Sigurd B, Enk B, Nielsen H, Jacobsen L, Svendsen TL, Hoegholm A, Munter H, Kaufmann P, Haunso S, Grande P, Eriksen C, Nielsen HH, Jurlander B, Pinborg T, Pindborg J, Tost H, Christiansen BD, Oppenhagen M, Egede F, Hvidt S, Kjaerby T, Lemming L, Klausen I, Miettinen TA, Vanhanen H, Strandberg TE, Holtta K, Luomanmaki H, Pekuri T, Vuorinen A, Pasternack A, Oksa H, Siitonen L, Rimpi R, Kesaniemi YA, Lilja M, Korhonen T, Rantala A, Rantala M, Savolainen M, Ukkola O, Laine L, Virkkala L, Lehto S, et al. Lancet. 1994;344:1383–1389. [Google Scholar]; b Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, McKillop JH, Packard CJ. N Engl J Med. 1995;333:1301–1307. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]; c Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, Brown L, Warnica JW, Arnold JMO, Wun CC, Davis BR, Braunwald E. N Engl J Med. 1996;335:1001–1009. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 18.Koskinas KC, Windecker S, Raber L. Trends in Cardiovascular Medicine. 2015 doi: 10.1016/j.tcm.2015.05.004. in press. [DOI] [PubMed] [Google Scholar]
- 19.Chaipichit N, Krska J, Pratipanawatr T, Jarernsiripornkul N. Int J Clin Phar. 2015;37:355–364. doi: 10.1007/s11096-015-0068-5. [DOI] [PubMed] [Google Scholar]
- 20.Cederberg H, Stancakova A, Yaluri N, Modi S, Kuusisto J, Laakso M. Diabetologia. 2015;58:1109–1117. doi: 10.1007/s00125-015-3528-5. [DOI] [PubMed] [Google Scholar]
- 21.Salmoirago EB, Hovey KM, Andrews CA, Robinson JG, Johnson K, Wassertheil CSS, Crawford S, Qi L, Martin LW, Ockene J, Manson JE. BMJ Open. 2015;5 doi: 10.1136/bmjopen-2014-007075. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.a Gaudino M, Crea F, Cammertoni F, Massetti M. Ann Thorac Surg. 2015;99:1479–1485. doi: 10.1016/j.athoracsur.2014.11.045. [DOI] [PubMed] [Google Scholar]; b Davierwala PM, Mohr FW. Int J Surg. 2015;16:133–139. doi: 10.1016/j.ijsu.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 23.Sipahi I, Akay MH, Dagdelen S, Blitz A, Alhan C. JAMA Intern Med. 2014;174:223–230. doi: 10.1001/jamainternmed.2013.12844. [DOI] [PubMed] [Google Scholar]
- 24.Kang SH, Park KW, Kang DY, Lim WH, Park KT, Han JK, Kang HJ, Koo BK, Oh BH, Park YB, Kandzari DE, Cohen DJ, Hwang SS, Kim HS. Eur Heart J. 2014;35:1147. doi: 10.1093/eurheartj/eht570. [DOI] [PubMed] [Google Scholar]
- 25.Giustino G, Baber U, Sartori S, Mehran R, Mastoris I, Kini AS, Sharma SK, Pocock SJ, Dangas GD. J Am Coll Cardiol. 2015;65:1298–1310. doi: 10.1016/j.jacc.2015.01.039. [DOI] [PubMed] [Google Scholar]
- 26.a Stefanini GG, Kalesan B, Serruys PW, Heg D, Buszman P, Linke A, Ischinger T, Klauss V, Eberli F, Wijns W, Morice MC, Di Mario C, Corti R, Antoni D, Sohn HY, Eerdmans P, van Es GA, Meier B, Windecker S, Juni P. Lancet. 2011;378:1940–1948. doi: 10.1016/S0140-6736(11)61672-3. [DOI] [PubMed] [Google Scholar]; b Byrne RA, Kastrati A, Massberg S, Wieczorek A, Laugwitz KL, Hadamitzky M, Schulz S, Pache J, Fusaro M, Hausleiter J, Schomig A, Mehilli J Investigators IT. J Am Coll Cardiol. 2011;58:1325–1331. doi: 10.1016/j.jacc.2011.06.027. [DOI] [PubMed] [Google Scholar]; c Stettler C, Wandel S, Allemann S, Kastrati A, Morice MC, Schomig A, Pfisterer ME, Stone GW, Leon MB, de Lezo JS, Goy JJ, Park SJ, Sabate M, Suttorp MJ, Kelbaek H, Spaulding C, Menichelli M, Vermeersch P, Dirksen MT, Cervinka P, Petronio AS, Nordmann AJ, Diem P, Meier B, Zwahlen M, Reichenbach S, Trelle S, Windecker S, Juni P. Lancet. 2007;370:937–948. doi: 10.1016/S0140-6736(07)61444-5. [DOI] [PubMed] [Google Scholar]
- 27.Gruntzig A, Vetter W, Meier B, Kuhlmann U, Lutolf U, Siegenthaler W. Lancet. 1978;1:801–802. doi: 10.1016/s0140-6736(78)93000-3. [DOI] [PubMed] [Google Scholar]
- 28.a de Feyter PJ, de Jaegere PPT, Serruys PW. American Heart Journal. 1994;127:643–651. doi: 10.1016/0002-8703(94)90675-0. [DOI] [PubMed] [Google Scholar]; b Sigwart U, Urban P, Golf S, Kaufmann U, Imbert C, Fischer A, Kappenberger L. Circulation. 1988;78:1121–1127. doi: 10.1161/01.cir.78.5.1121. [DOI] [PubMed] [Google Scholar]; c Bauters C, Meurice T, Hamon M, McFadden E, Lablanche JM, Bertrand ME. Cardiovasc Res. 1996;31:835–846. [PubMed] [Google Scholar]
- 29.Chandrasekar B, Tanguay JF. J Am Coll Cardiol. 2000;35:555–562. doi: 10.1016/s0735-1097(99)00596-3. [DOI] [PubMed] [Google Scholar]
- 30.Clowes AW, Reidy MA, Clowes MM. Lab Invest. 1983;49:208–215. [PubMed] [Google Scholar]
- 31.a Mueller RL, Sanborn TA. American Heart Journal. 1995;129:146–172. doi: 10.1016/0002-8703(95)90055-1. [DOI] [PubMed] [Google Scholar]; b Iqbal J, Gunn J, Serruys PW. Br Med Bull. 2013;106:193–211. doi: 10.1093/bmb/ldt009. [DOI] [PubMed] [Google Scholar]
- 32.Pache J, Kastrati A, Mehilli J, Schuhlen H, Dotzer F, Hausleiter J, Fleckenstein M, Neumann FJ, Sattelberger U, Schmitt C, Muller M, Dirschinger J, Schomig A. J Am Coll Cardiol. 2003;41:1283–1288. doi: 10.1016/s0735-1097(03)00119-0. [DOI] [PubMed] [Google Scholar]
- 33.O’Brien B, Carroll W. Acta Biomater. 2009;5:945–958. doi: 10.1016/j.actbio.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 34.Moliterno DJ. N Engl J Med. 2005;353:724–727. doi: 10.1056/NEJMe058140. [DOI] [PubMed] [Google Scholar]
- 35.O’Connell BM, McGloughlin TM, Walsh MT. Biomed Eng Online. 2010;9:12. doi: 10.1186/1475-925X-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.a Spaulding C, Daemen J, Boersma E, Cutlip DE, Serruys PW. N Engl J Med. 2007;356:989–997. doi: 10.1056/NEJMoa066633. [DOI] [PubMed] [Google Scholar]; b Muldowney JAS, Stringham JR, Levy SE, Gleaves LA, Eren M, Piana RN, Vaughan DE. Arterioscler Thromb Vasc Biol. 2007;27:400–406. doi: 10.1161/01.ATV.0000254677.12861.b8. [DOI] [PubMed] [Google Scholar]
- 37.Kim U, Park JS, Lee SH, Shin DG, Kim YJ. J Korean Med Sci. 2013;28:396–401. doi: 10.3346/jkms.2013.28.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Erne P, Schier M, Resink TJ. Cardiovasc Interv Radiol. 2006;29:11–16. doi: 10.1007/s00270-004-0341-9. [DOI] [PubMed] [Google Scholar]
- 39.Waksman R. The Journal of Invasive Cardiology. 2006;18:70–75. [PubMed] [Google Scholar]
- 40.Peuster M, Wohlsein P, Brugmann M, Ehlerding M, Seidler K, Fink C, Brauer H, Fischer A, Hausdorf G. Heart. 2001;86:563–569. doi: 10.1136/heart.86.5.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seitz J-M, Durisin M, Goldman J, Drelich J. Advanced Healthcare Materials. 2015 doi: 10.1002/adhm.201500189. in press. [DOI] [PubMed] [Google Scholar]
- 42.Witte F. Acta Biomater. 2010;6:1680–1692. doi: 10.1016/j.actbio.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 43.Caiazzo G, Kilic ID, Fabris E, Serdoz R, Mattesini A, Foin N, De Rosa S, Indolfi C, Di Mario C. Cardiology. 2015 doi: 10.1016/j.ijcard.2015.07.101. in press. [DOI] [PubMed] [Google Scholar]
- 44.a Grube E, Sonoda S, Ikeno F, Honda Y, Kar S, Chan C, Gerckens U, Lansky AJ, Fitzgerald PJ. Circulation. 2004;109:2168–2171. doi: 10.1161/01.CIR.0000128850.84227.FD. [DOI] [PubMed] [Google Scholar]; b Tamai H, Igaki K, Kyo E, Kosuga K, Kawashima A, Matsui S, Komori H, Tsuji T, Motohara S, Uehata H. Circulation. 2000;102:399–404. doi: 10.1161/01.cir.102.4.399. [DOI] [PubMed] [Google Scholar]
- 45.Serruys PW, Chevalier B, Dudek D, Cequier A, Carrie D, Iniguez A, Dominici M, van der Schaaf RJ, Haude M, Wasungu L, Veldhof S, Peng L, Staehr P, Grundeken MJ, Ishibashi Y, Garcia-Garcia HM, Onuma Y. Lancet. 2015;384:43–54. doi: 10.1016/S0140-6736(14)61455-0. [DOI] [PubMed] [Google Scholar]
- 46.Di Mario C, Caiazzo G. Lancet. 2015;384:10–12. doi: 10.1016/S0140-6736(14)61636-6. [DOI] [PubMed] [Google Scholar]
- 47.Nishio S, Kosuga K, Igaki K, Okada M, Kyo E, Tsuji T, Takeuchi E, Inuzuka Y, Takeda S, Hata T, Takeuchi Y, Kawada Y, Harita T, Seki J, Akamatsu S, Hasegawa S, Bruining N, Brugaletta S, de Winter S, Muramatsu T, Onuma Y, Serruys PW, Ikeguchi S. Circulation. 2012;125:2343–2352. doi: 10.1161/CIRCULATIONAHA.110.000901. [DOI] [PubMed] [Google Scholar]
- 48.Yamawaki T, Shimokawa H, Kozai T, Miyata K, Higo T, Tanaka E, Egashira K, Shiraishi T, Tamai H, Igaki K, Takeshita A. Journal of the American College of Cardiology. 1998;32:780–786. doi: 10.1016/s0735-1097(98)00312-x. [DOI] [PubMed] [Google Scholar]
- 49.a Tamai H, Igaki K, Kyo E, Kosuga K, Kosuga K, Kawashima A, Matsui S, Matsui S, Komori H, Tsuji T, Tsuji T, Motohara S, Uehata H, Uehata H. Circulation. 2000;25:399–404. doi: 10.1161/01.cir.102.4.399. [DOI] [PubMed] [Google Scholar]; b Nishio S, Kosuga K, Igaki K, Okada M, Okada M, Kyo E, Tsuji T, Tsuji T, Takeuchi E, Inuzuka Y, Inuzuka Y, Takeda S, Hata T, Hata T, Takeuchi Y, Kawada Y, Kawada Y, Harita T, Seki J, Seki J, Akamatsu S, Hasegawa S, Hasegawa S, Bruining N, Brugaletta S, Brugaletta S, de Winter S, Muramatsu T, Muramatsu T, Onuma Y, Serruys PW, Serruys Pw, Ikeguchi S. Circulation. 2012;125:2343–2353. doi: 10.1161/CIRCULATIONAHA.110.000901. [DOI] [PubMed] [Google Scholar]
- 50.Tamai H, Igaki K, Tsuji T, Kyo E, Kosuga K, Kawashima A, Matsui S, Komori H, Motohara S, Uehata H, Takeuchi E. Journal of Interventional Cardiology. 1999;12:443–450. [Google Scholar]
- 51.Hietala EM, Salminen Us, Stahls A, Valimaa T, Valimaa T, Maasilta P, Tormala P, Tormala P, Nieminen MS, Harjula AL, Harjula AL. J Vasc Res. 2001;38:361–369. doi: 10.1159/000051067. [DOI] [PubMed] [Google Scholar]
- 52.Vogt F, Stein A, Rettemeier G, Krott N, Hoffmann R, Dahl Jv, Bosserhoff A-K, Michaeli W, Hanrath P, Weber C, Blindt R. European Heart Journal. 2004;25:1330–1340. doi: 10.1016/j.ehj.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 53.Peuster M, Hesse C, Schloo T, Fink C, Beerbaum P, Schnakenburg C. Biomaterials. 2006;27:4955–4962. doi: 10.1016/j.biomaterials.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 54.Waksman R, Pakala R, Baffour R, Seabron R, Seabron R, Hellinga D, Tio FO, Tio FO. J Interv Cardiol. 2008;21:15–20. doi: 10.1111/j.1540-8183.2007.00319.x. [DOI] [PubMed] [Google Scholar]
- 55.Di Mario C, Griffiths H, Goktekin O, Peeters N, Peeters N, Verbist J, Bosiers M, Bosiers M, Deloose K, Heublein B, Heublein B, Rohde R, Kasese V, Kasese V, Ilsley C, Erbel R, Erbel R. J Interv Cardiol. 2004;17:391–395. doi: 10.1111/j.1540-8183.2004.04081.x. [DOI] [PubMed] [Google Scholar]
- 56.Waksman R, Pakala R, Kuchulakanti PK, Baffour R, Baffour R, Hellinga D, Seabron R, Seabron R, Tio FO, Wittchow E, Wittchow E, Hartwig S, Harder C, Harder C, Rohde R, Heublein B, Heublein B, Andreae A, Waldmann K-H, Waldmann KH, Haverich A. Catheter Cardiovasc Interv. 2006;68:607–617. doi: 10.1002/ccd.20727. [DOI] [PubMed] [Google Scholar]
- 57.Waksman R, Pakala R, Okabe T, Hellinga D, Hellinga D, Chan R, Tio MO, Tio MO, Wittchow E, Hartwig S, Hartwig S, Waldmann K-H, Harder C, Harder C. J Interv Cardiol. 2007;20:367–372. doi: 10.1111/j.1540-8183.2007.00272.x. [DOI] [PubMed] [Google Scholar]
- 58.Zartner P, Cesnjevar R, Singer H, Weyand M, Weyand M. Catheter Cardiovasc Interv. 2005;66:590–594. doi: 10.1002/ccd.20520. [DOI] [PubMed] [Google Scholar]
- 59.Boland EL, Shine R, Kelly N, Sweeney CA, McHugh PE. Annals of biomedical engineering. 2015 doi: 10.1007/s10439-015-1413-5. [DOI] [PubMed] [Google Scholar]
- 60.Onuma Y, Serruys PW. Circulation. 2011;123:779–797. doi: 10.1161/CIRCULATIONAHA.110.971606. [DOI] [PubMed] [Google Scholar]
- 61.a Witte F, Fischer J, Nellesen J, Crostack HA, Kaese V, Pisch A, Beckmann F, Windhagen H. Biomaterials. 2006;27:1013–1018. doi: 10.1016/j.biomaterials.2005.07.037. [DOI] [PubMed] [Google Scholar]; b Willumeit R, Fischer J, Feyerabend F, Hort N, Bismayer U, Heidrich S, Mihailova B. Acta Biomater. 2011;7:2704–2715. doi: 10.1016/j.actbio.2011.03.004. [DOI] [PubMed] [Google Scholar]; c Bowen PK, Drelich J, Goldman J. Acta Biomater. 2014;10:1475–1483. doi: 10.1016/j.actbio.2013.11.021. [DOI] [PubMed] [Google Scholar]; d Bowen PK, Drelich A, Drelich J, Goldman J. J Biomed Mater Res A. 2014 [Google Scholar]
- 62.Zheng YF, Gu XN, Witte F. Mater Sci Eng R-Rep. 2014;77:1–34. [Google Scholar]
- 63.Bowen PK, Guillory R, II, Shearier ER, Seitz JM, Drelich J, Bocks ML, Zhao F, Goldman J. Mater Sci Eng C. 2015 doi: 10.1016/j.msec.2015.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Garg S, Serruys PW. J Am Coll Cardiol. 2010;56:S43–S78. doi: 10.1016/j.jacc.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 65.Hermawan H. Biodegradable Metals: From Concept to Applications. Springer Science & Business Media; 2012. [Google Scholar]
- 66.Balcon R, Beyar R, Chierchia S, Scheerder I, Hugenholtz P, Kiemeneij F, Meier B, Meyer J, Monassier J, Wijns W. Eur Heart J. 1997;18:1536–1547. doi: 10.1093/oxfordjournals.eurheartj.a015133. [DOI] [PubMed] [Google Scholar]
- 67.Moravej M, Mantovani D. International journal of molecular sciences. 2011;12:4250–4270. doi: 10.3390/ijms12074250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Werkhoven RJ, Sillekens WH, van Lieshout JBJM. TMS Annual Meeting - Magnesium 2011. The Minerals, Metals, and Materials Society; San Diego, CA: 2011. [Google Scholar]
- 69.Witte F, Hort N, Feyerabend F, Vogt C. In: Corrosion of Magnesium Alloys. Song G, editor. Woodhead Publishing Ltd; Philadelphia, PA: 2011. pp. 416–417. [Google Scholar]
- 70.Poncin P, Proft J. In: Medical Device Materials: Proceedings of the Materials & Processes for Medical Devices Conference. Shrivastava S, editor. ASM International; Materials Park, Ohio: 2004. pp. 253–259. [Google Scholar]
- 71.Poncin P, Profit J. In: Materials & Processes for Medical Devices. Shrivastava S, editor. ASM International; Materials Park, OH: 2004. pp. 253–259. [Google Scholar]
- 72.Damien Kenny ZMH. Interventional Cardiology. 2015;7:245–255. [Google Scholar]
- 73.Lewitus D, Vogelstein Rj, Zhen G, Choi Y-S, Choi YS, Kohn J, Harshbarger S, Harshbarger S, Jia X. Trans Neural Syst Rehabil Eng. 2015;19:204–212. doi: 10.1109/TNSRE.2010.2098047. [DOI] [PubMed] [Google Scholar]
- 74.A. International, West Conshohocken, PA, USA, 2008.
- 75.Mani G, Feldman MD, Patel D, Agrawal CM. Biomaterials. 2007;28:1689–1710. doi: 10.1016/j.biomaterials.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 76.Hermawan H, Dube D, Mantovani D. Adv Mater Res. 2007;15 [Google Scholar]
- 77.Schinhammer M, Hänzi AC, Löffler JF, Uggowitzer PJ. Acta Biomater. 2010;6:1705–1713. doi: 10.1016/j.actbio.2009.07.039. [DOI] [PubMed] [Google Scholar]
- 78.a Schinhammer M, Pecnik CM, Rechberger F, Hänzi AC, Löffler JF, Uggowitzer PJ. Acta Materialia. 2012;60:2746–2756. [Google Scholar]; b Schinhammer M, Steiger P, Moszner F, Löffler JF, Uggowitzer PJ. Materials Science and Engineering: C. 2013;33:1882–1893. doi: 10.1016/j.msec.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 79.Moravej M, Prima F, Fiset M, Mantovani D. Acta Biomaterialia. 2010;6:1726–1735. doi: 10.1016/j.actbio.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 80.Liu B, Zheng YF. Acta Biomater. 2011;7:1407–1420. doi: 10.1016/j.actbio.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 81.Nie FLZY, Wei SC, Hu C, Yang G. Biomed Mater. 2010;5 doi: 10.1088/1748-6041/5/6/065015. [DOI] [PubMed] [Google Scholar]
- 82.Hermawan H, Moravej M, Dubé D, Fiset M, Mantovani D. Adv Mater Res. 2006;15–17:113–118. [Google Scholar]
- 83.Gu X-N, Zheng Y-F. Frontiers of Materials Science in China. 2010;4:111–115. [Google Scholar]
- 84.Lévesque J, Hermawan H, Dubé D, Mantovani D. Acta Biomaterialia. 2008;4:284–295. doi: 10.1016/j.actbio.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 85.Witte F, Fischer J, Nellesen J, Crostack H-A, Kaese V, Pisch A, Beckmann F, Windhagen H. Biomaterials. 2006;27:1013–1018. doi: 10.1016/j.biomaterials.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 86.Rong-chang Z, Jun C, Dietzel W, Hort N, Kainer K. Trans Nonferrous Met Soc China. 2007;17:166–170. [Google Scholar]
- 87.a Hänzi AC, Gerber I, Schinhammer M, Löffler JF, Uggowitzer PJ. Acta Biomaterialia. 2010;6:1824–1833. doi: 10.1016/j.actbio.2009.10.008. [DOI] [PubMed] [Google Scholar]; b Hanzi ACS, AS, Uggowitzer PJ. Mater Sci Forum. 2009:618–619. [Google Scholar]
- 88.a Zhang S, Zhang X, Zhao C, Li J, Song Y, Xie C, Tao H, Zhang Y, He Y, Jiang Y, Bian Y. Acta Biomater. 2010;6:626–640. doi: 10.1016/j.actbio.2009.06.028. [DOI] [PubMed] [Google Scholar]; b Kubasek J, Vojtech D. Journal of materials science Materials in medicine. 2013;24:1615–1626. doi: 10.1007/s10856-013-4916-3. [DOI] [PubMed] [Google Scholar]
- 89.Zhang E, Yin D, Xu L, Yang L, Yang K. Materials Science and Engineering: C. 2009;29:987–993. [Google Scholar]
- 90.a Kannan MB, Raman RKS. Biomaterials. 2008;29:2306–2314. doi: 10.1016/j.biomaterials.2008.02.003. [DOI] [PubMed] [Google Scholar]; b Li Z, Gu X, Lou S, Zheng Y. Biomaterials. 2008;29:1329–1344. doi: 10.1016/j.biomaterials.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 91.Alexy RD, Levi DS. Biomed Res Int. 2013;2013:137985. doi: 10.1155/2013/137985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Capodanno D, Gori T, Nef H, Latib A, Mehilli J, Lesiak M, Caramanno G, Naber C, Di Mario C, Colombo A, Capranzano P, Wiebe J, Araszkiewicz A, Geraci S, Pyxaras S, Mattesini A, Naganuma T, Munzel T, Tamburino C. EuroIntervention. 2014 doi: 10.4244/EIJY14M07_11. [DOI] [PubMed] [Google Scholar]
- 93.a Serruys PW, Chevalier B, Dudek D, Cequier A, Carrie D, Iniguez A, Dominici M, van der Schaaf RJ, Haude M, Wasungu L, Veldhof S, Peng L, Staehr P, Grundeken MJ, Ishibashi Y, Garcia-Garcia HM, Onuma Y. Lancet. 2014 doi: 10.1016/S0140-6736(14)61455-0. [DOI] [PubMed] [Google Scholar]; b Di Mario C, Caiazzo G. Lancet. 2014 [Google Scholar]
- 94.Mani G, Feldman M, Patel D, Agrawal C. Biomaterials. 2007;28:1689–1710. doi: 10.1016/j.biomaterials.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 95.Liu B, Zheng YF, Ruan L. Mater Lett. 2011;65:540–543. [Google Scholar]
- 96.Pierson D, Edick J, Tauscher A, Pokorney E, Bowen P, Gelbaugh J, Stinson J, Getty H, Lee CH, Drelich J, Goldman J. J Biomed Mater Res B. 2012;100B:58–67. doi: 10.1002/jbm.b.31922. [DOI] [PubMed] [Google Scholar]
- 97.a Hermawan H, Dube D, Mantovani D. J Biomed Mater Res A. 2010;93:1–11. doi: 10.1002/jbm.a.32224. [DOI] [PubMed] [Google Scholar]; b Hermawan H, Alamdari H, Mantovani D, Dube D. Powder Metall. 2008;51:38–45. [Google Scholar]
- 98.McGregor DB, Baan RA, Partensky C, Rice JM, Rice JM, Wilbourn JD. Eur J Cancer. 2000;36:307–313. doi: 10.1016/s0959-8049(99)00312-3. [DOI] [PubMed] [Google Scholar]
- 99.a Aghion E, Levy G, Ovadia S. Journal of materials science Materials in medicine. 2012;23:805–812. doi: 10.1007/s10856-011-4536-8. [DOI] [PubMed] [Google Scholar]; b Hort N, Huang Y, Fechner D, Störmer M, Blawert C, Witte F, Vogt C, Drücker H, Willumeit R, Kainer KU, Feyerabend F. Acta Biomater. 2010;6:1714–1725;. doi: 10.1016/j.actbio.2009.09.010. [DOI] [PubMed] [Google Scholar]; c Li L, Gao J, Wang Y. Surf Coat Technol. 2004;185:92–98. [Google Scholar]
- 100.Song G. Corros Sci. 2007;49:1696–1701. [Google Scholar]
- 101.Wang Y, Wei M, Gao J. Mater Sci Eng C. 2009;29:1311–1316. [Google Scholar]
- 102.Sanschagrin A, Tremblay R, Angers R, Dubé D. Mater Sci Eng A. 1996;220:69–77. [Google Scholar]
- 103.Seitz JM, Durisin M, Goldman J, Drelich JW. Adv Healthc Mater. 2015 doi: 10.1002/adhm.201500189. [DOI] [PubMed] [Google Scholar]
- 104.Vallee BL, Falchuk KH. Physiological Reviews. 1993;73:79–118. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- 105.Jones MM, Schoenheit JE, Weaver AD. Toxicol Appl Pharmacol. 1979;49:41–44. doi: 10.1016/0041-008x(79)90274-6. [DOI] [PubMed] [Google Scholar]
- 106.Trumbo P, Yates AA, Schlicker S, Poos M. J Am Diet Assoc. 2001;101:294–301. doi: 10.1016/S0002-8223(01)00078-5. [DOI] [PubMed] [Google Scholar]
- 107.A. f. T. S. a. D. R. D. o. T. a. E. Medicine, editor. Atlanta, GA, USA: 2005. [Google Scholar]
- 108.Plum LM, Rink L, Haase H. Int J Environ Res Public Health. 2010;7:1342–1365. doi: 10.3390/ijerph7041342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.a Rohrs LC. AMA Arch Ind Health. 1957;16:42–47. [PubMed] [Google Scholar]; b Brown JJ. Br J Radiol. 1988;61:327–329. doi: 10.1259/0007-1285-61-724-327. [DOI] [PubMed] [Google Scholar]
- 110.Blanc P, Wong H, Berstein MS, Boushey HA. Ann Intern Med. 1991;114:930–936. doi: 10.7326/0003-4819-114-11-930. [DOI] [PubMed] [Google Scholar]
- 111.Brown MA, Thom JV, Orth GL, Cova P, Juarez J. Arch Environ, Health. 1964;8:657–660. doi: 10.1080/00039896.1964.10663736. [DOI] [PubMed] [Google Scholar]
- 112.Ogiso T, Moriyama K, Sasaki S, Ishimura Y, Minato A. Chem Pharm Bull (Tokyo) 1974;22:55–60. doi: 10.1248/cpb.22.55. [DOI] [PubMed] [Google Scholar]
- 113.Igic PG, Lee E, Harper W, Roach KW. Mayo Clinic Protoc. 2002;77:713–716. doi: 10.4065/77.7.713. [DOI] [PubMed] [Google Scholar]
- 114.Fiske DN, McCoy HE, Kitchens CS. Am J Hematol. 1994;46:147–150. doi: 10.1002/ajh.2830460217. [DOI] [PubMed] [Google Scholar]
- 115.a Kumar N, Gross JB, Ahlskog E. Neurology. 2003;61:273–274. doi: 10.1212/01.wnl.0000073542.02761.5f. [DOI] [PubMed] [Google Scholar]; b Prodan CI, Holland NR, Wisdom PJ, Burstein SA, Bottomley S. Neurology. 2002;59:1453–1456. doi: 10.1212/01.wnl.0000032497.30439.f6. [DOI] [PubMed] [Google Scholar]
- 116.Greenberg SA, Briemberg HR. J Neurol. 2004;251:11–114. doi: 10.1007/s00415-004-0263-0. [DOI] [PubMed] [Google Scholar]
- 117.Lansdown ABG. Fd Chem Toxic. 1991;29:57–64. doi: 10.1016/0278-6915(91)90063-d. [DOI] [PubMed] [Google Scholar]
- 118.a Agren MS. Dermatologica. 1990;180:36–39. [PubMed] [Google Scholar]; b Agren MS, Krusell M, Franzen L. Acta Dermato Venereol. 1991;71:330–333. [PubMed] [Google Scholar]
- 119.Stromberg HE, Agren MS. Br J Dermatol. 1984;111:461–468. doi: 10.1111/j.1365-2133.1984.tb06610.x. [DOI] [PubMed] [Google Scholar]
- 120.a Wuehler SE, Peerson JM, Brown JH. Public Health Nutr. 2005;8:812–819. doi: 10.1079/phn2005724. [DOI] [PubMed] [Google Scholar]; b Briefel RR, Bialostosky K, Kennedy-Stephenson J, McDowell MA, Ervin RB, Wright JD. J Nutr. 2000;130:1367S–1373S. doi: 10.1093/jn/130.5.1367S. [DOI] [PubMed] [Google Scholar]
- 121.Hambidge KM, Krebs NF. J of Nutr. 2007;137:1101–1105. doi: 10.1093/jn/137.4.1101. [DOI] [PubMed] [Google Scholar]
- 122.Beyersmann D. Mat-wiss u Werkstofftech. 2003;33:764–769. [Google Scholar]
- 123.Tapiero H, Tew KD. Biomed Pharmaco-other. 2003;57:399–411. doi: 10.1016/s0753-3322(03)00081-7. [DOI] [PubMed] [Google Scholar]
- 124.Calesnick B, Dinan AM. Am Fam Physician. 1988;37:267–270. [PubMed] [Google Scholar]
- 125.Mocchegiani E, Muzzioli M, Giacconi R. Trends Pharmacol Sci. 2000;21:205–208. doi: 10.1016/s0165-6147(00)01476-0. [DOI] [PubMed] [Google Scholar]
- 126.Maret W. J Nutri. 2003;133:S1460–S1462. [Google Scholar]
- 127.Falchuk K. Mol Cell Biochem. 1998;188:41–48. [PubMed] [Google Scholar]
- 128.a Davis SR, McMahon RJ, Cousins RJ. J Nutri. 1998;128:825–831. doi: 10.1093/jn/128.5.825. [DOI] [PubMed] [Google Scholar]; b Cousins RJ. Proc Nutr Soc. 1985;57:307–311. doi: 10.1079/pns19980045. [DOI] [PubMed] [Google Scholar]
- 129.Henning B, Toborek M, McClain CJ. Nutrition. 1998;12:711–717. doi: 10.1016/s0899-9007(96)00125-6. [DOI] [PubMed] [Google Scholar]
- 130.Dreosti IE. Mutat Res. 2001;475:161–167. doi: 10.1016/s0027-5107(01)00067-7. [DOI] [PubMed] [Google Scholar]
- 131.a Verhaegh GW, Parat MO, Richard MJ, Hainaut P. Mol Carcinog. 1998;21:205–214. doi: 10.1002/(sici)1098-2744(199803)21:3<205::aid-mc8>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]; b Palecek E, Brazdova M, Cernocka H, Vlk D, Brazda V, Vojtesek B. Oncogene. 1999;17/18:3617–3625. doi: 10.1038/sj.onc.1202710. [DOI] [PubMed] [Google Scholar]
- 132.Chimienti F, Seve M, Richard S, Mathieu J, Favier A. Biochem Pharmac. 2001;62:51–62. doi: 10.1016/s0006-2952(01)00624-4. [DOI] [PubMed] [Google Scholar]
- 133.a Truong-Tran AQ, Carter J, Ruffin RE, Zalewski PD. Biometals. 2001;14:315–330. doi: 10.1023/a:1012993017026. [DOI] [PubMed] [Google Scholar]; b Brown AM, Kristal BS, Effron MS, Shestopalov AI, Ullucci PA, Sheu KF, Blass JP, Cooper AJ. J Biol Chem. 2000;275:13441–13447. doi: 10.1074/jbc.275.18.13441. [DOI] [PubMed] [Google Scholar]; c Sheline CT, Behrens MM, Chioi DW. J Neurosci. 2000;20:3139–3146. doi: 10.1523/JNEUROSCI.20-09-03139.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cummings JE, Kovacic JP. J Vet Emerg Crit Care. 2009;19:215–240. doi: 10.1111/j.1476-4431.2009.00418.x. [DOI] [PubMed] [Google Scholar]
- 135.Black J. Biological Performance of Materials: Fundamentals of Biocompatibility. 3. Marcel Dekker; New York: 1999. [Google Scholar]
- 136.Bowen PK, Drelich J, Goldman J. Adv Mater. 2013;25:2577–2582. doi: 10.1002/adma.201300226. [DOI] [PubMed] [Google Scholar]
- 137.a Krezel A, Maret W. Journal of biological inorganic chemistry : JBIC : a publication of the Society of Biological Inorganic Chemistry. 2006;11:1049–1062. doi: 10.1007/s00775-006-0150-5. [DOI] [PubMed] [Google Scholar]; b Krezel A, Maret W. J Am Chem Soc. 2007;129:10911–10921. doi: 10.1021/ja071979s. [DOI] [PubMed] [Google Scholar]; c Maret W. Experimental gerontology. 2008;43:363–369. doi: 10.1016/j.exger.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 138.Zhang XG. Corrosion and Electrochemistry of Zinc. Springer; New York: 1996. [Google Scholar]
- 139.Porter FC. Corrosion Resistance of Zinc and Zinc Alloys. Marcel Dekker; New York: 1994. [Google Scholar]
- 140.a Dietrich HG, Johnston J. J Am Chem Soc. 1927;49:1419–1431. [Google Scholar]; b Rieichle RA, McCurdy KG, Hepler LG. Can J Chem. 1975;53:3841–3845. [Google Scholar]
- 141.Zberg B, Uggowitzer PJ, Loffler JF. Nature materials. 2009;8:887–891. doi: 10.1038/nmat2542. [DOI] [PubMed] [Google Scholar]
- 142.Bowen PK, Drelich J, Goldman J. Acta Biomater. 2014;10:1475–1483. doi: 10.1016/j.actbio.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 143.Thomas S, Cole IS, Gonzalez-Garcia Y, Chen M, Musameh M, Mol JMC, Terryn H, Birbilis N. Journal of Applied Electrochemistry. 2014 [Google Scholar]
- 144.Thomas S, Birbilis N, Venkatraman MS, Cole IS. Corrosion. 2012;68:015009. [Google Scholar]
- 145.Bowen PK, Guillory R, II, Shearier ER, Seitz J-M, Drelich J, Bocks ML, Zhao F, Goldman J. Materials Science & Engineering C - Materials for Biological Applications. 2015;56:467–472. doi: 10.1016/j.msec.2015.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Guillory RJ, II, Bowen PK, Shearier ER, Hopkins S, Gillete A, Drelich J, Goldman J. Acta Biomater. 2015 submitted. [Google Scholar]
- 147.Werkhoven RJ, Sillekens WH, van Lieshout JBJM. In: Magnesium Technology 2011. Sillekens WH, Agnew SR, Neelameggham NR, Mathaudhu SN, editors. The Minerals, Metals & Materials Society; 2011. pp. 419–424. [Google Scholar]
- 148.Song G. Corros Sci. 2007;49:1696–1701. [Google Scholar]
- 149.Ashby MF. Materials Selection in Mechanical Design. 3. Elsevier Butterworth-Heinemann; Oxford: 2005. [Google Scholar]
- 150.Barnhurst RJ. Properties and Selection: Nonferrous Alloys and Special-Purpose Materials. ASM International; Materials Park, Ohio, USA: 1990. [Google Scholar]
- 151.a Vojtech D, Kubasek J, Serak J, Novak P. Acta Biomater. 2011;7:3515–3522. doi: 10.1016/j.actbio.2011.05.008. [DOI] [PubMed] [Google Scholar]; b Dambatta MS, Izman S, Kurniawan D, Farahany S, Yahaya B, Hermawan H. Mater Design. 2015;85:431–437. [Google Scholar]; c Gong H, Wang K, Strich R, Zhou JG. J Biomed Mater Res B. 2015 doi: 10.1002/jbm.b.33341. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Li HF, Xie XH, Zheng YF, Cong Y, Zhou FY, Qiu KJ, Wang X, Chen SH, Huang L, Tian L, Qin L. Sci Rep. 2015;5:10719. doi: 10.1038/srep10719. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Li H, Yang H, Zheng Y, Zhou F, Qiu K, Wang X. Mater Design. 2015;83:95–102. [Google Scholar]; f Liu X, Sun J, Yang Y, Pu Z, Zheng Y. Mater Lett. 2015 [Google Scholar]
- 152.Bowen PK, Drelich A, Drelich J, Goldman J. J Biomed Mater Res A. 2015;103:341–349. doi: 10.1002/jbm.a.35179. [DOI] [PubMed] [Google Scholar]
- 153.Dreher M, Anderson S. ASTM-FDA workshop on absorbable medical devices: Lessons learned from correlations of bench testing and clinical performance. Department of Health and Human Services, Food and Drug Administration; Silver Spring, MD: 2012. [Google Scholar]
- 154.Törne K, Larsson M, Norlin A, Weissenrieder J. J Biomed Mater Res B. 2015 doi: 10.1002/jbm.b.33458. [DOI] [PubMed] [Google Scholar]
- 155.a Kirkland NT, Birbilis N, Staiger MP. Acta Biomater. 2012;8:925–936. doi: 10.1016/j.actbio.2011.11.014. [DOI] [PubMed] [Google Scholar]; b Kirkland NT, Birbilis N. Magnesium Biomaterials: Design, Testing, and Best Practice. Springer; Heidelberg: 2014. [Google Scholar]
- 156.Raval AN, Telep JD, Guttman MA, Ozturk C, Jones M, Thompson RB, Wright VJ, Schenke WH, DeSilva R, Aviles RJ, Raman VK, Slack MC, Lederman RJ. Circulation. 2005;112:699–706. doi: 10.1161/CIRCULATIONAHA.105.542647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Schenck JF. Medical Physics. 1996;23:815–850. doi: 10.1118/1.597854. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.