Abstract
Background
Pneumonia, a known complication of coronary artery bypass (CABG) surgery, significantly increases a patient’s risk of morbidity and mortality. While not well characterized, red blood cell transfusions (RBC) may increase a patient’s risk of pneumonia. We describe the relationship between RBC transfusion and post-operative pneumonia after CABG surgery.
Methods
A total of 16,182 consecutive patients underwent isolated CABG surgery between 2011 and 2013 at one of 33 hospitals in the state of Michigan. We used multivariable logistic regression to estimate the odds of pneumonia associated with the use or number (0, 1, 2, 3, 4, 5, >6) of RBC units. We adjusted for predicted risk of mortality, pre-operative hematocrit, history of pneumonia, cardiopulmonary bypass duration and medical center. We confirmed the strength and direction of these relationships among selected clinical subgroups in a secondary analysis.
Results
576 (3.6%) patients developed pneumonia and 6,451 (39.9%) received RBC transfusions. There was a significant association between any RBC transfusion and pneumonia (ORadj 3.4, p<0.001). There was a dose-response between number of units and odds of pneumonia, ptrend<0.001. Patients receiving only 2 units of RBCs had twofold (ORadj 2.1, p<0.001) increased odds of pneumonia. These findings were consistent across clinical subgroups.
Conclusions
We found a significant, volume-dependent association between an increasing number of RBCs and odds of pneumonia, which persisted after adjusting for pre-operative patient characteristics. Clinical teams should explore opportunities for preventing a patient’s risk of RBC transfusions, including reducing hemodilution or adopting a lower transfusion threshold in a stable patient.
Keywords: blood management, conservation, consequences, infection, coronary artery bypass grafts, CABG, complications
INTRODUCTION
Nearly half of patients undergoing coronary artery bypass grafting (CABG) surgery are exposed to red blood cell (RBC) products. While large volumes (3+ units) of transfusions are typically given to preserve life in cases of acute blood loss, smaller quantities may be potentially avoidable. Indeed, research suggests that patients exposed to 1–2 units of RBCs may have a 16% increase in risk adjusted mortality.1
For every 20 patients who undergo cardiac surgery, one will develop a major infection, with pneumonia being the most common.2 Such rates of pneumonia and other healthcare-associated infections after CABG surgery have been shown to vary considerably across medical centers – a finding not accounted for by differences in patient case mix.3 Prior studies have suggested that exposure to allogeneic blood may explain some of this variance.4 Findings from a recent multi-center study suggested a 23% increased hazard of infection within 65 days of cardiac surgery associated with every 1 unit increase in RBC transfusions.2 The strength of this relationship was most evident for pneumonia and bloodstream infection. While large, this study included a heterogeneous population (i.e. a mix of cardiac surgical procedures) observed through year 2010. Given that this was a volunteer population and the response rate was not available, we intended to explore this hypothesis in more depth, capturing a contemporaneous series of consecutive patients undergoing CABG surgery over a 3-year time period.
We leveraged a prospective, contemporaneous multi-institutional cohort study to characterize the relationship between the use and number of RBC transfusions and post-operative pneumonia after isolated CABG surgery.
METHODS
This study was approved by the Institutional Review Board (IRB) of the University of Michigan Health System for the Michigan Society of Thoracic and Cardiovascular Surgeons Quality Collaborative.
Patient Population
The Michigan Society of Thoracic and Cardiovascular Surgeons Quality Collaborative (MSTCVS-QC) is a surgeon-led, voluntary, multidisciplinary group consisting of all 33 hospitals that perform cardiac surgical procedures on adults in the state of Michigan.5 All programs use the Society of Thoracic Surgeons (STS) data collection form and submit data on a quarterly basis to both the STS database and the MSTCVS-QC data warehouse, which specifically includes other cardiac surgical data including perfusion, readmission and specific mortality data. Participating sites are routinely audited for data validity and accuracy as part of the MSTCVS-QC audit system, with sites on average demonstrating excellent overall scores (>98% data accuracy).
We included 16,182 patients undergoing isolated CABG surgery at any of 33 medical centers in Michigan between 2011 and 2013.
Measures
The utilization of non-autologous RBC transfusions (intra- or post-operatively) were determined.
The primary outcome for this analysis was post-operative pneumonia according to STS criteria. Pneumonia is captured in the STS database when a physician or physician extender has documented the diagnosis in the medical record based on symptoms (positive sputum cultures, trans tracheal fluid, bronchial washings) and radiologic evidence (e.g. chest radiograph diagnostic of pulmonary infiltrates).
Statistical Analysis
Standard statistical tests were used, including chi-square tests for categorical data, and two-sided Wilcoxon rank-sum tests for non-normally distributed continuous variables. Trends in patient characteristics, processes of care, and clinical outcomes were tested using the Cuzick non-parametric test of trend.6
Relative risks and odds ratios were computed for univariate comparisons of patient and disease characteristics against RBC transfusion use and post-operative pneumonia occurrence, respectively.
A multivariable logistic regression model was used to estimate the effect of the use and volume of RBC transfusions on post-operative pneumonia. For this analysis, we modeled each patient’s exposure to RBC transfusions as: no transfusion, 1 unit, 2 units, 3 units, 4 units, 5 units, or ≥ 6 units. In this model, covariates based on clinical relevance included the patient’s pre-operative risk of mortality (Society of Thoracic Surgeon’s PROM), pre-operative Hct, pre-operative pneumonia, and medical center. Dummy variables were used to account for medical center.
In sensitivity analysis, we explored the effect of transfusions and pneumonia among distinct clinical subgroups: age (<60, 60–69, ≥70), sex, acuity (elective, urgent, emergent), chronic obstructive pulmonary disease, smoking (no history, smoking within 1 year, smoking within 2 weeks), and use of any other blood products (fresh frozen plasma, platelets, cryoprecipitate, and factor VIIa).
Statistical analyses were performed using Stata 13.0 (College Station, TX). The two-tailed tests were considered significant at p<0.05.
RESULTS
Of the 16,182 patients, the age ranged from 25–95 years, 26.6% were female, 13.6% had a body mass index ≥ 37kg/m2, 18.9% had smoked within 2 weeks of surgery, 2.7% had a recent history of pneumonia, 12.6% had moderate-severe chronic lung disease, 44.8% had diabetes, 12.7% congestive heart failure, 16.9% peripheral arterial disease, 25.5% had pre-operative hematocrit<36%, 3.0% had a previous cardiac surgical operation, 14.8% had an ejection fraction<40%, 60.9% were urgent, 96.5% had the appropriate type and 99.2% the appropriate timing of pre-operative antibiotics.
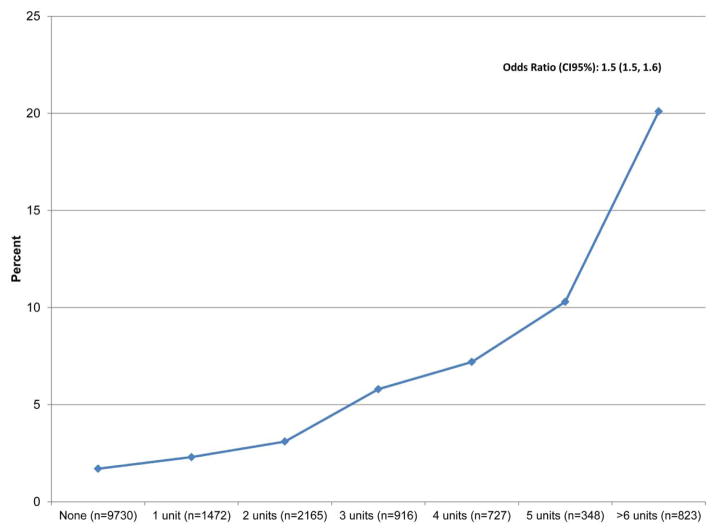
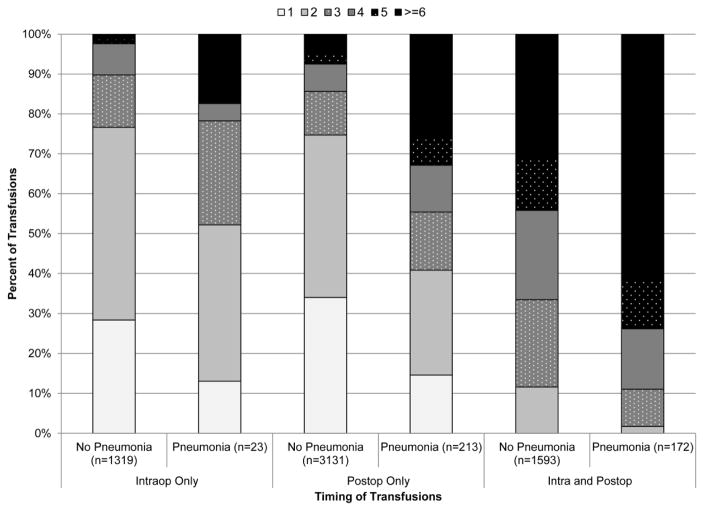
Red blood cells were transfused among 39.9% of patients: 1 unit (9.1%), 2 units (13.4%), 3 units (5.7%), 4 units (4.5%), 5 units (2.5%), and ≥6 units (5.1%), Figure 1. 8.3% of patients received transfusions intra-operatively, 20.7% post-operatively, and 10.9% intra- and postoperatively. Post-operative pneumonia developed among 3.6% of patients. Patients receiving small quantities of RBCs (i.e. 1 or 2 units) were transfused more often only in the post-operative setting, p<0.001. (Figure 2) Patients exposed to larger quantities of RBCs were more likely to receive transfusions in the intra- and post-operative settings.
Figure 1.
Rate of Pneumonia by Number of RBC Units After Isol CABG Surgery
Figure 2.
Distribution of Transfusions by Timing and Post-operative Pneumonia
Patients transfused with RBCs were more likely to be older, female, have greater body mass index, have a history of pneumonia, moderate-severe chronic lung disease, diabetes, congestive heart failure, peripheral arterial disease, lower pre-operative hematocrit, have had a previous operation, lower ejection fraction, and greater acuity. (Table 1) They were less likely to have smoked within two weeks of surgery and appropriate selection of antibiotics.
Table 1.
Univariate characteristics of the study population by risk of transfusion or post-operative pneumonia
| Variable | No Transfusion | Transfusion | RR | CI 95% | ptrend | No Pneumonia | Pneumonia | OR | CI 95% | ptrend |
|---|---|---|---|---|---|---|---|---|---|---|
| Observations | 9,731 | 6451 | 15,606 | 576 | ||||||
| 39.9% | 3.6% | |||||||||
| Demographics | ||||||||||
| Patient Age | ||||||||||
| <60 | 34.0 | 20.6 | Ref | 28.7 | 27.8 | Ref | ||||
| 60–69 | 38.1 | 33.5 | 1.29 | (1.20,1.38) | 36.5 | 31.4 | 0.89 | (0.72,1.10) | ||
| 70+ | 27.9 | 45.9 | 1.82 | (1.71,1.95) | 34.9 | 40.8 | 1.21 | (0.99,1.48) | ||
| <0.001 | 0.04 | |||||||||
| Female sex, % | 16.4 | 41.9 | 1.99 | (1.90,2.10) | <0.001 | 26.5 | 29.5 | 1.16 | (0.97,1.40) | |
| Body Mass Index | ||||||||||
| <25 | 13.1 | 23.5 | Ref | 17.1 | 20.1 | Ref | ||||
| 25–29.9 | 36.9 | 35.8 | 0.72 | (0.68,0.77) | 36.7 | 29.5 | 0.68 | (0.52,0.87) | ||
| ≥ 30.0 | 50.0 | 40.7 | 0.65 | (0.61,0.69) | 46.2 | 50.4 | 0.93 | (0.74,1.16) | ||
| <0.001 | 0.711 | |||||||||
| Smoking History, % | ||||||||||
| No Smoking Hx | 67.5 | 70.8 | Ref | 69.3 | 56.3 | Ref | ||||
| Smoking within 1 Year | 12.0 | 12.7 | 1.00 | (0.93,1.08) | 12.2 | 12.9 | 1.29 | (1.00,1.67) | ||
| Smoking within 2 Wks | 20.5 | 16.5 | 0.85 | (0.79,0.91) | 18.5 | 30.9 | 2.06 | (1.71,2.49) | ||
| <0.001 | <0.001 | |||||||||
| Pneumonia, % | ||||||||||
| No Hx | 93.4 | 90.6 | Ref | 92.5 | 85.7 | Ref | ||||
| Recent | 1.8 | 4.0 | 1.52 | (1.32,1.75) | 2.6 | 6.3 | 2.66 | (1.80,3.94) | ||
| Remote | 4.8 | 5.4 | 1.09 | (0.97,1.23) | 4.9 | 8.0 | 1.77 | (1.25,2.50) | ||
| <0.001 | <0.001 | |||||||||
| Comorbid Disease, % | ||||||||||
| Chronic Lung Disease, Mod-Sev | 10.1 | 16.3 | 1.36 | (1.27,1.45) | 12.1 | 25.2 | 2.44 | (2.01, 2.97) | ||
| Diabetes Mellitus | 41.6 | 49.6 | 1.21 | (1.15,1.27) | 44.5 | 53.3 | 1.42 | (1.21,1.68) | ||
| CHF | 8.1 | 19.7 | 1.67 | (1.58,1.78) | 12.3 | 23.8 | 2.22 | (1.82,2.71) | ||
| Periperhal Arterial Disease | 13.2 | 22.5 | 1.43 | (1.34,1.51) | 16.4 | 29.2 | 2.10 | (1.74,2.52) | ||
| Hct, last preop | ||||||||||
| <36 | 11.4 | 46.9 | 4.47 | (4.10,4.88) | 25.1 | 36.8 | 1.84 | (1.45,2.33) | ||
| 36–39 | 27.1 | 29.2 | 2.54 | (2.32,2.79) | 28.0 | 25.5 | 1.14 | (0.88,1.47) | ||
| 40–42 | 30.0 | 14.6 | 1.49 | (1.34,1.65) | 24.1 | 19.4 | 1.01 | (0.77,1.33) | ||
| 43+ | 31.5 | 9.3 | Ref | 22.8 | 18.2 | Ref | ||||
| <0.001 | <0.001 | |||||||||
| 2.13 | 4.28 | |||||||||
| Previous operation, % | 1.45 | (1.29,1.64) | 2.97 | 3.3 | 1.11 | (0.70,1.77) | ||||
| Ejection Fraction | ||||||||||
| <40% | 21.2 | 18.6 | 1.32 | (1.23,1.42) | 14.4 | 25.8 | 2.28 | (1.81,2.88) | ||
| 40–9% | 15.7 | 17.3 | 1.11 | (1.03,1.19) | 16.2 | 19.5 | 1.53 | (1.20,1.96) | ||
| 50–9% | 35.7 | 30.6 | 0.96 | (0.90,1.02) | 33.9 | 26.8 | 1.01 | (0.80,1.26) | ||
| 60%+ | 27.4 | 33.5 | Ref | 35.5 | 27.9 | Ref | ||||
| <0.001 | <0.001 | |||||||||
| Acuity, % | ||||||||||
| Elective | 39.4 | 29.9 | Ref | 36.0 | 25.5 | Ref | ||||
| Urgent | 58.3 | 64.8 | 0.94 | (0.88,1.01) | 60.8 | 64.9 | 1.51 | (1.24,1.83) | ||
| Emergent/Salvage | 2.3 | 5.3 | 1.34 | (1.27,1.42) | 3.2 | 9.6 | 4.15 | (3.01,5.74) | ||
| <0.001 | <0.001 | |||||||||
| Antibioticsa, % | ||||||||||
| Appropriate Selection | 97.1 | 95.6 | 0.79 | (0.70,0.89) | 96.5 | 95.8 | 0.81 | (0.53,1.23) | ||
| Appropriate Timing | 99.3 | 99.1 | 0.89 | (0.68,1.16) | 99.2 | 99.1 | 0.86 | (0.35,2.12) | ||
Patients who had a contraindication to antibiotics were removed from the denominator RR = Relative Risk, OR = Odds Ratio
Patients developing pneumonia were more likely to be older, and have greater body mass index, have a history of smoking, pneumonia, moderate-severe chronic lung disease, diabetes, congestive heart failure, peripheral arterial disease, lower pre-operative hematocrit, lower ejection fraction, and greater acuity. (Table 1)
Patients exposed to RBC transfusions had a significant 3.4-fold increased odds of pneumonia (ORadj 3.4, p<0.001). The odds of pneumonia increased with the transfusion of more RBC units, ptrend<0.001, Table 2. These finding were quantitatively and qualitatively similar when explored in our sensitivity analyses, Table 3.
Table 2.
Crude and adjusted odds of post-operative pneumonia by use and volume of red blood cell transfusions
| Variable | OR | CI95% | p-value |
|---|---|---|---|
| Crude | |||
| Any Transfusion | 3.8 | (3.2, 4.6) | <0.001 |
| Number of RBC Transfusions | |||
| None | Ref | ||
| 1 | 1.4 | (0.93, 2.0) | 0.12 |
| 2 | 1.9 | (1.4, 2.5) | <0.001 |
| 3 | 3.5 | (2.5, 4.8) | <0.002 |
| 4 | 4.4 | (3.2, 6.0) | <0.003 |
| 5 | 6.6 | (4.5, 9.6) | <0.004 |
| 6+ | 14.3 | (11.4, 17.9) | <0.005 |
| ptrend | <0.001 | ||
| Adjusteda | |||
| Number of RBC Transfusions | |||
| None | Ref | ||
| 1 | 1.5 | (1.0, 2.1) | 0.06 |
| 2 | 2.0 | (1.5, 2.8) | <0.001 |
| 3 | 3.7 | (2.6, 5.3) | <0.001 |
| 4 | 5.1 | (3.5, 7.3) | <0.001 |
| 5 | 7.9 | (5.2, 12.1) | <0.001 |
| 6+ | 14.3 | (10.6, 19.2) | <0.001 |
| ptrend | <0.001 | ||
| Predicted Risk of Mortality | 27.5 | (5.6, 136.7) | <0.001 |
| Hct, last preop | 1.0 | (1.0, 1.1) | <0.001 |
| Pneumonia, % | |||
| No Hx | Ref | ||
| Recent | 1.7 | (1.1, 2.7) | 0.02 |
| Remote | 1.4 | (1.0, 2.1) | 0.09 |
| Cardiopulmonary Bypass Duration (min) | 1.00 | (1.0, 1.0) | 0.51 |
Adjusted for: STS predicted risk of mortality, pre-operative Hct, pre-operative pneumonia, perfusion time, medical center Hospital-level estimates are not included ROC for adjusted model: 0.81
Table 3.
Adjusted odds ratios of pneumonia for increasing units of transfusions
| Number of Perioperative Transfusion Units | ||||||||
|---|---|---|---|---|---|---|---|---|
| No units (Reference) | 1 | 2 | 3 | 4 | 5 | ≥ 6 | Trenda (CI95%) | |
| Age | ||||||||
| <60 | 1.5 | 1.1 | 2.4 | 5.8 | 6.8 | 8.8 | 1.5 (1.3, 1.6) | |
| 60–69 | 0.8 | 2.7 | 4.8 | 5.5 | 7.2 | 18.6 | 1.6 (1.5, 1.8) | |
| >=70 | 2.1 | 2.5 | 4.7 | 5.7 | 10.4 | 18.5 | 1.6 (1.5, 1.7) | |
| Sex | ||||||||
| Males | 1.7 | 2.7 | 4.6 | 7.7 | 11.0 | 15.6 | 1.6 (1.5, 1.7) | |
| Females | 1.0 | 1.0 | 2.1 | 2.3 | 3.6 | 11.0 | 1.5 (1.4, 1.7) | |
| Acuity | ||||||||
| Elective | 1.3 | 1.9 | 4.3 | 4.3 | 5.7 | 14.9 | 1.6 (1.4, 1.7) | |
| Urgent | 1.6 | 2.0 | 2.7 | 4.7 | 7.3 | 13.9 | 1.6 (1.5, 1.6) | |
| Emergent/Salvage | NA | 2.3 | 12.8 | 8.1 | 10.9 | 14.9 | 1.6 (1.3, 1.9) | |
| Chronic Obstructive Pulmonary Disease | ||||||||
| No | 1.3 | 2.0 | 3.9 | 4.6 | 8.3 | 13.4 | 1.6 (1.5, 1.6) | |
| Yes | 2.1 | 2.1 | 2.7 | 7.3 | 6.0 | 17.1 | 1.6 (1.4, 1.8) | |
| Smoking | ||||||||
| No Smoking Hx | 1.5 | 2.3 | 4.1 | 6.6 | 10.1 | 16.1 | 1.6 (1.5, 1.7) | |
| Smoking within 1 Year | 1.1 | 0.9 | 3.1 | 1.8 | 6.1 | 11.5 | 1.5 (1.3, 1.7) | |
| Smoking within 2 Wks | 1.7 | 2.3 | 3.8 | 5.1 | 5.1 | 15.7 | 1.5 (1.4, 1.7) | |
| Other Blood Products | ||||||||
| No | 1.6 | 1.9 | 3.1 | 4.4 | 7.1 | 22.1 | 1.6 (1.5, 1.7) | |
| Yes | 0.3 | 1.4 | 2.3 | 2.3 | 3.4 | 3.9 | 1.3 (1.2, 1.5) | |
Adjusted for: predicted risk of mortality, pre-operative Hct, pre-operative pneumonia, perfusion time, medical center
Odds ratio reflecting the relative odds of pneumonia per 1 unit increase in red blood cells
DISCUSSION
In Michigan, pneumonia occurs after CABG surgery in 1 of every 28 patients, although is 1 of every 8 patients among those receiving perioperative RBC transfusions. These findings are important for at least two reasons. First, our study reflects the experience of the entire isolated CABG surgical population in the state of Michigan (exclusive of patients receiving cardiac surgical care at federal facilities). Thus, unlike trials, our study does not suffer from selection bias.7 Second, we report a dose-response relationship between RBC transfusions and incidence of post-operative pneumonia. Importantly, patients receiving only 2 units of RBCs had a twofold increased odd of pneumonia, even after adjusting for baseline risk, duration of the cardiopulmonary bypass period and medical center. These findings suggest a potential strategy for reducing a patients’ risk of pneumonia, namely through blood conservation.
Prior studies in and outside of cardiac surgery have shed light on the relationship between RBC transfusions and infections.8–10 Taylor and colleagues undertook a study of adult patients admitted to a single institution between 2001–2003.8 Nosocomial infection and other adverse outcomes were prospectively tracked, and rates compared by use of RBC transfusions. Patients receiving transfusions had higher rates of infections (14.3% vs. 5.8%, p<0.001), including pneumonia (7.2% vs. 3.4%). This finding was consistent across strata of increasing pre-operative risk, p<0.001. Interestingly, the number of RBC units was an independent risk factor for infections, p=0.005. Murphy and colleagues used a single-center institutional database to assess the impact of RBC transfusions on clinical outcomes and costs among adult cardiac surgical patients.9 Nadir hematocrit occurring during the first 12 hours post-operatively was collected, as was the number of RBC units transfused. A propensity score was derived to estimate the probability for a transfusion, and then subsequently used to estimate the risk of any of 2 composite endpoints: infection (respiratory infection, wound infection or septicemia) or ischemia (myocardial infarction, stroke, renal). The authors found that the use of a RBC transfusion was associated with a 3.7-fold increased odds of infection, and 4.1-fold increased odds of ischemic injury. In both cases, they also found a dose-dependent relationship with the number of RBC units transfused.
Prior studies have documented immunomodulation secondary to leukocytes within allogeneic blood products.11 While still not clearly understood, transfusion-associated immunomodulation (TRIM) may occur secondary to the accumulation of white blood cells and plasma (among others) in stored RBC units. It is thought that allogeneic white blood cells may cause immunosuppression among recipients of RBC transfusions. TRIM has been associated with bacterial infection, multi-organ injury and mortality.12 Leukoreduction of these stored units have been consistently associated with low rates of non-hemolytic febrile transfusion reactions.13–15 Hebert and colleagues evaluated the impact of leukoreduction across 23 Canadian hospitals subsequent to a universal prestorage leukoreduction program.16 Outcomes evaluated included mortality, infections, fever and antibiotic use among 14,786 patients (6,982 during a period prior to the implementation, and 7,804 after the program’s implementation) who received RBC transfusions secondary to cardiac surgery, repair from hip fracture, or during their intensive care unit stay following a surgical intervention or trauma. Adjusted in-hospital mortality rates were lower (ORadj 0.87) after leukoreduction (7.03% vs. 6.19%, p=0.04). The direction and magnitude were similar, albeit not significant, among cardiac surgical patients (OR 0.88, p=0.20). There was a small, yet protective, effect for suspected (ORadj 0.94, p=0.21) and confirmed infection (ORadj 0.97, p=0.63) secondary to the leukoreduction program. The program was also associated with a significantly lower risk of fever (ORadj 0.86, p<0.001) and antibiotic usage (ORadj 0.90, p=0.03). We were unable to assess the rate, and therefore impact, of RBC leukoreduction on risk of post-operative pneumonia.
We recognize some limitations to our present study. First, we recognize that similar to other observational studies, we cannot rule out the effect of unmeasured confounding. We employed standard statistical approaches, including multivariable regression to adjust for differences in pre-operative characteristics, cardiopulmonary bypass duration and institution. Second, while we do not have information concerning the indication for the RBC transfusion, we adjusted for the patient’s pre-operative Hct as a proxy of the patient’s oxygen carrying capacity at the time of the operation. Transfusion of large quantities of RBCs is likely for volume replacement secondary to active bleeding. Nonetheless, patients receiving small quantities of RBCs (e.g. 2 units), which may be more discretionary in nature, had a 2-fold increased odds of post-operative pneumonia. Third, we did not collect information concerning the use of prestorage leukoreduction of RBC transfusions nor the length of storage.
Given the implications associated with increased exposure to transfusions, our findings suggest that clinical teams should consider opportunities for minimizing a patient’s exposure to RBC units, principally through increasing a patient’s oxygen carrying capacity. In a recent meta-analysis, Rohde found no statistically significant difference in risk of infection associated with a restrictive transfusion strategy [restrictive vs. liberal, RR 1.30 (CI95% 0.85, 1.97)].17 Nonetheless, other strategies may exist to reduce the delivery of RBC transfusions. Clinical teams may consider discharging stable inpatients to increase their pre-operative hemoglobin level. In the most recent version of its blood management guidelines, the Society of Thoracic Surgeons found Class IIa, Level B evidence to support the use of pre-operative erythropoietin (EPO) plus iron several days prior to surgery among patients presenting with anemia or those at high risk for post-operative anemia.18 Authors of this guideline recommendation recognized the need for clinical teams to weigh the benefit associated with EPO against the risk of thromboembolic events and hypertension, as well as the cost associated with its use.
Other strategies found to be reasonable in lowering RBC transfusions include the use of: mini circuits to reduce prime volume (Class I, level A), modified ultrafiltration (Class I, level A), antifibrinolytics (Class IIa, level B), centrifugation of salvaged blood (Class IIa, Level A), and multidisciplinary blood management teams (Class IIa, Level B). Databases such as the PERForm registry (http://www.performregistry.org/) are well equipped to assist clinical teams in tracking and evaluating the use of these and other strategies. Clinical teams should consider employing these practices as part of a broader blood management program and assess their association with the risk of post-operative pneumonia and other sequelae.
In this large, statewide, observational study, we found a significant, volume-dependent association between an increasing number of RBCs and subsequent onset of pneumonia, even after adjustment. Our findings suggest that reduction of transfusions (through blood conservation strategies, including reducing hemodilution or adopting a lower transfusion threshold in a stable patient) may provide an opportunity for reducing the risk of post-operative pneumonia.
Acknowledgments
The authors thank Katie Wopinsky for her editorial review.
Funding Sources: This project was supported by grant numbers R01HS022535 and R03HS022909 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality.
Footnotes
Presented at the 51st Annual Meeting of the Society of Thoracic Surgeons, San Diego, CA, January 24–28, 2015.
Disclosures
The authors have no relevant financial disclosures.
References
- 1.Surgenor SD, DeFoe GR, Fillinger MP, et al. Intraoperative red blood cell transfusion during coronary artery bypass graft surgery increases the risk of postoperative low-output heart failure. Circulation. 2006;114:I43–48. doi: 10.1161/CIRCULATIONAHA.105.001271. [DOI] [PubMed] [Google Scholar]
- 2.Horvath KA, Acker MA, Chang H, et al. Blood transfusion and infection after cardiac surgery. The Annals of thoracic surgery. 2013;95:2194–2201. doi: 10.1016/j.athoracsur.2012.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shih T, Zhang M, Kommareddi M, et al. Center-level variation in infection rates after coronary artery bypass grafting. Circulation Cardiovascular quality and outcomes. 2014;7:567–573. doi: 10.1161/CIRCOUTCOMES.113.000770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rogers MA, Blumberg N, Saint S, Langa KM, Nallamothu BK. Hospital variation in transfusion and infection after cardiac surgery: A cohort study. BMC medicine. 2009;7:37. doi: 10.1186/1741-7015-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prager RL, Armenti FR, Bassett JS, et al. Cardiac surgeons and the quality movement: The michigan experience. Seminars in thoracic and cardiovascular surgery. 2009;21:20–27. doi: 10.1053/j.semtcvs.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Cuzick J. A wilcoxon-type test for trend. Statistics in medicine. 1985;4:87–90. doi: 10.1002/sim.4780040112. [DOI] [PubMed] [Google Scholar]
- 7.Rothman KJ, Greenland S, Lash TL. Modern epidemiology. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 8.Taylor RW, O'Brien J, Trottier SJ, et al. Red blood cell transfusions and nosocomial infections in critically ill patients. Critical care medicine. 2006;34:2302–2308. doi: 10.1097/01.CCM.0000234034.51040.7F. quiz 2309. [DOI] [PubMed] [Google Scholar]
- 9.Murphy GJ, Reeves BC, Rogers CA, Rizvi SI, Culliford L, Angelini GD. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. 2007;116:2544–2552. doi: 10.1161/CIRCULATIONAHA.107.698977. [DOI] [PubMed] [Google Scholar]
- 10.Allou N, Bronchard R, Guglielminotti J, et al. Risk factors for postoperative pneumonia after cardiac surgery and development of a preoperative risk score*. Critical care medicine. 2014;42:1150–1156. doi: 10.1097/CCM.0000000000000143. [DOI] [PubMed] [Google Scholar]
- 11.Blajchman MA. The clinical benefits of the leukoreduction of blood products. The Journal of trauma. 2006;60:S83–90. doi: 10.1097/01.ta.0000199537.09201.7b. [DOI] [PubMed] [Google Scholar]
- 12.Vamvakas EC, Blajchman MA. Deleterious clinical effects of transfusion-associated immunomodulation: Fact or fiction? Blood. 2001;97:1180–1195. doi: 10.1182/blood.v97.5.1180. [DOI] [PubMed] [Google Scholar]
- 13.Paglino JC, Pomper GJ, Fisch GS, Champion MH, Snyder EL. Reduction of febrile but not allergic reactions to rbcs and platelets after conversion to universal prestorage leukoreduction. Transfusion. 2004;44:16–24. doi: 10.1046/j.0041-1132.2004.00608.x. [DOI] [PubMed] [Google Scholar]
- 14.Yazer MH, Podlosky L, Clarke G, Nahirniak SM. The effect of prestorage wbc reduction on the rates of febrile nonhemolytic transfusion reactions to platelet concentrates and rbc. Transfusion. 2004;44:10–15. doi: 10.1046/j.0041-1132.2003.00518.x. [DOI] [PubMed] [Google Scholar]
- 15.King KE, Shirey RS, Thoman SK, Bensen-Kennedy D, Tanz WS, Ness PM. Universal leukoreduction decreases the incidence of febrile nonhemolytic transfusion reactions to rbcs. Transfusion. 2004;44:25–29. doi: 10.1046/j.0041-1132.2004.00609.x. [DOI] [PubMed] [Google Scholar]
- 16.Hebert PC, Fergusson D, Blajchman MA, et al. Clinical outcomes following institution of the canadian universal leukoreduction program for red blood cell transfusions. Jama. 2003;289:1941–1949. doi: 10.1001/jama.289.15.1941. [DOI] [PubMed] [Google Scholar]
- 17.Rohde JM, Dimcheff DE, Blumberg N, et al. Health care-associated infection after red blood cell transfusion: A systematic review and meta-analysis. Jama. 2014;311:1317–1326. doi: 10.1001/jama.2014.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Society of Thoracic Surgeons Blood Conservation Guideline Task F. Ferraris VA, Brown JR, et al. 2011 update to the society of thoracic surgeons and the society of cardiovascular anesthesiologists blood conservation clinical practice guidelines. The Annals of thoracic surgery. 2011;91:944–982. doi: 10.1016/j.athoracsur.2010.11.078. [DOI] [PubMed] [Google Scholar]