Abstract
Polysialic acid (PSA), a large, linear glycan composed of 8 to over 100 α2,8-linked sialic acid residues, modulates development of the nervous system by enhancing cell migration, axon pathfinding, synaptic targeting and by regulating differentiation of progenitor cells. PSA also functions in developing and adult immune systems and is a signature of many cancers. In this study we identified vinorelbine, a semi-synthetic third generation vinca alkaloid, and epirubicin, an anthracycline and 4′-epimer of doxorubicin, as PSA mimetics. Similar to PSA, vinorelbine and epirubicin bind to the PSA-specific monoclonal antibody 735 and compete with the bacterial analogue of PSA, colominic acid in binding to monoclonal antibody 735. Vinorelbine and epirubicin stimulate neurite outgrowth of cerebellar neurons via the neural cell adhesion molecule (NCAM), via myristoylated alanine-rich C kinase substrate, and via fibroblast growth factor receptor, signaling through Erk pathways. Furthermore, the two compounds enhance process formation of Schwann cells and migration of cerebellar neurons in culture, and reduce migration of astrocytes after injury. These novel results show that the structure and function of PSA can be mimicked by the small organic compounds vinorelbine and epirubicin, thus raising the possibility to retarget drugs used in treatment of cancers to nervous system repair.
Keywords: Polysialic acid, neural cell adhesion molecule, vinorelbine, epirubicin, neurite outgrowth, migration
INTRODUCTION
Polysialic acid (PSA) is a negatively charged carbohydrate polymer consisting of α2,8-linked N-acetylneuraminic acid units with a large hydration volume and it is important for neural cell migration, axon pathfinding and synaptic targeting during development of the nervous system (Rutishauser 2008; Hildebrandt and Dityatev 2015). In the adult nervous system, the expression of PSA becomes restricted to regions of neuronal and glial plasticity where it enables synaptic plasticity (Rutishauser 2008; Bonfanti and Theodosis 2009; Senkov et al 2012). The major carrier of PSA in the nervous system is the neural cell adhesion molecule NCAM and less prominent carriers of PSA are SynCAM-1, the polysialyltransferase ST8SiaII, neuropilin-2 and the scavenger receptor CD36 (Hildebrandt and Dityatev 2015; Mühlenhoff et al 2013). A transient re-expression of PSA in neurons and glial cells was detected in different lesion models using adult animals (Brezun and Daszuta 2000; Bonfanti 2006). When PSA was re-introduced into the adult nervous system by implanting PSA-overexpressing Schwann cells into the spinal cord after injury, these cells exhibited improved migration and promoted axonal regeneration, remyelination and functional recovery (Lavdas et al. 2006; Luo et al. 2011; Ghosh et al. 2012). In mice expressing one of the polysialyltransferases responsible for generation of PSA under control of a glial-specific promoter, the proportion of successfully mono-(re)innervated motor endplates in the foot pad muscle was significantly increased (Jungnickel et al 2012). Altered PSA levels were found to be associated with various neuropathological conditions (El Maarouf and Rutishauser 2005), including chronic stress (Senkov et al. 2006), Alzheimer's disease (Mikkonen et al. 1999; Strekalova et al. 2006), schizophrenia (Barbeau et al. 1995; Gilabert-Juan et al. 2012) and temporal lobe epilepsy (Mikkonen et al. 1998; Pekcec et al. 2007). In the context of cancer, PSA is aberrantly re-expressed on many tumors (Falconer et al. 2012) and may promote invasion (Suzuki et al. 2005). Additionally, PSA was suggested to be useful to extend circulation time and improve therapeutic efficacy when used as the basis of drug carrier systems (Gregoriadis et al. 2001; Bader et al. 2011). Micelles of PSA grafted with polycaprolactone and filled with cyclosporine A, a therapeutic used in the treatment of rheumatoid arthritis, were shown to be taken up by synovial fibroblasts through a non-receptor mediated form of endocytosis and partitioning of cyclosporine A into the membrane (Wilson et al. 2014).
To exploit the beneficial functions of PSA for nervous system repair we searched for small organic compounds that mimic PSA structurally and functionally. Several studies have been reported that use a polysialylation based approach to increase the pharmacokinetic stability of peptides and proteins in biological fluids (Byrne et al. 2007), but high expression of sialidases like Neu4 or Neu1 in the central nervous system (Takahashi et al. 2012; Sumida et al. 2015) make PSA vulnerable to cleavage and degradation. These enzymes are not only present intracellularly, but were also shown to be secreted via exosomes in the brain, to be present at the cell surface and to be active not only at pH 4.5 but also at pH 7.2, the pH of the extracellular environment (Nan et al. 2007; Sumida et al. 2015). The small PSA mimicking organic compounds with considerable half-life in the blood and no cleavage site for sialidases may thus prove to be superior over native PSA. In the last years molecular mimetics have been shown to display superior affinity and metabolic stability in comparison to natural compounds (Magnani and Ernst, 2009). PSA mimicking peptides which were developed by screening of phage display libraries with PSA specific monoclonal antibodies (Torregrossa et al. 2004; Mehanna et al. 2009) were shown to promote functional recovery and plasticity after nervous system injury (Marino et al. 2009; Mehanna et al. 2009, 2010). In the present study, we have identified novel roles of vinorelbine, a semi-synthetic vinca alkaloid, commercially available as Navelbine® (Pierre Fabre Medicament, Boulogne, France) to treat several forms of cancer, and epirubicin, an anthracycline and 4′-epimer of doxorubicin, commercially available as Ellence® (Pfizer Pharmaceuticals, New York, USA) to treat breast cancer, as PSA mimetics and tested their functionality in vitro using neuronal and glial cultures. Our results show that vinorelbine and epirubicin influence the behavior of neuronal and glial cells in a manner similar to colominic acid and PSA.
MATERIALS AND METHODS
Animals
C57BL/6J mice of either sex were used as wild-type mice and obtained from the central breeding facility of the University Hospital Hamburg-Eppendorf. NCAM-deficient (−/−) mice (Cremer et al. 1994) on the C57BL/6J background and their wild-type littermates were used for cell culture experiments. Mice were kept under standard laboratory conditions with food and water supply ad libitum and with an artificial 12 h light/dark cycle. All experiments were conducted in accordance with the “Principles of laboratory animal care” (NIH publication No. 85-23, revised in 1985), the German and European Community laws on protection of experimental animals, and all procedures used were approved by the responsible committee of the State of Hamburg (permission number ORG 679). Two-day-old Wistar rats were used for primary cortical cell culture. Animal care and procedures were followed in accordance with the guidelines of the Animal Ethical Committee, Guru Nanak Dev University, Amritsar, India (permission number 226/CPCSEA).
The paper was written in compliance with the ARRIVE guidelines for reports on animal research.
Antibodies and chemicals
In the following, purchased reagents are indicated with their companies in brackets: colominic acid, catalase, Dulbecco's modified Eagle's medium (DMEM) (Sigma-Aldrich, St. Louis, MO, USA); the PSA mimicking peptide (NTHTDPYIYPID) with and without biotin label (Mehanna et al. 2009), the myristoylated alanine-rich C kinase substrate (MARCKS)-effector domain (ED) peptide (KKKKKRFSFKKSFKLSGFSFKKNKK) and the MARCKS-ED control peptide (KKKKKRASAKKSAKLSGASAKKNKK) (Theis et al. 2013) (Schafer-N, Copenhagen, Denmark); vinorelbine ditartrate ((2β,3β,4β,5α,12R,19α)-4-(acetyloxy)-6,7-didehydro-15-[(2R,6R,8S)-4-ethyl-1,3,6,7,8,9-hexahydro-8-(methoxycarbonyl)-2,6-methano-2H-azecino[4,3-b]indol-8-yl]-3-hydroxy-16-methoxy-1-methylaspidospermidine-3-carboxylic acid methyl ester; vinorelbine), epirubicin hydrochloride ((8S,10S)-10-[(3-amino-2,3,6-trideoxy-α-L-arabinohexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(2-hydroxyacetyl)-1-methoxy-5,12-naphthacenedione hydrochloride; epirubicin), nocodazole ([5-(2-thienylcarbonyl)-1H-benzimidazol-2-yl]carbonic acid methyl ester) and nitrendipine (5-O-ethyl-3-O-methyl-2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate) (Tocris Bioscience, Bristol, UK); PD166866 (1-(2-Amino-6-(3,5-dimethoxyphenyl)pyrido [2,3-d]pyrimidin-7-yl)-3-tert-butyl urea; fibroblast growth factor receptor tyrosine kinase inhibitor, CAS 192705-79-6 and CAS 865362-74-9 (extracellular regulated kinase Erk inhibitor II) (Millipore, Schwalbach, Germany). O-phenylenediamine dihydrochloride, m-maleimido-benzoyl-N-hydroxysuccinimidylester, stable peroxidase buffer (Thermo Scientific, Dreieich, Germany); Alexa or Cy coupled secondary antibodies (Jackson ImmunoResearch, Newmarket, UK or Life Technologies, Darmstadt, Germany); HRP coupled secondary antibodies for Western blots (Jackson Immunoresearch or GeNei). PSA-specific monoclonal antibody 735 and endoneuraminidase N (EndoN) (kind gifts of R. Gerardy-Schahn, Department of Biochemistry, Institute for Cellular Chemistry, Hannover Medical School, Hannover, Germany); 2B2 anti-NCAM antibody (Kleene et al. 2010); anti-PSA antibody (Millipore); anti-NCAM antibody (Sigma-Aldrich).
Screening of a compound library and identification of PSA mimetics by ELISA
To identify PSA mimicking compounds, the NIH Clinical Collection 1 Library (446 small molecules with a history of application in human clinical trials) was screened as described (Loers et al. 2014). Colominic acid (CA) was coupled to catalase by reductive amination, and peptides were coupled to catalase via their terminal cysteine using the cross-linker m-maleimido-benzoyl-N-hydroxysuccinimidylester (Wang et al. 2011). The number of coupled glycans and peptides per catalase molecule were determined by ELISA and Western blotting and showed, in average, 2.3 coupled glycan chains or peptides per catalase molecule. In brief, PSA mimicking peptide or CA coupled to catalase (3 μg/mL; 25 μL/well) were incubated with antibody 735 (0.1 μg/mL; 25 μL/well) with PBS, PBS containing 1% DMSO as solvent control, or PBS containing 1% DMSO with 10 μM of compounds from the library. As a positive control, antibody 735 was incubated with the PSA mimicking peptide (NTHTDPYIYPID; 10 μM). After incubation with secondary antibody, binding of 735 antibody was quantified using an ELISA reader (EnVision with Plateworks software, Perkin Elmer, Waltham, MA, USA). Experiments were repeated three times to reliably identify the hit compounds.
Neurite outgrowth, neuronal migration and process formation of Schwann cells
Primary cultures of cerebellar neurons, cerebellar explants, dorsal root ganglion (DRG) neurons or Schwann cells were prepared from cerebella, dorsal root ganglia or sciatic nerves of 7-day-old C57BL/6J or NCAM-deficient mice (Loers et al. 2005; Jakovcevski et al. 2009; Lieberoth et al. 2009; Mehanna et al. 2009); motoneurons were prepared from spinal cords of E14 mice (Simova et al. 2006); cortical neurons were prepared from cortices of 16-day-old mouse embryos (Jara et al. 2006); hippocampal neurons and astrocytes were prepared from early neonatal mice (Wang et al. 2011) and neurons from cortices of rats were prepared as described (Alho et al 1988; Favaron et al 1988). Staining of neuronal cultures against βIII-tubulin and glial fibrillary acidic protein (GFAP) verified that more than 98% of the isolated hippocampal and cerebellar cells were neurons. Schwann cells and DRG neurons were identified by their characteristic spindle shaped form (Schwann cells) or by their size and dendritic tree morphology (DRG neurons). Primary cells were treated with compounds at the indicated concentrations 1 hour after seeding. In experiments with the MARCKS-ED peptide, control peptide, Erk inhibitor and fibroblast growth factor receptor (FGFR) tyrosine kinase inhibitor and EndoN, peptides (20 μg/ml), enzymes (2.5 μg/ml) or inhibitors (1 μM for Erk inhibitor 100 nM for FGFR inhibitor) were added to the cultures 2 h before application of colominic acid or compounds and were kept in the medium during the experimental time period, with exception of stimulation with colominic acid where fresh medium was added together with the glycan. Neurite or process lengths and migration of cerebellar neurons were quantified as described (Loers et al. 2005; Jakovcevski et al. 2009; Mehanna et al. 2009). Schwann cell processes and neurites with lengths of at least one cell body diameter were evaluated and total neurite or process lengths per cell were determined from 50 cells in each of two wells per experiment. At least three independent experiments were performed per condition. Explants were prepared from cerebella of 6-day-old C57BL/6J mice as described (Jakovcevski et al. 2009) and treated with compounds at the indicated concentrations 16 h after isolation.
Scratch injury assay, immunocytofluorescence staining, Western blot analysis and competition ELISA
For procedures see supplementary materials and methods.
Molecular modeling of PSA, vinorelbine and epirubicin
A model of PSA bound to the surface of antibody 735 was constructed using the information provided by Evans et al. (1995) and the eight residue segment PSA docked into antibody 735 was constructed and manipulated as described (Bushman et al. 2014; Loers et al. 2014). We carried out to two complementary modeling approaches to characterize the similarity between the putative PSA mimetics and PSA. First, in order to model the structural and chemical ‘shape’ similarity, we compared vinorelbine and epirubicin to a pre-existing 8mer helical model of PSA, based on evidence that the longer helical structure of PSA is functionally significant. Second, we carried out modeling vinorelbine and epirubicin directly onto the structure of monoclonal antibody 735. We used the older Evans model (Evans et al. 1995) rather than the newer Nagae model (Nagae et al. 2013) to be in line with previous docking experiments (Bushman et al. 2014; Loers et al. 2014) and because the antibody structure in both models is close to identical (Nagae et al. 2013).
The 2D structures of vinorelbine and epirubicin from PubChem and OpenEye OMEGA (http://www.eyesopen.com/omega) were used to enumerate all possible low-energy 3D conformations for this compound. OpenEye ROCS (http://www.eyesopen.com/rocs) was used to carry out shape- and chemistry-based matching of each conformation with the PSA template to identify the 3D conformation for vinorelbine and epirubicin that most closely matched PSA. Vinorelbine and epirubicin were also docked to the variable region of the antibody 735 structure using Schrödinger Glide in XP mode (Friesner et al. 2006) to identify putative interactions with antibody 735.
Statistical analysis
The significance of values was determined by one-way analysis of variance (ANOVA) with Dunnett's post-hoc test or two-way repeated measures ANOVA followed by Tukey's post hoc test were appropriate. One-way ANOVA with Holm-Sidak post-hoc test was used for immunofluorescence and immunoblot intensity analysis using Sigma Stat for Windows (version 3.5, Systat Software, Inc, San Jose, CA). Values are expressed as means ± SEM from at least three independent experiments and differences were considered significant at p < 0.05.
RESULTS
Vinorelbine and epirubicin bind to anti-PSA antibody 735
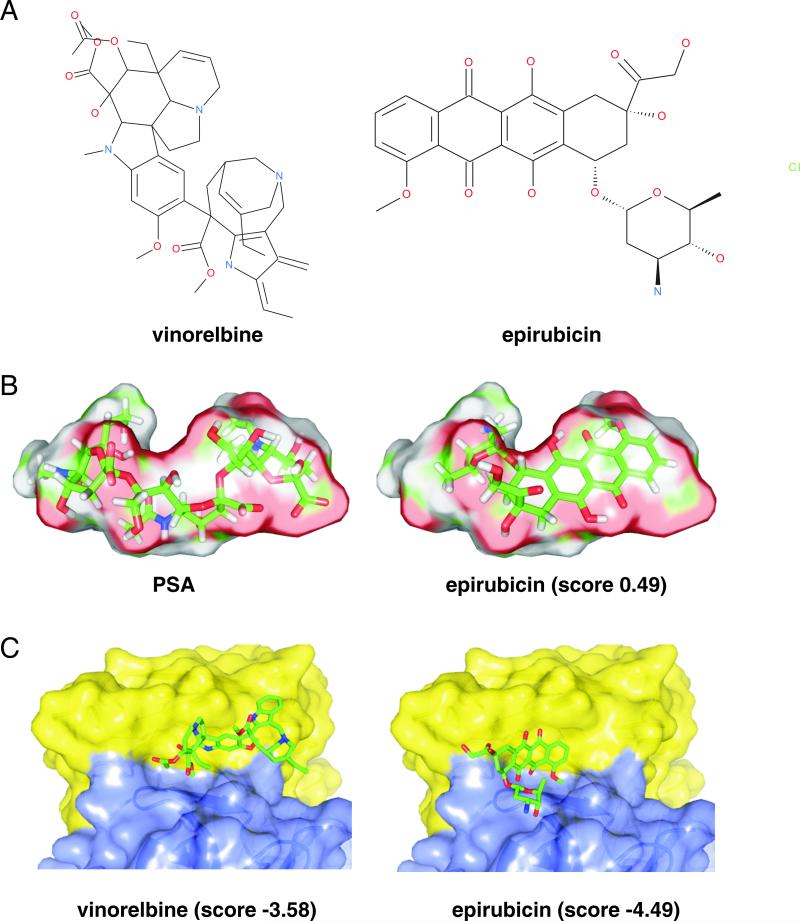
To identify novel PSA mimetics the NIH Clinical Collection 1 Library was screened for compounds that inhibit binding of the PSA mimetic peptide to the PSA receptor site of antibody 735. Vinorelbine ditartrate, a semi-synthetic third generation vinca alkaloid, and epirubicin hydrochloride, an anthracycline and 4′-epimer of doxorubicin, were identified via this screen as a potential PSA mimetics (Fig. 1A).
Fig. 1. Structure models of PSA and vinorelbine and epirubicin.
(A) Chemical structures of vinorelbine and epirubicin. (B) Configuration of PSA, shown as a surface, superimposed with the 3D structure of epirubicin (right) showing that epirubicin can adopt a conformation compatible with that of PSA when bound to antibody 735. No significant structural similarity with vinorelbine is observed. (C) Structure models of complexes between antibody 735 and vinorelbine (left) or epirubicin (right) suggest that the two compounds bind to a hydrophobic region that appears critical for interactions with PSA.
Since PSA is a very large negatively charged molecule, we were further interested to elucidate how the small organic molecules can mimic this glycan. The structures of vinorelbine and epirubicin were therefore compared with the predicted helical conformation of the PSA 8mer (Evans et al. 1995) (Fig. 1B) by a rapid overlay of chemical structures (ROCS). The comparison revealed a conformation of epirubicin that matched the PSA shape obtained from the structural model of antibody 735 (Evans et al. 1995). The overall similarity score obtained for epirubicin was 0.49 on a scale of 0 to 2. This indicates that although epirubicin does not contain a high degree of similarity with PSA it can adopt a shape compatible with the van der Waals volume of the PSA binding conformation within antibody 735. However, for vinorelbine no conformation matching the PSA shape could be found.
We then generated putative vinorelbine-antibody 735 and epirubicin-antibody 735 model structures using the docking software Glide (Friesner et al. 2006). The structure of the free Fab fragment of antibody 735 predicted by Evans et al. (1995) used for the comparisons is almost identical to a more recently determined structure of the antigen-bound single chain fragment variable of antibody 735 determined by Nagae et al. (2013), leading to the expectation that modeling by docking either structure would produce very similar results. A comparison of the models of the PSA/antibody 735 and vinorelbine-antibody 735 or epirubicin-antibody 735 complexes (Fig. 1C) suggests that vinorelbine and epirubicin bind in vicinity of the region of antibody 735 that contains numerous hydrophobic residues that are critical for interaction with PSA.
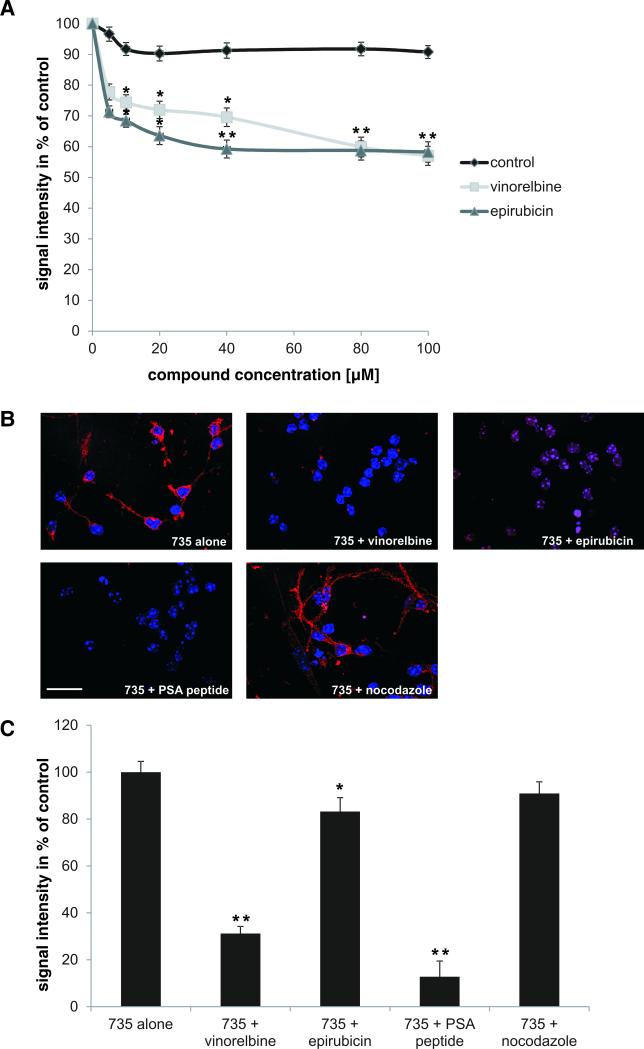
To confirm the results from the initial screen, a competition ELISA was performed with different concentrations of vinorelbine and epirubicin and nitrendipine as negative control. Vinorelbine and epirubicin inhibited binding of antibody 735 to catalase carrying PSA mimicking peptide (data not shown) and colominic acid. This inhibition was concentration dependent. Maximal inhibition was obtained at approximately 80 μM (Fig. 2). At all tested concentrations, nitrendipine was not able to impede binding of antibody 735 to colominic acid or the PSA peptide (Fig. 2A). When cerebellar neurons were stained with PSA antibody 735 in the absence or presence of vinorelbine, epirubicin or PSA mimicking peptide, binding of the PSA antibody was markedly reduced by vinorelbine and PSA mimicking peptide (31% of control and 12.8% of control, respectively), decreased in the presence of epirubicin (83% of control) and unchanged in the presence of control compound nocodazole, showing that vinorelbine, epirubicin and PSA mimicking peptide bind to PSA antibody 735 and thereby reduce PSA antibody binding to native PSA at the cell surface (Fig. 2B,C).
Fig. 2. Vinorelbine and epirubicin compete with colominic acid for binding to the PSA-specific antibody 735.
(A) Colominic acid coupled to catalase was immobilized in 384-wells and incubated with antibody 735 in the presence of vinorelbine (light gray line) or epirubicin (dark grey line) at 100 nM – 100 μM concentrations (means ± SEM). The signal from antibody binding to colominic acid was set to 100%. Vinorelbine and epirubicin compete with colominic acid for binding to antibody 735 in a concentration dependent manner reaching a maximal effect at 40 to 80 μM concentrations. * p < 0.05, ** p < 0.005; two-way analysis of variance (ANOVA) followed by Tukey's post hoc testing. (B) Representative images of cerebellar neurons stained with PSA antibody mAb 735 (Cy3; red) alone or in the presence of vinorelbine or epirubicin, PSA peptide (positive control) or nocodazole (negative control). Nuclei are shown in blue. Scale bar: 50 μm. (C) Histogram showing the quantification of immunostainings from 50 cells (means + SEM). * p < 0.05, ** p < 0.005 (one-way ANOVA).
Vinorelbine and epirubicin induce neurite outgrowth and Schwann cell process formation
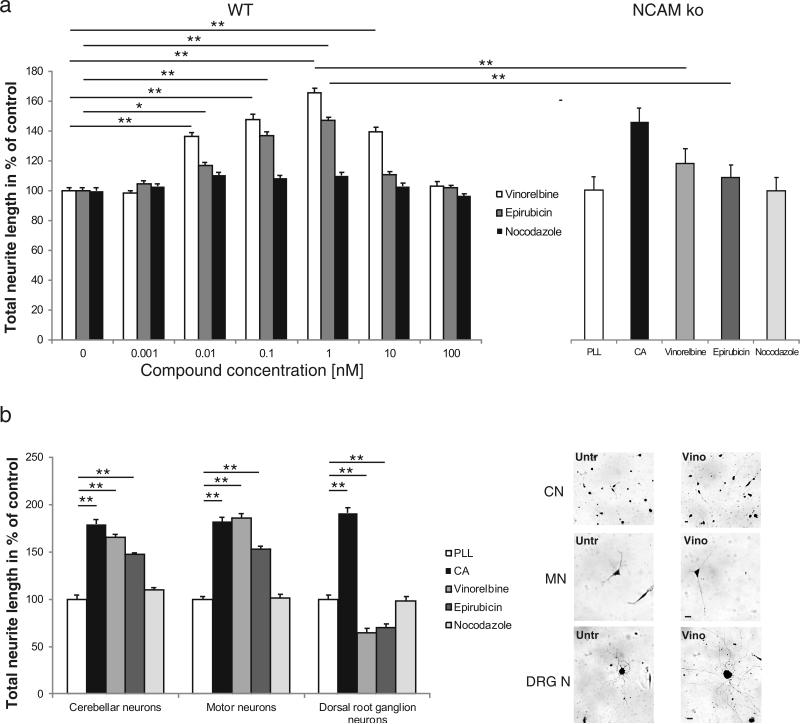
To investigate if vinorelbine and epirubicin are able to functionally mimic PSA, we determined the process outgrowth of PSA-responsive neurons and glial cells. Application of 0.001 to 100 nM vinorelbine and epirubicin to wild-type cerebellar granule neurons led to a concentration dependent increase in neurite length from 16% (epirubicin) or 36% (vinorelbine) at 0.01 nM compound concentration to 48% or 65% (1 nM) of the neurite length of control cells only treated with vehicle control or control compound nocodazole, which is also a cell cycle inhibitor acting on tubulin and microtubules similar to vinorelbine and epirubicin (Fig. 3A). Interestingly, vinorelbine and epirubicin failed to induce neuritogenesis in NCAM-deficient cerebellar neurons (Fig. 3A). At all tested compound concentrations (0.001 to 100 nM) no alteration in the outgrowth of NCAM-deficient cerebellar neurons were detected (1 nM: Fig. 3A; all other concentrations: data not shown). We hence conclude that the PSA mimetics might act via NCAM at the neuronal cell surface.
Fig. 3. Vinorelbine and epirubicin stimulate neurite outgrowth from wild-type neurons.
(A) Vinorelbine and epirubicin but not nocodazole stimulate concentration dependent neurite outgrowth from wild-type cerebellar neurons (left). Neurite outgrowth from NCAM-deficient cerebellar neurons in the absence (poly-L-lysine; PLL) or presence of colominic acid (CA), vinorelbine, epirubicin and nocodazole (small organic compounds at 1 nM concentration, CA at 30 μg/ml; right). (B) Representative images of cerebellar neurons, motor neurons and dorsal root ganglion (DRG) neurons grown in the absence (untr) or presence of vinorelbine (vino). Scale bars: 20 μm. The bar diagram shows the comparison of neurite lengths of cerebellar, motor and dorsal root ganglion (DRG) neurons in the absence (poly-L-lysine; PLL) or presence of colominic acid (CA), vinorelbine, epirubicin and nocodazole (small organic compounds at 1 nM concentration, CA at 30 μg/ml). (A-B) Data represent means of neurite lengths per cell + SEM as compared with PLL from three independent experiments. Asterisks denote significant differences from control. ** p < 0.001; one-way or two-way (3A left panel) ANOVA followed by Tukey's post hoc testing.
Comparison of the neuritogenic effects of vinorelbine, epirubicin and the PSA analog colominic acid revealed that the stimulatory effects of vinorelbine and colominic acid were similar, reaching 1.8-fold of control value with colominic acid and 1.6-fold of control value with vinorelbine (Fig. 3B). Epirubicin was slightly less potent with maximally 1.5-fold of control value. To determine if similar effects of vinorelbine and epirubicin are observed with other PSA-responsive neurons, motor neurons and dorsal root ganglion neurons were cultured in the presence of colominic acid, vinorelbine, epirubicin and nocodazole (Fig. 3B). Colominic acid and vinorelbine stimulated neuritogenesis of motor neurons by 1.8-fold, epirubicin stimulated neuritogenesis by 1.5-fold, whereas the nocodazole control had no effect (Fig. 3B). Interestingly, colominic acid stimulated neuritogenesis of dorsal root ganglion neurons by 1.9-fold, but vinorelbine and epirubicin failed to induce neuritogenesis at nM compound concentrations and even lead to a reduction in neurite elongation (Fig. 3B).
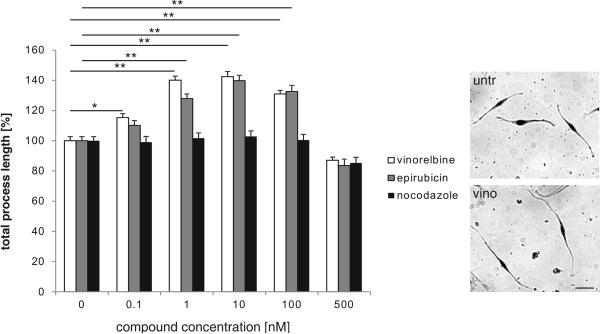
Similar to cerebellar neurons, process formation of Schwann cells was increased by vinorelbine and epirubicin in a concentration dependent manner with maximal stimulation (1.4-fold of control) at 10 nM concentration, whereas nocodazole used as negative control was ineffective (Fig. 4). These results show that the tested PSA mimetics stimulate neurite outgrowth of cerebellar neurons and motor neurons as well as process formation of Schwann cells. Using NCAM-deficient neurons revealed that neuritogenesis appears to be mediated via NCAM present at the cell surface of wild-type cells.
Fig. 4. Vinorelbine and epirubicin stimulate process formation of wild-type Schwann cells.
Representative images of Schwann cells grown in the absence (untr) or presence of 10 nM vinorelbine (vino). Scale bar: 20 μM. Histogram shows that vinorelbine and epirubicin but not nocodazole stimulate concentration dependent process formation of Schwann cells. Data represent means of process lengths per cell + SEM as compared with PLL from three independent experiments. Asterisks denote significant differences from control. * p < 0.01, ** p < 0.001; two-way ANOVA followed by Tukey's post hoc testing.
Vinorelbine and epirubicin stimulate neurite outgrowth via myristoylated alanine-rich C kinase substrate
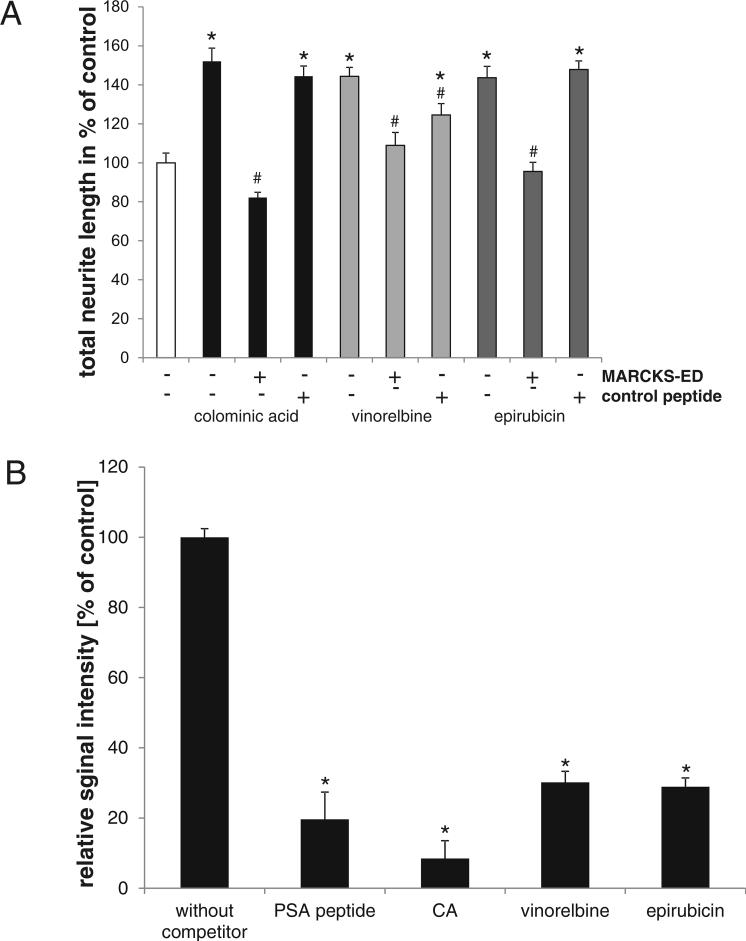
Theis et al. (2013) demonstrated that disruption of the interaction of PSA with MARCKS by addition of a peptide comprising the effector domain of MARCKS (MARCKS-ED) abolished PSA-induced enhancement of neurite outgrowth. To investigate whether vinorelbine- and epirubicin-mediated neurite outgrowth also depend on MARCKS, we performed neurite outgrowth experiments using the MARCKS-ED peptide. In the presence of the MARCKS-ED peptide, but not the control peptide, colominic acid, epirubicin and vinorelbine did not stimulate neurite outgrowth (Fig. 5A). To get further insights into interaction of the compounds with the PSA binding partner MARCKS, we treated cerebellar explants with compounds and the MARCKS-ED peptide. Explants treated with vinorelbine or epirubicin and the MARCKS-ED peptide showed reduced surface expression of PSA as compared to the control groups treated with MARCKS scrambled peptide (Supplementary Fig. S1).
Fig. 5. Vinorelbine and epirubicin bind with and stimulate neurite outgrowth via MARCKS.
(A) Neurite lengths of cerebellar neurons in the presence of 1 nM vinorelbine, 1 nM epirubicin and 30 μg/ml colominic acid, and the MARCKS-ED and control peptides (20 μg/ml). Data represent mean values of neurite lengths per cell + SEM as compared with PLL from three independent experiments. Asterisks denote significant differences from control, * p < 0.001; one-way analysis of variance (ANOVA). Hatches denote differences within a group, # p < 0.001. (B) MARCKS-ED was incubated with PBS (without competitor), colominic acid (CA), vinorelbine or epirubicin followed by addition of PSA mimicking peptide coupled to biotin. Binding of PSA mimicking peptide was detected with Streptavidin-HRP. Means + SD from triplicates and three independent experiments are shown. * p < 0.05.
To investigate if the compounds directly bind to the effector domain of MARCKS as shown for colominic acid (Theis et al. 2013), we performed ELISA experiments and show that the compounds compete with the PSA mimicking peptide for binding to the effector domain of MARCKS (Fig. 5B), indicating that the compounds directly bind to MARCKS. No binding to the control peptide was observed (data not shown).
These results suggest that vinorelbine and epirubicin interact with the PSA binding partner MARCKS and that this interaction is necessary to enhance the surface expression of PSA.
Vinorelbine and epirubicin induce neurite outgrowth via extracellular regulated kinase and the fibroblast growth factor receptor
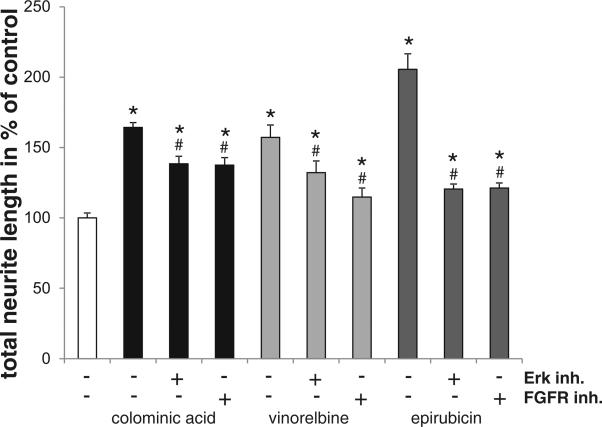
Homophilic NCAM-binding was shown to induce neurite outgrowth through pathways involving activation of FGFR and Erk (Ditlevsen et al. 2003, 2008). To investigate whether vinorelbine- and epirubicin-mediated neurite outgrowth does not only depend on the presence of NCAM at the cell surface but also on activation of the FGFR and Erk, we performed neurite outgrowth experiments using the Erk inhibitor CAS 865362-74-9 and the FGFR receptor tyrosine kinase inhibitor CAS 192705-79-6. In the presence of the FGFR and Erk inhibitors, the stimulatory effect of colominic acid, vinorelbine and epirubicin on neurite outgrowth was strongly reduced, but still higher than control values (Fig. 6). These results show that vinorelbine and epirubicin at least partially activate the FGF receptor and Erk pathways to enhance neurite outgrowth.
Fig. 6. Vinorelbine and epirubicin stimulate neurite outgrowth via FGF receptor and Erk signaling pathways.
Neurite length of cerebellar neurons grown in the presence of 1 nM vinorelbine, 1 nM epirubicin and colominic acid (CA at 30 μg/ml) and in the presence of the Erk inhibitor CAS 865362-74-9 (1 μM) and the FGFR receptor tyrosine kinase inhibitor CAS 192705-79-6 (100 nM). Data represent means of neurite lengths per cell + SEM as compared with PLL from three independent experiments. Asterisks denote significant differences from control, * p < 0.001; one-way ANOVA. Hatches denote differences within a group, # p < 0.001.
Vinorelbine and epirubicin increase migration of cerebellar neurons and decrease migration of astrocytes
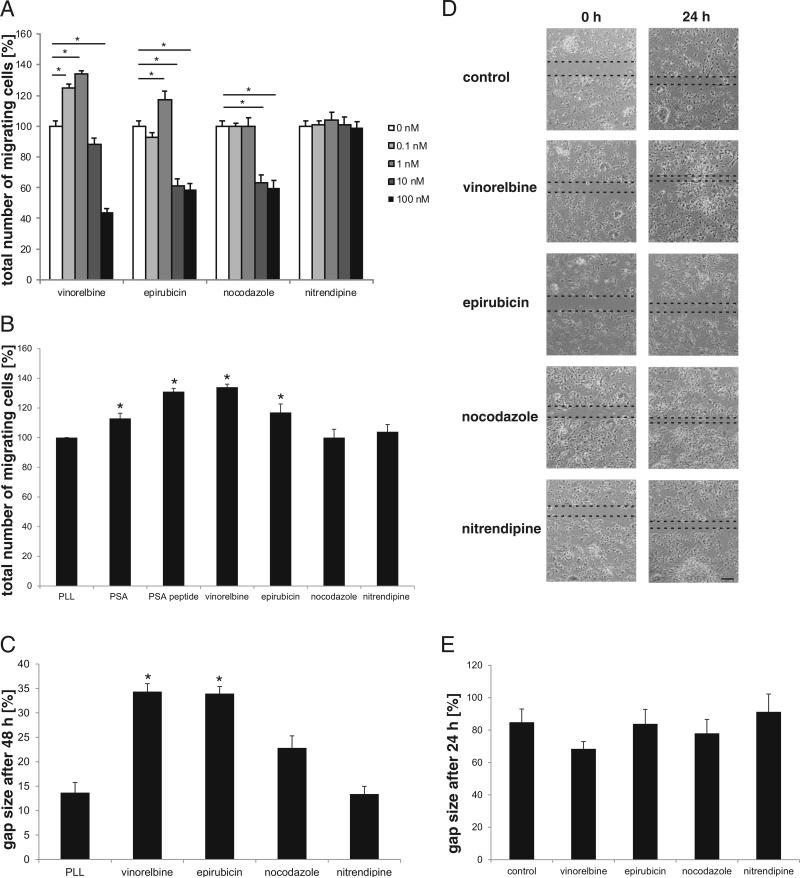
Since ectopic expression or overexpression of PSA by Schwann cells leads to enhanced migration (Bachelin et al. 2010; Luo et al. 2011; Ghosh et al. 2012) and removal of PSA by endoneuraminidase N impairs migration of neural progenitor cells (Burgess et al. 2008) as well as neuronal migration in the embryonic cerebellum (Rieger et al. 2008), we examined if vinorelbine and epirubicin affect migration of cells out of cerebellar explants and migration of cultured astrocytes after scratch injury (Fig. 7). As seen for colominic acid, low concentrations of vinorelbine (up to 1 nM) enhanced migration of neurons out of cerebellar explants, but higher concentrations (100 nM) inhibited migration (Fig. 7A). Furthermore, most concentrations of epirubicin reduced migration of neurons out of cerebellar explants with exception of a 1 nM concentration, showing that vinorelbine and epirubicin are less effective than colominic acid in this parameter. Interestingly, 10 nM epirubicin did not enhance the migration of cortical neurons after scratch injury. Although the values were not statistically significantly different from control values, a 1.5-fold increase in migration of cortical neurons was observed after treatment with vinorelbine (Fig. 7D, E). Since vinorelbine is known to stimulate microtubule depolymerization and to block mitotic progression at G2-M cell cycle and epirubicin intercalates with the DNA and generates oxygen radicals, it is likely that their PSA-mimicking effect and stimulatory action on migrating neurons is overridden by these known inhibitory effects in several of the investigated cell types. This assumption is supported by the observed inhibitory effect of nocodazole, a microtubule inhibitor, which was used as control (Fig. 7A, B).
Fig.7. Vinorelbine and epirubicin increase migration of neurons out of cerebellar explants and of cortical neurons, and inhibit migration of astrocytes.
(A, B) Migration of cells out of cerebellar explants was determined 32 h after compound application (0.1 to 100 nM concentrations (A), 1 nM (B)) and the total number of migrating cells was determined. (C) Confluent monolayers of wild-type astrocytes were scratched resulting in a gap of approximately 800 μm (gap width was set to 100%). Closure of the gap was measured by inverted phase-contrast microscopy from 0 to 48 h. Histograms show data representing mean values + SEM from three independent experiments. Asterisks denote significant differences from control. * p< 0.01; one-way ANOVA. (D, E) Monolayers of cortical neurons were injured by applying a scratch and treated with 10 nM vinorelbine, 10 nM epirubicin, and the control compounds 10 nM nocodazole and nitrendipine or vehicle control (control). Phase contrast images are shown for 0 h and 24 h after scratching. Scale bar = 200 μm. Histograms represent the gap widths in percent of the original gap width (gap size at 0 h was set to 100%) (means+ SEM, from three independent experiments).
Vinorelbine and epirubicin slowed down the migration of astrocytes after scratch injury like application of colominic acid does. The control compound nocodazole also slowed down migration of astrocytes after injury although less so than colominic acid and the PSA mimetics (Fig. 7C). Nitrendipine, which was used as second control compound, did not reduce or slow down the migration of astrocytes after injury. These results show that vinorelbine and epirubicin inhibit migration of astrocytes after injury in a similar extent as colominic acid and that they partially stimulate migration of neurons.
Vinorelbine and epirubicin enhance endogenous PSA expression on cultured neurons but removal of PSA does not impede neurite outgrowth
In a previous study on the PSA mimetic 5-nonyloxytryptamine we showed that treatment of neurons with this compound also enhanced expression of PSA and NCAM on neurons (Loers et al. 2014). Therefore, we investigated if vinorelbine and epirubicin also alter the expression of PSA and its major carrier NCAM on cerebellar neurons. Levels of endogenous PSA were enhanced after treatment with 10 nM vinorelbine and epirubicin, but no changes in levels of endogenous NCAM were observed (Supplementary Fig. S2). In addition, treatment of astrocytes with vinorelbine and epirubicin did not change the levels of PSA, and treatment with vinorelbine reduced NCAM expression by 36%. Epirubicin treatment also reduced levels of PSA, although non-significantly by only 12% (Supplementary Fig. S3). On astrocytes nocodazole only slightly enhanced PSA expression and did not change expression of NCAM.
To investigate if the presence and upregulation of PSA is necessary for vinorelbine and epirubicin to stimulate neurite outgrowth, we removed endogenous PSA by EndoN treatment before addition of compounds and colominic acid and determined neurite outgrowth (Supplementary Fig. S4). In the experiments with compounds EndoN was also present during the entire culture period, so as to counteract the upregulation of endogenous PSA by compound addition. Pre-treatment of cerebellar neurons with EndoN and presence of EndoN during compound stimulation only led to a 4.1%, 8.8% or 9.1% reduction in neurite lengths, when compared to values obtained with vinorelbine, epirubicin and colominic acid without treatment with EndoN (Supplementary Fig. S4).
These results show that the microtubule inhibitors vinorelbine and nocodazole as well as the topoisomerase inhibitor epirubicin alter the expression of PSA at the surface of these cells, but this upregulation of PSA and the presence of PSA at the cell surface are not necessary for stimulation of neurite outgrowth by colominic acid as well as vinorelbine and epirubicin.
DISCUSSION
In the current study we analyzed the PSA mimicking potential of epirubicin and vinorelbine (Supplementary Table 1) which are FDA (U.S. Food and Drug Administration) approved drugs for the treatment of cancers. Vinorelbine, a semi-synthetic third generation vinca alkaloid, stimulates microtubule depolymerization and mitotic spindle destruction at high concentration whereas at lower concentrations, it is able to block mitotic progression at G2-M phase. Its main targets are tubulin and microtubules, but it was also shown to inhibit the stability of the lipid bilayer membranes (Moudi et al. 2013). Mechanism of action of the amphiphilic anthracycline epirubicin, the 4′-epimer of doxorubicin, is through intercalation of DNA, DNA strand breakage, inhibition of topoisomerase II activity by stabilizing the DNA-topoisomerase II complex, generation of oxygen and other free radicals, resulting in interference with DNA, RNA, and protein synthesis and its cytocidal activity (Khasraw et al. 2012). In agreement with the results on PSA, epirubicin and vinorelbine bind to the PSA-specific monoclonal antibody 735 and compete with the PSA mimetic peptide or colominic acid for binding to this antibody. These small organic compounds can adopt a conformation that is compatible with the structure of PSA, as shown by modeling of PSA and epirubicin or vinorelbine into the antigen binding pocket of antibody 735. That PSA, vinorelbine and epirubicin can adopt similar conformations and thus may bind to the same cellular receptors is strengthened by the fact that all bind to histones: PSA binds to extracellularly localized histone H1 (Mishra et al. 2010), vinorelbine binds to several histones (Rabbani-Chadegani et al. 2009) and epirubicin binds to histone H3 (Khan et al. 2012). Furthermore, vinorelbine and epirubicin induce similar cellular responses as colominic acid in cell culture. When applied at concentrations in the picomolar to low nanomolar range, vinorelbine and epirubicin stimulate neuritogenesis of cerebellar neurons and motor neurons as well as process formation of Schwann cells to a similar extent as colominic acid. Interestingly, in contrast to cerebellar neurons, outgrowth from DRG neurons was reduced in the presence of vinorelbine and epirubicin but enhanced in the presence of colominic acid. Different responses of sensory neurons to additives have been previously observed: CD24, a highly glycosylated protein, stimulates outgrowth from cerebellar neurons and inhibits outgrowth from DRG neurons. This difference was induced by interaction of CD24 with different co-receptors of the cell adhesion molecule L1 (Lieberoth et al. 2009). These results suggest that different neuronal cell types express different PSA receptors and that co-signaling pathways differ between these cells. The different response of DRG neurons to colominic acid versus vinorelbine and epirubicin may be due to the fact that apart from influencing the NCAM mediated homophilic and heterophilic interactions, PSA also regulates the local concentration of neurotrophins (Durbec et al. 2001; Ono et al. 2012) which is likely not achieved with the compounds which are not cell surface-bound. Interaction of PSA with soluble factors depends on its degree of polymerization (Kanato et al. 2008; Ono et al. 2012) and colominic acid like PSA consists of a large number of repetitive units of sialic acid. In contrast, the PSA mimetics are small hydrophobic compounds which do not contain any repetitive units and may therefore not be able to provide suitable steric constraints which are required to interact with neurotrophins and cell surface receptors other than NCAM.
To understand by which mechanisms the PSA mimetics may exert their function, several pathways stimulated by NCAM or PSA were analyzed. NCAM-deficient cerebellar neurons were not affected by the PSA mimicking compounds, suggesting that these compounds act on cerebellar neurons specifically through an NCAM-dependent pathway. The observed effects of colominic acid on NCAM-deficient cerebellar neurons is likely due to the high degree of polymerization of colominic acid which may allow it to interact with and mediate neurite outgrowth additionally through soluble factors and cell surface receptors other than NCAM. Similar results were obtained in previous experiments: the PSA mimetic 5-nonyloxytryptamine stimulated neurite outgrowth of NCAM-deficient hippocampal neurons, but not of motor neurons, and the PSA mimetic tegaserod enhanced outgrowth of motor neurons and cerebellar neurons by 2-fold, whereas stimulation of DRG neurons was only 1.5-fold (Bushman et al. 2014; Loers et al. 2014). In agreement with the observation that PSA interacts with the effector domain of MARCKS at and/or within the plane of the membrane and addition of a peptide containing the effector domain of MARCKS abolished the PSA-mediated neurite outgrowth of hippocampal neurons (Theis et al. 2013), we here show that vinorelbine and epirubicin compete with PSA-mimicking peptide for binding to the MARCKS-ED peptide and that application of MARCKS-ED peptide abolishes vinorelbine- and epirubicin-mediated neurite outgrowth and reduces surface expression of PSA in cerebellar explants. These results show that the PSA mimicking compounds not only depend on the presence of NCAM at the cell surface but also on the interaction with MARCKS. Removal of endogenous PSA by digestion with endoneuraminidase N reduces vinorelbine and epirubicin stimulated neurite outgrowth only by 5-10%, suggesting that compounds do not rely on the presence of PSA on the cell surface. Furthermore, stimulation of neurite outgrowth by colominic acid as well as the PSA mimetics was reduced in the presence of an FGF receptor tyrosine kinase inhibitor and an Erk inhibitor. Similar results were obtained using NIH-3T3 cells (Li et al. 2011). PSA-stimulated migration was paralleled by activation of the FGFR and its downstream signaling components, phospholipase C, focal adhesion kinase and Erk1/2, again confirming that the PSA mimetics depend on co-stimulation of the FGFR and Erk kinase pathways to enhance neurite outgrowth as had been shown for NCAM and PSA-NCAM.
Recently, several carbohydrate and glycomimetic based approaches have emerged in search of potential candidates to stimulate neural repair. With PSA being involved in multiple clinical conditions ranging from influenza virus infections (Rameix-Welti et al. 2009), cancer (Falconer et al. 2012) to nervous system disorders (Mikkonen et al. 1999; Atz et al. 2007; Varea et al. 2007; Tsoory et al. 2008; Brennaman and Maness 2010; Gilabert-Juan et al. 2012; Varea et al. 2012), it is a possible neuroprotective compound. Also, since PSA is important for migration of cells and pathfinding by axons, neuron-glia plasticity, and spatial learning and memory, it is a potential candidate for regulating not only development, but also neuroprotection in acute and chronic neurological conditions (Parkash and Kaur 2005; Bonfanti 2006; Rutishauser 2008; Franceschini et al. 2010; Kumar et al. 2012).
As PSA promotes dynamic cell interactions, which are essential for plasticity of axons and their associated glia and is essential for the induction and reversal of changes in axonal as well as glial morphology (Monlezun et al. 2005), we studied the effect of the PSA mimicking compounds on Schwann cells and astrocytes. Vinorelbine and epirubicin enhance process formation of Schwann cells and may thus serve for repair after peripheral nerve injury. That PSA is beneficial for regeneration is underscored by the findings that PSA overexpressing Schwann cells are more motile and improve regeneration after spinal cord injury (Lavdas et al. 2006; Luo et al. 2011; Ghosh et al. 2012). Ectopic expression of polysialylated NCAM promotes adult macaque Schwann cell migration and improves their integration into cultured astrocytes. When transplanted into mice with focally induced demyelination, these cells showed accelerated recruitment to the lesion site and not only enhanced interaction with reactive astrocytes when exiting the graft, but also enhanced ‘chain-like’ migration along the dorsal midline (Bachelin et al. 2010). Proliferation and migration of reactive astrocytes is implicated in formation of the glial scar which may inhibit or support regeneration (Sofroniew and Vinters 2010). Therefore, the ability of epirubicin and vinorelbine to reduce migration of astrocytes after scar injury indicates their potential application to enhance regeneration after injury in the central nervous system. Furthermore, we were interested to know whether the observed effects of the test compounds were due to their PSA mimicking property or their ability to enhance the expression of endogenous PSA. The test compounds enhanced the expression of endogenous PSA levels on cerebellar neurons, but did not change the expression of NCAM by these cells. The stimulation of neurite outgrowth via MARCKS and the FGF receptor which acts via Erk signaling pathways also strengthen the evidence that vinorelbine and epirubicin act as PSA mimetics.
Supplementary Material
Acknowledgements
The authors are very grateful to Dr. Rita Gerady-Schahn for donation of the antibody 735 and EndoN, Markus Wolf and Ute Bork for excellent technical assistance, Eva Kronberg for excellent animal care, and Philip Gribbon for help with the screening setup. Vedangana Saini thanks the Council of Scientific and Industrial Research (CSIR) India for a Senior Research Fellowship and the DAAD for a six-month research scholarship in Germany. Infrastructure provided by University Grants Commission (UGC), India, under the UPE and CPEPA schemes and Department of Biotechnology (DBT), India, under the DISC facility is highly acknowledged. Melitta Schachner thanks the NIH for support.
Funding
This work was supported by the BMBF and ICMR (Indo-German Research Project 10/050 to Melitta Schachner and Gurcharan Kaur), the U.S. Army Medical Research and Materiel Command Clinical and Rehabilitative Medicine Research Program (to Anders Wallqvist and Sidhartha Chaudhury) and NIH grant RO1 NS078385-01A1 (to Melitta Schachner).
Abbreviations
- CA
colominic acid
- DAPI
4',6-diamidino-2-phenylindole
- DMEM
Dulbecco's modified Eagle's medium
- DMSO
dimethyl sulfoxide
- DRG
dorsal root ganglia
- ED
effector domain
- epirubicin
(8S,10S)-10-[(3-amino-2,3,6-trideoxy-α-L-arabino-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(2-hydroxyacetyl)-1-methoxy-5,12-naphthacenedione hydrochloride
- Erk
extracellular regulated kinase
- FGFR
fibroblast growth factor receptor
- HRP
horse radish peroxidase
- MARCKS
myristoylated alanine rich C kinase substrate
- NCAM
neural cell adhesion molecule
- nitrendipine
5-O-ethyl-3-O-methyl-2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate
- nocodazole
[5-(2-thienylcarbonyl)-1H-benzimidazol-2-yl]carbonic acid methyl ester
- PBS
phosphate buffered saline solution
- PBST
phosphate buffered saline solution with 0.01% Tween-20
- PSA
polysialic acid
- TBS
Tris buffered saline solution
- TBST
Tris buffered saline solution with 0.1% Tween-20
- vinorelbine
(2β,3β,4β,5α,12R,19α)-4-(acetyloxy)-6,7-didehydro-15-[(2R,6R,8S)-4-ethyl-1,3,6,7,8,9-hexahydro-8-(methoxycarbonyl)-2,6-methano-2H-azecino[4,3-b]indol-8-yl]-3-hydroxy-16-methoxy-1-methylaspidospermidine-3-carboxylic acid methyl ester
Footnotes
ARRIVE guidelines have been followed:
Yes => if No, skip complete sentence => if Yes, insert “All experiments were conducted in compliance with the ARRIVE guidelines.”
Conflicts of interest: none => if ‘none’, insert “The authors have no conflict of interest to declare.” => otherwise insert info unless it is already included
Competing Interests
The authors declare that no competing interests exist. The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the U.S. Army or the U.S. Department of Defense. This paper has been approved for public release with unlimited distribution.
REFERENCES
- Alho H, Ferrarese C, Vicini S, Vaccarino F. Subsets of GABAergic neurons in dissociated cell cultures of neonatal rat cerebral cortex show co-localization with specific modulator peptides. Brain Res. 1988;467:193–204. doi: 10.1016/0165-3806(88)90023-5. [DOI] [PubMed] [Google Scholar]
- Atz ME, Rollins B, Vawter MP. NCAM1 association study of bipolar disorder and schizophrenia: polymorphisms and alternatively spliced isoforms lead to similarities and differences. Psychiatr. Genet. 2007;17:55–67. doi: 10.1097/YPG.0b013e328012d850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachelin C, Zujovic V, Buchet D, Mallet J, Baron-Van Evercooren A. Ectopic expression of polysialylated neural cell adhesion molecule in adult macaque Schwann cells promotes their migration and remyelination potential in the central nervous system. Brain. 2010;133:406–420. doi: 10.1093/brain/awp256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader RA, Silvers AL, Zhang N. Polysialic acid-based micelles for encapsulation of hydrophobic drugs. Biomacromolecules. 2011;12:314–320. doi: 10.1021/bm1008603. [DOI] [PubMed] [Google Scholar]
- Barbeau D, Liang JJ, Robitaille Y, Quirion R, Srivastava LK. Decreased expression of the embryonic form of the neural cell adhesion molecule in schizophrenic brains. Proc. Natl. Acad. Sci. USA. 1995;92:2785–2789. doi: 10.1073/pnas.92.7.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfanti L. PSA-NCAM in mammalian structural plasticity and neurogenesis. Prog. Neurobiol. 2006;80:129–164. doi: 10.1016/j.pneurobio.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Bonfanti L, Theodosis DT. Polysialic acid and activity-dependent synapse remodeling. Cell Adh. Migr. 2009;3:43–50. doi: 10.4161/cam.3.1.7258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennaman LH, Maness PF. NCAM in neuropsychiatric and neurodegenerative disorders. Adv. Exp. Med. Biol. 2010;663:299–317. doi: 10.1007/978-1-4419-1170-4_19. [DOI] [PubMed] [Google Scholar]
- Brezun JM, Daszuta A. Serotonergic reinnervation reverses lesion-induced decreases in PSA-NCAM labeling and proliferation of hippocampal cells in adult rats. Hippocampus. 2000;10:37–46. doi: 10.1002/(SICI)1098-1063(2000)10:1<37::AID-HIPO4>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Burgess A, Wainwright SR, Shihabuddin LS, Rutishauser U, Seki T, Aubert I. Polysialic acid regulates the clustering, migration, and neuronal differentiation of progenitor cells in the adult hippocampus. Dev. Neurobiol. 2008;68:1580–1590. doi: 10.1002/dneu.20681. [DOI] [PubMed] [Google Scholar]
- Bushman J, Mishra B, Ezra M, Gul S, Schulze C, Chaudhury S, Ripoll D, Wallqvist A, Kohn J, Schachner M, Loers G. Tegaserod mimics the neurostimulatory glycan polysialic acid and promotes nervous system repair. Neuropharmacol. 2014;79:456–466. doi: 10.1016/j.neuropharm.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne B, Donohoe CG, O'Kennedy R. Sialic acids: carbohydrate moieties that influence the biological and physical properties of biopharmaceutical proteins and living cells. Drug Discov. Today. 2007;12:319–326. doi: 10.1016/j.drudis.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Cremer H, Lange R, Christoph A, Plomann M, Vopper G, Roes J, Brown R, Baldwin S, Kraemer P, Scheff S. Inactivation of the N-CAM gene in mice results in size reduction of the olfactory bulb and deficits in spatial learning. Nature. 1994;367:455–459. doi: 10.1038/367455a0. [DOI] [PubMed] [Google Scholar]
- Ditlevsen DK, Køhler LB, Pedersen MV, Risell M, Kolkova K, Meyer M, Berezin V, Bock E. The role of phosphatidylinositol 3-kinase in neural cell adhesion molecule-mediated neuronal differentiation and survival. J. Neurochem. 2003;84:546–556. doi: 10.1046/j.1471-4159.2003.01538.x. [DOI] [PubMed] [Google Scholar]
- Ditlevsen DK, Owczarek S, Berezin V, Bock E. Relative role of upstream regulators of Akt, ERK and CREB in NCAM- and FGF2-mediated signalling. Neurochem. Int. 2008;53:137–147. doi: 10.1016/j.neuint.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Durbec P, Cremer H. Revisiting the function of PSA-NCAM in the nervous system. Mol. Neurobiol. 2001;24:53–64. doi: 10.1385/MN:24:1-3:053. [DOI] [PubMed] [Google Scholar]
- El Maarouf A, Rutishauser U. Polysialic acid in adult brain plasticity. In: Fukuda M, Rutishauser U, Schnaar RL, editors. Neuroglycobiology. Oxford Univ. Press; London: 2005. pp. 39–57. [Google Scholar]
- Evans SV, Sigurskjold BW, Jennings HJ, Brisson JR, To R, Tse WC, Altman E, Frosch M, Weisgerber C, Kratzin HD, et al. Evidence for the extended helical nature of polysaccharide epitopes. The 2.8 A resolution structure and thermodynamics of ligand binding of an antigen binding fragment specific for alpha-(2-->8)-polysialic acid. Biochemistry. 1995;34:6737–6744. doi: 10.1021/bi00020a019. [DOI] [PubMed] [Google Scholar]
- Falconer RA, Errington RJ, Shnyder SD, Smith PJ, Patterson LH. Polysialyltransferase: a new target in metastatic cancer. Curr. Cancer Drug Targets. 2012;12:925–939. doi: 10.2174/156800912803251225. [DOI] [PubMed] [Google Scholar]
- Favaron M, Manev H, Alho H, Bertolino M, Ferret B, Guidotti A, Costa E. Gangliosides prevent glutamate and kainate neurotoxicity in primary neuronal cultures of neonatal rat cerebellum and cortex. Proc. Natl. Acad. Sci. USA. 1988;85:7351–7355. doi: 10.1073/pnas.85.19.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschini I, Desroziers E, Caraty A, Duittoz A. The intimate relationship of gonadotropin-releasing hormone neurons with the polysialylated neural cell adhesion molecule revisited across development and adult plasticity. Eur. J. Neurosci. 2010;32:2031–2041. doi: 10.1111/j.1460-9568.2010.07517.x. [DOI] [PubMed] [Google Scholar]
- Friesner RA, Murphy RB, Repasky MP, Frye LL, Greenwood JR, Halgren TA, Sanschagrin PC, Mainz DT. Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J. Med. Chem. 2006;49:6177–6196. doi: 10.1021/jm051256o. [DOI] [PubMed] [Google Scholar]
- Gilabert-Juan J, Varea E, Guirado R, Blasco-Ibáñez JM, Crespo C, Nácher J. Alterations in the expression of PSA-NCAM and synaptic proteins in the dorsolateral prefrontal cortex of psychiatric disorder patients. Neurosci. Lett. 2012;530:97–102. doi: 10.1016/j.neulet.2012.09.032. [DOI] [PubMed] [Google Scholar]
- Ghosh M, Tuesta LM, Puentes R, Patel S, Melendez K, El Maarouf A, Rutishauser U, Pearse DD. Extensive cell migration, axon regeneration, and improved function with polysialic acid-modified Schwann cells after spinal cord injury. Glia. 2012;60:979–992. doi: 10.1002/glia.22330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoriadis G, Fernandes A, Mital M, McCormack B. Polysialic acids: potential in improving the stability and pharmacokinetics of proteins and other therapeutics. Cell. Mol. Life Sci. 2001;57:1964–1969. doi: 10.1007/PL00000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt H, Dityatev A. Polysialic acid in brain development and synaptic plasticity. Top. Curr. Chem. 2015;(366):55–96. doi: 10.1007/128_2013_446. 2013 May 30 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Jakovcevski I, Siering J, Hargus G, Karl N, Hoelters L, Djogo N, Yin S, Zecevic N, Schachner M, Irintchev A. Close homologue of adhesion molecule L1 promotes survival of Purkinje and granule cells and granule cell migration during murine cerebellar development. J. Comp. Neurol. 2009;513:496–510. doi: 10.1002/cne.21981. [DOI] [PubMed] [Google Scholar]
- Jara HJ, Singh BB, Floden AM, Combs CK. Tumor necrosis factor alpha stimulates NMDA receptor activity in mouse cortical neurons resulting in ERK-dependent death. J. Neurochem. 2006;100:1407–1420. doi: 10.1111/j.1471-4159.2006.04330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungnickel J, Eckhardt M, Haastert-Talini K, Claus P, Bronzlik P, Lipokatic-Takacs E, Maier H, Gieselmann V, Grothe C. Polysialyltransferase overexpression in Schwann cells mediates different effects during peripheral nerve regeneration. Glycobiology. 2012;22:107–115. doi: 10.1093/glycob/cwr113. [DOI] [PubMed] [Google Scholar]
- Kanato Y, Kitajima K, Sato C. Direct binding of polysialic acid to a brain-derived neurotrophic factor depends on the degree of polymerization. Glycobiology. 2008;18:1044–1053. doi: 10.1093/glycob/cwn084. [DOI] [PubMed] [Google Scholar]
- Khan SN, Danishuddin M, Varshney B, Lal SK, Khan AU. Inhibition of N-terminal lysines acetylation and transcription factor assembly by epirubicin induced deranged cell homeostasis. PLoS One. 2012;7:e51850. doi: 10.1371/journal.pone.0051850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasraw M, Bell R, Dang C. Epirubicin: is it like doxorubicin in breast cancer? A clinical review. Breast. 2012;21:142–149. doi: 10.1016/j.breast.2011.12.012. [DOI] [PubMed] [Google Scholar]
- Kleene R, Mzoughi M, Joshi G, Kalus I, Bormann U, Schulze C, Xiao M-F, Dityatev A, Schachner M. NCAM-induced neurite outgrowth depends on binding of calmodulin to NCAM and on nuclear import of NCAM and fak fragments. J. Neurosci. 2010;30:10784–10798. doi: 10.1523/JNEUROSCI.0297-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Parkash J, Kataria H, Kaur G. Enzymatic removal of polysialic acid from neural cell adhesion molecule interrupts gonadotropin releasing hormone (GnRH) neuron-glial remodeling. Mol. Cell. Endocrinol. 2012;348:95–103. doi: 10.1016/j.mce.2011.07.040. [DOI] [PubMed] [Google Scholar]
- Lavdas AA, Franceschini I, Dubois-Dalcq M, Matsas R. Schwann cells genetically engineered to express PSA show enhanced migratory potential without impairment of their myelinating ability in vitro. Glia. 2006;53:868–878. doi: 10.1002/glia.20340. [DOI] [PubMed] [Google Scholar]
- Li J, Dai G, Cheng YB, Qi X, Geng MY. Polysialylation promotes neural cell adhesion molecule-mediated cell migration in a fibroblast growth factor receptor-dependent manner, but independent of adhesion capability. Glycobiology. 2011;21:1010–1018. doi: 10.1093/glycob/cwr020. [DOI] [PubMed] [Google Scholar]
- Lieberoth A, Splittstoesser F, Katagihallimath N, Jakovcevski I, Loers G, Ranscht B, Karagogeos D, Schachner M, Kleene R. Lewis(x) and alpha2,3-sialyl glycans and their receptors TAG-1, Contactin, and L1 mediate CD24-dependent neurite outgrowth. J. Neurosci. 2009;29:6677–6690. doi: 10.1523/JNEUROSCI.4361-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loers G, Chen S, Grumet M, Schachner M. Signal transduction pathways implicated in neural recognition molecule L1 triggered neuroprotection and neuritogenesis. J. Neurochem. 2005;92:1463–1476. doi: 10.1111/j.1471-4159.2004.02983.x. [DOI] [PubMed] [Google Scholar]
- Loers G, Saini V, Mishra B, Papastefanaki F, Lutz D, Chaudhury S, Ripoll DR, Wallqvist A, Gul S, Schachner M, Kaur G. Nonyloxytryptamine mimics polysialic acid and modulates neuronal and glial functions in cell culture. J. Neurochem. 2014;128:88–100. doi: 10.1111/jnc.12408. [DOI] [PubMed] [Google Scholar]
- Luo J, Bo X, Wu D, Yeh J, Richardson PM, Zhang Y. Promoting survival, migration, and integration of transplanted Schwann cells by over-expressing polysialic acid. Glia. 2011;59:424–34. doi: 10.1002/glia.21111. [DOI] [PubMed] [Google Scholar]
- Marino P, Norreel JC, Schachner M, Rougon G, Amoureux MC. A polysialic acid mimetic peptide promotes functional recovery in a mouse model of spinal cord injury. Exp. Neurol. 2009;219:163–174. doi: 10.1016/j.expneurol.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Magnani JL, Ernst B. Glycomimetic drugs - a new source of therapeutic opportunities. Discov. Med. 2009;8:247–252. [PubMed] [Google Scholar]
- Mehanna A, Mishra B, Kurschat N, Schulze C, Bian C, Loers G, Irintchev A, Schachner M. Polysialic acid glycomimetics promote myelination and functional recovery after peripheral nerve injury in mice. Brain. 2009;132:1449–1462. doi: 10.1093/brain/awp128. [DOI] [PubMed] [Google Scholar]
- Mehanna A, Jakovcevski I, Acar A, Xiao M, Loers G, Rougon G, Irintchev A, Schachner M. Polysialic acid glycomimetic promotes functional recovery and plasticity after spinal cord injury in mice. Mol. Ther. 2010;18:34–43. doi: 10.1038/mt.2009.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkonen M, Soininen H, Kälviänen R, Tapiola T, Ylinen A, Vapalahti M, Paljärvi L, Pitkänen A. Remodeling of neuronal circuitries in human temporal lobe epilepsy: increased expression of highly polysialylated neural cell adhesion molecule in the hippocampus and the entorhinal cortex. Ann. Neurol. 1998;44:923–934. doi: 10.1002/ana.410440611. [DOI] [PubMed] [Google Scholar]
- Mikkonen M, Soininen H, Tapiola T, Alafuzoff I, Miettinen R. Hippocampal plasticity in Alzheimer's disease: changes in highly polysialylated NCAM immunoreactivity in the hippocampal formation. Eur. J. Neurosci. 1999;11:1754–1764. doi: 10.1046/j.1460-9568.1999.00593.x. [DOI] [PubMed] [Google Scholar]
- Mishra B, von der Ohe M, Schulze C, Bian S, Makhina T, Loers G, Kleene R, Schachner M. Functional role of the interaction between polysialic acid and extracellular histone H1. J. Neurosci. 2010;30:12400–12413. doi: 10.1523/JNEUROSCI.6407-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monlezun S, Ouali S, Poulain DA, Theodosis DT. Polysialic acid is required for active phases of morphological plasticity of neurosecretory axons and their glia. Mol. Cell. Neurosci. 2005;29:516–524. doi: 10.1016/j.mcn.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Moudi M, Go R, Yien CY, Nazre M. Vinca alkaloids. Int. J. Prev. Med. 2013;4:1231–1235. [PMC free article] [PubMed] [Google Scholar]
- Mühlenhoff M, Rollenhagen M, Werneburg S, Gerardy-Schahn R, Hildebrandt H. Polysialic acid: versatile modification of NCAM, SynCAM 1 and neuropilin-2. Neurochem. Res. 2013;38:1134–1143. doi: 10.1007/s11064-013-0979-2. [DOI] [PubMed] [Google Scholar]
- Nagae M, Ikeda A, Hane M, Hanashima S, Kitajima K, Sato C, Yamaguchi Y. Crystal structure of anti-polysialic acid antibody single chain Fv fragment complexed with octasialic acid: insight into the binding preference for polysialic acid. J. Biol. Chem. 2013;288:33784–33796. doi: 10.1074/jbc.M113.496224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan X, Carubelli I, Stamatos NM. Sialidase expression in activated human T lymphocytes influences production of IFN-gamma. J. Leukoc. Biol. 2007;81:284–296. doi: 10.1189/jlb.1105692. [DOI] [PubMed] [Google Scholar]
- Ono S, Hane M, Kitajima K, Sato C. Novel regulation of fibroblast growth factor 2 (FGF2)-mediated cell growth by polysialic acid. J. Biol. Chem. 2012;287:3710–3722. doi: 10.1074/jbc.M111.276618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkash J, Kaur G. Neuronal-glial plasticity in gonadotrophin releasing hormone release in adult female rats: role of the polysialylated form of neural cell adhesion molecule. J. Endocrinol. 2005;186:397–409. doi: 10.1677/joe.1.06156. [DOI] [PubMed] [Google Scholar]
- Pekcec A, Mühlenhoff M, Gerardy-Schahn R, Potschka H. Impact of the PSA-NCAM system on pathophysiology in a chronic rodent model of temporal lobe epilepsy. Neurobiol. Dis. 2007;27:54–66. doi: 10.1016/j.nbd.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Rabbani-Chadegani A, Chamani E, Hajihassan Z. The effect of vinca alkaloid anticancer drug, vinorelbine, on chromatin and histone proteins in solution. Eur. J. Pharmacol. 2009;613:34–38. doi: 10.1016/j.ejphar.2009.04.040. [DOI] [PubMed] [Google Scholar]
- Rameix-Welti MA, Zarantonelli ML, Giorgini D, Ruckly C, Marasescu M, van der Werf S, Alonso JM, Naffakh N, Taha MK. Influenza A virus neuraminidase enhances meningococcal adhesion to epithelial cells through interaction with sialic acid-containing meningococcal capsules. Infect. Immun. 2009;77:3588–3595. doi: 10.1128/IAI.00155-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger S, Volkmann K, Köster RW. Polysialyltransferase expression is linked to neuronal migration in the developing and adult zebrafish. Dev. Dyn. 2008;237:276–285. doi: 10.1002/dvdy.21410. [DOI] [PubMed] [Google Scholar]
- Rutishauser U. Polysialic acid in the plasticity of the developing and adult vertebrate nervous system. Nat. Rev. Neurosci. 2008;9:26–35. doi: 10.1038/nrn2285. [DOI] [PubMed] [Google Scholar]
- Senkov O, Sun M, Weinhold B, Gerardy-Schahn R, Schachner M, Dityatev A. Polysialylated neural cell adhesion molecule is involved in induction of long-term potentiation and memory acquisition and consolidation in a fear-conditioning paradigm. J. Neurosci. 2006;26:10888–109898. doi: 10.1523/JNEUROSCI.0878-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senkov O, Tikhobrazova O, Dityatev A. PSA-NCAM: synaptic functions mediated by its interactions with proteoglycans and glutamate receptors. Int. J. Biochem. Cell Biol. 2012;44:591–595. doi: 10.1016/j.biocel.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Simova O, Irintchev A, Mehanna A, Liu J, Dihné M, Bächle D, Sewald N, Loers G, Schachner M. Carbohydrate mimics promote functional recovery after peripheral nerve repair. Ann. Neurol. 2006;6:430–437. doi: 10.1002/ana.20948. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strekalova H, Buhmann C, Kleene R, Eggers C, Saffell J, Hemperly J, Weiller C, Müller-Thomsen T, Schachner M. Elevated levels of neural recognition molecule L1 in the cerebrospinal fluid of patients with Alzheimer disease and other dementia syndromes. Neurobiol. Aging. 2006;27:1–9. doi: 10.1016/j.neurobiolaging.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Sumida M, Hane M, Yabe U, Shimoda Y, Pearce OM, Kiso M, Miyagi T, Sawada M, Varki A, Kitajima K, Sato C. Rapid trimming of cell surface polysialic acid (PolySia) by exovesicular sialidase triggers release of preexisting surface neurotrophin. J. Biol. Chem. 2015;290:13202–13214. doi: 10.1074/jbc.M115.638759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Nakayama J, Suzuki A, Angata K, Chen S, Sakai K, Hagihara K, Yamaguchi Y, Fukuda M. Polysialic acid facilitates tumor invasion by glioma cells. Glycobiology. 2005;15:887–894. doi: 10.1093/glycob/cwi071. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Mitoma J, Hosono M, Shiozaki K, Sato C, Yamaguchi K, Kitajima K, Higashi H, Nitta K, Shima H, Miyagi T. Sialidase NEU4 hydrolyzes polysialic acids of neural cell adhesion molecules and negatively regulates neurite formation by hippocampal neurons. J. Biol. Chem. 2012;287:14816–14826. doi: 10.1074/jbc.M111.324186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theis T, Mishra B, von der Ohe M, Loers G, Prondzynski M, Pless O, Blackshear PJ, Schachner M, Kleene R. Functional role of the interaction between polysialic acid and myristoylated alanine-rich C kinase substrate at the plasma membrane. J. Biol. Chem. 2013;288:6726–6742. doi: 10.1074/jbc.M112.444034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torregrossa P, Buhl L, Bancila M, Durbec P, Schafer C, Schachner M, Rougon G. Selection of poly-alpha 2,8-sialic acid mimotopes from a random phage peptide library and analysis of their bioactivity. J. Biol. Chem. 2004;279:30707–30714. doi: 10.1074/jbc.M403935200. [DOI] [PubMed] [Google Scholar]
- Tsoory M, Guterman A, Ritcher-Levin G. Exposure to stressors during juvenility disrupts development related alterations in the PSA-NCAM to NCAM expression ratio: potential relevance for mood and anxiety disorders. Neuropsychopharmacol. 2008;33:378–393. doi: 10.1038/sj.npp.1301397. [DOI] [PubMed] [Google Scholar]
- Varea E, Castillo-Gomez E, Gomez Climent MA, Blasco-Ibanez JM, Crespo C, Martinez-Guijarro FJ, Nacher J. Chronic antidepressant treatment induces contrasting patterns of synaptophysin and PSA-NCAM expression in different regions of adult rat telencephalon. Eur. Neuropsychopharmol. 2007;17:546–557. doi: 10.1016/j.euroneuro.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Varea E, Guirado R, Gilabert-Juan J, Marti U, Castillo-Gomez E, Blasco-Ibanez JM, Crespo C, Nacher J. Expression of PSA-NCAM and synaptic proteins in the amygdala of psychiatric disorder patients. J. Psychiatr. Res. 2012;46:189–197. doi: 10.1016/j.jpsychires.2011.10.011. [DOI] [PubMed] [Google Scholar]
- Wang S, Cesca F, Loers G, Schweizer M, Buck F, Benfenati F, Schachner M, Kleene R. Synapsin I is an oligomannose-carrying glycoprotein, acts as an oligomannose-binding lectin, and promotes neurite outgrowth and neuronal survival when released via glia-derived exosomes. J. Neurosci. 2011;31:7275–7290. doi: 10.1523/JNEUROSCI.6476-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DR, Zhang N, Silvers AL, Forstner MB, Bader RA. Synthesis and evaluation of cyclosporine A-loaded polysialic acid-polycaprolactone micelles for rheumatoid arthritis. Eur. J. Pharm. Sci. 2014;51:146–156. doi: 10.1016/j.ejps.2013.09.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.