Abstract
A growing number of environmental insults have been shown to induce epigenetic effects that persist across generations. For instance, paternal preconception exposures to ethanol or stress have independently been shown to exert such intergenerational effects. Since ethanol exposure is a physiological stressor that activates the hypothalamic-pituitary-adrenal (HPA) axis, we hypothesized that paternal ethanol exposure would impact stress responsivity of offspring. Adult male mice were exposed to chronic intermittent vapor ethanol or control conditions for 5 weeks before being mated with ethanol-naïve females to produce ethanol (E)- and control (C)-sired offspring. Adult male and female offspring were tested for plasma corticosterone (CORT) levels following acute restraint stress and the male offspring were further examined for stress-evoked 2-bottle choice ethanol drinking. Paternal ethanol exposure blunted plasma CORT levels following acute restraint stress selectively in male offspring; females were unaffected. In a stress-evoked ethanol-drinking assay, there was no effect of stress on ethanol consumption. However, C-sired males exhibited increased total fluid intake (polydipsia) in response to stress while E-sired males were resistant to this stress-induced phenotype. Taken together, these data suggest that paternal ethanol exposure imparts stress hyporesponsivity to male offspring.
Keywords: epigenetics, corticosterone, alcohol drinking, intergenerational, polydipsia, HPA axis
Introduction
Epigenetic inheritance has been gaining acceptance as a plausible explanation for transmission of complex behavioral traits across generations (Bohacek & Mansuy, 2013; Vassoler & Sadri-Vakili, 2014). Several studies have shown that paternal preconception exposures to stress (Dietz et al., 2011; Gapp et al., 2014; Rodgers, Morgan, Bronson, Revello, & Bale, 2013) or addictive substances (Byrnes, Johnson, Schenk, & Byrnes, 2012; Vassoler, Johnson, & Byrnes, 2013; Vassoler, White, Schmidt, Sadri-Vakili, & Pierce, 2013) can impart adaptive behavioral phenotypes to offspring. Similarly, various chronic paternal ethanol exposures induce intergenerational phenotypes (see Finegersh, Rompala, Martin, & Homanics, 2015 for review). Recently, we reported that exposing adult male mice to vapor ethanol over 5 weeks prior to mating with ethanol-naïve females conferred attenuated 2-bottle choice ethanol-drinking behavior and increased sensitivity to an anxiolytic dose of ethanol selectively in male offspring (Finegersh & Homanics, 2014). The neurobiological mechanisms underlying these effects of paternal ethanol on intergenerational ethanol-modulated behaviors are unknown.
One neurobiological system that is overactivated by ethanol abuse is the hypothalamus-pituitary-adrenal (HPA) axis endocrine stress response pathway. Ethanol acutely engages the HPA axis (Rivier, 2014) and the transition to ethanol dependence is characterized by sustained HPA-axis tolerance to ethanol and other stressors (Stephens & Wand, 2012). Interestingly, non-ethanol dependent individuals with a family history of alcoholism also show aberrant HPA-axis responsivity to stress or ethanol exposure (Dai, Thavundayil, & Gianoulakis, 2002; Evans, Greaves-Lord, Euser, Franken, & Huizink, 2012; Schuckit, 1988; Sorocco & Ferrell, 2006). While there is evidence that maternal ethanol exposure during gestation impacts HPA-axis responsivity in offspring (Govorko, Bekdash, Zhang, & Sarkar, 2012), it is not known whether preconception ethanol dependence causally impacts stress responsivity in the next generation. Notably, paternal chronic stress exposures have been found to blunt HPA-axis responsivity in offspring (Pisu et al., 2013; Rodgers et al., 2013). Therefore, we hypothesized that paternal ethanol similarly impacts intergenerational stress responsivity. As stress is a major risk factor for excessive and problematic ethanol drinking (Becker, Lopez, & Doremus-Fitzwater, 2011; Koob et al., 2014), this has significant implications for intergenerational ethanol-drinking behavior.
In the current study, we test the hypothesis that paternal ethanol exposure blunts HPA-axis responsivity to acute stress and alters stress-induced ethanol-drinking behaviors. Our findings suggest that paternal ethanol exposure prior to conception may have an underappreciated impact on stress responsivity in the next generation.
Materials and methods
All experiments were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh and were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Eight-week-old, ethanol-naïve, C57BL/6J (B6) and Strain 129S1/SvImJ mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and CD-1 mice were purchased from Charles River Laboratories (Burlington, MA). Unless otherwise specified, specific pathogen-free mice were group-housed in individually ventilated micro-isolater cages under 12-h light/dark cycles and had ad libitum access to food and water.
Paternal ethanol exposure
The paternal ethanol-exposure model was modified slightly from previously published methods (Finegersh & Homanics, 2014). Briefly, group-housed 8-week-old, B6 male mice were exposed to vapor ethanol (E) or room air control conditions (C) for 8 h/day (0900 to 1700), 5 days/week (M-F) for 5 weeks. Notably, this method has been optimized to produce stable blood ethanol concentrations (BECs) without the use of an alcohol dehydrogenase inhibitor. Immediately after the final ethanol exposures of week 5, each E- and C-exposed male was mated in the home cage of two 8-week-old Strain 129S1/SvIMJ ethanol-naïve female mice for 48 h. Breeding was limited to 48 h to minimize the influence of paternal ethanol exposure on maternal care. Strain 129S1/SvIMJ mice were chosen for mothers in accordance with our published paternal preconception ethanol-exposure breeding scheme (Finegersh & Homanics, 2014). Moreover, this breeding scheme was used in a relevant study which found that paternal stress experience impacts HPA-axis reactivity in offspring (Rodgers et al., 2013). Two different paternal ethanol cohorts were used to produce all offspring used in the current study. Cohort 1 contributed mice to the acute restraint and HPA-axis responsivity assay while cohort 2 contributed offspring for the stress-evoked ethanol-drinking experiments. Sires were weighed weekly and BECs were measured following the final exposure of each week. Ethanol in plasma was measured with an Analox Ethanol Analyzer (AM1, Analox Instruments, London, UK).
Acute restraint stress and measurement of plasma corticosterone (CORT)
Twelve-week-old male and female E- and C-sired offspring were subjected to a 15-min restraint stress exposure. All animals were tested between 3 and 5 h after lights-on (10:00 AM to 12:00 noon). Briefly, mice were removed from group housing and restrained in conical plastic tubes with several air hole perforations near the animal’s head and an opening for the tail. After the 15-min restraint, each mouse was returned to a single novel cage until the 90-min time point. Only one mouse was tested per group-housed cage to avoid pre-stressing any test animals. Tail blood (<10 μL) was collected with heparin-coated capillary tubes (Drummond, Broomall, PA) at time points 0, 15, 30, and 90 min from the onset of restraint. Blood samples were centrifuged for 10 min at 5000 rpm to separate plasma for measurement of CORT with an enzyme immunoassay (Enzo Life Sciences, Farmingdale, NY). Statistical analysis was performed by repeated-measures ANOVA and Bonferroni post hoc tests where appropriate. For both males and females in Experiment 1, mice were derived from 6 E-sired and 6 C-sired litters with no more than two mice selected per litter.
Chronic variable stress (CVS) with 2-bottle free-choice ethanol drinking
Mice were first acclimated to 2-bottle free-choice ethanol drinking. E- and C-sired male offspring were single-housed and habituated to two sipper tubes filled with water. After one week, one tube was filled with escalating ethanol concentrations of 2 and 4% for 4 days at each concentration, followed by 8% ethanol for the remainder of testing. Baseline 2-bottle free-choice ethanol drinking continued at 8% for 3 weeks before the onset of stress. Baseline drinking measures used in the study were obtained over the final 8 days preceding stress. Tube position was changed daily to control for side preference.
CVS
Following acclimation to 2-bottle free-choice drinking and the baseline 2-bottle choice ethanol-drinking period, mice were exposed to CVS and drinking behavior was measured. Over the 4-week CVS period, each week began with 3 consecutive days of the same unique stress exposure (described below). Each stress exposure occurred between 1400 and 1700 h during the light cycle. Two-bottle free-choice ethanol drinking was ongoing daily throughout and between each stress exposure period. Statistical analysis of 2-bottle choice drinking behavior was performed using two-way repeated-measures ANOVA and Bonferroni post hoc tests where appropriate. One outlier (defined here as mean ± 2 SD over at least 2 weeks of testing) was removed from the E-sired group. Male mice used in this test were derived from 6 E-sired litters and 6 C-sired litters with no more than two mice selected per litter.
CVS week 1: social-defeat stress
Test mice were introduced to the home cage of a 10-month-old outbred CD-1 male aggressor mouse. All aggressors were retired breeders and screened for reliable attack behavior prior to use in the CVS experiment using published methods (Golden, Covington, Berton, & Russo, 2011). Body weights for aggressors were at least 25% greater than those for each test mouse. After the aggressor mouse completed one 3–5-sec attack, the test mouse was isolated in a wire cup within the aggressor cage for another 30 min before being returned to the home cage, where 24-h 2-bottle choice drinking was continued. The social-defeat procedure was repeated for two additional days, each time with new pairings of aggressor and test mice.
CVS week 2: forced-swim stress
The forced-swim stressor was completed in a 12-cm diameter glass cylinder filled with 23 °C water. Each mouse was placed in the cylinder for a 5-min period. Following the test, mice were briefly dried and placed under a heating lamp for 3 min before being returned to their home cages.
CVS week 3: predator urine
The predator-odor stress test was performed in the home cage within a fume hood with the cage cover removed. Four single-housed mice were tested simultaneously for 15 min with two folded filter papers soaked with 1 mL of fox urine (Tink’s Red Fox-P ®, Covington, GA) placed just outside each cage, flanking each side.
CVS week 4: restraint stress
Restraint stress was conducted as described above except the stress lasted for 30 min and no tail blood samples were collected.
Results
Paternal ethanol exposure
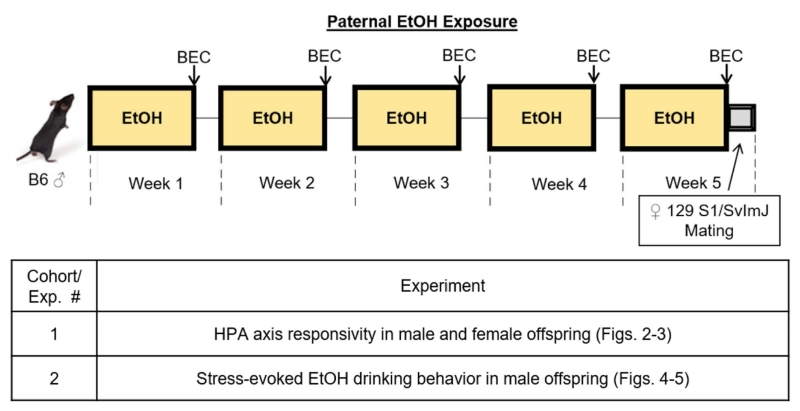
As illustrated in Fig. 1, B6 males were exposed to chronic intermittent ethanol vapor (E-sires) or room air conditions (C-sires) for 5 weeks. Two separate cohorts were used to produce offspring for each intergenerational experiment (Fig. 1).
Fig. 1. Paternal ethanol exposure and offspring experiments.
For paternal chronic exposure to ethanol, two cohorts of male mice were used in a chronic intermittent vapor ethanol paradigm, persisting over 5 weeks. Each week was comprised of 5 consecutive days of ethanol (E-sires) or room air (C-sires) exposures (8 h/day) followed by a 48-h abstinence period. Following the final exposure on week 5, males were bred with 129S1/SvlmJ ethanol-naïve females to produce male and female offspring from ethanol-exposed males (E-sired) or room air controls (C-sired). Male and female offspring were tested in Experiment 1 while only male offspring were examined in Experiment 2.
Experiment 1
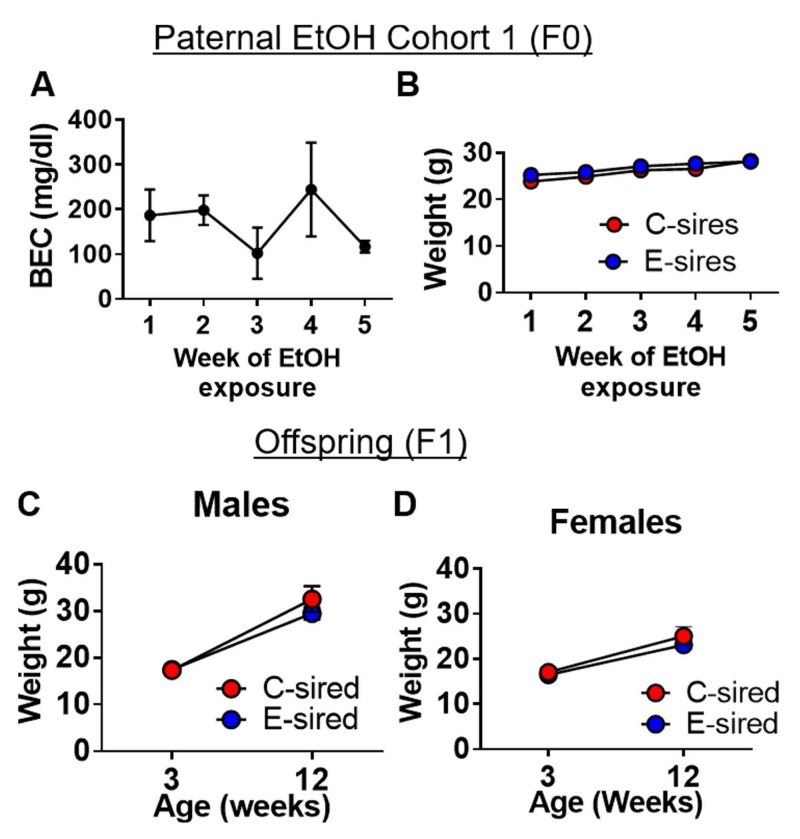
The average BEC across all weeks of paternal ethanol exposure was 169.7 ± 26.5 mg/dL (Fig. 2A). There was a significant effect of time on body weight for both E-sires and C-sires (F[4,20] = 33.8, p < 0.001, Fig. 2B). There was no effect for exposure and no exposure × time interaction. These results indicate that animals gained weight and there was no difference in weight between E-sires and C-sires over the course of the exposure period.
Fig. 2. Parental ethanol exposure cohort 1.
Cohort 1 produced male and female offspring for Experiment 1. (A) BECs of E-sires were measured at the end of each of the 5 weeks of ethanol vapor exposure. (B) Body weight did not differ between groups (n = 3/4 C-sired/E-sired). Moreover, neither (C) male offspring (n = 6/9, C-sired/E-sired) nor (D) female offspring (n = 6/7, C-sired/E-sired) body weights were altered by paternal ethanol at weaning or time of Experiment 1 testing. Data presented as mean ± SEM. In panel B, SEMs are obscured by the symbols.
E-sired and C-sired male offspring were analyzed for differences in body weight at weaning and at the time of testing. This analysis revealed significant effects of age (F[1,13] = 117.6, p < 0.01, Fig. 2C) with no effect of sire and no age × sire interaction; similarly, analysis of female offspring body weights revealed a significant effect for age (F[1,11] = 64.8; p < 0.001, Fig. 2D), but no effect of sire and no age × sire interaction. Thus, the E-sired male and female offspring examined in Experiment 1 did not differ in body weight vs. C-sired controls.
Paternal ethanol blunted acute HPA-axis responsivity selectively in male offspring
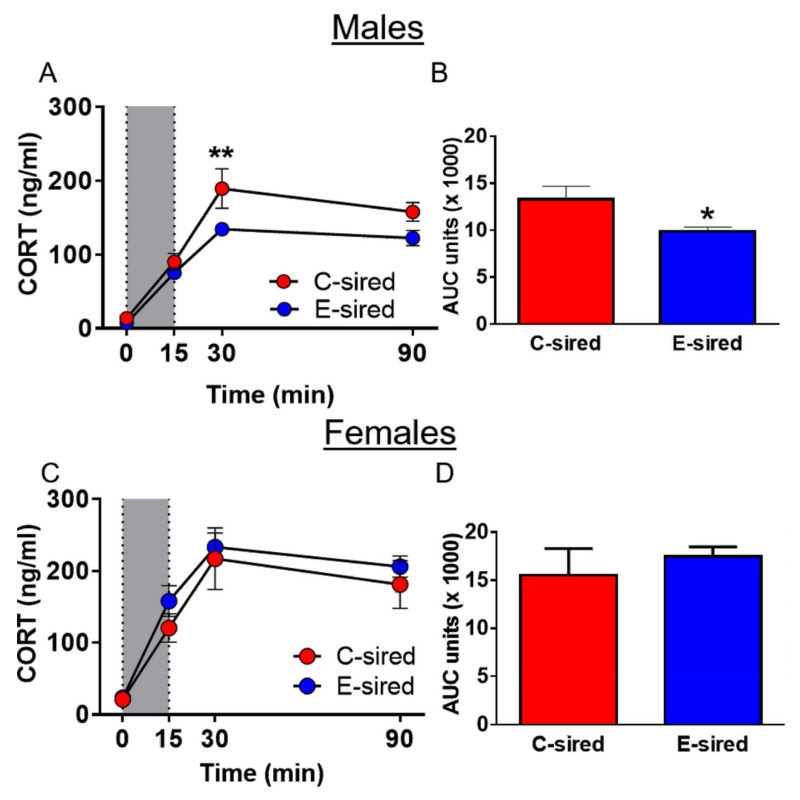
Plasma CORT levels were measured in response to an acute 15-min restraint stress at time points 0, 15, 30, and 90 min from the onset of restraint. For male offspring, there was a significant effect of time (F[3,39] = 110.8, p < 0.0001), sire (F[1,13] = 7.4, p < 0.05), and a time × sire interaction (F[3,39] = 2.9, p < 0.05) on CORT levels. Bonferroni post hoc tests revealed that E-sired CORT levels were reduced at the 30-min time point (p < 0.01) vs. C-sired male controls (Fig. 3A). Moreover, area under the curve (AUC) analysis revealed a reduced CORT response in E-sired vs. C-sired males (t[13] = 2.7, p < 0.05, Fig. 3B). Testing of female offspring revealed a significant effect of time (F[3,33] = 54.7, p < 0.001, Fig. 3C), but no effect of sire, no time × sire interaction, and no difference in AUC (Fig. 3D).
Fig. 3. Paternal ethanol exposure blunts HPA-axis reactivity to acute restraint stress selectively in male offspring.
(A) The CORT response to 15 min of restraint stress (shaded column) was significantly blunted in E-sired males (n = 8) at the 30-min time point vs. C-sired males (n = 6). (B) Area under the curve (AUC) analysis revealed a significant reduction in the CORT response to stress in E-sired males vs C-sired males. (C) E-sired (n = 7) and C-sired (n = 6) females revealed no significant differences in CORT levels following acute restraint stress. (D) There was no difference in the CORT response to acute restraint between E-sired and C-sired females as measured using AUC analysis. Data presented as mean ± SEM. **=p < 0.01, *=p <0.05.
Experiment 2
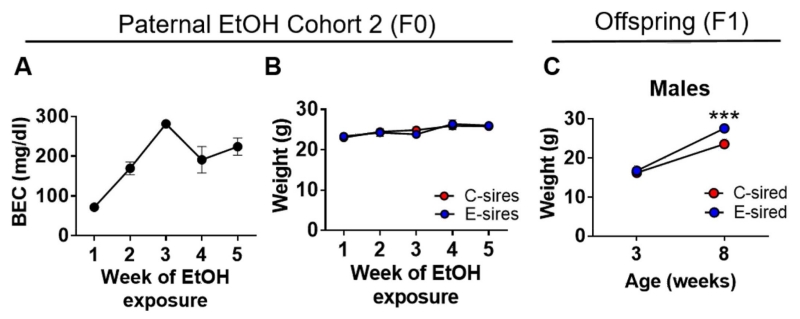
The average BEC across all weeks of paternal ethanol exposure was 187.6 ± 34.6 mg/dL (Fig. 4A). There was a significant effect of time on body weight (F[4,28] = 16.7, p < 0.001, Fig. 4B), but no effect of exposure or the exposure × time interaction. Male offspring of these mice were examined for body weight at weaning and at adulthood adults before testing. There was a significant effect of age (F[1,18] = 268.1, p < 0.001), sire (F[1,18] = 12.40, p < 0.01) and an age × sire interaction (F[1,18] = 9.8, p < 0.01). Bonferroni post hoc analysis revealed a significant increase in the body weight of E-sired males vs. C-sired males at adulthood (8 weeks) (p < 0.001, Fig. 4C). Thus, E-sired male offspring used in Experiment 2 exhibited increased body weight, consistent with our previous findings (Finegersh & Homanics, 2014).
Fig. 4. Paternal ethanol exposure cohort 2.
Paternal ethanol cohort 2 produced male offspring for Experiment 2. (A) BECs of E-sires were measured at the end of each of the 5 weeks of ethanol vapor exposure. (B) There was no effect of paternal ethanol exposure on body weight (n = 5/4, C-sired/E-sired). (C) E-sired male offspring (n = 10) showed increased body weight vs. C-sired males (n = 10). Data presented as mean ± SEM. ***=p < 0.001.
Paternal ethanol exposure did not affect stress-evoked ethanol-drinking behavior in male offspring
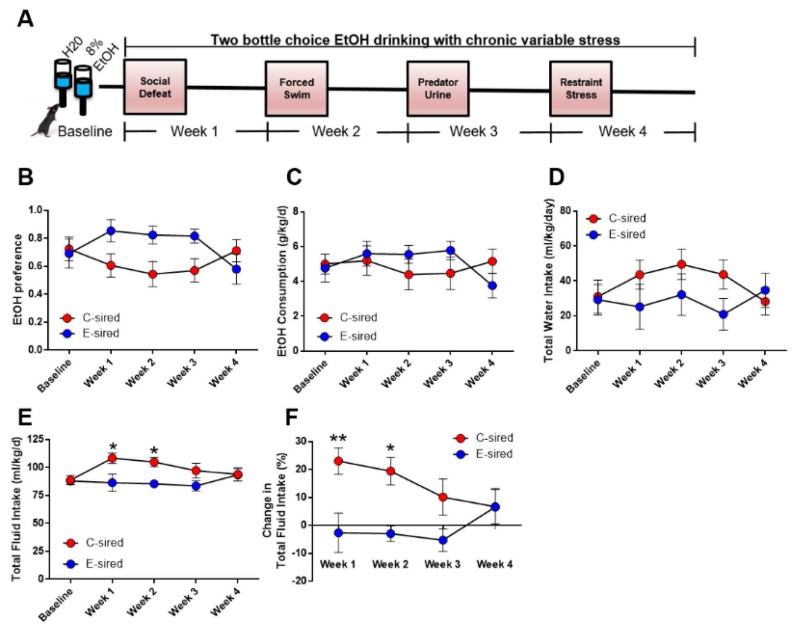
Given our finding in Experiment 1 that E-sired male offspring exhibited blunted CORT responses to acute stress, we hypothesized that E-sired males would be resistant to stress-evoked ethanol-drinking behavior. To test this, we stabilized baseline 2-bottle choice 8% ethanol drinking in E- and C-sired male offspring over one month before exposing them to a 4-week CVS paradigm while concurrently measuring ethanol-drinking behavior (Fig. 5A). For ethanol preference, there was a significant CVS × sire interaction (F[4,68] = 3.4, p < 0.05, Fig. 5B). However, Bonferroni post hoc tests did not reveal significant differences between E-sired vs. C-sired males during any week of testing. Moreover, there was no effect of sire, CVS, or CVS × sire on ethanol consumption or water intake (Figs. 5C and 5D).
Fig. 5. Paternal ethanol exposure does not impact intergenerational stress-evoked ethanol drinking, but prevents stress-induced polydipsia in male offspring.
(A) After one month of 2-bottle choice ethanol drinking at 8% (w/vol), ethanol drinking was measured throughout 4 weeks of CVS, entailing three consecutive exposures to a unique stressor that changed each week. (B) E-sired males (n = 9) exhibited no change in ethanol preference vs. C-sired mice (n = 10) during CVS Weeks 1, 2, and 3. (C & D) There was no significant effect of CVS on ethanol consumption or water intake. (E) E-sired males showed reduced total fluid intake vs. C-sired males at weeks 1 and 2. (F) E-sired males were resistant to an increase in total fluid intake from baseline levels (% of baseline) at CVS weeks 1 and 2. Data presented as mean ± SEM. *=p < 0.05, **=p < 0.01.
E-sired males are resistant to stress-induced excessive fluid intake
For total fluid intake, there was a significant CVS × sire interaction (F[4,68] = 3.4, p < 0.01) and no effect of sire or CVS alone (Fig. 5E). Bonferroni post hoc test revealed significantly increased total fluid intake in C-sired males vs. E-sired males during week 1 and week 2 (p < 0.05). Moreover, there was a significant effect of sire (F[1,17] = 7.3, p < 0.05) and a CVS × sire interaction (F[3,51] = 3.6, p < 0.05) for change in total fluid intake from baseline (Fig. 5F). Bonferroni post hoc test revealed that C-sired males showed a greater increase in total fluid intake during weeks 1 (p < 0.01) and 2 (p < 0.05) vs. E-sired males.
Discussion
Paternal preconception ethanol exposure has previously been shown to have various effects on offspring including reduced testosterone levels (Abel, 1989), decreased grooming (Abel, 1991), reduced organ weight (Abel, 1993), increased immobility time in a forced-swim test (Abel & Bilitzke, 1990), thickening of the cerebral cortex (Jamerson, Wulser, & Kimler, 2004), impaired working memory (Kim et al., 2014) and, most recently, alterations in ethanol sensitivity and drinking preference (Finegersh & Homanics, 2014). In the current study, our results add to this literature with the novel finding that paternal ethanol exposure imparts blunted stress responsivity to adult male offspring. Paternal ethanol exposure resulted in reduced plasma CORT in response to acute restraint stress and resistance to stress-induced excessive fluid intake. Altered HPA-axis function is implicated in the etiology of alcohol-use disorder and numerous other psychiatric conditions (Clarke et al., 2008; Pariante & Lightman, 2008). Thus, the observed effects of paternal ethanol exposure on stress responsivity in the next generation may have broad human health implications.
The hypothesis that paternal ethanol exposure would alter HPA-axis function in offspring was based on two important lines of evidence. First, the HPA axis is strongly implicated in the neuropathophysiology of alcoholism, and deficits are frequently observed in individuals with a family history of alcoholism (Dai et al., 2002; Schuckit, Gold, & Risch, 1987; Stephens & Wand, 2012). Secondly, paternal pharmacological and behavioral stress exposures have been shown to impact stress-related behavior in offspring (Crews et al., 2012; Dietz et al., 2011; Gapp et al., 2014; Pisu et al., 2013). Indeed, our finding that paternal ethanol exposure blunts HPA-axis responsivity in male offspring is remarkably similar to a recent study that found the same blunted CORT phenotype following acute restraint stress in offspring of fathers exposed to a 6-week CVS paradigm (Rodgers et al., 2013). Ethanol is a potent physiological stressor, activating the HPA axis in rodents during both forced ethanol exposures as well as during voluntary ethanol drinking (reviewed in Rivier, 2014). Therefore, it is possible that the stress associated with chronic ethanol exposure may be important for the intergenerational phenotypes observed in E-sired male offspring. Additional studies are needed to explore whether paternal stress exposures, such as the chronic stress paradigm used in Rodgers et al. (2013), can similarly impact intergenerational ethanol-drinking behavior and behavioral sensitivity to ethanol.
Notably, the effects of paternal ethanol exposure on the CORT response to acute stress were sex-specific, consistent with our previous findings for ethanol-related phenotypes (Finegersh & Homanics, 2014). Indeed, intergenerational and transgenerational studies have found sex-specific effects of paternal preconception exposures across generations in adult rodents (Franklin et al., 2010; Vassoler, White, et al., 2013). However, it does not appear that intergenerationally altered HPA-axis responsivity is an entirely sex-specific phenomenon, as paternal chronic variable stress blunts the CORT response to acute restraint stress in both male and female offspring (Rodgers et al., 2013).The complex epigenetic mechanisms underlying sex-specific vs. sex-independent intergenerational phenotypes remain to be elucidated. Furthermore, it is important to note that the female estrus cycle influences HPA-axis activity (Kalil, Leite, Carvalho-Lima, & Anselmo-Franci, 2013), perhaps masking any observable deficits. Future experiments will need to control for hormonal variance to determine whether sex-specific blunted HPA-axis responsivity is dependent on sex differences in gonadal signaling.
Acute administration of CORT promotes ethanol-drinking behavior, and inhibition of CORT by adrenalectomy decreases ethanol consumption (Fahlke, Hard, & Hansen, 1996). Therefore, we hypothesized that E-sired male offspring, having shown blunted HPA-axis responsivity, would exhibit attenuated stress-evoked ethanol-drinking behavior. Surprisingly, we found that E-sired males did not differ in ethanol-drinking preference or consumption vs. C-sired males. However, there was a large difference between E-sired and C-sired males in total fluid intake. Specifically, C-sired males exhibited a robust polydipsia-like (excessive fluid intake) phenotype during weeks 1 and 2 of CVS that was absent in E-sired male offspring.
Although stress is generally assumed to increase ethanol drinking, rodent studies have produced inconsistent results (Becker et al., 2011). In the present study, C-sired control mice did not show a significant stress-evoked increase in ethanol-drinking behavior. The relationship between stress and ethanol drinking is complex and several variables including type of stress, strain, sex, and time course can ultimately influence the direction and magnitude of ethanol-drinking behavior (Spanagel, Noori, & Heilig, 2014). Future experiments will aim to validate and employ a specific model for stress-escalated ethanol drinking, such as chronic stress preceding ethanol consumption (Lopez, Doremus-Fitzwater, & Becker, 2011; Rodriguez-Arias et al., 2014).
Increased total fluid intake following chronic stress such as social defeat is an adjunctive phenotype in mice referred to as stress-induced polydipsia (i.e., excessive thirst) (Golden et al., 2011). Stress-induced polydipsia is a hallmark behavioral phenotype observed in male mice following chronic and subchronic social defeat (Golden et al., 2011; Goto et al., 2014). CORT inhibition has been shown to block polydipsia-induced drinking behavior, suggesting an important role for HPA-axis activity (Strekalova et al., 2011). Therefore, resistance to polydipsia-like drinking also supports the conclusion that E-sired males are hyporesponsive to stress. Between Experiments 1 and 2, we have shown that at the endocrine and behavioral levels and following acute (restraint) or chronic (4 weeks of CVS) stress, paternal ethanol exposure imparts stress hyporesponsivity phenotypes to adult male offspring.
It is worth discussing certain limitations to the current study. It is possible that maternal care differed between groups based on altered maternal-paternal interactions during E-sire and C-sire mating (Mashoodh, Franks, Curley, & Champagne, 2012). Indeed, altered maternal care can influence HPA-axis responsivity (Champagne & Meaney, 2001). However, the breeding period was limited to just two nights and there were no differences in E-sired and C-sired male body weights at the time of weaning, suggesting that maternal care was not significantly different between E-sired and C-sired litters. Another limitation is that breeding occurred immediately after ethanol exposure. Thus, it is unclear whether the intergenerational effects of paternal ethanol exposure are specific to preconception chronic ethanol exposure and not in part dependent on acute intoxication or withdrawal at the time of mating. Therefore, future experiments will delay the period between paternal ethanol exposure and breeding.
Previously we reported that paternal ethanol increased body weight selectively in male offspring (Finegersh & Homanics, 2014). While we replicated this phenotype in Experiment 2, we did not observe this effect in the male offspring of Experiment 1. These conflicting results may be due to the differences in BECs between the two exposures and need to be further examined. Notably though, in spite of these weight differences between offspring cohorts, we still observed stress hyporesponsivity phenotypes in each E-sired male cohort. Thus, the finding of blunted HPA-axis responsivity in Experiment 1 (in the absence of significant weight differences) supports the interpretation that resistance to stress-induced polydipsia-like behavior observed in E-sired males of Experiment 2 is unlikely to depend on increased body weight.
We also did not observe decreased ethanol-drinking preference in E-sired males as previously published (Finegersh & Homanics, 2014). This may be a result of differences in the drinking paradigm employed between the two studies. Baseline measures were taken here after 3 weeks of habituation to unlimited access 2-bottle choice drinking with 8% ethanol and water. In the study by Finegersh and Homanics (2014), drinking behavior was measured without prior habituation. Moreover, differences were no longer detectable at 12% ethanol which was measured 2 weeks into ethanol drinking. Thus, this may reflect that baseline differences do not persist with extended unlimited-access 2-bottle choice drinking. Additional experiments are needed to fully characterize the reduced ethanol-drinking phenotype in E-sired males, including the use of additional drinking models such as the limited-access drinking in the dark paradigm commonly employed to model binge-like ethanol consumption in mice (Thiele & Navarro, 2014).
How might paternal ethanol exposure impart stress hyporesponsivity and an altered ethanol phenotype in offspring? Multiple studies have found changes in DNA methylation (Dias & Ressler, 2014; Govorko et al., 2012) or chromatin regulation (Vassoler, White, et al., 2013) in sperm that are associated with altered behaviors in the next generation. Interestingly, two recent studies have implicated sperm RNAs in germ line transmission of intergenerational stress-related behaviors (Gapp et al., 2014; Rodgers et al., 2013). Remarkably, the intergenerational effects were recapitulated by injecting RNAs isolated from the sperm of stressed males into fertilized oocytes (Gapp et al., 2014; Rodgers, Morgan, Leu, & Bale, 2015), suggesting this epigenetic mechanism may underlie intergenerational transmission of stress-related behaviors. It is conceivable that paternal ethanol may similarly alter epigenetic marks in sperm to causally impact stress responsivity in the next generation.
In summary, we have shown that paternal ethanol exposure confers stress hyporesponsivity to male offspring. Alterations in stress responsivity were observed at both the endocrine and behavioral levels. Identifying heritable mechanisms that mediate vulnerability and resilience to chronic stress has major implications for the development of novel prevention and treatment strategies for psychiatric disease and addiction. Therefore, future studies will aim to identify ethanol-induced epigenetic factors in sperm that confer altered stress-related phenotypes to male offspring.
Highlights.
Paternal alcohol blunts acute HPA axis responsivity selectively in male offspring
Paternal alcohol has no effect on stress-evoked ethanol drinking in male offspring
Paternal alcohol prevents stress-induced behavior (polydipsia-like fluid intake) in male offspring
Acknowledgments
We would like to thank Carolyn Ferguson and Daniel Farrell for providing expert technical assistance. This work was supported by grants from the National Institute on Alcohol Abuse and Alcoholism awarded to GEH (R37AA010422) and AF (AA021632).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel EL. Paternal and maternal alcohol consumption: effects on offspring in two strains of rats. Alcoholism: Clinical and Experiemental Research. 1989;13:533–541. doi: 10.1111/j.1530-0277.1989.tb00373.x. [DOI] [PubMed] [Google Scholar]
- Abel EL. Paternal alcohol consumption affects grooming response in rat offspring. Alcohol. 1991;8:21–23. doi: 10.1016/0741-8329(91)91168-2. [DOI] [PubMed] [Google Scholar]
- Abel EL. Rat offspring sired by males treated with alcohol. Alcohol. 1993;10:237–242. doi: 10.1016/0741-8329(93)90042-m. [DOI] [PubMed] [Google Scholar]
- Abel EL, Bilitzke P. Paternal alcohol exposure: paradoxical effect in mice and rats. Psychopharmacology (Berl) 1990;100:159–164. doi: 10.1007/BF02244399. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF, Doremus-Fitzwater TL. Effects of stress on alcohol drinking: a review of animal studies. Psychopharmacology (Berl) 2011;218:131–156. doi: 10.1007/s00213-011-2443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohacek J, Mansuy IM. Epigenetic inheritance of disease and disease risk. Neuropsychopharmacology. 2013;38:220–236. doi: 10.1038/npp.2012.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes JJ, Johnson NL, Schenk ME, Byrnes EM. Cannabinoid exposure in adolescent female rats induces transgenerational effects on morphine conditioned place preference in male offspring. Journal of Psychopharmacology. 2012;26:1348–1354. doi: 10.1177/0269881112443745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne F, Meaney MJ. Like mother, like daughter: evidence for non genomic transmission of parental behavior and stress responsivity. Progress in Brain Research. 2001;133:287–302. doi: 10.1016/s0079-6123(01)33022-4. [DOI] [PubMed] [Google Scholar]
- Clarke TK, Treutlein J, Zimmermann US, Kiefer F, Skowronek MH, Rietschel M, et al. HPA-axis activity in alcoholism: examples for a gene-environment interaction. Addiction Biology. 2008;13:1–14. doi: 10.1111/j.1369-1600.2007.00084.x. [DOI] [PubMed] [Google Scholar]
- Crews D, Gillette R, Scarpino SV, Manikkam M, Savenkova MI, Skinner MK. Epigenetic transgenerational inheritance of altered stress responses. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:9143–9148. doi: 10.1073/pnas.1118514109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X, Thavundayil J, Gianoulakis C. Response of the hypothalamic-pituitary-adrenal axis to stress in the absence and presence of ethanol in subjects at high and low risk of alcoholism. Neuropsychopharmacology. 2002;27:442–452. doi: 10.1016/S0893-133X(02)00308-1. [DOI] [PubMed] [Google Scholar]
- Dias BG, Ressler KJ. Parental olfactory experience influences behavior and neural structure in subsequent generations. Nature Neuroscience. 2014;17:89–96. doi: 10.1038/nn.3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz DM, Laplant Q, Watts EL, Hodes GE, Russo SJ, Feng J, et al. Paternal transmission of stress-induced pathologies. Biological Psychiatry. 2011;70:408–414. doi: 10.1016/j.biopsych.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans BE, Greaves-Lord K, Euser AS, Franken IH, Huizink AC. The relation between hypothalamic-pituitary-adrenal (HPA) axis activity and age of onset of alcohol use. Addiction. 2012;107:312–322. doi: 10.1111/j.1360-0443.2011.03568.x. [DOI] [PubMed] [Google Scholar]
- Fahlke C, Hård E, Hansen S. Facilitation of ethanol consumption by intracerebroventricular infusions of corticosterone. Psychopharmacology (Berl) 1996;127:133–139. doi: 10.1007/BF02805986. [DOI] [PubMed] [Google Scholar]
- Finegersh A, Homanics GE. Paternal alcohol exposure reduces alcohol drinking and increases behavioral sensitivity to alcohol selectively in male offspring. PLoS One. 2014;9:e99078. doi: 10.1371/journal.pone.0099078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegersh A, Rompala GR, Martin DI, Homanics GE. Drinking beyond a lifetime: New and emerging insights into paternal alcohol exposure on subsequent generations. Alcohol. 2015;49:461–470. doi: 10.1016/j.alcohol.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TB, Russig H, Weiss IC, Gräff J, Linder N, Michalon A, et al. Epigenetic transmission of the impact of early stress across generations. Biological Psychiatry. 2010;68:408–415. doi: 10.1016/j.biopsych.2010.05.036. [DOI] [PubMed] [Google Scholar]
- Gapp K, Jawaid A, Sarkies P, Bohacek J, Pelczar P, Prados J, et al. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nature Neuroscience. 2014;17:667–669. doi: 10.1038/nn.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SA, Covington HE, 3rd, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nature Protocols. 2011;6:1183–1191. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto T, Kubota Y, Tanaka Y, Iio W, Moriya N, Toyoda A. Subchronic and mild social defeat stress accelerates food intake and body weight gain with polydipsia-like features in mice. Behavioural Brain Research. 2014;270:339–348. doi: 10.1016/j.bbr.2014.05.040. [DOI] [PubMed] [Google Scholar]
- Govorko D, Bekdash RA, Zhang C, Sarkar DK. Male germline transmits fetal alcohol adverse effect on hypothalamic proopiomelanocortin gene across generations. Biological Psychiatry. 2012;72:378–388. doi: 10.1016/j.biopsych.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamerson PA, Wulser MJ, Kimler BF. Neurobehavioral effects in rat pups whose sires were exposed to alcohol. Brain Research. Developmental Brain Research. 2004;149:103–111. doi: 10.1016/j.devbrainres.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Kalil B, Leite CM, Carvalho-Lima M, Anselmo-Franci JA. Role of sex steroids in progesterone and corticosterone response to acute restraint stress in rats: sex differences. Stress. 2013;16:452–460. doi: 10.3109/10253890.2013.777832. [DOI] [PubMed] [Google Scholar]
- Kim P, Choi CS, Park JH, Joo SH, Kim SY, Ko HM, et al. Chronic exposure to ethanol of male mice before mating produces attention deficit hyperactivity disorder-like phenotype along with epigenetic dysregulation of dopamine transporter expression in mouse offspring. Journal of Neuroscience Research. 2014;92:658–670. doi: 10.1002/jnr.23275. [DOI] [PubMed] [Google Scholar]
- Koob GF, Buck CL, Cohen A, Edwards S, Park PE, Schlosburg JE, et al. Addiction as a stress surfeit disorder. Neuropharmacology. 2014;76(Pt B):370–382. doi: 10.1016/j.neuropharm.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, Doremus-Fitzwater TL, Becker HC. Chronic social isolation and chronic variable stress during early development induce later elevated ethanol intake in adult C57BL/6J mice. Alcohol. 2011;45:355–364. doi: 10.1016/j.alcohol.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashoodh R, Franks B, Curley JP, Champagne FA. Paternal social enrichment effects on maternal behavior and offspring growth. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(Suppl 2):17232–17238. doi: 10.1073/pnas.1121083109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends in Neurosciences. 2008;31:464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Pisu MG, Garau A, Olla P, Biggio F, Utzeri C, Dore R, et al. Altered stress responsiveness and hypothalamic-pituitary-adrenal axis function in male rat offspring of socially isolated parents. Journal of Neurochemistry. 2013;126:493–502. doi: 10.1111/jnc.12273. [DOI] [PubMed] [Google Scholar]
- Rivier C. Role of hypothalamic corticotropin-releasing factor in mediating alcohol-induced activation of the rat hypothalamic-pituitary-adrenal axis. Frontiers in Neuroendocrinology. 2014;35:221–233. doi: 10.1016/j.yfrne.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL. Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. The Journal of Neuroscience. 2013;33:9003–9012. doi: 10.1523/JNEUROSCI.0914-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers AB, Morgan CP, Leu NA, Bale TL. Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:13699–13704. doi: 10.1073/pnas.1508347112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Arias M, Navarrete F, Blanco-Gandia MC, Arenas MC, Bartoll-Andrés A, Aguilar MA, et al. Social defeat in adolescent mice increases vulnerability to alcohol consumption. Addiction Biology. 2014;21:87–97. doi: 10.1111/adb.12184. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Reactions to alcohol in sons of alcoholics and controls. Alcoholism: Clinical and Experimental Research. 1988;12:465–470. doi: 10.1111/j.1530-0277.1988.tb00228.x. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Gold E, Risch C. Plasma cortisol levels following ethanol in sons of alcoholics and controls. Archives of General Psychiatry. 1987;44:942–945. doi: 10.1001/archpsyc.1987.01800230022005. [DOI] [PubMed] [Google Scholar]
- Sorocco KH, Ferrell SW. Alcohol use among older adults. The Journal of General Psychology. 2006;133:453–467. doi: 10.3200/GENP.133.4.453-467. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Noori HR, Heilig M. Stress and alcohol interactions: animal studies and clinical significance. Trends in Neurosciences. 2014;37:219–227. doi: 10.1016/j.tins.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Stephens MA, Wand G. Stress and the HPA axis: role of glucocorticoids in alcohol dependence. Alcohol Research. 2012;34:468–483. doi: 10.35946/arcr.v34.4.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strekalova T, Couch Y, Kholod N, Boyks M, Malin D, Leprince P, et al. Update in the methodology of the chronic stress paradigm: internal control matters. Behavioral and Brain Functions. 2011;7:9. doi: 10.1186/1744-9081-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, Navarro M. “Drinking in the dark” (DID) procedures: a model of binge-like ethanol drinking in non-dependent mice. Alcohol. 2014;48:235–241. doi: 10.1016/j.alcohol.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassoler FM, Johnson NL, Byrnes EM. Female adolescent exposure to cannabinoids causes transgenerational effects on morphine sensitization in female offspring in the absence of in utero exposure. Journal of Psychopharmacology. 2013;27:1015–1022. doi: 10.1177/0269881113503504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassoler FM, Sadri-Vakili G. Mechanisms of transgenerational inheritance of addictive-like behaviors. Neuroscience. 2014;264:198–206. doi: 10.1016/j.neuroscience.2013.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassoler FM, White SL, Schmidt HD, Sadri-Vakili G, Pierce RC. Epigenetic inheritance of a cocaine-resistance phenotype. Nature Neuroscience. 2013;16:42–47. doi: 10.1038/nn.3280. [DOI] [PMC free article] [PubMed] [Google Scholar]