Abstract
Bone marrow stromal cells (BMSCs) have been reported to exert potential neuroprotective properties in models of neurotrauma, although precise mechanisms underlying their benefits are poorly understood. Despite this lack of knowledge, several clinical trials have been initiated using these cells. To determine whether local mechanisms mediate BMSC neuroprotective actions, we grafted allogeneic BMSCs to sites of severe, compressive spinal cord injury (SCI) in Sprague-Dawley rats. Cells were administered 48 h after the original injury. Additional animals received allogeneic MSCs that were genetically modified to secrete brain-derived neurotrophic factor (BDNF) to further determine whether a locally administered neurotrophic factor provides or extends neuroprotection. When assessed 2 months post-injury in a clinically relevant model of severe SCI, BMSC grafts with or without BDNF secretion failed to improve motor outcomes. Thus, allogeneic grafts of BMSCs do not appear to act through local mechanisms, and future clinical trials that acutely deliver BMSCs to actual sites of injury within days are unlikely to be beneficial. Additional studies should address whether systemic administration of BMSCs alter outcomes from neurotrauma.
Key words: : bone marrow stromal cells, brain-derived neurotrophic factor, neuroprotection, severe spinal cord compression
Introduction
There are no effective therapies for spinal cord injury (SCI). Several previous reports indicate that bone marrow stromal cell (BMSC) transplantation into experimental models of SCI generate modest locomotor recovery and enhanced tissue sparing.1–10 Based on such reports, clinical trials of BMSC transplantation for SCI have already been conducted11,12 and several more are in progress (Clinicaltrials.gov). Yet relatively little is known regarding mechanisms underlying potential therapeutic effects of BMSC. Modification of systemic immune responses,13–15 trophic secretion,16–18 or, less likely, stem cell-related mechanisms19 have been cited as possible mediators of beneficial outcomes.
If BMSCs act through neuroprotective mechanisms to improve outcomes after SCI, the only practical human donor cell source would consist of allogeneic BMSCs. That is, cells would need to be rapidly administered to influence secondary injury mechanisms that are targets of neuroprotection, and autologous cells could not be prepared within a time frame of less than several weeks. Human BMSCs are routinely banked and human leukocyte antigen (HLA)-typed for human use20 as a means of reconstituting bone marrow in patients who have undergone marrow ablation, and constitute an available cell source for human clinical trials of neuroprotection. Of note, the medical literature indicates that allogeneic bone marrow cells transplanted into humans typically survive for a period of only several weeks, and then host endogenous bone marrow cells reconstitute the hematopoietic system.21–23 The transient nature of allogeneic BMSC transplant survival presents an opportunity for application to neurotrauma: allogeneic BMSC grafts could be used to transiently deliver candidate therapeutics to sites of SCI. For example, delivery of brain-derived neurotrophic factor (BDNF) to sites of SCI promotes axonal growth24–26 and, in some reports, improves functional outcomes27,28; however long-term secretion of BDNF results in spasticity25,29,30 or pain.29,31 Genetic manipulation of BMSCs to overexpress and secrete BDNF could represent a means of transient therapeutic delivery if the grafted cells eventually die, providing a sub-acute period of growth factor delivery to enable neuroprotection while potentially avoiding adverse consequences of long-term expression.
In the present study, we addressed two topics related to BMSC transplantation after SCI. First, we determined whether BMSC transplantation achieves neuroprotection via local cellular mechanisms by implanting allogeneic donor cells directly into sites of severe compressive SCI. We hypothesized that if cells secrete neuroprotective molecules, they will reduce lesion size and improve functional outcomes. Second, taking advantage of the transient survival of allogeneic BMSC transplants, we examined whether BMSCs genetically engineered to over-express BDNF can further augment tissue sparing and functional recovery. To enhance clinical relevance, we used a model of severe T8 spinal cord compression because most human injuries are severe in degree32,33; BMSCs have not previously been tested in models of severe rodent SCI that are most likely to predict benefit in clinical trials,34 and surprisingly, BMSCs have gone on to human clinical trials without such supportive rodent data. We now report that subacute implants of allogeneic BMSC grafts to sites of severe SCI fail to improve motor or autonomic outcomes, suggesting that their local administration is unlikely to merit clinical translation.
Methods
Experimental design
Fifty-four adult female Sprague-Dawley rats (10–14 weeks old, 200–250 g) were experimental subjects. This strain of rat is outbred, so any non-self cell transplants would eventually be rejected by the immune system. Three groups of animals were studied. All groups underwent T8 severe forceps compression lesions (n = 12 animals per group) followed 48 h later by one of three possible treatments: Group 1 received implants of allogeneic BMSCs into the lesion site, genetically engineered to express green fluorescent protein (GFP); Group 2 received implants of allogeneic BMSCs into the lesion site, genetically engineered to express human BDNF and GFP; and Group 3 received lesions only followed by injection of PBS 48 h later. Animals then underwent weekly Basso, Beattie, and Bresnahan (BBB) and sensory testing and were sacrificed 10 weeks post-lesion. In addition, four animals underwent T8 severe compression lesions and were sacrificed 48 h later for histological assessment of the lesion cavity, to understand the nature of the lesion cavity at the time of cell grafting in other animals. To access the survival of BMSC transplants through early post-grafting time-points, when they are hypothesized to enhance neuroprotection, 24 animals received BMSC transplants expressing either BDNF or GFP (n = 4 per group) at 48 h post-compression, and were sacrificed 3 days, 7 days, or 14 days post-transplant (5 days, 9 days, or 16 days post-compression, respectively).
BMSC isolation and cell preparation
Rat primary BMSCs were isolated according to the method of Azizi and colleagues.35 Briefly, adult Sprague-Dawley rats were deeply anesthetized with a combination (2 mL/kg) of ketamine (25 mg/mL), xylazine (1.3 g/mL), and acepromazine (0.25 mg/mL), and the tibias and femurs were dissected free of overlying muscle tissue. The ends of each bone were removed and 5 mL of BMSC culture media was injected into the central canal of the bone to extrude the marrow. Bone marrow aspirates were gently triturated and plated in BMSC media at a density of 5 × 105 cells/cm2. Cells were maintained at 37°C in a mixture of 20% O2 and 5% CO2. Media was changed every day for 5 days and at that time stromal cells had adhered to the flask. BMSCs were expanded to passage 3, then infected with lentivirus expressing either green fluorescent protein (GFP) or BDNF-ires-GFP, as described previously.7 GFP+ cells were separated by flow cytometry to >95% purity. BMSCs were further expanded in BMSC media contained Minimum Essential Medium alpha +2 mM GlutaMAX +1× penicillin/streptomycin (all from Life Technologies), supplemented with 20% heat-inactivated fetal bovine serum (Hyclone). Prior to grafting, BDNF secretion was determined by two-site BDNF enzyme-linked immunosorbent assay (ELISA).36 Basal levels of BDNF secretion from control BMSCs was determined by ELISA to be 34 ng/106 cells/day for GFP cells and 177 ng/106 cells/day for BDNF-ires-GFP infected cells.
In vitro characterization
For BMSC immunocytochemistry, passage 7 cells were incubated with alexa-647 conjugated mouse anti-rat CD90 (5 ug/mL; BioLegend), for 30 min at 37°C. Cells were then washed with phosphate-buffered saline (PBS) and fixed in 3.7% formaldehyde for 20 min at 37°C. Following PBS washes, cells were permeabilized in 100% methanol for 10 min at room temperature. Cells were washed and blocked in 5% goat serum for 30 min at room temperature. Rabbit anti-GFP (0.5 μg/mL; Life Technologies) was added and the cells incubated overnight at 4°C. Cells were washed and incubated in a mixture of alexa-488 conjugated goat anti-rabbit (2 μg/mL; Life Technologies) and 4′,6-diamidino-2-phenylindole (DAPI; 1 μg/mL; Sigma-Aldrich), for 1 h at room temperature. Cells were washed and immediately imaged.
For flow cytometry, cells were dissociated with 1 mM EDTA (Life Technologies) and incubated with alexa-647 conjugated mouse anti-rat CD90, CD29, CD45 and CD11b (10 μg/ml: BioLegend) for 20 min at 37°C. Cells were washed in PBS and fixed in a 2% formaldehyde/PBS solution on ice. Background staining was determined with cells incubated without antibodies or cells incubated with alexa-647 mouse Fc fragments. At least 50,000 singlet events were analyzed on a FACSCANTO II flow cytometry system (BD Biosciences).
Surgery
National Institutes of Health (NIH) guidelines for laboratory animal care and safety were strictly followed. All surgery was performed under anesthesia with a combination (2 mL/kg) of ketamine (25 mg/mL), xylazine (1.3 g/mL), and acepromazine (0.25 mg/mL).
For spinal cord compression lesions, blunt dissection was used to expose the T8 and T9 dorsal processes. Without laminectomy, a blunted, non-graded #55 forceps was lowered between the T8 and T9 vertebral columns and the forceps tips were entirely closed for 1 sec from the lateral aspects of the spinal cord. The muscles were sutured and the skin stapled. Rats were given ampicillin (20 mg/kg, subcutaneously) and banamine (1 mg/kg, subcutaneously), and the bladder was manually expressed twice a day.
Two days post-compression, the animals were re-anesthetized with isoflurane gas (instead of ketamine, to reduce potential toxicity from repeated ketamine exposure and to enable more rapid awakening) placed in a spinal stereotax (Kopf) and a laminectomy of the T8 vertebra was performed. Immediately prior to transplantation, BMSCs (passage 7) were concentrated by centrifugation at 200 g for 4 min and re-suspended in 5 μL of PBS. The lesion was identified by a visible hematoma and a total volume of 5 μL (500,000 cells) was injected directly into the lesion site using a Picospritzer II (General Valve). Cells were injected into three points (one point at midline and two points 0.5 mm lateral of midline). Control animals received injections of PBS containing no cells. The muscles were sutured, the skin stapled, and the rats were maintained for in a 37°C incubator until awake and alert. Rats were given daily subcutaneous injections for 3 days of a 3 mL solution containing lactated Ringer's, ampicillin (20 mg/kg), and banamine (1 mg/kg). Bladder was manually expressed twice daily until reflex voiding returned (10–14 days post-compression).
Behavior testing
Prior to SCI, all rats were acclimated to the tester and the testing apparatus. Rat bladders were voided, then 10 min later the animals were placed in the open field. Two independent observers blinded to group identity assessed locomotor activity for 4 min and the open-field score was determined using the 21-point BBB locomotor rating scale.37 No significant differences were observed when scoring the right and left hind paws at any time-point (analysis of variance [ANOVA], p = 0.9); therefore, the values were averaged.
Tissue preparation and immunohistochemistry
Rats were transcardially perfused with 0.9% NaCl2, followed by ice cold 4% paraformaldehyde (PFA). Spinal cords were removed and post-fixed overnight in 4% PFA, then transferred to 30% sucrose in 0.1 M phosphate buffer and cryoprotected for 3 days at 4°C. A 1 cm block of spinal cord tissue containing the lesion site was embedded in tissue cryoprotectant solution (Thermo Fisher) and frozen on dry ice. Horizontal tissue sections were cut on a cryostat set to 16 μm and thaw-mounted directly onto superfrost plus slides. A subset of slides were stained with 0.25% cresyl violet to label Nissl bodies or immunolabeled, as described below.
Fluorescent immunolabeling
For glial acidic fibrillary protein (GFAP) and/or green fluorescent protein (GFP) double labeling, slides were place in a slide staining rack (Thermo Fisher), rinsed with Tris-buffered saline (TBS), permeabilized with 100% methanol for 20 min, and rinsed with TBS. Non-specific antibody binding was then blocked for 1 h in a solution of 5% horse serum in 0.25% triton-x in TBS (TBS++). Slides were incubated in a mixture of mouse anti-GFAP (1 μg/mL; Millipore,) and rabbit anti-GFP (0.5 μg/mL; Life Technologies) overnight in TBS++. Following TBS washes, slides were incubated in a mixture of donkey anti-rabbit alexa-488 and donkey anti-mouse alexa-594 (4 μg/mL; Life Technologies) in TBS++ for 1 h at room temperature. All nuclei were counterstained with DAPI (1 μg/mL; Sigma-Aldrich) for 5 min. Slides were air-dried and cover-slipped with Fluoromount G (Southern Biotech).
Light level immunolabeling
For GFAP immunolabeling, mouse anti-GFAP (0.2 μg/mL; Millipore) was diluted in TBS++ and added to each slide and incubated overnight at 4°C. Following TBS washes, slides were incubated for 1 h in biotinylated horse anti-mouse immunoglobulin G (IgG; 7.5 μg/mL; Vector Labs) diluted in TBS++. Following TBS washes, slides were incubated for 1 h in ABC reagent (1:100 dilution; Vector Labs). Slides were transferred to a coplin jar and washed 3× in TBS. The horseradish peroxidase (HRP) reaction product was allowed to react for 10 min with a solution of 0.25 mg/mL 3,3'-diaminobenzidine (DAB), 0.04% NiCl2, 1.2% H2O2 in TBS. Slides were rinsed in TBS and dehydrated with a series of increasing ethanol concentrations to citrosolv. Finally, slides were cover-slipped with Entellan mounting solution.
For BDNF immunolabeling, slides were placed in a coplin jar and washed in TBS. Slides were then transferred to a 0.01M Tris-HCl, pH 9 solution pre-warmed to 80°C and incubated for 30 min. Slides were allowed to return to room temperature, then fixed in a solution of 2% paraformaldehyde/0.2% parabenzoquinone in 0.1 M PO4 for 5 min. Slides were washed in TBS, blocked in TBS++ for 1 h, then incubated for four nights at 4°C in a mixture of rabbit anti-BDNF (0.1 μg/mL; Chicago Proteintech) in TBS++. Following TBS washes, slides were incubated for 2 h in biotinylated horse anti-rabbit IgG (7.5 μg/mL; Vector Labs) diluted in TBS++. Following TBS washes, slides were incubated for 1 h in ABC reagent (1:100 dilution; Vector Labs). Slides were transferred to a coplin jar and washed 3× in TBS. The HRP reaction product was allowed to react for 10 min with a solution of 0.25 mg/mL DAB, 0.04% NiCl2, 1.2% H2O2 in TBS. Slides were rinsed in TBS and dehydrated with a series of increasing ethanol concentrations to citrosolv. Finally, slides were cover-slipped with Entellan mounting solution.
Imaging
Both light level and fluorescent images were acquired with an Olympus AX70 microscope equipped with an Optronics Microfire A/R digital camera. Images were imported into Photoshop CS2 and minimally altered for brightness and contrast.
Lesion size quantification
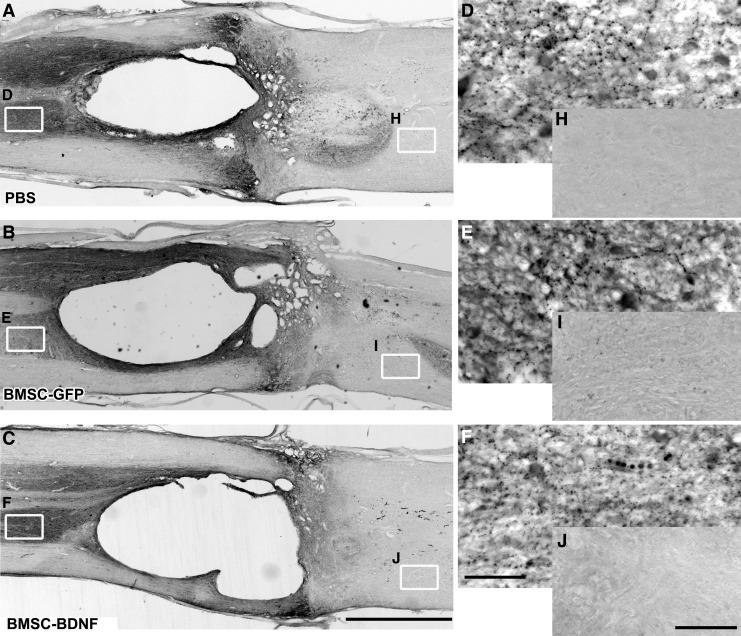
GFAP immunolabeled horizontal sections containing the entire lesion site were used for estimation of lesion size. Lack of GFAP immunolabeling in six sections spaced every 300 μm was outlined using StereoInvestigator software (MicroBrightField). Live images were received from an Olympus Optical OLY-200 video camera mounted on an Olympus Optical BX60 microscope fitted with a Ludl 99S000 XYZ motorized stage. The lesion volume index was calculated by summing the lesion area from all six sections. The mean lesion volume index was calculated for each group.
Glial scar quantification
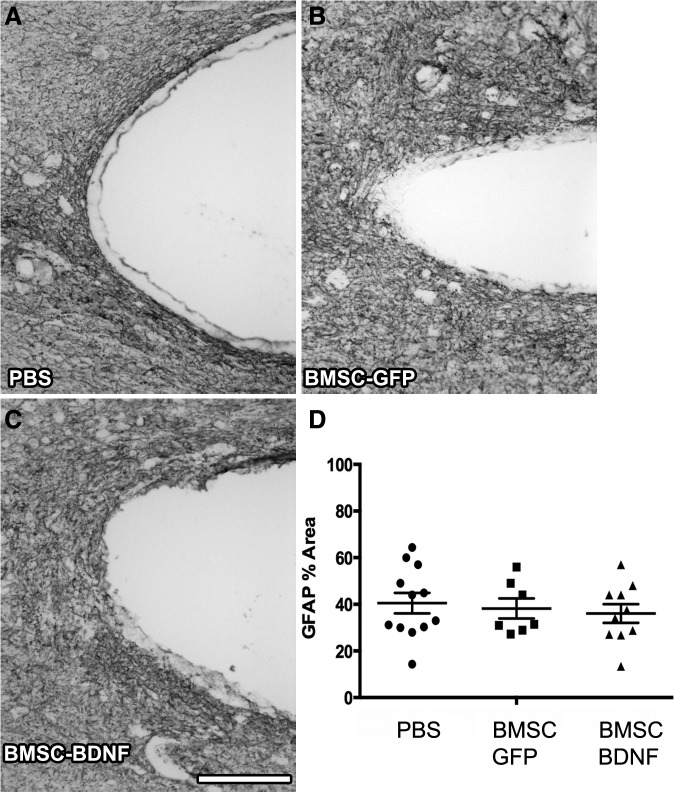
Images magnified 20 ×were acquired of GFAP immunolabeled horizontal sections containing the rostral edge of the lesion cavity. Images were imported into ImageJ (NIH) and a region of interest outlined 100 μm from the lesion cavity. Percent of area occupied by GFAP immunolabeling was quantified in six serial sections spaced 300 μm apart. The mean percent of GFAP area was calculated for each group.
Statistical analysis
In all quantification procedures observers were blinded to the nature of the experimental manipulations. For histological outcomes, multiple group comparisons were made using one-way ANOVA with a designated significance level of 95%. Post hoc differences were tested by Fisher's least square difference. For behavioral outcomes, multiple group comparisons were made by repeated measures two-way ANOVA (group vs. time, BBB and sensory threshold testing) or one-way ANOVA (conditioned place preference testing) with a significance level of 95% followed by Fisher's post hoc test. Data are presented as mean ± standard error of the mean (SEM). The final numbers of animals per group that were assessed were n = 7 in the BMSC-GFP group, n = 10 in the BMSC-BDNF group, and n = 12 in the PBS control group. Animal numbers were reduced from the original n = 12 per group for the following reasons: five died in the BMSC-GFP group due to excessive weight loss and bladder infections, and two died in the BMSC-BDNF group due to bladder infections.
Results
In vitro characterization of bone marrow stromal cells
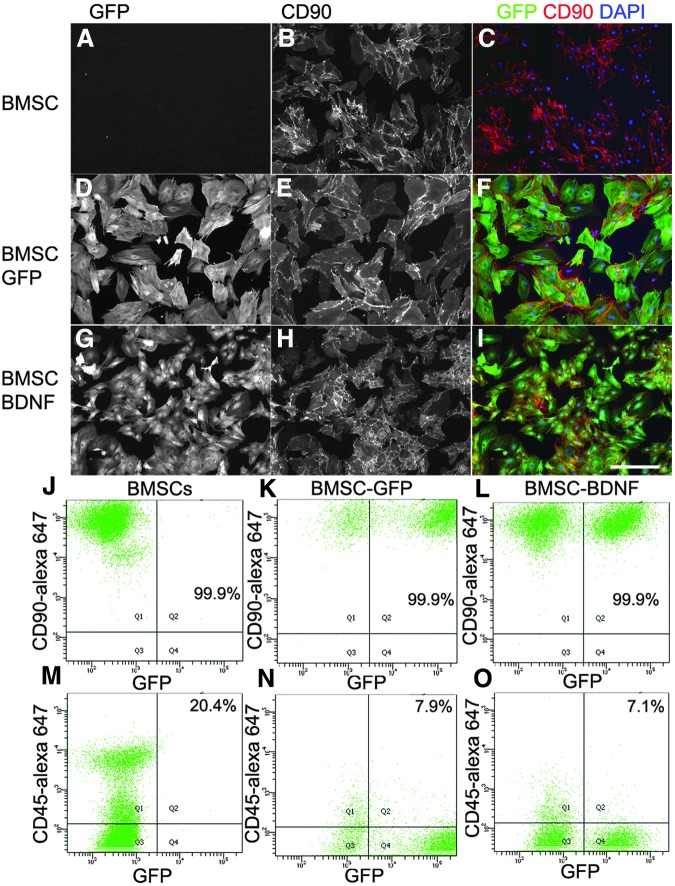
Immunocytochemistry of non-transduced, GFP-expressing and BDNF-expressing BMSCs showed similar cell surface expression of CD90 (thy 1.1), a characteristic marker of rat BMSCs (Fig. 1).38 Flow cytometry demonstrate that neither lentiviral infection nor BDNF expression changed the percentage of cells immunolabeled by the BMSCs markers CD90 or CD29 (see Table 1). With the media used in this study, the presence of <20% CD45+ and/or CD11b+ cells is to be expected.38
FIG. 1.
In vitro characterization of bone marrow stromal cells (BMSCs). (A-I) Immunolabeling of BMSCs for green fluorescent protein (GFP) and CD90 with or without infection of GFP or brain-derived neurotrophic factor (BDNF)-ires-GFP lentivirus. Flow cytometry quantification of CD90 (J-L) and CD45 (M-O) expression on BMSCs with or without lentiviral infection. Scale bar: 100 μm. DAPI, 4′,6-diamidino-2-phenylindole. Color image is available online at www.liebertpub.com/neu
Table 1.
FACS Analysis of In Vitro BMSCs
| Sample | CD90+ | CD29+ | CD45+ | CD11b+ |
|---|---|---|---|---|
| BMSC | 99.9% | 99.9% | 20.4% | 27.0% |
| BMSC-GFP | 99.9% | 99.9% | 7.9% | 13.8% |
| BMSC-BDNF | 99.9% | 99.9% | 7.1% | 17.9% |
FACS,; BMSC, bone marrow stromal cell; GFP, green fluorescent protein; BDNF, brain-derived neurotrophic factor.
Minimal tissue sparing 2 days following severe thoracic compression lesion
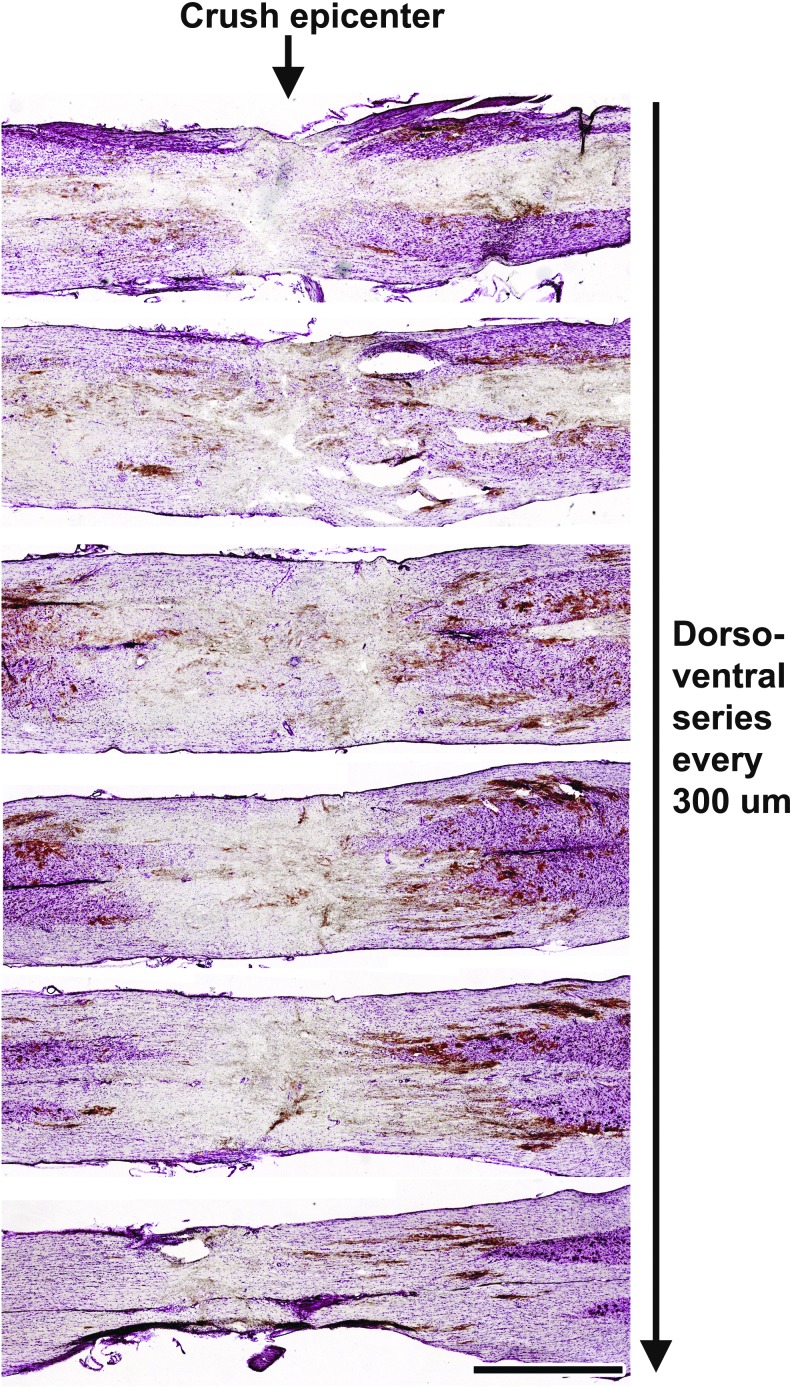
To examine the nature of the T8 severe compression site at the time of BMSC transplantation, we examined four animals 2 days post-lesion. At this time-point, the lesion site contained cellular debris and a hematoma within the lesion epicenter (Fig. 2). A cystic lesion cavity had not yet formed, as expected, but there was attenuation of parenchymal density that appeared compatible with an ability to graft cells into the lesion site, confirmed at later time-points (see below). Most white matter appeared directly injured by the lesion, with possible minimal sparing in only the most ventrally located sections (Fig. 2).
FIG. 2.
Morphology of lesion site at time of grafting. Nissl-stained series of horizontal sections 2 days following severe spinal cord compression between the T8 and T9 vertebrae. Images show the morphology of the lesion epicenter prior to bone marrow stromal cell injections. Scale bar: 1 mm. Color image is available online at www.liebertpub.com/neu
Bone marrow stromal cells survive and fill the lesion cavity 3 days post-grafting
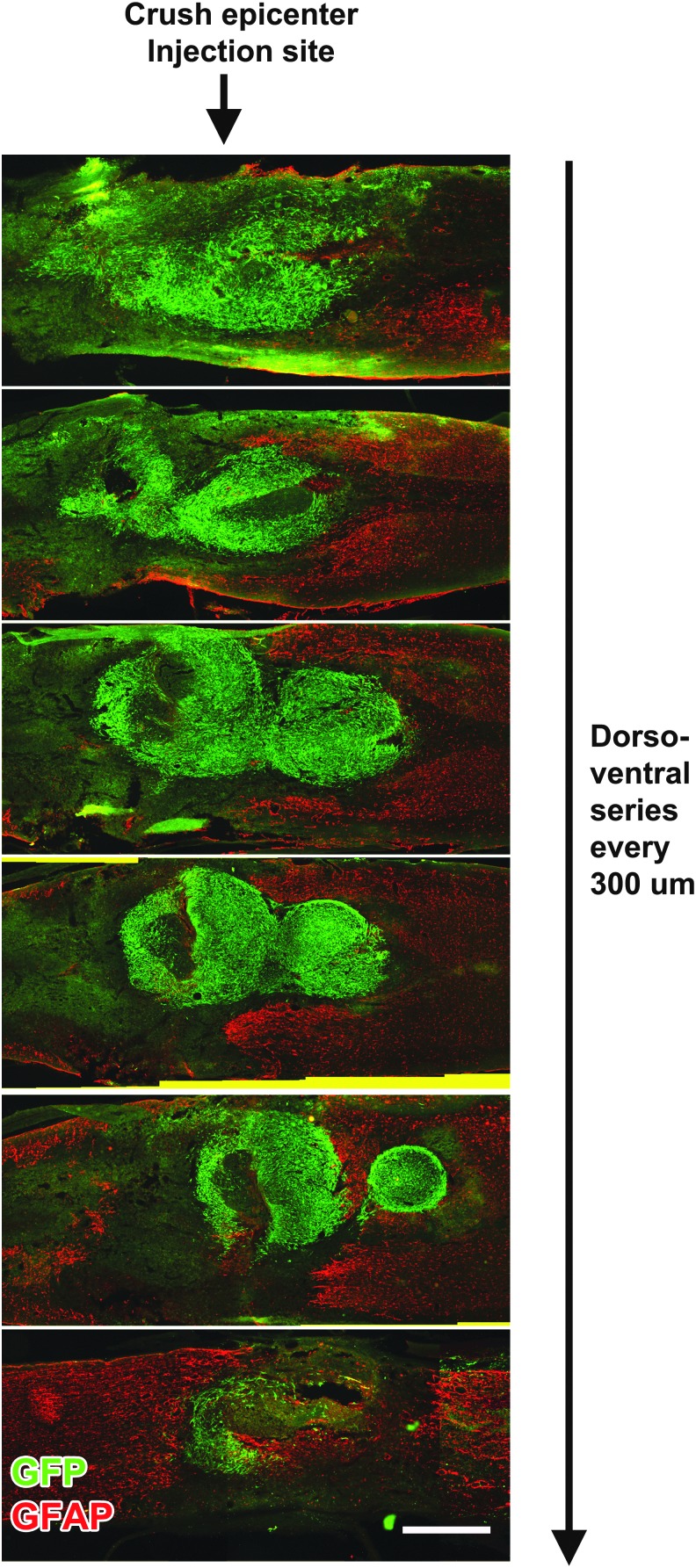
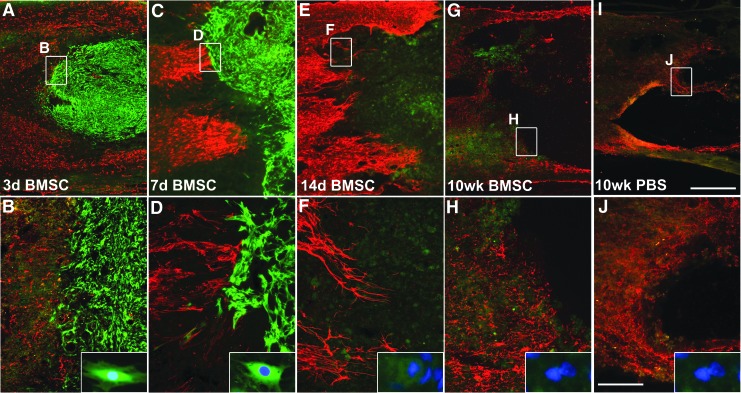
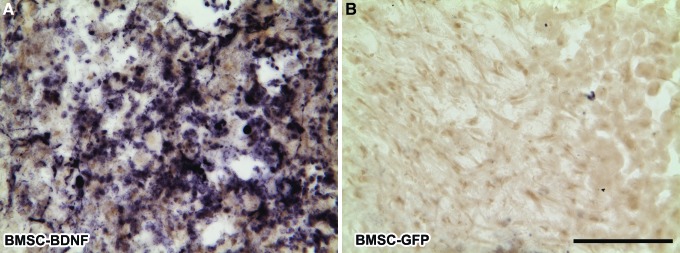
When examined 3 days post-grafting (5 days post-lesion), GFP-immunoreactive BMSCs extensively filled the lesion site (Fig. 3). BMSCs remained present in the lesion site at 7 days post-grafting (9 days post-lesion). However, at 14 days and 10 weeks post-grafting, allogeneic BMSCs were no longer present within the lesion site (Fig. 4); this finding is consistent with previous reports that allogeneic BMSC transplants do not survive for prolonged time periods in vivo.39 BDNF immunolabeling confirmed expression of BDNF by grafted cells at this 3-day time-point, indicating that BDNF was locally available through early time-points post-injury (Fig. 5), when it might support neuroprotection. Thus, allogeneic BMSCs survive early grafting and express BDNF within subacute lesion sites, but are not present at prolonged time-points. This experimental paradigm thereby generates a transient cellular fill of the lesion site and, in the case of cells genetically engineered to secrete BDNF, allows transient delivery of the growth factor BDNF.
FIG. 3.
Survival of bone marrow stromal cells (BMSCs) 3 days post-grafting. Horizontal series, spaced 300 μm apart, immunolabeled for green fluorescent protein (GFP)+ BMSCs and glial acidic fibrillary protein (GFAP). Animals were injected with GFP+ BMSCs 2 days following severe compression and perfused 3 days later. Scale bar: 1 mm. Color image is available online at www.liebertpub.com/neu
FIG. 4.
Survival of bone marrow stromal cells (BMSCs) within potential neuroprotective window. Immunolabeling of grafts of green fluorescent protein (GFP)-expressing BMSCs implanted 2 days following severe compression and examined at several time-points thereafter. Red, glial acidic fibrillary protein label; blue, 4′,6-diamidino-2-phenylindole nuclei label. (A-D) Three and 7 days post-transplantation, GFP+ BMSCs survive and fill T8 lesion site. (E-H) Two and 10 weeks post-transplantation, GFP+ BMSCs are no longer detectable. (E, F) Lesion-only subjects at 10 weeks. Scale bars: A,C,E, 1 mm; B,D,F, 250 μm. PBS, phosphate-buffered saline. Color image is available online at www.liebertpub.com/neu
FIG. 5.
Brain-derived neurotrophic factor (BDNF) expression. BDNF-transduced bone marrow stromal cells (BMSCs) express BDNF 3 days after grafting. (A) BDNF protein is detected within BDNF-transduced BMSC grafts and surrounding areas, but (B) not within control BMSC grafts lacking the BDNF complementary DNA. Scale bar: 100 μm. GFP, green fluorescent protein. Color image is available online at www.liebertpub.com/neu
Acute BMSC grafts do not improve locomotor outcomes
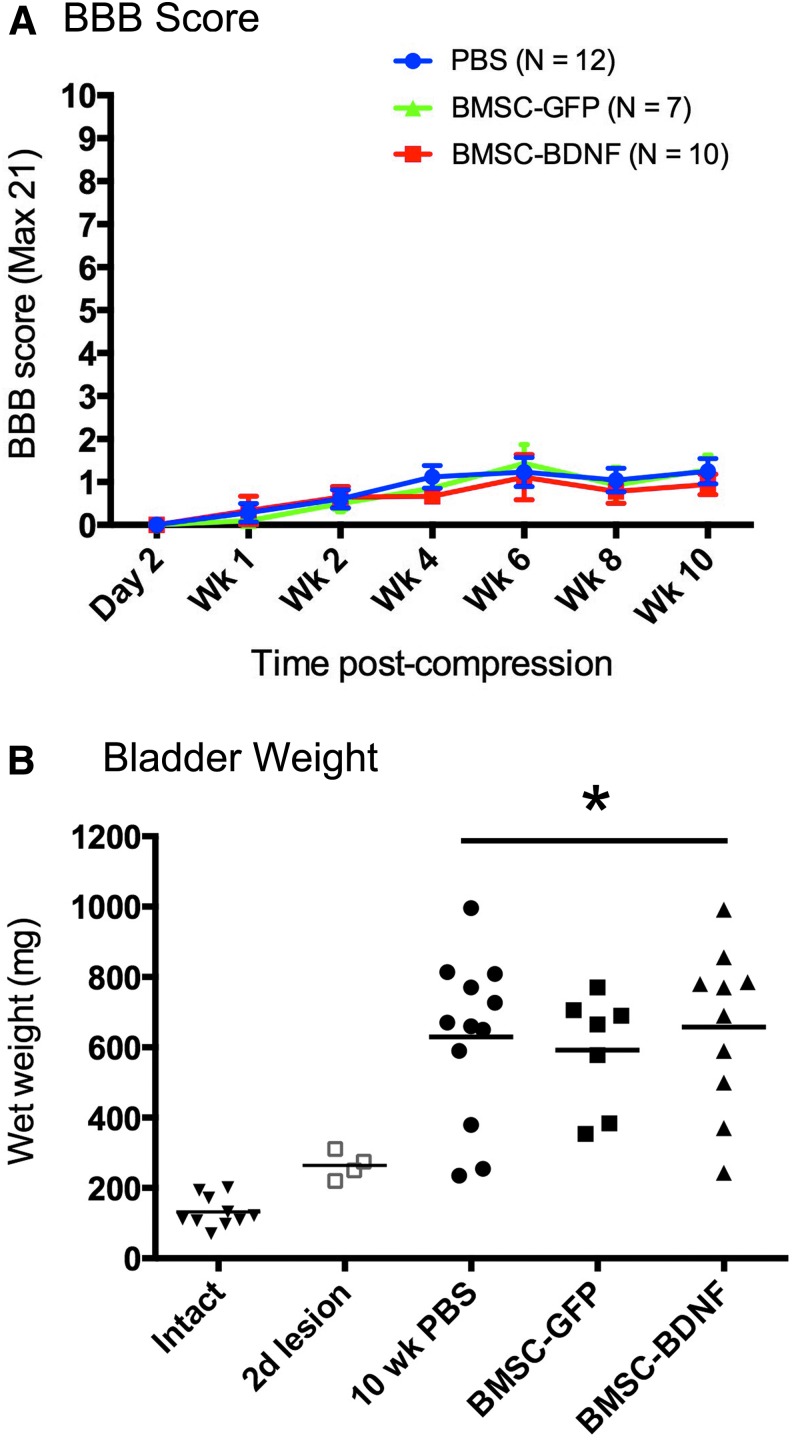
This study employed a severe spinal cord compression lesion to more closely model the most common form of human SCI, severe injury.40 Of 36 animals that underwent T8 severe compression lesions, 29 survived the planned 10-week period of in-life analysis, as follows: Group 1, GFP, n = 7; Group 2, BDNF, n = 10; Group 3, PBS control, n = 12. This final group of 29 subjects was used for behavioral and anatomical analysis. On the 21-point BBB open field locomotor scale, control PBS-injected rats exhibited severe deficits, recovering to a mean BBB score of only 1.24 ± 0.34 (mean ± SEM; n = 12) by week 10 after injury (Fig. 6A). Neither group of grafted animals exhibited a significant difference in outcome, compared with lesioned controls (Fig. 6A; ANOVA, p = 0.9).
FIG. 6.
Motor and autonomic outcomes. (A) Open field Basso, Beattie, and Bresnahan (BBB) scoring following T8 severe compression. No group differences are evident (p = 0.99, analysis of variance [ANOVA]). (B) Bladder weight following T8 severe compression. There are no differences among lesioned groups, which significantly differ from intact animals and animal's 2 days post-lesion (p < 0.05, ANOVA). PBS, phosphate-buffered saline; BMSC, bone marrow stromal cell; GFP, green fluorescent protein; BDNF; brain-derived neurotrophic factor. Color image is available online at www.liebertpub.com/neu
Acute BMSC grafts do not support recovery of bladder function
Another dimension of neuroprotection that can be measured in experimental paradigms of SCI is bladder function. SCI is typically followed by flaccid expansion of the bladder muscle as a consequence of denervation,26 with a consequent increase in weight. Neuroprotective therapies may prevent this expansion.27,28,29 At the conclusion of functional testing, animals were sacrificed and bladder weights were examined. Severe compression lesions resulted in a significant increase in bladder weight (ANOVA, p < 0.05; Fig. 6B). All lesioned groups exhibited equal increases in bladder weight without evidence of protection from this outcome (Fig. 6B).
Acute BMSC grafts do not reduce lesion size or attenuate glial scar
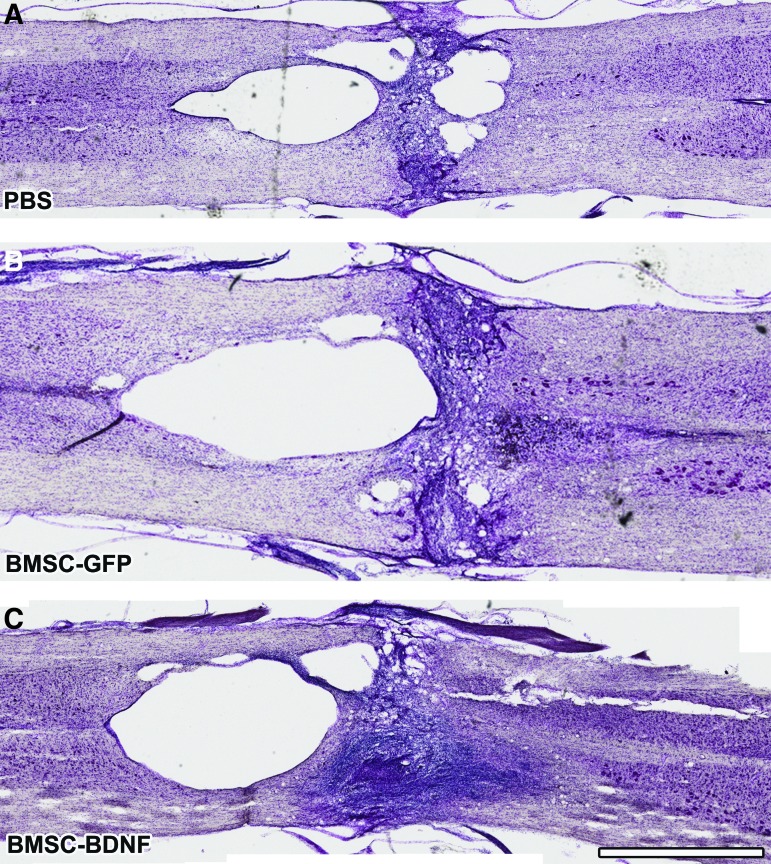
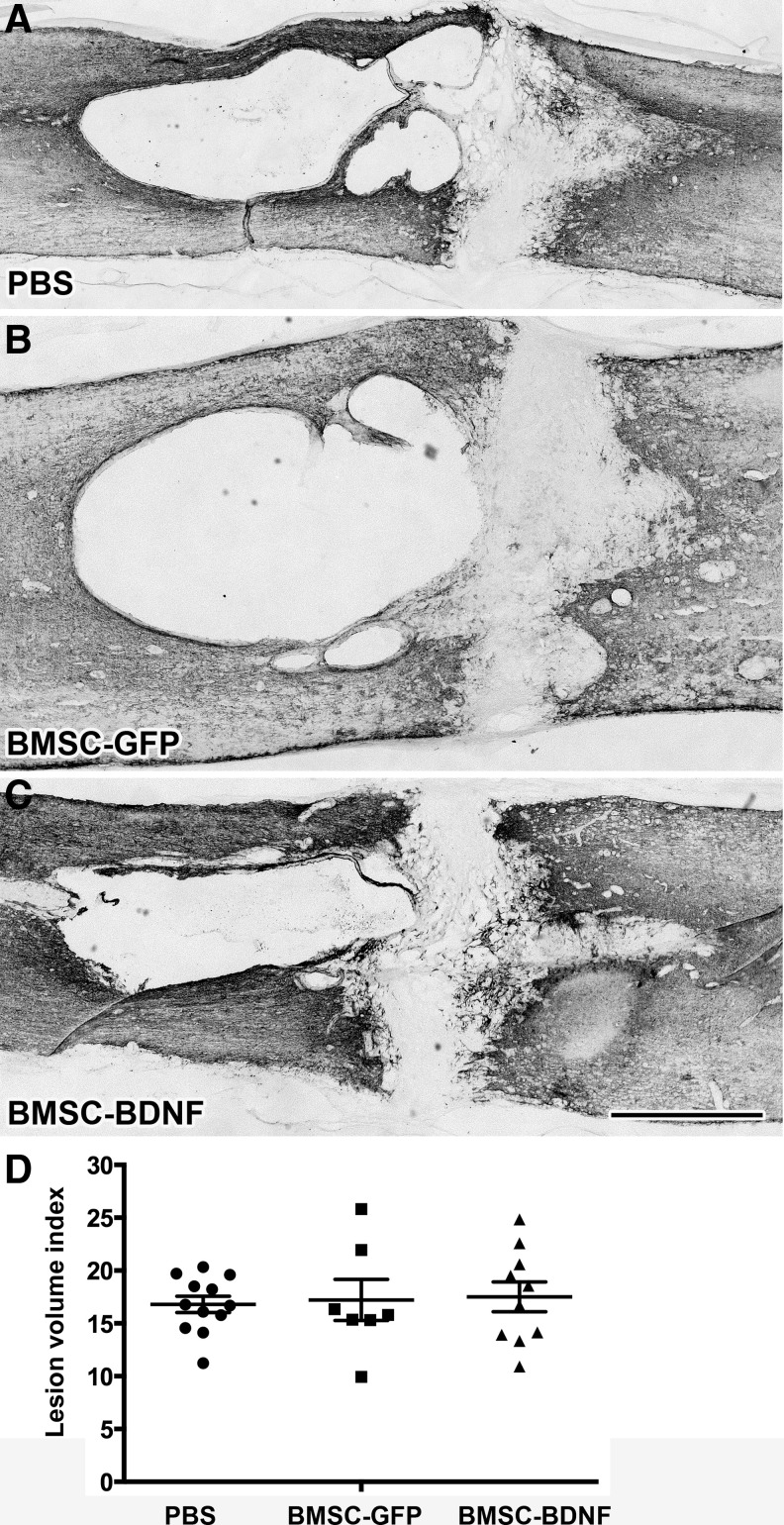
Cresyl violet stains and GFAP-immunolabeling performed 10 weeks post-compression demonstrated cavitation and cellular infiltration surrounding the lesion (Fig. 7 and Fig. 8). Quantification of lesion size through a series of 1-in-6 GFAP-labeled sections showed no significant difference in lesion size among groups (Fig. 8D). Quantification of GFAP immunolabeling at the rostral edge of the lesion site showed no significant difference in glial scar density among groups (Fig. 9).
FIG. 7.
Lesion site after 10 weeks. Nissl-stained series of horizontal sections, 10 weeks following severe T8 compression. Cavitation is evident in all groups and surviving bone marrow stromal cells (BMSCs) are not present within the lesion site. Scale bar: 1 mm. Color image is available online at www.liebertpub.com/neu
FIG. 8.
Tissue sparing after 10 weeks. Light-level immunolabeling for glial acidic fibrillary protein (GFAP) outlines lesion border. (A-C) No qualitative difference in lesion size is evident among groups. (D) Quantification of lesion size indicates no quantitative difference between the groups (p = 0.92, analysis of variance). Scale bar: 1 mm.
FIG. 9.
Glial scar after 10 weeks. Light-level immunolabeling for glial acidic fibrillary protein defines glial scar at lesion border. (A-C) No qualitative difference in glial scar deposition evident among groups. (D) Quantification of glial scar in a 100 μm-thick region in host tissue, measured from the lesion cavity border, indicates no quantitative difference between the groups (p = 0.75 ANOVA). Scale bar: 1 mm. PBS, phosphate-buffered saline; BMSC, bone marrow stromal cell; GFP, green fluorescent protein; BDNF, brain-derived neurotrophic factor.
Acute BMSC grafts do not increase serotonergic axons below lesion
Light level immunolabeling for serotonin (5-HT) performed 10 weeks post-compression demonstrated a high density of labeling rostral to the lesion site and immediately adjacent to the lesion epicenter. However, no 5-HT labeled axons were observed caudal to the lesion site in any group (Fig. 10).
FIG. 10.
Serotonergic axons are not present below the lesion. (A-C) Light level immunolabeling for serotonin (5-HT) demonstrates high density of labeling rostral to the compression site, and a lack of labeling caudally in all groups at 10 weeks post-lesion. (D-F) Higher magnification images of 5-HT labeling rostral and (G-I) caudal to the lesion. Scale bars: A-C, 1 mm; D-F, 150 μm; H-J, 100 μm. PBS, phosphate-buffered saline; BMSC, bone marrow stromal cell; GFP, green fluorescent protein; BDNF; brain-derived neurotrophic factor.
Discussion
Preceding studies in the scientific literature indicate that grafts of BMSCs improve functional and anatomical outcomes after experimental SCI,6,41 leading to clinical trials that have yielded inconsistent findings.11,12 However, a substantial problem in translating pre-clinical findings from rodent models of SCI to human clinical trials is the fact that many experimental therapies for SCI use only moderate injury severity models, whereas most humans sustain severe injuries.32,33 Severe injury models in rodents appear a priori to offer a more valuable tool for identifying therapies that could merit clinical translation.34
In this study, we employed a severe compression lesion model to determine whether allogeneic grafts of BMSCs locally to injury sites could improve functional outcomes. We succeeded in generating severe lesions with minimal functional recovery below the level of the lesion; animals exhibited only thin bands of spared white matter, as is often encountered after human injury.42–44 Using this model, we now find no beneficial effect of allogeneic BMSC grafts on functional outcomes after SCI. The preceding literature indicates that BMSC grafts may confer neuroprotection in moderate models of SCI, a finding observed whether BMSCs were injected locally into sites of SCI1,28,45–47 intrathecally48 or systemically.49,50 Mechanisms that might confer neuroprotection in these less severe lesion models include either local neuroprotective effects, or alternatively systemic alterations in immune responses that reduce inflammation in the spinal cord.15 Our findings suggest that the magnitude of neuroprotection provided by a local cell graft will be insufficient to improve outcomes after more severe injuries.
In addition, several studies report that neurotrophic factors are neuroprotective when tested in models of incomplete or moderate contusive SCI.51–54 However, we now find that grafts of allogeneic BMSCs transduced to secrete BDNF are not neuroprotective when delivered two days after severe SCI.
We chose to use an allogeneic grafting protocol for several reasons. First, only an allogeneic cell source is practical as an acute or subacute intervention after SCI; culturing autologous BMSCs would require weeks in the clinical setting and is not a practical translational option for acute or subacute SCI. Second, HLA-characterized banks of BMSCs already exist in major academic medical centers for human grafting (for bone marrow replacement), allowing practical grafting of more immune-tolerant cells among individuals. The use of outbred Sprague-Dawley rats models the type of allografting that would be encountered in a clinical setting. Third, even HLA-matched cell grafts in humans largely die off within weeks to months of grafting, to be replaced by progeny that are derived from the host.21–23,55 There are potential major advantages to an eventual loss of grafted cells to a site of SCI over time: first, a potential immune stimulus would not persist over time. Second, cells genetically modified to secrete a growth factor will express only transiently over the time period of cell survival, avoiding potential adverse effects of persistent growth factor expression, such as spasticity41 or pain.56 Indeed, in this study, we found that allogeneic cells readily survived grafting for several days but were no longer detectable 10 weeks after grafting. However, this grafting paradigm did not confer neuroprotection or improvement in functional outcomes.
Negative outcomes of studies must be interpreted with appropriate caution. We grafted cells 48 h after injury, a time-point that may have missed an earlier window of opportunity for neuroprotection. We note, however, that human clinical trials of BMSC grafting in SCI have grafted cells at time-points of 5 and 10 days post-injury; thus, our experimental paradigm was representative of these subacute time-points explored clinically57,58 We grafted cells directly into lesion epicenters, as undertaken in several previous studies,1,28,45,46 whereas other studies have grafted cells intrathecally48 or systemically49,50; it is possible that these alternative routes of administration may be effective where local administration is not. It is also possible that delivery of an alternative cell type, such as a Schwann cell, might be successful whereas a BMSC is not, or that an alternative neuroprotective factor such as NT-359 or erythropoietin60,61 might be effective. With these important caveats in mind, our findings support the conclusion that 2-day delayed intraparenchymal injection of wild-type or BDNF-expressing BMSCs is unlikely to represent a useful translational therapy for SCI.
We propose that translational programs consider the use of severe rodent injury models as a useful predictor of potential clinical benefit, a point raised in a recent review.34 Our study identified a divergence in efficacy outcomes in partial lesion models compared to our severe model, and the negative clinical trial to date11 is compatible with the concept that a severe rodent lesion model represent the best predictor of clinical outcome.
Acknowledgments
We thank Nicole Armstrong for technical assistance. This study was supported by the Department of Defense Congressionally Directed Medical Research Program: Spinal Cord Injury Research Program (CDMRP-SCIRP), the Veterans Administration, the NIH (NS42291), the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation and the Bernard and Anne Spitzer Charitable Trust.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Nandoe Tewarie R.D., Hurtado A., Levi A.D., Grotenhuis J.A., and Oudega M. (2006). Bone marrow stromal cells for repair of the spinal cord: towards clinical application. Cell Transplant. 15, 563–577 [DOI] [PubMed] [Google Scholar]
- 2.Vaquero J. and Zurita M. (2009). Bone marrow stromal cells for spinal cord repair: a challenge for contemporary neurobiology. Histol. Histopathol. 24, 107–116 [DOI] [PubMed] [Google Scholar]
- 3.Vaquero J. and Zurita M. (2011). Functional recovery after severe CNS trauma: current perspectives for cell therapy with bone marrow stromal cells. Prog. Neurobiol. 93, 341–349 [DOI] [PubMed] [Google Scholar]
- 4.Tetzlaff W., Okon E.B., Karimi-Abdolrezaee S., Hill C.E., Sparling J.S., Plemel J.R., Plunet W.T., Tsai E.C., Baptiste D., Smithson L.J., Kawaja M.D., Fehlings M.G., and Kwon B.K. (2011). A systematic review of cellular transplantation therapies for spinal cord injury. J. Neurotrauma 28, 1611–1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wright K.T., El Masri W., Osman A., Chowdhury J., and Johnson W.E. (2011). Concise review: Bone marrow for the treatment of spinal cord injury: mechanisms and clinical applications. Stem Cells 29, 169–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vawda R. and Fehlings M.G. (2013). Mesenchymal cells in the treatment of spinal cord injury: current & future perspectives. Curr. Stem Cell Res. Ther. 8, 25–38 [DOI] [PubMed] [Google Scholar]
- 7.Forostyak S., Jendelova P., and Sykova E. (2013). The role of mesenchymal stromal cells in spinal cord injury, regenerative medicine and possible clinical applications. Biochimie 95, 2257–2270 [DOI] [PubMed] [Google Scholar]
- 8.Chopp M., Zhang X.H., Li Y., Wang L., Chen J., Lu D., Lu M., and Rosenblum M. (2000). Spinal cord injury in rat: treatment with bone marrow stromal cell transplantation. Neuroreport 11, 3001–3005 [DOI] [PubMed] [Google Scholar]
- 9.Paul C., Samdani A.F., Betz R.R., Fischer I., and Neuhuber B. (2009). Grafting of human bone marrow stromal cells into spinal cord injury: a comparison of delivery methods. Spine 34, 328–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nandoe Tewarie R.D., Hurtado A., Ritfeld G.J., Rahiem S.T., Wendell D.F., Barroso M.M., Grotenhuis J.A., and Oudega M. (2009). Bone marrow stromal cells elicit tissue sparing after acute but not delayed transplantation into the contused adult rat thoracic spinal cord. J. Neurotrauma 26, 2313–2322 [DOI] [PubMed] [Google Scholar]
- 11.Karamouzian S., Nematollahi-Mahani S.N., Nakhaee N., and Eskandary H. (2012). Clinical safety and primary efficacy of bone marrow mesenchymal cell transplantation in subacute spinal cord injured patients. Clin. Neurol. Neurosurg. 114, 935–939 [DOI] [PubMed] [Google Scholar]
- 12.Saito F., Nakatani T., Iwase M., Maeda Y., Murao Y., Suzuki Y., Fukushima M., and Ide C. (2012). Administration of cultured autologous bone marrow stromal cells into cerebrospinal fluid in spinal injury patients: a pilot study. Restor. Neurol. Neurosci. 30, 127–136 [DOI] [PubMed] [Google Scholar]
- 13.Bai L., Lennon D.P., Eaton V., Maier K., Caplan A.I., Miller S.D., and Miller R.H. (2009). Human bone marrow-derived mesenchymal stem cells induce Th2-polarized immune response and promote endogenous repair in animal models of multiple sclerosis. Glia 57, 1192–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang M., Wei X., Li J., Heine L.A., Rosenwasser R., and Iacovitti L. (2010). Changes in host blood factors and brain glia accompanying the functional recovery after systemic administration of bone marrow stem cells in ischemic stroke rats. Cell Transplant. 19, 1073–1084 [DOI] [PubMed] [Google Scholar]
- 15.Glenn J.D. and Whartenby K.A. (2014). Mesenchymal stem cells: Emerging mechanisms of immunomodulation and therapy. World J. Stem Cells 6, 526–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen X., Katakowski M., Li Y., Lu D., Wang L., Zhang L., Chen J., Xu Y., Gautam S., Mahmood A., and Chopp M. (2002). Human bone marrow stromal cell cultures conditioned by traumatic brain tissue extracts: growth factor production. J. Neurosci. Res. 69, 687–691 [DOI] [PubMed] [Google Scholar]
- 17.Hawryluk G.W., Mothe A., Wang J., Wang S., Tator C., and Fehlings M.G. (2012). An in vivo characterization of trophic factor production following neural precursor cell or bone marrow stromal cell transplantation for spinal cord injury. Stem Cells Dev. 21, 2222–2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahmood A., Lu D., and Chopp M. (2004). Intravenous administration of marrow stromal cells (MSCs) increases the expression of growth factors in rat brain after traumatic brain injury. J. Neurotrauma 21, 33–39 [DOI] [PubMed] [Google Scholar]
- 19.Steffenhagen C., Dechant F.X., Oberbauer E., Furtner T., Weidner N., Kury P., Aigner L., and Rivera F.J. (2012). Mesenchymal stem cells prime proliferating adult neural progenitors toward an oligodendrocyte fate. Stem Cells Dev. 21, 1838–1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stroncek D.F., Sabatino M., Ren J., England L., Kuznetsov S.A., Klein H.G., and Robey P.G. (2014). Establishing a bone marrow stromal cell transplant program at the National Institutes of Health Clinical Center. Tissue Eng. Part B Rev. 20, 200–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Novitzky N. and Davison G.M. (2001). Immune reconstitution following hematopoietic stem-cell transplantation. Cytotherapy 3, 211–220 [DOI] [PubMed] [Google Scholar]
- 22.Kalwak K., Gorczynska E., Toporski J., Turkiewicz D., Slociak M., Ussowicz M., Latos-Grazynska E., Krol M., Boguslawska-Jaworska J., and Chybicka A. (2002). Immune reconstitution after haematopoietic cell transplantation in children: immunophenotype analysis with regard to factors affecting the speed of recovery. Br. J. Haematol. 118, 74–89 [DOI] [PubMed] [Google Scholar]
- 23.Williams K.M. and Gress R.E. (2008). Immune reconstitution and implications for immunotherapy following haematopoietic stem cell transplantation. Best Pract. Res. Clin. Haematol. 21, 579–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu P., Jones L.L., and Tuszynski M.H. (2005). BDNF-expressing marrow stromal cells support extensive axonal growth at sites of spinal cord injury. Exper. Neurol. 191, 344–360 [DOI] [PubMed] [Google Scholar]
- 25.Lu P., Blesch A., Graham L., Wang Y., Samara R., Banos K., Haringer V., Havton L., Weishaupt N., Bennett D., Fouad K., and Tuszynski M.H. (2012). Motor axonal regeneration after partial and complete spinal cord transection. J. Neurosci. 32, 8208–8218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao M., Lu P., Bednark B., Lynam D., Conner J.M., Sakamoto J., and Tuszynski M.H. (2013). Templated agarose scaffolds for the support of motor axon regeneration into sites of complete spinal cord transection. Biomaterials 34, 1529–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gransee H.M., Zhan W.Z., Sieck G.C., and Mantilla C.B. (2015). Localized delivery of brain-derived neurotrophic factor-expressing mesenchymal stem cells enhances functional recovery following cervical spinal cord injury. J. Neurotrauma 32, 185–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sasaki M., Radtke C., Tan A.M., Zhao P., Hamada H., Houkin K., Honmou O., and Kocsis J.D. (2009). BDNF-hypersecreting human mesenchymal stem cells promote functional recovery, axonal sprouting, and protection of corticospinal neurons after spinal cord injury. J. Neurosci. 29, 14932–14941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boyce V.S., Park J., Gage F.H., and Mendell L.M. (2012). Differential effects of brain-derived neurotrophic factor and neurotrophin-3 on hindlimb function in paraplegic rats. Eur. J. Neurosci. 35, 221–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fouad K., Bennett D.J., Vavrek R., and Blesch A. (2013). Long-term viral brain-derived neurotrophic factor delivery promotes spasticity in rats with a cervical spinal cord hemisection. Front. Neurol. 4, 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trang T., Beggs S., and Salter M.W. (2011). Brain-derived neurotrophic factor from microglia: a molecular substrate for neuropathic pain. Neuron Glia Biol. 7, 99–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zariffa J., Kramer J.L., Fawcett J.W., Lammertse D.P., Blight A.R., Guest J., Jones L., Burns S., Schubert M., Bolliger M., Curt A., and Steeves J.D. (2011). Characterization of neurological recovery following traumatic sensorimotor complete thoracic spinal cord injury. Spinal Cord 49, 463–471 [DOI] [PubMed] [Google Scholar]
- 33.Lee B.A., Leiby B.E., and Marino R.J. (2014). Neurological and functional recovery after thoracic spinal cord injury. J. Spinal Cord Med. 2014. December 18; Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwon B.K., Soril L.J., Bacon M., Beattie M.S., Blesch A., Bresnahan J.C., Bunge M.B., Dunlop S.A., Fehlings M.G., Ferguson A.R., Hill C.E., Karimi-Abdolrezaee S., Lu P., McDonald J.W., Muller H.W., Oudega M., Rosenzweig E.S., Reier P.J., Silver J., Sykova E., Xu X.M., Guest J.D., and Tetzlaff W. (2013). Demonstrating efficacy in preclinical studies of cellular therapies for spinal cord injury—how much is enough? Exper. Neurol. 248, 30–44 [DOI] [PubMed] [Google Scholar]
- 35.Azizi S.A., Stokes D., Augelli B.J., DiGirolamo C., and Prockop D.J. (1998). Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats—similarities to astrocyte grafts. Proc. Natl. Acad. Sci. U. S. A. 95, 3908–3913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conner J.M. and Varon S. (1996). Characterization of antibodies to nerve growth factor: assay-dependent variability in the cross-reactivity with other neurotrophins. J. Neurosci. Methods 65, 93–99 [DOI] [PubMed] [Google Scholar]
- 37.Basso D.M., Beattie M.S., and Bresnahan J.C. (1995). A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 12, 1–21 [DOI] [PubMed] [Google Scholar]
- 38.Harting M., Jimenez F., Pati S., Baumgartner J., and Cox C., Jr. (2008). Immunophenotype characterization of rat mesenchymal stromal cells. Cytotherapy 10, 243–253 [DOI] [PubMed] [Google Scholar]
- 39.Swanger S.A., Neuhuber B., Himes B.T., Bakshi A., and Fischer I. (2005). Analysis of allogeneic and syngeneic bone marrow stromal cell graft survival in the spinal cord. Cell Transplant. 14, 775–786 [DOI] [PubMed] [Google Scholar]
- 40.Burns A.S., Marino R.J., Flanders A.E., and Flett H. (2012). Clinical diagnosis and prognosis following spinal cord injury. Handb. Clin. Neurol. 109, 47–62 [DOI] [PubMed] [Google Scholar]
- 41.Dasari V.R., Veeravalli K.K., and Dinh D.H. (2014). Mesenchymal stem cells in the treatment of spinal cord injuries: a review. World J. Stem Cells 6, 120–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koskinen E., Brander A., Hakulinen U., Luoto T., Helminen M., Ylinen A., and Ohman J. (2013). Assessing the state of chronic spinal cord injury using diffusion tensor imaging. J. Neurotrauma 30, 1587–1595 [DOI] [PubMed] [Google Scholar]
- 43.Fleming J.C., Norenberg M.D., Ramsay D.A., Dekaban G.A., Marcillo A.E., Saenz A.D., Pasquale-Styles M., Dietrich W.D., and Weaver L.C. (2006). The cellular inflammatory response in human spinal cords after injury. Brain 129, 3249–3269 [DOI] [PubMed] [Google Scholar]
- 44.Chang Y., Jung T.D., Yoo D.S., and Hyun J.K. (2010). Diffusion tensor imaging and fiber tractography of patients with cervical spinal cord injury. J. Neurotrauma 27, 2033–2040 [DOI] [PubMed] [Google Scholar]
- 45.Ankeny D.P., McTigue D.M., and Jakeman L.B. (2004). Bone marrow transplants provide tissue protection and directional guidance for axons after contusive spinal cord injury in rats. Exp. Neurol. 190, 17–31 [DOI] [PubMed] [Google Scholar]
- 46.Ritfeld G.J., Patel A., Chou A., Novosat T.L., Castillo D.G., Roos R.A., and Oudega M. (2015). The role of brain-derived neurotrophic factor in bone marrow stromal cell-mediated spinal cord repair. Cell Transplant. 2015. January 9; Epub ahead of print [DOI] [PubMed] [Google Scholar]
- 47.Sandner B., Ciatipis M., Motsch M., Soljanik I., Weidner N., and Blesch A. (2015). Limited functional effects of subacute syngeneic bone marrow stromal cell transplantation after rat spinal cord contusion injury. Cell transplant. 2015. March 25; Epub ahead of print, [DOI] [PubMed] [Google Scholar]
- 48.Ohta M., Suzuki Y., Noda T., Ejiri Y., Dezawa M., Kataoka K., Chou H., Ishikawa N., Matsumoto N., Iwashita Y., Mizuta E., Kuno S., and Ide C. (2004). Bone marrow stromal cells infused into the cerebrospinal fluid promote functional recovery of the injured rat spinal cord with reduced cavity formation. Exp. Neurol. 187, 266–278 [DOI] [PubMed] [Google Scholar]
- 49.Cizkova D., Rosocha J., Vanicky I., Jergova S., and Cizek M. (2006). Transplants of human mesenchymal stem cells improve functional recovery after spinal cord injury in the rat. Cell. Mol. Neurobiol. 26, 1167–1180 [DOI] [PubMed] [Google Scholar]
- 50.Osaka M., Honmou O., Murakami T., Nonaka T., Houkin K., Hamada H., and Kocsis J.D. (2010). Intravenous administration of mesenchymal stem cells derived from bone marrow after contusive spinal cord injury improves functional outcome. Brain Res. 1343, 226–235 [DOI] [PubMed] [Google Scholar]
- 51.Kim D.H., Gutin P.H., Noble L.J., Nathan D., Yu J.S., and Nockels R.P. (1996). Treatment with genetically engineered fibroblasts producing NGF or BDNF can accelerate recovery from traumatic spinal cord injury in the adult rat. Neuroreport 7, 2221–2225 [DOI] [PubMed] [Google Scholar]
- 52.McTigue D.M., Horner P.J., Stokes B.T., and Gage F.H. (1998). Neurotrophin-3 and brain-derived neurotrophic factor induce oligodendrocyte proliferation and myelination of regenerating axons in the contused adult rat spinal cord. J. Neurosci. 18, 5354–5365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mitsui T., Fischer I., Shumsky J.S., and Murray M. (2005). Transplants of fibroblasts expressing BDNF and NT-3 promote recovery of bladder and hindlimb function following spinal contusion injury in rats. Exp. Neurol. 194, 410–431 [DOI] [PubMed] [Google Scholar]
- 54.Park W.B., Kim S.Y., Lee S.H., Kim H.W., Park J.S., and Hyun J.K. (2010). The effect of mesenchymal stem cell transplantation on the recovery of bladder and hindlimb function after spinal cord contusion in rats. BMC Neurosci. 11, 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li J.M. and Waller E.K. (2004). Donor antigen-presenting cells regulate T-cell expansion and antitumor activity after allogeneic bone marrow transplantation. Biol. Blood Marrow Transplant. 10, 540–551 [DOI] [PubMed] [Google Scholar]
- 56.Vanelderen P., Rouwette T., Kozicz T., Roubos E., Van Zundert J., Heylen R., and Vissers K. (2010). The role of brain-derived neurotrophic factor in different animal models of neuropathic pain. Eur. J. Pain 14, 473 e471–e479 [DOI] [PubMed] [Google Scholar]
- 57.Sykova E., Homola A., Mazanec R., Lachmann H., Konradova S.L., Kobylka P., Padr R., Neuwirth J., Komrska V., Vavra V., Stulik J., and Bojar M. (2006). Autologous bone marrow transplantation in patients with subacute and chronic spinal cord injury. Cell Transplant. 15, 675–687 [DOI] [PubMed] [Google Scholar]
- 58.Geffner L.F., Santacruz P., Izurieta M., Flor L., Maldonado B., Auad A.H., Montenegro X., Gonzalez R., and Silva F. (2008). Administration of autologous bone marrow stem cells into spinal cord injury patients via multiple routes is safe and improves their quality of life: comprehensive case studies. Cell Transplant. 17, 1277–1293 [DOI] [PubMed] [Google Scholar]
- 59.Cui X., Chen L., Ren Y., Ji Y., Liu W., Liu J., Yan Q., Cheng L., and Sun Y.E. (2013). Genetic modification of mesenchymal stem cells in spinal cord injury repair strategies. Biosci. Trends 7, 202–208 [PubMed] [Google Scholar]
- 60.Mofidi A., Bader A., and Pavlica S. (2011). The use of erythropoietin and its derivatives to treat spinal cord injury. Mini Rev. Med. Chem. 11, 763–770 [DOI] [PubMed] [Google Scholar]
- 61.Jin W., Ming X., Hou X., Zhu T., Yuan B., Wang J., Ni H., Jiang J., Wang H., and Liang W. (2014). Protective effects of erythropoietin in traumatic spinal cord injury by inducing the Nrf2 signaling pathway activation. J. Trauma Acute Care Surg. 76, 1228–1234 [DOI] [PubMed] [Google Scholar]