Abstract

A novel series of piperazinylquinoline derivatives were discovered as respiratory syncytial virus (RSV) fusion inhibitors by the ligand-based screening approach. Among 3,000 hits, 1-amino-3-[[2-(4-phenyl-1-piperidyl)-4-quinolyl]amino]propan-2-ol (7) was proven to be active against the RSV long (A) strain. The anti-RSV activity was improved by converting piperidine to benzylcarbonyl substituted piperazine. The basic side chain was also found to be crucial for anti-RSV activity. The selected analogues, 45 and 50, demonstrated anti-RSV activities up to EC50 = 0.028 μM and 0.033 μM, respectively. A direct anti-RSV effect was confirmed by a plaque reduction assay and a fusion inhibition assay. Both 45 and 50 showed promising DMPK properties with good oral bioavailability, and could potentially lead to novel therapeutic agents targeting the RSV fusion process.
Keywords: Respiratory syncytial virus (RSV), antiviral, fusion inhibitors, quinoline, piperazine
Human respiratory syncytial virus (RSV) is a negative-sense single-stranded RNA virus, which belongs to the family of Paramyxoviridae.1 Human RSV is the most common cause of acute upper and lower respiratory tract infection in infants and young children. RSV can also lead to severe diseases in certain patient populations.2 Almost all children can be infected by RSV at least once by age of three.3 The protection from human immunity against RSV infection is incomplete. In normal adults and elder children, RSV infection is mild and mainly associated with upper respiratory tract symptoms. In young children and immunocompromised adults, severe RSV infection often leads to bronchiolitis and pneumonia with an increased chance of morbidity or mortality.3,4 Furthermore, sequela of severe RSV infection at a young age is recurrent wheezing or asthma.5 For the populations with high-risk factors for lower respiratory tract infections, such as premature birth, congenital heart disease, chronic pulmonary diseases, and immunocompromised conditions, the RSV-related mortality rate becomes higher.6,7
There is no available vaccine for RSV infection, despite many attempts in inactivated subunits and live-attenuated vaccination approaches. Virazole, the aerosol formulation comprising ribavirin, is currently the only approved antiviral therapy for RSV infection. However, it is rarely used in the clinic, due to potential side effects and limited efficacy.8 Palivizumab, which was approved for prophylaxis in high-risk infants in 1998, is a humanized monoclonal antibody against RSV fusion protein.9 Unfortunately, Palivizumab shows no efficacy in treatment of established RSV infection. As such, safe and effective therapy for RSV infection is an unmet medical need.
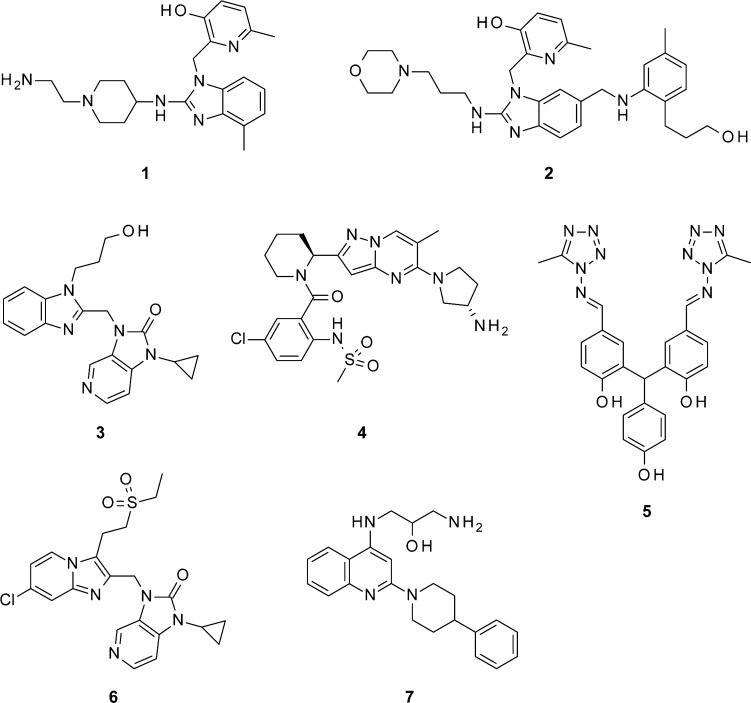
RSV Fusion (F) protein is a surface glycoprotein on the viral envelope which, together with the G surface glycoprotein, mediates viral entry into a host cell.10 The F protein drives the fusion process between viral and host cellular membranes and also promotes syncytia formation. Inhibition of viral entry and spread by targeting on the RSV F protein is emerging as a promising treatment for RSV infected patients. Small molecule RSV F protein inhibitors (Figure 1) have strong potential to decrease the duration and severity of respiratory symptoms, and the subsequent risk of prolonged hospitalization and complications.
Figure 1.
Selected RSV fusion inhibitors under development.
In the last two decades, a number of highly potent and structurally different RSV fusion protein inhibitors have been reported.11−14 Several of these compounds have successfully progressed to late stages of preclinical optimization, but only a selected few have entered early clinical development.15 JNJ-2408068 (1) is a small-molecule antiviral with a high potency (picomolar activity) and a low cytotoxicity in a wide range of cells. Unfortunately, its long tissue retention time in several species created a cause for concern.12 TMC-353121 (2) showed picomolar anti-RSV activity in vitro and efficacy in inhibiting viral replication of RSV, both prophylactically and therapeutically.13 BMS-433771 (3) is the first fusion inhibitor to demonstrate oral bioavailability.14 Unfortunately, it appears that further development was not pursued for the reason of business interests. Recently, GS-5806 (4) achieved proof-of-concept in human RSV challenge studies and showed significantly reduced viral load and clinical symptoms.16 In addition, VP-14637 (5), a poor orally bioavailable anti-RSV agent, is being developed as a dry powder inhaled product MDT-637 in Phase I trials with good tolerance in healthy human subjects.17
Thus, there is a clear unmet medical need to develop an orally available drug to prevent and treat RSV infection. Our RSV inhibition program first led to the discovery of imidazopyridine 6 according to the established docking studies.18 In our endeavors to develop highly potent fusion inhibitors, we identified a novel class of piperazinylquinolines as RSV fusion inhibitors by a similarity-based virtual screening approach. MOS19 and ROCS20 were used in parallel to screen the Roche Smart library (more than one million small molecules) for potential RSV fusion inhibitors. The ligands JNJ-2408068 (1), TMC-353121 (2), and BMS-433771 (3) were chosen as reference compounds for the virtual screening. Each method chose the best 1,000 similar compounds to every reference compound in the library based on similarity scores, which resulted in total 6,000 similar hits for 3 reference compounds. After removing the duplicated structures, less than 3,000 compounds were finally selected for anti-RSV activity in reduction of cytopathic effect (CPE) assay. Among these molecules, a drug-like molecule, 1-amino-3-[[2-(4-phenyl-1-piperidyl)-4-quinolyl]amino]propan-2-ol (7), was identified as a potent anti-RSV hit.
The antiviral activity was evaluated by the cytopathic effect reduction assay, which was induced by the RSV long strain of virus replication in HEp-2 human lung epithelial carcinoma cells.21 Compound 7 showed an EC50 of 0.759 uM against the RSV long (A) strain, suggesting that 7 was a good starting point for further exploration.
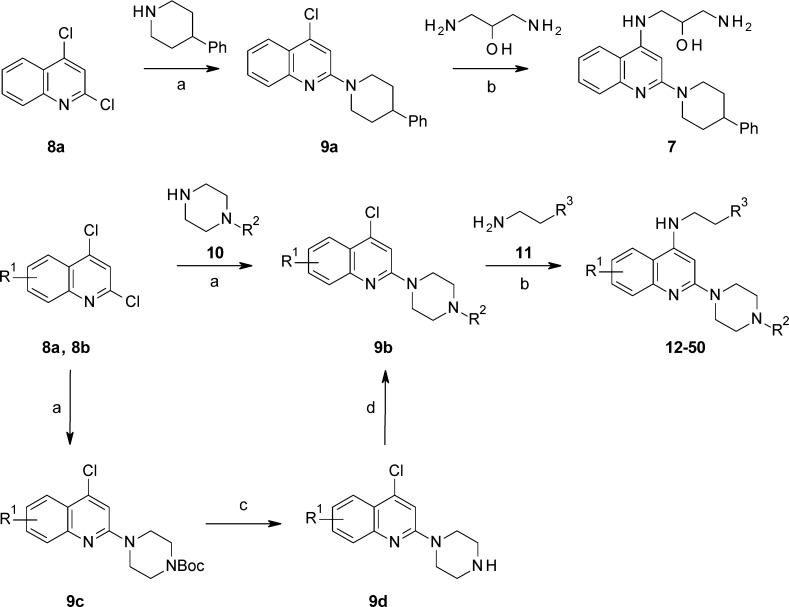
The general synthesis of 7 and piperazinylquinoline derivatives is summarized in Scheme 1. The reaction commenced with commercially available 2,4-dichloroquinoline (8a and 8b), which was coupled with 4-phenylpiperidine or piperazines 10 in refluxing toluene to generate 4-chloro-2-piperidylquinoline 9a or 4-chloro-2-piperazinylquinoline 9b, followed by coupling with 1,3-diaminopropan-2-ol or 11 under microwave irradiation to afford 7 or piperazinylquinoline derivatives in modest yields. Alternatively, 4-chloro-2-piperazinylquinoline 9b can also be synthesized from 9c by deprotection of Boc with HCl/EtOAc, followed by substitution of 9d with different R2 groups in good yields.
Scheme 1. Synthesis of Piperazinylquinolines.
Reagents and conditions: (a) 10, tert-butyl piperazine-1-carboxylate or 4-phenylpiperidine, DIPEA, toluene, reflux; (b) 11 or 1,3-diaminopropan-2-ol, neat, microwave, 100 to 160 °C; (c) HCl/EtOAc, 5 °C; (d) R2Cl, TEA, DCM, 0 °C to r.t..
Our preliminary structure activity relationship (SAR) studies surrounding 7 suggested that the piperidyl linker could be important for conformational control. Thus, we designed piperazinyl analogues, such as N′-[2-(4-phenylpiperazin-1-yl)-4-quinolyl]ethane-1,2-diamine (12), which could help the conformation hopefully to retain antiviral activity, to improve metabolic stability, and to conveniently introduce substituents (R2). Additionally, replacement of the aminoalcohol head portion with a simple ethyl amine did not cause a remarkable decrease of the activity.
When a Cl was introduced on the N-phenyl ring of the piperazine group (R2, Table 1), it was found that only the meta-Cl substitution (14) maintained the potency, but increased cytotoxicity, as measured by CC50, was observed. The ortho-Cl (13) and para-chloro (15) exhibited reduced potency compared to 12. Interestingly, the replacement of the phenyl group with an acetyl can slightly improve the potency (16). Unfortunately, further elongation of the amide, such as in 17 and 19, exhibited loss of potency (EC50 = 3.090 μM and 1.884 μM, respectively), except for 18, which kept the potency (EC50 = 0.847 μM). The c-hexyl amide 20 showed much weaker activity (EC50 = 9.240 μM). To our surprise, the terminal phenyl analogues 21–23 were all tolerated. Especially for 22, which showed 10-fold activity improvement compared to 7 (EC50 = 0.079 μM, CC50 > 100 μM). In contrast, methanesulfonamide 24 and benzenesulfonamide 25 both showed much weaker activity than the corresponding acetamide 16 and benzamide 21.
Table 1. Antiviral Activity of Compounds 7 and 12–25a.
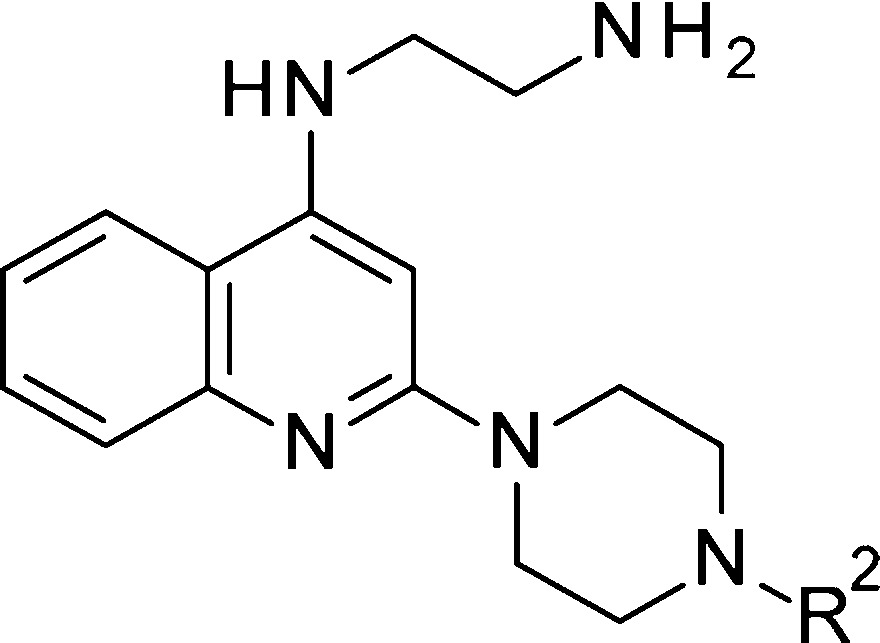
| compds | R2 | EC50(μM) | CC50 (μM) |
|---|---|---|---|
| 7 | 0.759 | 20.80 | |
| 12 | Ph | 0.865 | 20.80 |
| 13 | 2-Cl-Ph | 2.014 | 20.80 |
| 14 | 3-Cl-Ph | 0.977 | 6.40 |
| 15 | 4-Cl-Ph | >100 | 6.60 |
| 16 | COCH3 | 0.317 | >100 |
| 17 | COCH2CH3 | 3.090 | >100 |
| 18 | COCH(CH3)2 | 0.847 | >100 |
| 19 | COCH2CH(CH3)2 | 1.884 | >100 |
| 20 | CO(c-Hexyl) | 9.240 | >100 |
| 21 | COPh | 0.570 | >100 |
| 22 | COCH2Ph | 0.079 | >100 |
| 23 | COCH2CH2Ph | 0.464 | >100 |
| 24 | SO2CH3 | 2.272 | >100 |
| 25 | SO2Ph | 1.166 | 20.50 |
EC50: the concentration of compound that reduced 50% of the cytopathic effect of RSV. Long strain infected HEp-2 cells. CC50: the concentration of compound that manifests cytotoxicity toward 50% of uninfected HEp-2 cells. Values are means of at least two experiments performed in consecutive weeks.17
According to the new insight of the distinct impact of 2-phenylacetamide on potency improvement, a number of substituted phenylacetamide analogues 26–42 bearing piperazine were synthesized as shown in Table 2.
Table 2. Antiviral Activity of Compounds 26–42.
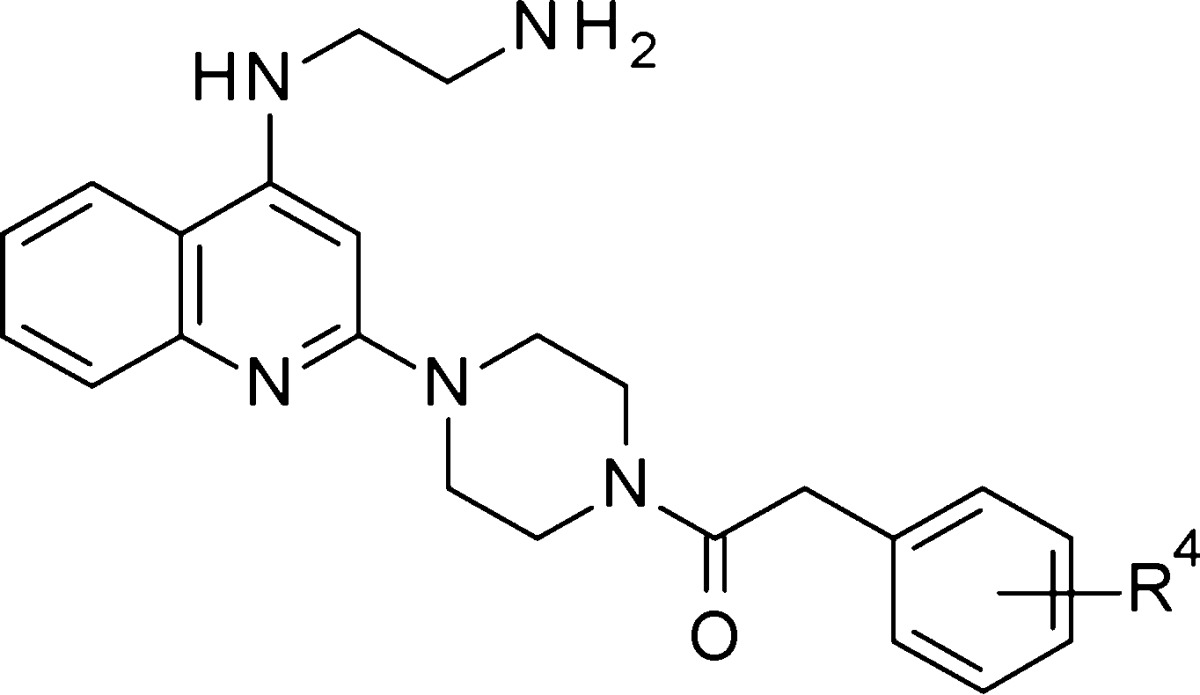
| compds | R4 | EC50 (μM) | CC50 (μM) |
|---|---|---|---|
| 26 | 2-Cl | 0.077 | >100 |
| 27 | 3-Cl | 0.066 | >100 |
| 28 | 4-Cl | 0.058 | >100 |
| 29 | 2-F | 0.089 | >100 |
| 30 | 3-F | 0.080 | >100 |
| 31 | 4-F | 0.095 | >100 |
| 32 | 2-CH3 | 0.029 | >100 |
| 33 | 3-CH3 | 0.059 | >100 |
| 34 | 4-CH3 | 0.142 | >100 |
| 35 | 2-OCH3 | 0.056 | >100 |
| 36 | 3-OCH3 | 0.098 | >100 |
| 37 | 4-OCH3 | 0.679 | >100 |
| 38 | 3-CN | 0.225 | >100 |
| 39 | 3-CF3 | 0.563 | >100 |
| 40 | 3-F-4-F | 0.255 | >100 |
| 41 | 3-Cl-4-Cl | 0.220 | 6.68 |
| 42 | 3-F-5-F | 0.264 | >100 |
The substitutions at the phenyl ring could tolerate broader modification, and exhibit good RSV inhibitory activity. Some of the results were presented in Table 2. The chloro and fluoro substitutions at different positions of the phenyl ring showed similar potency (26 to 31). Surprisingly, the 2-methyl substitution resulted in a further increased activity (32, EC50 = 0.029 μM). However, the methyl substitutions at the meta- and para-positions exhibited decreased potency (33 and 34). The same trend was observed in methoxy substitution (35 to 37). In comparison to an electron donating group, cyano and trifluoromethyl substitutions at the meta-position led to a slight loss of activity. In addition, no activity difference was observed between the difluoro (40 and 42) and dichloro (41) substitutions on the phenyl ring, but cytotoxicity was observed in 41.
In an attempt to further understand the SAR, we tested additional analogues with substitutions at quinoline (R1), the head portion (R3), and the benzylic position (R5 and R6). The SAR was summarized in Table 3. To block a potential metabolic hot spot, small alkyl groups were introduced at the benzylic position (43 to 49). The molecule with a single methyl substituent (43) was less potent than 22. gem-Dimethyl substitution at the same position (45) resulted in nearly 3-fold potency improvement compared to 22. In contrast, the gem-diethyl analogue 46 showed weaker activity than 22. Subsequently, the cyclic substituents were investigated. The most potent analogue (47) was identified (EC50 = 0.017 μM). Further enlarging the size of the ring led to an activity loss (49).
Table 3. Antiviral Activity of Compounds 43–56.
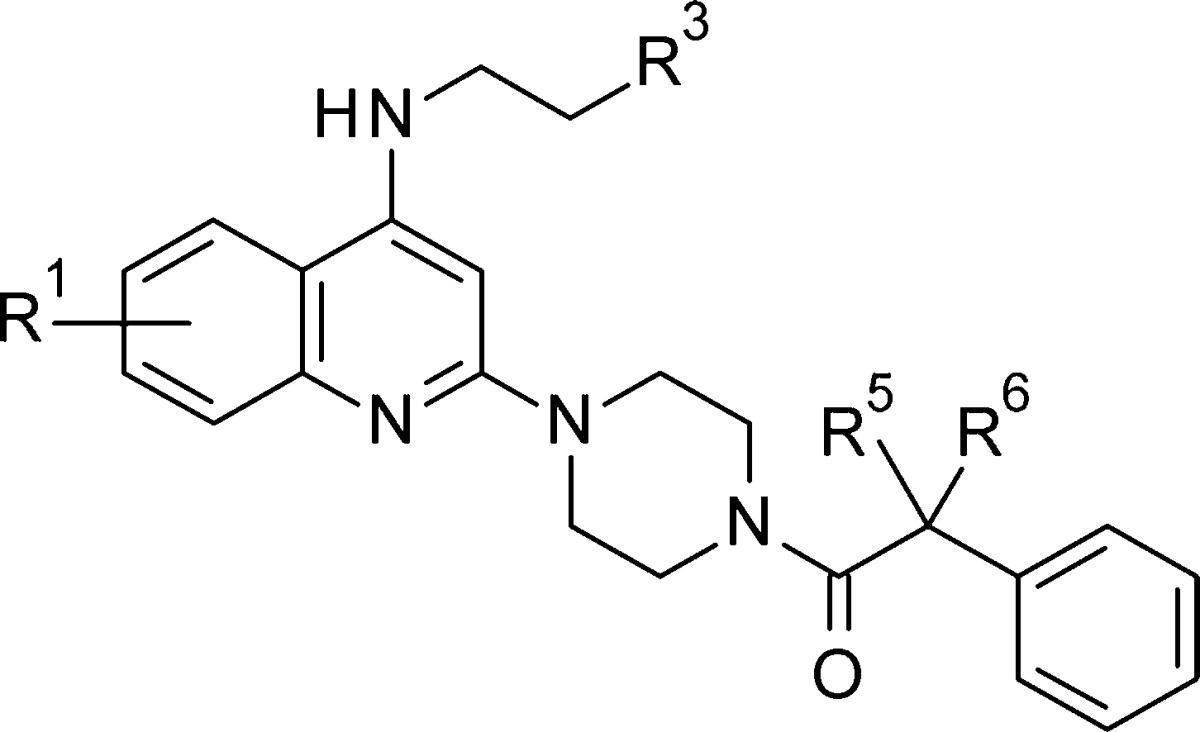
| compds | R1 | R3 | R5 | R6 | EC50 (μM) | CC50 (μM) |
|---|---|---|---|---|---|---|
| 43 | H | NH2 | CH3 | H | 0.091 | >100 |
| 44 | H | NH2 | Et | H | 0.556 | >100 |
| 45 | H | NH2 | CH3 | CH3 | 0.028 | >100 |
| 46 | H | NH2 | Et | Et | 0.624 | >100 |
| 47 | H | NH2 | (CH2)2 | 0.017 | >100 | |
| 48 | H | NH2 | (CH2)3 | 0.086 | >100 | |
| 49 | H | NH2 | (CH2)4 | 3.820 | >100 | |
| 50 | H | NHMe | CH3 | CH3 | 0.033 | >100 |
| 51 | H | NMe2 | CH3 | CH3 | 0.929 | 55.18 |
| 52 | H | NHAc | CH3 | CH3 | 15.580 | >100 |
| 53 | H | OH | CH3 | CH3 | 0.322 | >100 |
| 54 | 5-Cl | NH2 | CH3 | CH3 | 7.955 | 20.83 |
| 55 | 6-Cl | NH2 | CH3 | CH3 | 0.099 | 20.83 |
| 56 | 7-Cl | NH2 | CH3 | CH3 | 2.189 | 20.89 |
The basic amine at the head portion is crucial for anti-RSV activity. Reducing the basicity of the terminal amine by acylation (52) led to a more than 550-fold reduction of anti-RSV activity (EC50 = 15.58 μM) compared to 45. When the terminal basic NH2 of 45 was replaced by OH (53), the anti-RSV activity dropped more than 10-fold (EC50 = 0.322 μM). N-Methylation of 45 was allowed. The obtained compound 50 showed a similar activity (EC50 = 0.033 μM) as 45. But the more bulky terminal tertiary amine was not well tolerated (compound 51, EC50 = 0.929 μM).
Finally, we studied the Cl walk on the 5-, 6-, or 7- position of quinoline. The order of anti-RSV activity was 6-Cl > 7-Cl > 5-Cl.
A plaque reduction assay was performed to determine the direct anti-RSV effect. Compound 45 inhibits RSV replication with IC50 at lower than 100 nM concentration (Figure SI-1). Compound 50 can also directly inhibit RSV replication although it was less potent compared with compound 45 (Figure SI-1). Both compound 45 and 50 were proven to be RSV fusion inhibitors, which can prevent the fusion process mediated by RSV fusion protein in RSV-F expressing cell lines (Figure SI-2).
The DMPK properties of selected piperazinylquinoline-based RSV fusion inhibitors were evaluated in male Wistar rats by intravenous and oral administration. Analogues 45 and 50 showed high plasma clearance in rats despite their good microsomal stability (Table 4). On the other hand, both analogues had good oral bioavailability of >40%, in part due to the good solubility (LYSA > 400 μg/mL) and permeability (PAMPA values of 45 and 50 are 0.74 × 10–6 cm/s and 1.37 × 10–6 cm/s). The half-life of compound 45 is 3 h with a high volume of distribution (36.9 L kg–1). Furthermore, analogue 50, with a terminal methyl substitution, exhibited a longer half-life (7.3 h) and a higher volume of distribution (67.9 L kg–1). It is possible that the basic terminal aminoethyl moiety could play a role in the observed tissue retention.22
Table 4. DMPK Profiles of RSV Fusion Inhibitorsa,b.
| 45 | 50 | |
|---|---|---|
| HLM (mL min–1 kg–1) | 8.4 | 5.7 |
| RLM (mL min–1 kg–1) | 16.6 | 18.0 |
| CL (mL min–1 kg–1) | 222.0 | 142.6 |
| Vss (L kg–1) | 36.9 | 67.9 |
| T1/2 (h) | 3.0 | 7.3 |
| F (%) | 57.0 | 41.0 |
Scaled intrinsic clearance of compounds in human liver microsome (HLM) and rat liver microsome (RLM). Experiments were run in duplicate.
The single-dose pharmacokinetics (SDPK) study in male Wistar rats was carried out according to the standard procedures. Major parameters, including plasma clearance (CL), T1/2 (i.v.), Vss (i.v.), and oral bioavailability (F) are reported.
In the preparation of this manuscript, the crystal structures of SM RSV F fusion inhibitors in complex with RSV F glycoprotein were published.23 The SM RSV fusion inhibitors, such as JNJ-2408068 and BMS-433771, were reported to bind to a trisymmetric pocket in the central cavity of RSV F at the prefusion state.24 The stabilized prefusion conformation by SM RSV fusion inhibitor blocked further conformational change of RSV fusion and prevented the fusion process. The authors hypothesized that all SM RSV fusion inhibitors might bind to the same binding pocket to prevent the fusion process. Since our piperazinylquinoline molecules were identified as RSV F inhibitors, they were assumed to bind to the same trisymmetric pocket on the RSV fusion protein. The docking models with compound 7 (hit) and compound 45 were presented in Figure SI-3 and Figure SI-4. It was found that the quinoline ring and the piperidine ring of compound 7 or the piperazine ring of compound 45 could form the hydrophobic interaction with the residues of Phe140 and Phe488, respectively. And the positively charged terminal amine was found to form a salt bridge with Asp486, which is critical for the binding. These findings matched the key points discussed in the paper. Also other findings, such as the quinoline ring in a shallow and closed binding area and the terminal phenyl ring in the exposed area can explain the SAR of piperazinylquinoline molecules. To confirm whether our piperazinylquinoline compounds bind to the trisymmetric pocket at the prefusion state, we need to obtain the cocrystal structure, which is already in our plan.
In summary, a novel series of piperazinylquinoline anti-RSV fusion protein inhibitors were discovered by a ligand-based screening. Starting from hit 7, elongation of R2 by insertion of ethanone between piperazine and Ph improved the potency by 9-fold (22 vs 7). Further optimization of R2 led to the most potent compounds, 45 and 50. It was also proven that the basic terminal groups were crucial for anti-RSV activity. 45 and 50 showed potent antiviral activities in plaque reduction. Both of them can also potently inhibit cell–cell fusion in RSV-F expressing cells, which indicated that they were RSV fusion inhibitors. They also showed promising DMPK properties. In conclusion, the orally bioavailable piperazinylquinolines were discovered as a lead series with potential to evolve into anti-RSV drugs.
Acknowledgments
We are grateful to Guozhi Tang for comments on this manuscript. We also thank Rong Zhao, Yuxia Zhang, Yi Zhang, Hongxia Qiu, Xiaoyue Wu, and Sheng Zhong for carrying out the physicochemical, metabolic stability, and PK studies.
Glossary
Abbreviations
- HLM
human liver microsomal test
- LYSA
lyophilization solubility assay
- DMPK
drug metabolism and pharmacokinetics
- MLM
mouse liver microsomal test
- RSV
respiratory syncytial virus
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.5b00234.
Virtual screening, biological assays, synthetic procedures, and analytical data for selected compounds and docking model (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Borchers A. T.; Chang C.; Gershwin M. E.; Gershwin L. J. Respiratory syncytial virus-a comprehensive review. Clin. Rev. Allergy Immunol. 2013, 45, 331–379. 10.1007/s12016-013-8368-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough A. Respiratory Syncytial Virus Infection: Clinical Features, Management and Prophylaxis. Curr. Opin. Pulm. Med. 2002, 8, 214–217. 10.1097/00063198-200205000-00011. [DOI] [PubMed] [Google Scholar]
- Piedimonte G.; Perez M. K. Alternative mechanisms for respiratory syncytial virus (RSV) infection and persistence: could RSV be transmitted through the placenta and persist into developing fetal lungs?. Curr. Opin. Pharmacol. 2014, 16, 82–88. 10.1016/j.coph.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright M.; Piedimonte G. Respiratory syncytial virus prevention and therapy: past, present, and future. Pediatr. Pulmonol. 2011, 46, 324–347. 10.1002/ppul.21377. [DOI] [PubMed] [Google Scholar]
- Sigurs N. Epidemiologic and clinical evidence of a respiratory syncytial viruss reactive airways disease link. Am. J. Respir. Crit. Care Med. 2001, 163 (no 3), S2–6. 10.1164/ajrccm.163.supplement_1.2011109. [DOI] [PubMed] [Google Scholar]
- Falsey A. R.; Hennessey P. A.; Formica M. A.; Cox C.; Walsh E. E. Respiratory syncytial virus infection in elderly and high-risk adults. N. Engl. J. Med. 2005, 352 (17), 1749–1759. 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- Boeckh M.; Englund J.; Li Y.; Miller C.; Cross A.; Fernandez H.; Kuypers J.; Kim H.; Gnann J.; Whitley R. Randomized controlled multicenter trial of aerosolized ribavirin for respiratory syncytial virus upper respiratory tract infection in hematopoietic cell transplant recipients. Clin. Infect. Dis. 2007, 44, 245–249. 10.1086/509930. [DOI] [PubMed] [Google Scholar]
- Ventre K.; Randolph A. G.. Ribavirin for respiratory syncytial virus infection of the lower respiratory tract in infants and young children. Cochrane Database Syst. Rev. 2010, DOI: 10.1002/14651858.CD000181.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The IMpact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics 1998, 102, 531–537. 10.1542/peds.102.3.531 [DOI] [PubMed] [Google Scholar]
- Zhao X.; Singh M.; Malashkevich V. N.; Kim P. S. Structural characterization of the human respiratory syncytial virus fusion protein core. Proc. Natl. Acad. Sci. U. S. A. 2000, 97, 14172–14177. 10.1073/pnas.260499197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding W. D.; Mitsner B.; Krishnamurthy G.; Aulabaugh A.; Hess C. D.; Zaccardi J.; Cutler M.; Feld B.; Gazumyan A.; Raifeld Y.; Antonia Nikitenko A.; Lang S. A.; Gluzman Y.; O’Hara B.; Ellestad G. A. Novel and specific respiratory syncytial virus inhibitors that target virus fusion. J. Med. Chem. 1998, 41, 2671–2675. 10.1021/jm980239e. [DOI] [PubMed] [Google Scholar]
- Andries K.; Moeremans M.; Gevers T.; Willebrords R.; Sommen C.; Lacrampe J.; Janssens F.; Wyde P. R. Substituted benzimidazoles with nanomolar activity against respiratory syncytial virus. Antiviral Res. 2003, 60, 209–219. 10.1016/j.antiviral.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Bonfanti J. F.; Meyer C.; Doublet F.; Fortin J.; Muller P.; Queguiner L.; Gevers T.; Janssens P.; Szel H.; Willebrords R.; Timmerman P.; Wuyts K.; van Remoortere P.; Janssens F.; Wigerinck P.; Andries K. Selection of a respiratory syncytial virus fusion inhibitor clinical candidate. 2. Discovery of a morpholinopropylaminobenzimidazole derivative (TMC353121). J. Med. Chem. 2008, 51, 875–896. 10.1021/jm701284j. [DOI] [PubMed] [Google Scholar]
- Yu K. L.; Sin N.; Civiello R. L.; Wang X. A.; Combrink K. D.; Gulgeze H. B.; et al. Respiratory syncytial virus fusion inhibitors. Part 4: Optimization for oral bioavailability. Bioorg. Med. Chem. Lett. 2007, 17, 895–901. 10.1016/j.bmcl.2006.11.063. [DOI] [PubMed] [Google Scholar]
- Sun Z.; Pan Y.; Jiang S.; Lu L. Respiratory syncytial virus entry inhibitors targeting the F protein. Viruses 2013, 5, 211–225. 10.3390/v5010211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackman R. L.; Sangi M.; Sperandio D.; Parrish J. P.; Eisenberg E.; Perron M.; et al. Discovery of an oral respiratory syncytial virus (RSV) fusion inhibitor (GS-5806) and clinical proof of concept in a human RSV challenge study. J. Med. Chem. 2015, 58, 1630–1643. 10.1021/jm5017768. [DOI] [PubMed] [Google Scholar]
- Douglas J. L.; Panis M. L.; Ho E.; Lin K. Y.; Krawczyk S. H.; Grant D. M.; Cai R.; Swaminathan S.; Cihlar T. Inhibition of respiratory syncytial virus fusion by the small molecule VP-14637 via specific interactions with F protein. J. Virol. 2003, 77, 5054–5064. 10.1128/JVI.77.9.5054-5064.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S.; Hong D.; Wang B. X.; Zheng X. F.; Miao K.; Wang L. S.; Yun H. Y.; Gao L.; Zhao S. H.; Shen H. C. Discovery of imidazopyridine derivatives as highly potent respiratory syncytial virus fusion inhibitors. ACS Med. Chem. Lett. 2015, 6, 359–362. 10.1021/acsmedchemlett.5b00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. D.; Downs G. M.; Willett P.; Cook A. P. F. A hyperstructure model for chemical structure handling: generation and atom-by-atom searching of hyperstructures. J. Chem. Inf. Model. 1992, 32, 522–531. 10.1021/ci00009a020. [DOI] [Google Scholar]
- Hawkins P. C. D.; Skillman A. G.; Nicholls A. Comparison of shape-matching and docking as virtual screening tools. J. Med. Chem. 2007, 50, 74–82. 10.1021/jm0603365. [DOI] [PubMed] [Google Scholar]
- Cianci C.; Yu K.-L.; Combrink K.; Sin N.; Pearce B.; Wang A.; Civiello R.; Voss S.; Luo G.; Kadow K.; Genovesi E. V.; Venables B.; Gulgeze H.; Trehan A.; James J.; Lamb L.; Medina I.; Roach J.; Yang Z.; Zadjura L.; Colonno R.; Meanwell N.; Krystal M. Orally active fusion inhibitor of respiratory syncytial virus. Antimicrob. Agents Chemother. 2004, 48, 413–422. 10.1128/AAC.48.2.413-422.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. A.; Beaumont K.; Maurer T. S.; Di L. Volume of distribution in drug design. J. Med. Chem. 2015, 58, 5691–5698. 10.1021/acs.jmedchem.5b00201. [DOI] [PubMed] [Google Scholar]
- Battles M. B.; Langedijk J. P.; Furmanova-Hollenstein P.; Chaiwatpongsakorn S.; Costello H. M.; Kwanten L.; Vranckx L.; Vink P.; Jaensch S.; Jonckers T. H. M.; Koul A.; Arnoult E.; Peeples M. E.; Roymans D.; McLellan J. S. Molecular mechanism of respiratory syncytial virus fusion inhibitors. Nat. Chem. Biol. 2016, 12, 87–93. 10.1038/nchembio.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan J. S.; Chen M.; Leung S.; Graepel K. W.; Du X.; Yang Y.; Zhou T.; Baxa U.; Yasuda E.; Beaumont T.; Kumar A.; Modjarrad K.; Zheng Z.; Zhao M.; Xia N.; Kwong P. D.; Graham B. S. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science 2013, 340, 1113–1117. 10.1126/science.1234914. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.