Abstract
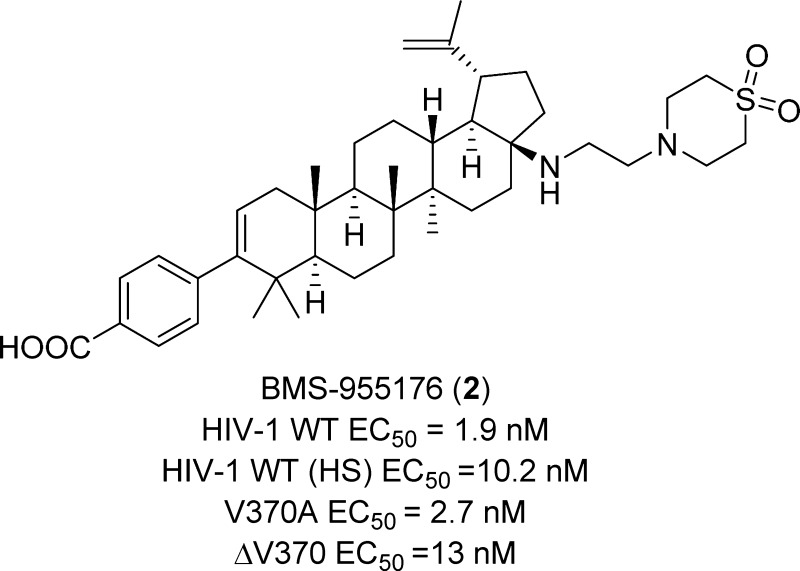
HIV-1 maturation inhibition (MI) has been clinically validated as an approach to the control of HIV-1 infection. However, identifying an MI with both broad polymorphic spectrum coverage and good oral exposure has been challenging. Herein, we describe the design, synthesis, and preclinical characterization of a potent, orally active, second generation HIV-1 MI, BMS-955176 (2), which is currently in Phase IIb clinical trials as part of a combination antiretroviral regimen.
Keywords: HIV-1, maturation inhibitors, BMS-955176, betulinic acid, triterpene, antiviral
HIV-1 infection can be effectively managed with current treatments that rely on long-term administration of multiple antiviral inhibitors designated as combination antiretroviral therapy (cART).1 Nevertheless, the development of novel agents with improved safety profiles and different mechanisms of action (MOA) is still highly desirable due to long-term toxicity and resistance development with current therapies. New ARTs would preferentially exhibit minimal drug–drug interactions with a relatively low once-daily dose to facilitate combinations with other agents as part of a fixed dose regimen.2
In the latter stages of the virus life cycle, the HIV-1 matrix (MA), capsid (CA), nucleocapsid (NC), and p6 proteins are released from the Gag polyprotein via multiple cleavages made by the viral protease. The final and rate-limiting step in this maturation process is cleavage at the CA-SP1 junction, which induces a major structural change within the virion, leading to the formation of the conical core that is characteristic of an infectious virion. Maturation inhibitors (MIs) prevent replication by binding near the CA-SP1 segment of the Gag polyprotein, specifically blocking only this final rate-limiting cleavage event, leading to the production of immature, noninfectious particles.3,4
Proof-of-concept for this MOA was demonstrated in a 10 day Phase I/II monotherapy trial with bevirimat,(1, Chart 1), an MI derived from betulinic acid (BA, 10).5,6 At the highest doses (150 and 250 mg), a maximal viral load reduction (VLR) of 0.72 log10 was observed. However, in the Phase IIa trial with highly experienced patients, only 45% of individuals responded to treatment with an HIV-1 RNA change from baseline of >0.5 log10, and only 34% with >1 log10 VLR.7 The restricted response to 1 was subsequently determined to be a consequence of naturally occurring polymorphic variation at Gag amino acid residues 369–371.8−10 The lack of polymorphic coverage in combination with difficulties in formulation ultimately led to a halt in the development of 1.11,12 Three other modified triterpenoid MIs have been evaluated in humans, but none of these compounds have advanced beyond Phase I clinical studies.13−15
Chart 1. Selected HIV-1 Maturation Inhibitors.
In order to drive the discovery of a second generation MI with broad polymorphic coverage, it was important to maintain activity against the key polymorphic variation at V370.16 Therefore, we chose the V370A- and ΔV370-containing viruses as the primary screening viruses due to their high prevalence in B and non-B subtypes, respectively, and their high level resistance to BVM.17 Additional improvements in a second generation MI would be lower binding to human serum components that decreased the effective potency of 1 (human serum (HS) binding >99%)17 and an improvement in physical properties that would allow for an appropriate formulation that could deliver targeted concentrations in vivo. Herein, we describe the discovery of BMS-955176 (2), an orally efficacious, second generation HIV-1 maturation inhibitor that is currently in Phase IIb clinical trials.18 The complete virology profile of BMS-955176 (2) is described in a companion manuscript, and resistance studies have been completed and will be published elsewhere.17
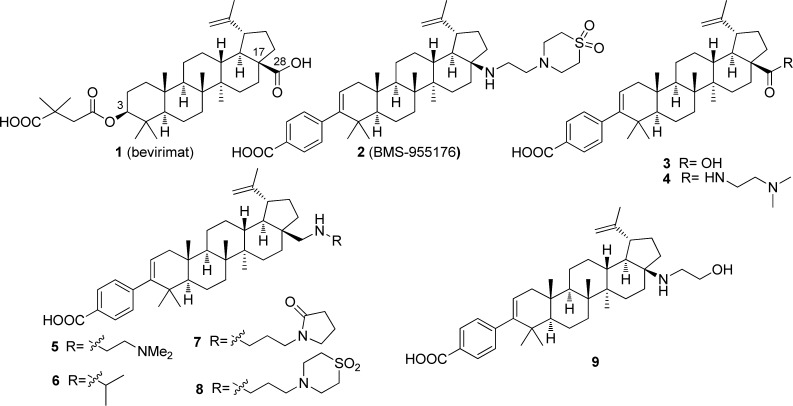
The description of the effect of Gag polymorphisms on the antiviral response to bevirimat (1) stimulated our interest in maturation inhibition as a target, since this identified a screening paradigm, but there was no evidence in the literature that structural modification of 1 could address the fundamental problem. As the first step in our study of this chemotype, we explored changes to the succinic acid-based side chain at C-3, a moiety that appeared to have largely been accepted as a pharmacophoric element, with a view to revealing key aspects of the topography of the carboxylic acid while providing new opportunities for structural modification.19,20 The benzoic acid derivative 3 emerged very quickly from that effort, exhibiting good antiviral activity against wild-type (WT) virus, a modest 7-fold shift in the presence of HS, and good oral exposure in rats (Table 1). The para-substitution pattern in 3 was critical for antiviral activity. However, potency against Gag V370A, the most prevalent polymorphism in the region of Gag associated with MI activity, was lacking.19
Table 1. HIV-1 Inhibition and Rat AUC0-6h of Compounds 1–9.
|
WT EC50(nM)a |
||||||
|---|---|---|---|---|---|---|
| WT | WT + HSb | V370A | ΔV370 | CC50(μM) | Rat AUC0–6h(nM*h)c | |
| 1 | 10 ± 11 | 1,291 ± 1,011 | 552 ± 633 | >10,000 | 16.8 ± 14.5 | 6,390 |
| 2 | 1.9 ± 1.8 | 10.2 ± 6 | 2.7 ± 1.5 | 13 ± 11 | 9.2 ± 4 | 4,132 ± 685d |
| 3 | 16 ± 13 | 105 ± 40 | 233 ± 305 | >6,000 | 27.2 ± 38.5 | 5,724 |
| 4 | 2.3 ± 1.3 | 43 ± 29 | 7.8 ± 8.8 | 31 ± 19 | 2.61 ± 1.78 | 1, 623 ± 240d |
| 5 | 2.2 ± 0.7 | 8.7 | 2.5 ± 2.2 | 20.6 ± 14 | 1.24 ± 0.16 | 153 |
| 6 | 2.2 | - | 12 | 61 | 2.36 | - |
| 7 | 0.7 ± 0.2 | - | 1.7 ± 0.7 | 9.9 ± 8.3 | 5.8 ± 0.8 | 1,044 |
| 8 | 1.0 ± 0.4 | 5.7 ± 3.1 | 2.5 ± 1.4 | 14.566 ± 8.4 | 3.5 ± 1.6 | 1,708 |
| 9 | 1.9 ± 1.2 | - | 9.9 ± 2.8 | 66 ± 53 | 8.48 ± 5.34 | 4,983 |
The antiviral activity of the compounds was assessed in a multiple cycle assay in MT-2 cells in 10% fetal bovine serum (FBS).
Compounds evaluated for serum effects in 10% FBS + 40% human serum except for 2 (10% FBS + 40% human serum + 27 mg/mL human serum albumin); values are means from experiments performed a minimum of three times (n ≥ 3).
Dose 5 mg/kg po, n = 2; see SI for vehicle information.
n = 3.
Attention was then directed toward derivatization of the C-28 position of 3, where an extensive series of analogues were prepared that incorporated a wide range of functionality. Amides such as 4, incorporating a basic side chain, provided excellent potency against both WT and V370A viruses, while maintaining a low human serum shift (∼4-fold). In addition, 4 exhibited an EC50 of 31 nM against the ΔV370 Gag polymorphism. However, 4 exhibited low oral exposure, attributed to a combination of poor solubility and low membrane permeability, precluding its further advancement.20 With further probing of this region, the dibasic C-28 amine 5 was found to retain good polymorphic coverage, but displayed even lower oral exposure than 4, while the more lipophilic monobasic isopropyl amine 6 exhibited 3- to 5-fold reduced activity toward the polymorphic viruses, demonstrating that a single amine installed close to the core was acceptable.21
Based on this discovery, the introduction of a nonbasic polar group in the side chain was probed with 7, which improved the antiviral profile of 6, with EC50 values of <10 nM against the 3 screening viruses (Table 1).22 However, while this combination of basicity and polarity afforded good polymorphic coverage, poor oral exposure in rats (AUC0–6h of 7 in rats =1,044 nM·h) remained an issue. The introduction of a moderately basic amine, 1,1-dioxidothiomorpholine heterocycle, pKa = 4.5,23 provided 8, which demonstrated targeted antiviral activity and improved oral exposure in rats, although still 2- to 3-fold less than for 1 and 3. We hypothesized that installing the more basic amine closer to the lipophilic core would have the effect of shielding the NH and may have a positive impact on the permeability properties of the molecule and, ultimately, on oral exposure. This concept was initially probed with the alcohol 9, which exhibited improved oral exposure (AUC0–6h in rats = 4,983 nM·h), although at the expense of reduced activity toward the polymorphic viruses, particularly ΔV370.24 With further experimentation, merging the C-17 amine with the 1,1-dioxidothiomorpholine heterocycle of 8 provided BMS-955176 (2), a compound with an optimal combination of antiviral potency and oral exposure. This compound exhibits excellent potency toward the key Gag mutations, with EC50 values of 1.9, 2.7, and 13 nM against WT, V370A, and ΔV370 viruses, respectively. Furthermore, following oral dosing of 5 mg/kg of BMS-955176 to rats, the AUC0–6h was 4,132 nM·h, demonstrating exposure comparable to 1 (Table 1). In the presence of HS, BMS-955176 exhibited a ∼5-fold reduction in antiviral activity, reflecting moderate serum binding (determined to be 84% using an ultracentrifugation method).17 The potent antiviral activity of 2 extended to a library of clinically relevant isolates (N = 87), representing sequence variability in the 362–370 Gag region of subtype B viruses, with a mean EC50 value of 3.9 ± 3.4 nM.17 In addition, other site directed Gag polymorphisms known to be resistant to 1 were introduced into a laboratory strain, with the results for a select group compiled in Table 2, illustrating that 2 retains activity toward all of these viruses.17 BMS-955176 was shown to be an MI by a series of mechanistic experiments which ruled out entry, reverse transcription, and protease as targets, while demonstrating that 2 acts late in the viral life cycle. The compound binds saturably and reversibly to HIV-1 Gag pseudoparticles and this binding is specifically displaced by 1. In addition, 2 specifically inhibits cleavage of CA/SP1 p25, resulting in inhibition of the production of capsid p24.17
Table 2. Antiviral Activity of Bevirimat (1) and BMS-955176 (2) toward HIV-1 Viruses Containing Naturally-Occurring Polymorphisms in the Gag Protein17,a.
|
EC50 |
||||||
|---|---|---|---|---|---|---|
| V362I | Q369H | V370M | V370A/ΔT371 | ΔV371A | ΔT371 | |
| bevirimat (1) | 74 ± 59 | 7.0 ± 2.0 | 1,810 ± 190 | 1,114 ± 119 | 40 ± 48 | 77 ± 97 |
| BMS-955176 (2) | 4.5 ± 2.2 | 1.9 ± 0.9 | 2.8 ± 0.3 | 3.6 ± 3.9 | 2.0 ± 0.1 | 7.3 ± 3.9 |
Values are means from experiments performed a minimum of three times (n ≥ 3).
The metabolic stability of 2 in vitro was high, with a t1/2 of >120 min when incubated in human, rat, and dog liver microsomes in the presence of NADPH, predicting low clearance in vivo. However, in monkey liver microsomes, 2 exhibited high metabolic turnover, with a t1/2 of only 12 min. It was not possible to accurately assess the in vitro permeability of 2 due to limited solubility under the assay conditions, but it is presumed to be satisfactory, since 2 demonstrated good oral exposure in rats, with an AUCtotal of 14,198 nM·h after a dose of 5 mg/kg (Table 3). In this experiment, the C24 of 2 was 128 nM, 21-fold higher than that of 1 under comparable circumstances. The oral bioavailability (%F) of 2 ranged from low in the dog and cynomologus monkey, reflecting the reduced metabolic stability in the latter species, to moderate in mice and rats, with long t1/2 values in all species tested. Allometric scaling and mean residence time methodology using data from all four species were used to predict the human PK profile of 2, which, combined with a targeted Ctrough value of 281 nM (3-fold the protein binding-adjusted EC90 vs the ΔV370 virus), indicated that 2 may be suitable for once-daily administration of reasonable low doses (≤120 mg). Efficacy at these doses has been confirmed in clinical studies.25 Safety profiling of BMS-955176 showed no evidence of mutagenicity in an Ames reverse mutation assay, and low potential for off-target liabilities based on evaluation in a panel of receptor, ion channel, and enzyme activity assays. There was also a low potential for drug–drug interactions based on CYP450 human microsomal inhibition assays.
Table 3. In Vivo Pharmacokinetic Properties of BMS-955176 in Mice,a Rats,b Dogs,c and Cynomolgus Monkeysd.
| F (%) | AUCtotal (nM·h) | C24 (nM) | IV t1/2 (h) | |
|---|---|---|---|---|
| Mouse | 27 | 28,912 | 256 ± 78 | 8.9 |
| Rat | 26 | 14,198 ± 1,083 | 128 ± 27 | 6.6 |
| Dog | 8.5 | 29,358 ± 16,436 | 559 ± 345 | 31.7 |
| Cyno | 3.9 | 995 ± 318 | 17.4 ± 10.9 | 8.6 |
Oral vehicle (solution): 90% polyethylene glycol 300 (PEG 300), 10% EtOH, n = 3; dose = 5 mg/kg PO.
Oral vehicle (solution): 84.5% PEG 300, 10% EtOH, 50.1 N NaOH and 0.5% Tween 80 (TW80), n = 3; dose = 5 mg/kg PO.
Oral vehicle (solution): 80% 50 mM citrate buffer; 20% sulfobutylether-beta-cyclodextrin (SBC), pH = 3, n = 3; dose = 1.85 mg/kg.
Oral vehicle (solution): 80% 50 mM citrate buffer; 20% SBC, pH = 3, n = 3; dose = 2 mg/kg.
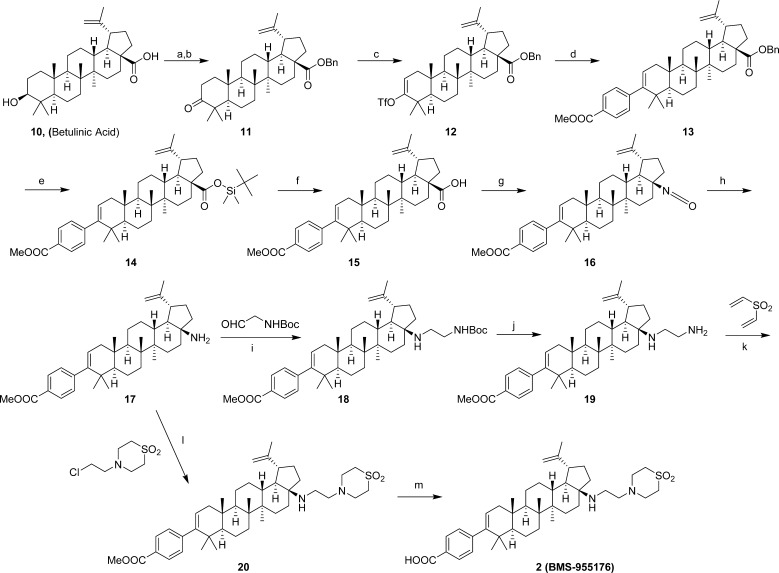
The synthesis of 2 (BMS-955176) is shown in Scheme 1.22 Commercially available 10 was treated with benzyl bromide in the presence of K2CO3, followed by oxidation with PCC to afford ketone 11, which was converted into the enol triflate 12. Suzuki coupling of 12 with (4-(methoxycarbonyl)phenyl)boronic acid afforded 13. The C-28 carboxylic acid in 13 was selectively deprotected using tert-butyldimethylsilane in the presence of Pd(OAc)2 to afford silyl ester 14, which upon treatment with TBAF generated 15. Curtius rearrangement of 15 using DPPA afforded the C-17 primary amine 17 in a process that could be carried out either in a single operation or stepwise via isolation of the corresponding C-28 isocyanate 16. Derivatization of the primary amine in 17 turned out to be a challenge, presumably due to a combination of steric hindrance and decreased nucleophilicity of the N atom caused by its proximity to the steroid core. A 3-step procedure was initially developed to install the side chain present in 2 via reductive amination of 17 using tert-butyl 2-oxoethylcarbamate in the presence of NaBH(OAc)3 and Ti(OiPr)4, which rendered 18. The BOC protecting group was removed using HCl to provide the C-17 primary amine 19. In situ trapping of 19 via a double Michael addition to divinylsulfone afforded 20, with an overall yield of 38% for the 3-step procedure. Ultimately, the reductive amination/hydrolysis/Michael addition sequence was replaced by a one-step procedure in which 17 was alkylated with 4-(2-chloroethyl)thiomorpholine 1,1-dioxide to afford 20 in 73% yield. This combination of base and solvent appeared to be optimal in order to achieve the complete conversion of 17 to 20. Final hydrolysis of 20 to unmask the carboxylic acid was performed with aqueous NaOH to afford 2 (BMS-955176). Overall, 2 was prepared in 10 steps from 10 with a total yield of 20%.
Scheme 1. Synthesis of 2 (BMS-955176).
Reagents and conditions: (a) K2CO3, BnBr, DMF, 60 °C, 3.5 h, 99%; (b) PCC, CH2Cl2, 6 h, 96%; (c) KHMDS, PhNTf2, THF, −78 °C, 4 h, 90%; (d) (4-(methoxycarbonyl)phenyl)boronic acid, Na2CO3, Pd(Ph3P)4, 1,4-dioxane/i-PrOH/H2O, reflux, 14.5 h, 68.1%; (e) TBDMSH, Pd(OAc)2, TEA, DCE, 60 °C, 2 h, 97%; (f) TBAF, H2O/1,4-dioxane, rt, 4 h, 99%; (g) DPPA, TEA, 1,4-dioxane, 100 °C, 5 h, 93%; (h) conc. HCl, THF, rt, 72 h, quant.; (i) Na(OAc)3BH, Ti(OiPr)4, DCE, rt, 19 h 79%; (j) HCl, 1,4-dioxane, rt, 4 h, 95%; (k) TEA, 1,4-dioxane/EtOH, 85 °C, 3 h, 91%; (l) K3PO4, KI, MeCN, 115–125 °C, 48 h, 73%; (m) NaOH, 1,4-dioxane, 78 °C, 3 h, 77%.
In conclusion, BMS-955176 is a potent HIV-1 inhibitor in cell culture that, unlike 1, exhibits broad spectrum antiviral effects that encompass the V370A- and ΔV370-containing polymorphic viruses. In addition, BMS-955176 exhibits low serum binding, which translates into a modest 5-fold effect on potency in vitro, and preclinical PK predictive of once-daily dosing in humans. In a Phase IIa clinical trial, 10-days of monotherapy with 2 administered daily to treatment-naive and treatment-experienced subjects infected with HIV-1 subtypes B or C was generally safe and well-tolerated and demonstrated >1 log10 reduction in viral RNA.18,25 BMS-955176 is currently being evaluated in a Phase IIb clinical study as a part of a treatment regimen with mechanistically different antiretroviral agents.
Acknowledgments
The authors would like to acknowledge the experimental support of the Bioanalytical group.
Glossary
Abbreviations
- AUC
area under the curve
- BA
betulinic acid
- CA
capsid
- cART
combination antiretroviral therapy
- Cyno
cynomologous monkey
- DCE
dichloroethane
- DCM
methylene chloride
- DME
1,2-dimethoxyethane
- DMF
dimethylformamide
- F
oral bioavailability
- FBS
fetal bovine serum
- HS
human serum
- KHMDS
potassium bis(trimethylsilyl)amide
- LANL
Los Alamos National Laboratory
- MA
matrix
- MI
maturation inhibitor
- MeOH
methanol
- MOA
mechanism of action
- NC
nucleocapsid
- NFV
nelfinavir
- PCC
pyridinium chlorochromate
- PEG
polyethylene glycol
- PK
pharmacokinetic
- PO
per os
- rt
room temperature
- SP
spacer peptide
- TBDMSH
tert-butyldimethylsilylhydride
- TEA
trimethylamine
- TFA
trifluoroacetic acid
- THF
tetrahydrofuran
- TW
tween
- VLR
viral load reduction
- WT
wild-type
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.6b00010.
Characterization data for key compounds and experimental procedures for the preparation of 2, as well as descriptions of biologic and pharmacokinetic assays (PDF)
# Deceased April eighth, 2016.
The authors declare the following competing financial interest: The authors were employees of Bristol-Myers Squibb at the time this work was completed.
Dedication
This article is dedicated to the memory of Beata Nowicka-Sans, who passed away on April 8th, 2016.
Supplementary Material
References
- Taiwo B.; Hicks C.; Eron J. Unmet therapeutic needs in the new era of combination antiretroviral therapy for HIV-1. J. Antimicrob. Chemother. 2010, 65, 1100–1107. 10.1093/jac/dkq096. [DOI] [PubMed] [Google Scholar]
- Pau A. K.; George J. M. Antiretroviral therapy. Infect. Dis. Clin. N. Am. 2014, 28, 371–402. 10.1016/j.idc.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundquist W. I.; Kräusslich H. G. HIV-1 assembly, budding, and maturation. Cold Spring Harbor Perspect. Med. 2012, 7, a006924. 10.1101/cshperspect.a006924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed E. O. HIV-1 assembly, release and maturation. Nat. Rev. Microbiol. 2015, 13, 484–496. 10.1038/nrmicro3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D. E.; Salzwedel K.; Allaway G. P. Bevirimat: a novel maturation inhibitor for the treatment of HIV-1 infection. Antiviral Chem. Chemother. 2008, 19, 107–113. 10.1177/095632020801900301. [DOI] [PubMed] [Google Scholar]
- Temesgen Z.; Feinberg J. E. Drug evaluation: bevirimat - HIV Gag protein and viral maturation inhibitor. Curr. Opin. Investig. Drugs 2006, 7, 759–765. [PubMed] [Google Scholar]
- Smith P. F.; Ogundele A.; Forrest J. W.; Salzweded K.; Doto J.; Allaway G. P.; Martin D. E. Phase I and II study of the safety, virologic effect, and pharmacokinetics/pharmacodynamics of single-dose 3-O-(3′3-dimethylsuccinyl)betulinic acid (bevirimat) against human immunodeficiency virus infection. Antimicrob. Agents Chemother. 2007, 51, 3574–3581. 10.1128/AAC.00152-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallister S.; Lalezari J.; Richmond G.; Thompson M.; Harrigan R.; Martin D.; Salzwedel K.; Allaway G. HIV-1 Gag polymorphisms determine treatment response to bevirimat (PA-457). Antiviral Ther. 2008, 13, A10. [Google Scholar]
- Van Baelen K. V.; Salzwedel K.; Rondelez E.; Veerle Van Eygen V. V.; De Vos S.; Verheyen A.; Steegen K.; Verlinden Y.; Allaway G. P.; Stuyver L. J. Susceptibility of human immunodeficiency virus type 1 to the maturation inhibitor bevirimat is modulated by baseline polymorphisms in Gag spacer peptide 1. Antimicrob. Agents Chemother. 2009, 53, 2185–2188. 10.1128/AAC.01650-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margot N. A.; Gibbs C. S.; Miller M. D. Phenotypic susceptibility to bevirimat among HIV-1 infected patient isolates without prior exposure to bevirimat. Antimicrob. Agents Chemother. 2010, 54, 2345–2353. 10.1128/AAC.01784-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob J.; Richards J.; Augustine J. G.; Milea J. S.. Liquid bevirimat dosage forms for oral administration. World Patent Application, WO 2009/042166 A1. November 5, 2009.
- Wainberg M. A.; Albert J. Can the further clinical development of bevirimat be justified?. AIDS 2010, 24, 773–774. 10.1097/QAD.0b013e328331c83b. [DOI] [PubMed] [Google Scholar]
- Kilgore N.; Reddick M.; Zuiderhof M.; Stanley D.; Nitz T.; Bullock P.; Allaway G.; Martin D.. Characterization of PA1050040, a second generation HIV-1 maturation inhibitor. Poster discussion: 4th IAS Conference on HIV Pathogenesis, Treatment and Prevention. Sydney, Australia July 22–25, 2007. Abstract no. MOPDX05.
- Baichwal V.; Austin H.; Brown B.; McKinnon R.; Yager K.; Kumar V.; Gerrish D.; Anderson M.; Carlson R.. Anti-viral characterization in vitro of a novel maturation inhibitor, MPC-9055. 16th Conference on Retroviruses and Opportunistic Infections, Montreal, Canada. February 8–11, 2009. Abstract #J-105.
- U.S. National Institutes of Health. A repeat dose pharmacokinetic (PK) and safety study of GSK2838232 with and without ritonavir (RTV) conducted in healthy subjects. https://www.clinicaltrials.gov/ct2/show/NCT02289495?term=GSK2838232&rank=1.
- Dang Z.; Ho P.; Zhu L.; Qian K.; Lee K.-H.; Huang L.; Chen C.-H. New betulinic acid derivatives for bevirimat-resistant human immunodeficiency virus type-1. J. Med. Chem. 2013, 56, 2029–2037. 10.1021/jm3016969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowicka-Sans B.; Protack T.; Lin Z.; Li Z.; Zhang S.; Samanta H.; Terry B.; Liu Z.; Chen Y.; Sin N.; Sit S.-Y.; Swidorski J. J.; Chen J.; Venables B. L.; Healy M.; Sun Y.; Meanwell N. A.; Cockett M.; Hanumegowda U.; Regueiro-Ren A.; Krystal M.; Dicker I. B. BMS-955176: identification and characterization of a second-generation HIV 1 maturation inhibitor with improved potency, anti-viral spectrum and Gag polymorphic coverage. Antimicrob. Agents Chemother. 2016, 10.1128/AAC.02560-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang C.; Schürmann D.; Shobotha C.; Sevinsky H.; Ravindran P.; Xiao H.; Ray N.; Krystal M.; Dicker I. B.; Lataillade M.. Antiviral activity/safety of a second generation HIV-1 maturation inhibitor. 22nd Conference on Retroviruses and Opportunistic Infections, Seattle, WA, February 23–26, 2015. Abstract #114LB.
- Liu Z.; Swidorski J. J.; Nowicka-Sans B.; Terry B.; Protack T.; Lin Z.; Samanta H.; Zhang S.; Li Z.; Rahematpura S.; Parker D. D.; Jenkins S.; Beno B. R.; Krystal M.; Meanwell N. A.; Dicker I. B.; Regueiro-Ren A. C-3 benzoic acid derivatives of C-3 deoxybetulinic acid and deoxybetuline as HIV-1 maturation inhibitors. Bioorg. Med. Chem. 2016, 24, 1757–1770. 10.1016/j.bmc.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Swidorski J. J.; Liu Z.; Sit S.-Y.; Chen J.; Chen Y.; Sin N.; Venables B. L.; Nowicka-Sans B.; Protack T.; Lin Z.; Terry B.; Zhang S.; Li Z.; Rahematpura S.; Parker D. D.; Jenkins S.; Krystal M.; Hanumegowda U.; Dicker I. B.; Meanwell N. A.; Regueiro-Ren A. Inhibitors of HIV-1 maturation: development of structure-activity relationships for C-28 amide derivatives of C-3 benzoic acid-modified triterpenoids. Bioorg. Med. Chem. Lett. 2016, 26, 1925–1930. 10.1016/j.bmcl.2016.03.019. [DOI] [PubMed] [Google Scholar]
- Urano E.; Alban S. D.; Mandt R.; Pauly G. T.; Sigano D. M.; Schneider J. P.; Martin D. E.; Nitz T. J.; Wild C. T.; Freed E. O. Antimicrob. Agents Chemother. 2016, 60, 190–197. 10.1128/AAC.02121-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regueiro-Ren A.; Swidorski J.; Sit S.; Chen Y.; Chen J.; Meanwell N. A.; Liu Z.; C-28 amines of C-3 modified betulinic acid derivatives as HIV maturation inhibitors. U.S. Patent 8,748,415, June 10, 2014.
- Martin R. E.; Plancq B.; Gavelle O.; Wagner B.; Fischer H.; Bendels S.; Müller K. ChemMedChem 2007, 2, 285–287. 10.1002/cmdc.200600265. [DOI] [PubMed] [Google Scholar]
- C-17 and C-3 modified triterpenoids with HIV maturation inhibitory activity. Regueiro-Ren A.; Liu Z.; Swidorski J.; Sin N.; Venables B. L.; Sit S.; Chen Y.; Chen J.; Meanwell N. A.; Modified C-3 betulinic acid derivatives as HIV maturation inhibitors. U.S. Patent 8,846,647, September 30, 2014.
- Hwang C.; Schartman R.; Sobotha C.; Boffito M.; Sevinsky H.; Ray N.; Ravindran P.; Xiao H.; Krystal M.; Dicker I.; Grasela D.; Lataillade M.. Overall antiviral activity and safety results from the Phase IIa proof-of-concept study (AI468002). 15th European AIDS Conference; October 21–24, 2015. Barcelona, Spain.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.