Abstract
A series of N1-azinylsulfonyl-3-(1,2,3,6,tetrahyrdopyridin-4-yl)-1H-indole derivatives was designed to obtain highly potent 5-HT6 receptor ligands. The study allowed for the identification of 25 (4-{[5-methoxy-3-(1,2,3,6-tetrahydropyridin-4-yl)-1H-indol-1-yl]sulfonyl}isoquinoline), a potent and selective 5-HT6 receptor antagonist. The selected compound, was evaluated in vivo in a novel object recognition (NOR) and forced swim (FST) tests in rats, demonstrating distinct pro-cognitive and antidepressant-like properties (MED = 1 mg/kg and 0.1 mg/kg, i.p., respectively). Compound SB-742457, used as comparator, reversed memory deficits in NOR task in similar doses, while in FST it was active in 10–30-fold higher dose (3 mg/kg). In contrast to SB-742457, which was active in Vogel test (MED = 3 mg/kg), compound 25 displayed no anxiolytic activity.
Keywords: Isoquinoline/quinoline sulfonamides, 5-HT6 receptor antagonist, Alzheimer’s disease, cognitive decline, novel object recognition task, forced swim test, Vogel test
The most devastating neurodegenerative form of dementia is Alzheimer’s disease (AD). The number of AD patients was estimated at 35.6 mln in 2012 and is expected to double every 20 years to 65.7 mln in 2030 and 115.4 in 2050. Current AD treatments provide only brief symptomatic relief and are based on the use of acetylcholinesterase inhibitors and NMDA receptor antagonist. One approach that has recently garnered significant interest is selective 5-HT6 receptor (5-HT6R) antagonism.1
The 5-HT6R belongs to the G-protein coupled receptor (GPCR) superfamily, and it stimulates adenylyl cyclase via Gs as well as extracellular-regulated kinase (ERK) signaling via the Src-family tyrosine kinase Fyn. Furthermore, 5-HT6R activates mTOR Complex 1 (mTORC1) in the prefrontal cortex, which is related to cognitive deficits,2 and the receptor constitutively activates Cdk5 to control cortical neurons migration and promote neurite growth.3
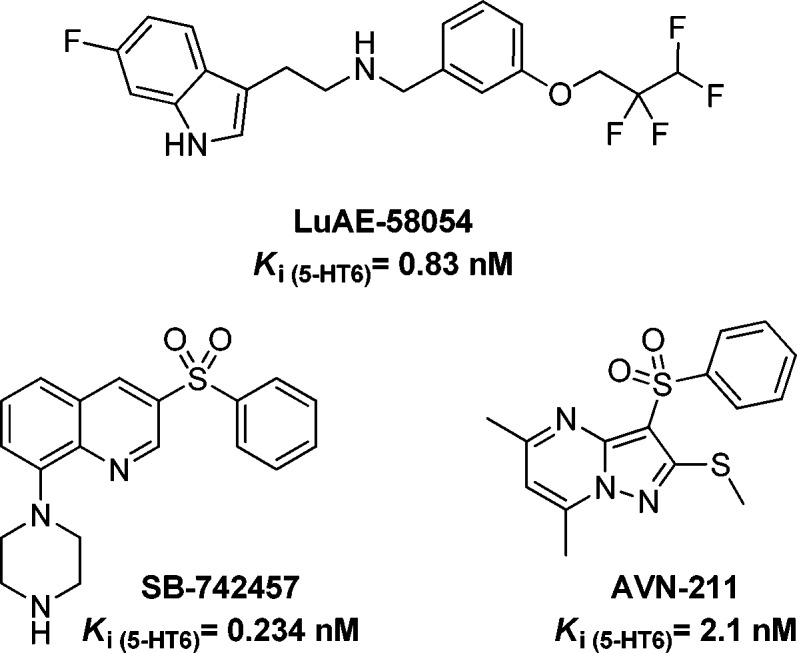
Blockade of 5-HT6R enhances cognitive performance in a broad range of tasks in rodents,4,5 and phase II clinical studies have demonstrated efficacy of 5-HT6R antagonists (Iladopirdine = LuAE58054, SB742457, and AVN-211, Figure 1) in conjunction with donepezil in mild-to-moderate AD patients. Currently, Iladopirdine is being evaluated in phase III clinical trials.6 The pro-cognitive effects of 5-HT6R antagonists are typically attributed to their ability to promote cortico-limbic release of acetylcholine, glutamate, and monoamines.7,8
Figure 1.
5-HT6R antagonists under clinical trials.
Further, 5-HT6R antagonists might be effective in the treatment of anxiety and depressive symptoms that often accompany AD. The results of in vivo tests have indicated that 5-HT6R antagonists produce antidepressant-like and anxiolytic-like responses in animal models.9
Almost exclusive localization of 5-HT6R in the cerebral cortex, nucleus accumbens, and hippocampus suggests that compounds, which selectively affect this receptor, might have relatively few peripheral side effects.
A vast group of 5-HT6R ligands is based on an indole scaffold.10,11 Structure–activity relationship studies within this class revealed that incorporating the tryptamine aminoethyl group into the constrained basic side chain of the tetrahydropyridynyl moiety and the sulfonylation at the N1 position on the indole scaffold is a good strategy for developing selective 5-HT6R antagonists. Many studies have investigated the effects of a kind of central aromatic core (indole, azaindole, indazole, benzoxazine, and tetrahydroquinoline), localization, and the nature of amino centers (tetrahydropyridine, piperazine, and pyrrolidine).12−14 However, the type of arylsulfonyl moiety has not been extensively explored considering azinyl (quinolinyl and isoquinolinyl) fragments.15
Herein we report on the synthesis of N1-azinylsulfonyl-3-(1,2,3,6-tetrahydropyridin-4-yl)-1H-indoles, in vitro biological evaluations, preliminary pharmacokinetic studies, and behavioral profiling in animal model of memory cognitive decline, i.e., novel object recognition (NOR) test. Since biochemical data suggest that neurotransmitters deficits in AD contribute to aggressive behavior, sleep disturbance, and depression, the compounds were also evaluated in the forced swim (FST) and Vogel tests to verify their potential antidepressant and anxiolytic properties.
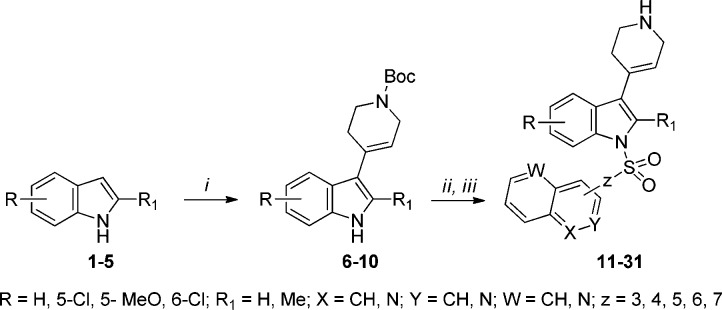
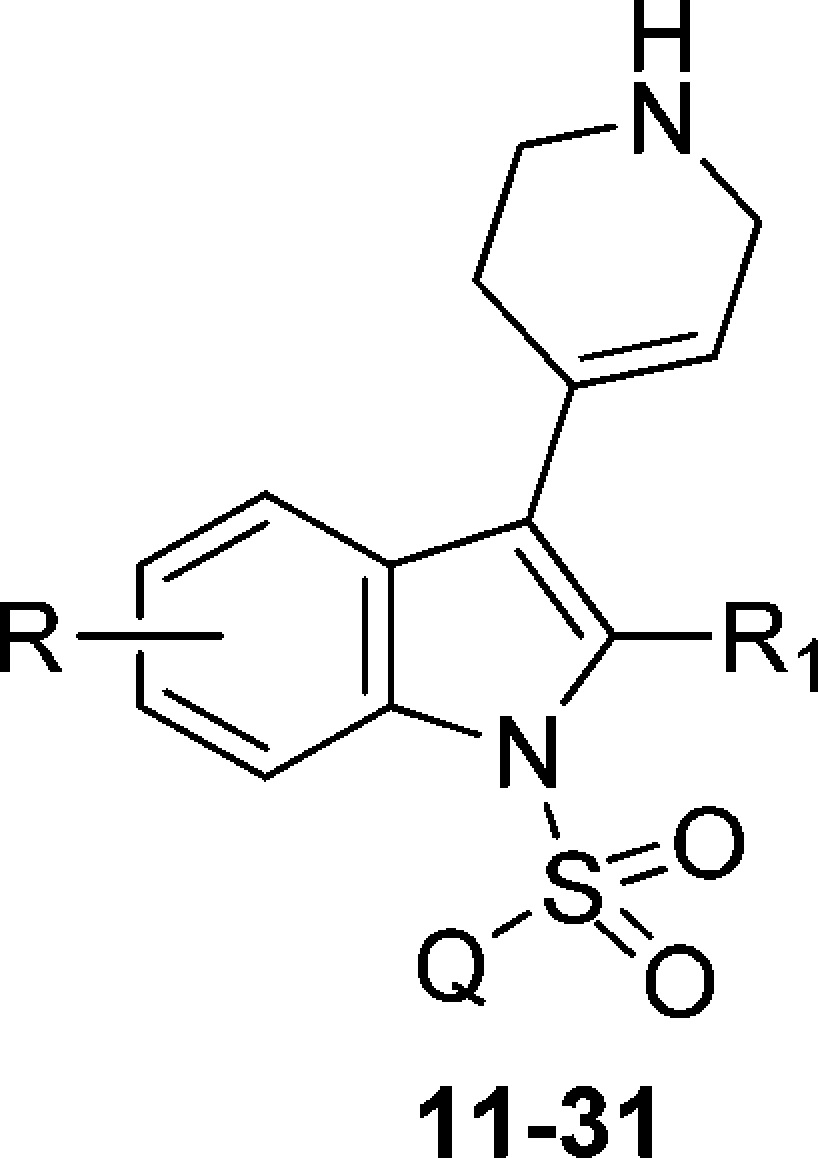
The final compounds 11–31 and 34–38 were synthesized as shown in Schemes 1 and 2. Condensation of differently substituted indole derivatives 1–5 with N-Boc-piperidone in the presence of potassium carbonate under refluxing methanol yielded intermediates 6–10. Next, strong, nonionic, basic conditions involving phosphazene base P1-t-Bu-tris(tetramethylene) (BTPP) in CH2Cl2 were employed for the N1-indole sulfonylation with the respective quinoline-/isoquinolinesulfonyl chlorides.16 Removal of the Boc-protecting group under stirring with a 1 N HCl solution in methanol gave the final compounds 11–31.
Scheme 1.
Reagents and conditions: i) KOH, MeOH, 60 °C, 12 h, 65–70%; ii) ArSO2Cl, BTPP, CH2Cl2, 0 °C, 2 h, 70–75%; iii) 1 N HCl in MeOH, r.t.
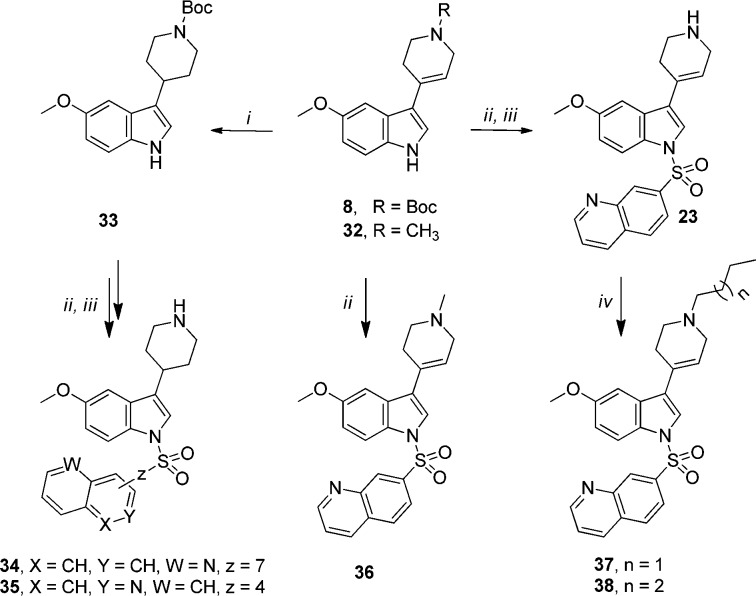
Scheme 2.
Reagents and conditions: i) H2, Pd/C, MeOH, r.t., 2 h, 75%; ii) ArSO2Cl, BTPP, CH2Cl2, 0 °C, 2 h, 73%; iii) 1 N HCl in MeOH, r.t.; iv) alkyl bromide, K2CO3, KI, acetone, 60 °C, 48 h, 63–67%.
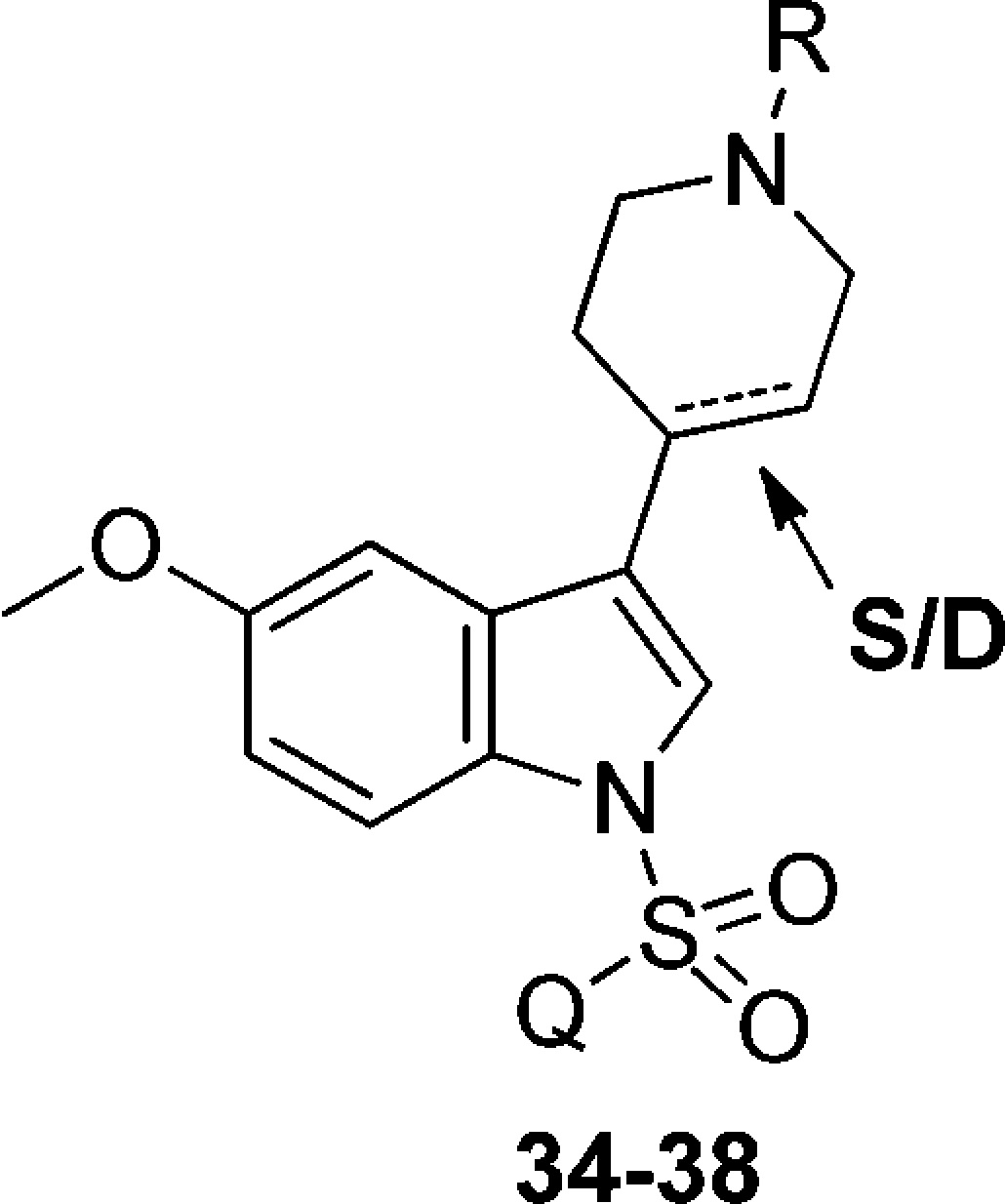
Alternatively, three-step procedure involving hydrogenation of intermediate 8 over palladium on carbon, followed by the treatment of intermediate 33 with azinylsulfonyl chloride and removal of Boc group in acidic conditions yielded piperidine analogues 34 and 35 (Scheme 2). However, condensation of 5-MeO-indole with N-methyl-piperidone resulted in intermediate 32, which was subsequently sulfonylated to obtain compound 36. Finally, the N-alkyl tetrahydropyridinyl analogues 37 and 38 were obtained by alkylation of compound 23 with the appropriate alkyl bromide under biphasic conditions.
N-Azinylsulfonyl derivatives 11–31 displayed high affinity for 5-HT6R (Table 1). Most importantly, replacement of naphthyl moiety present in compounds I and II(13) with azinyl fragment, apart from improvement of physicochemical properties (logD, logP, and pKa), has increased affinity for 5-HT6R. Thus, the contribution of the azinesulfonyl moiety to the compound affinity for 5-HT6R was subsequently investigated.
Table 1. Binding Data of the Synthesized Compounds 11–31 for 5-HT6R.
| compd | Q | R | R1 | 5-HT6Ki [nM]a |
|---|---|---|---|---|
| Ib | 2-napthtyl | H | H | 27 ± 6 |
| 11 | 3-quinolinyl | H | H | 13 ± 3 |
| 12 | 6-quinolinyl | H | H | 20 ± 4 |
| 13 | 7-quinolinyl | H | H | 7 ± 2 |
| 14 | 3-isoquinolinyl | H | H | 12 ± 1 |
| 15 | 4-isoquinolinyl | H | H | 5 ± 1 |
| 16 | 3-quinolinyl | 5-Cl | H | 11 ± 2 |
| 17 | 5-quinolinyl | 5-Cl | H | 11 ± 3 |
| 18 | 7-quinolinyl | 5-Cl | H | 9 ± 2 |
| 19 | 4-isoquinolinyl | 5-Cl | H | 18 ± 3 |
| IIb | 2-napthtyl | 5-MeO | H | 19 ± 2 |
| 20 | 3-quinolinyl | 5-MeO | H | 8 ± 1 |
| 21 | 5-quinolinyl | 5-MeO | H | 4 ± 1 |
| 22 | 6-quinolinyl | 5-MeO | H | 14 ± 3 |
| 23 | 7-quinolinyl | 5-MeO | H | 2 ± 0.4 |
| 24 | 3-isoquinolinyl | 5-MeO | H | 6 ± 1 |
| 25 | 4-isoquinolinyl | 5-MeO | H | 3 ± 0.2 |
| 26 | 3-quinolinyl | 6-Cl | H | 37 ± 5 |
| 27 | 6-quinolinyl | 6-Cl | H | 112 ± 9 |
| 28 | 7-quinolinyl | 6-Cl | H | 22 ± 2 |
| 29 | 3-isoquinolinyl | 6-Cl | H | 102 ± 12 |
| 30 | 7-quinolinyl | H | Me | 18 ± 3 |
| 31 | 3-isoquinolinyl | H | Me | 20 ± 2 |
Mean Ki values ± SEM based on three independent binding experiments.
Data from ref (13).
The type of azinesulfonamide moiety (position of the sulfonamide group in the α- or β-position of the azine moiety and the localization of the sulfonamide group in pyridine or benzene rings) affected the receptor affinity, but the quantitative influence of quinolinyl/isoquinolinyl moieties toward 5-HT6R was difficult to establish. However, the 7-quinolinyl fragment was the most preferential among the quinoline set, and 4-isoquinolinyl was more preferable than its 3-counterpart.
The effect of indole substitution on affinity for 5-HT6Rs was observed using 3-quinolinyl and 4-quinolinyl derivatives, which gave the following rank order: 5-MeO > 5-H > 5-chloro > 6-chloro. This is in contrast to data reported by Cole et al.13 where chloro substituent in position-6 of indole core was well tolerated. Further, introduction of methyl substituent in position-2 of the indole, slightly decreased affinity for 5-HT6 sites (11 vs 30, and 13 vs 31).
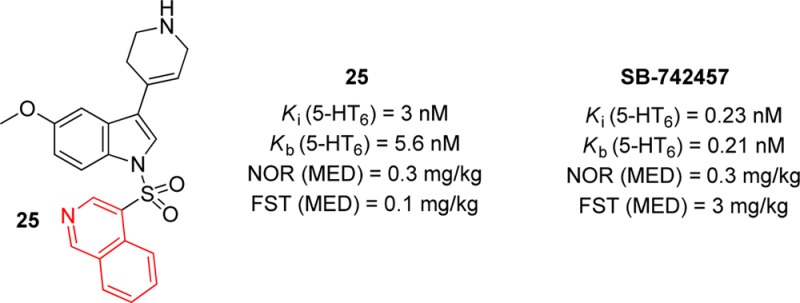
Yet another modification involving reduction of double bond in 1,2,3,6-tetrahydropyridin-4-yl giving N1-azinylsulfonyl-3-piperidin-4-yl derivatives, decreased affinity for 5-HT6Rs (23 vs 34, and 25 vs 35; Table 1, Table 2), which is in line with data reported by others.13
Table 2. Binding Data of the Synthesized Compounds 34–38 for 5-HT6R.
| compd | Q | R | S/D | 5-HT6Ki [nM]a |
|---|---|---|---|---|
| 34 | 7-quinolinyl | H | S | 30 ± 4 |
| 35 | 4-isoquinolinyl | H | S | 24 ± 4 |
| 36 | 7-quinolinyl | Me | D | 82 ± 10 |
| 37 | 7-quinolinyl | n-propyl | D | 247 ± 19 |
| 38 | 7-quinolinyl | n-butyl | D | 219 ± 24 |
Mean Ki values ± SEM based on three independent binding experiments.
Finally, as shown by the binding data outlined in Table 2, alkyl substituents at the tetrahydropyridine nitrogen atom were unfavorable. Introducing a small methyl substituent decreased affinity for 5-HT6R up to 11-fold, while compounds with unbranched alkyl substituents (n-propyl and n-butyl) displayed even 100-fold lower affinity. It thus seems that alkyl substituents in this position are not able to accommodate the restriction of the binding site.
Certain selected 5-HT6R ligands were subsequently examined in a panel of serotonin (5-HT1A, 5-HT2A, 5-HT7) and dopamine D2 receptors demonstrating at least 70-fold selectivity (Table 3).17
Table 3. Binding Data of the Selected Compounds for 5-HT6, 5-HT1A, 5-HT2A, 5-HT7, and D2Rs.
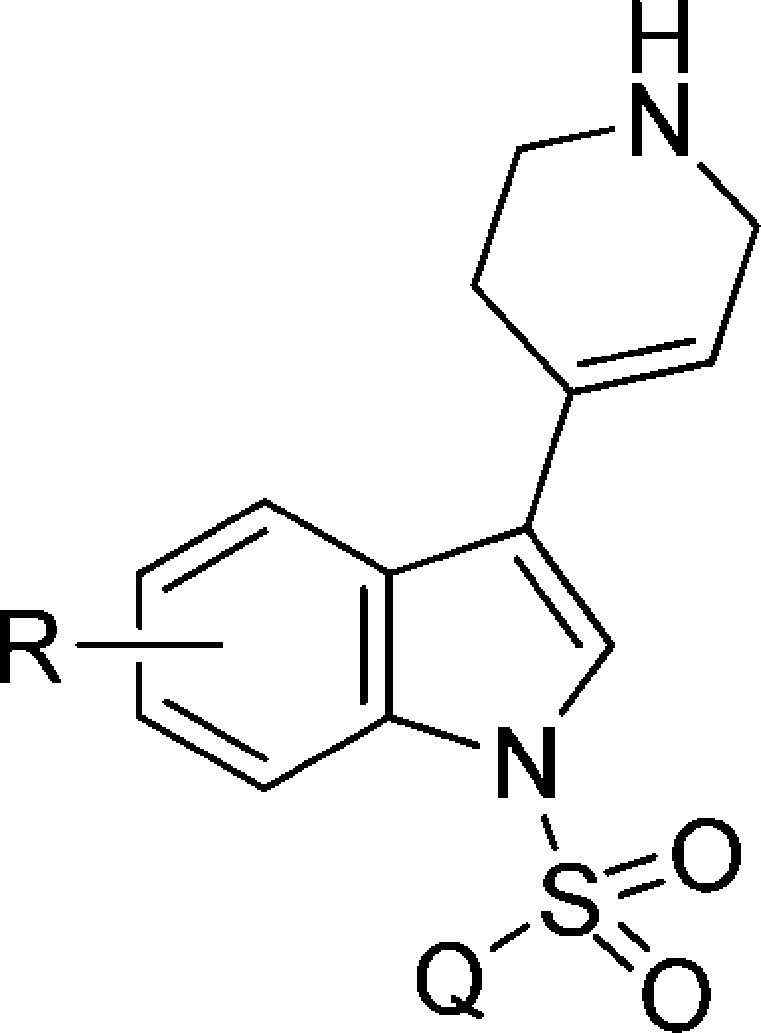
|
Ki [nM]a |
|||||||
|---|---|---|---|---|---|---|---|
| compd | Q | R | 5-HT6 | 5-HT1A | 5-HT2A | 5-HT7 | D2 |
| 12 | 6-quinolinyl | H | 20 | 1872 | 1412 | 6660 | 1410 |
| 18 | 7-quinolinyl | 5-Cl | 9 | 2467 | 2589 | 7322 | 1168 |
| 21 | 5-quinolinyl | 5-MeO | 4 | 2944 | 1552 | 2352 | 302 |
| 23 | 7-quinolinyl | 5-MeO | 2 | 5036 | 1003 | 6248 | 240 |
| 25 | 4-isoquinolinyl | 5-MeO | 3 | 5009 | 3355 | 7832 | 3319 |
Mean Ki values (SEM ± 27%) based on two independent binding experiments.
In the next step, compounds 23 and 25 with the highest affinity for 5-HT6R were investigated in vitro for their functional activity in cAMP assays, behaving as potent antagonists at h5-HT6Rs (Table 4).18 The same compounds were evaluated for their affinity for “off-target” receptor panel at CEREP and displayed weak affinity for adrenergic α1 and α2C, histamine H1, muscarinic M1 and M5, serotonin 5-HT2C receptors, and the serotonin transporter (SerT). Finally, neither 23 nor 25 displayed agonist properties at 5-HT2B receptors (<13% @ 1 μM) and did not bind at hERG (@ 10 and 50 μM). These results suggested a low risk that the tested compounds would evoke undesirable cardiovascular or CNS side effects. Interestingly, compounds 23 and 25 displayed high to moderate affinity for dopamine D3 receptors with Ki = 32 and 150 nM, respectively, but did not exhibit antagonist properties in an in vitro functional test.19
Table 4. Antagonist Activity of Selected Compounds 23 and 25 for 5-HT6R.
| 5-HT6 |
%inha |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| compd | Ki [nM] | Kb [nM]a | α1 | α 2C | H1 | M1 | M5 | 5-HT2C | SerT |
| 23 | 2 ± 0.4 | 4.4 | 41 | 18 | 9 | 11 | NT | 18 | 4 |
| 25 | 3 ± 0.2 | 5.6 | 40 | 21 | 15 | 0 | 3 | 15 | 6 |
Kb values and percentage of inhibition were determined at Eurofins Cerep.
Subsequently, the effects of the selected 5-HT6R antagonists 23 and 25 were determined on cytochrome P450 (CYP) enzyme catalytic activity in human liver microsomes and metabolic stability in rat in vitro assays (Table 5). Compounds 23 and 25 had no effect on CYP1A2, CYP2C9, CYP2C19, and CYP2D6, and moderately inhibited CYP3A4 activity. Compounds 23 and 25 were slowly metabolized in rat liver microsomes. Additionally, compounds 23 and 25 were not mutagenic in an Ames test (Table 1-SI).
Table 5. Interaction of Selected Compounds 23 and 25 with Cytochrome P450 and Their Metabolic Stability.
| assay type | 23 | 25 | |
|---|---|---|---|
| cytochrome P450 inhibition, IC50 [μM] | 1A2 | 100 | 100 |
| 2C9 | >100 | >100 | |
| 2C19 | >100 | >100 | |
| 2D6 | >100 | >100 | |
| 3A4 | 20 | 26 | |
| solubility [mg/mL] | pH 7.4 | >5 | >5 |
| pH 2.0 | >5 | >5 | |
| microsomal stability CLint [μL/min/mg] | rat | 18 | 10 |
Next, compounds 23 and 25 were submitted to the preliminary pharmacokinetic profiling after a single 3 mg/kg i.p. dose in male Wistar rats. Experiments carried out 15, 30, and 90 min after drug administration showed the highest concentrations at 30 min (21.9 and 18.6 ng/g of brain tissue for 23 and 25, respectively). The data indicated moderate blood–brain barrier penetration with a brain to plasma concentration ratio within 0.1–0.6 in the investigated time intervals, being higher for compound 25.
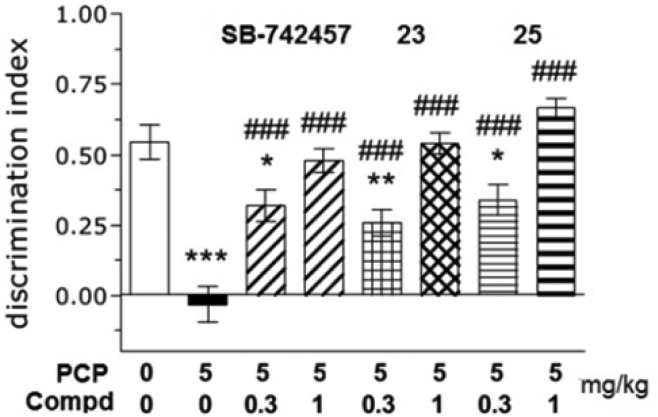
Many pieces of evidence suggest that 5-HT6R antagonists modulate cognitive processes and that their pro-cognitive properties may be beneficial for the treatment of Alzheimer’s disease. Thus, compounds 23 and 25 were tested in a NOR test and dose-dependently (MED 1 mg/kg, i.p.) ameliorated PCP-induced memory deficits in rats.20,21 Compound SB-742457 was used as a model selective 5-HT6 receptor antagonist and reversed memory deficits, and the effect reached statistical significance at doses ranging from 0.3–1 mg/kg (i.p.) (Figure 2).
Figure 2.
Effects of compounds 23 and 25 and SB-742457 in the novel-object recognition test in rats. Data are presented as the mean ± standard error of the mean of N = 7–12 animals per group. *p < 0.05 vs vehicle, **p < 0.01 vs vehicle, ***p < 0.001 vs vehicle, ###p < 0.001 vs PCP.
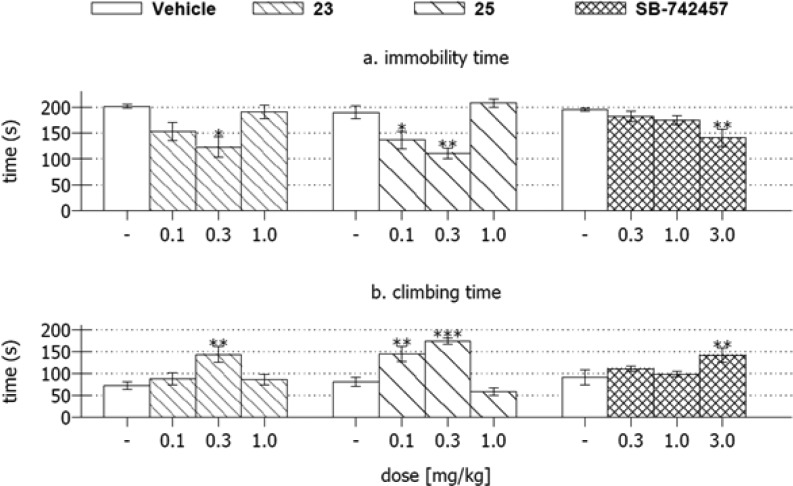
In the next step of behavioral evaluation, the potential antidepressant and anxiolytic activity of compounds 23 and 25 was evaluated in rats using a modified FST test22,23 and Vogel conflict drinking test,24 respectively. For comparison, the selective 5-HT6 receptor antagonist, SB-742457, was tested under the same conditions. Both 23 and 25 and SB-742457 significantly decreased the immobility time and increased the climbing time of rats in the forced swim test (Figure 3). The literature data indicated that 5-HT6R antagonists enhance the extracellular levels of dopamine (DA) and noradrenaline (NA) without altering serotonin (5-HT) neurotransmission in microdialysis in vivo study in rats.25 Moreover, the results of behavioral studies in rats demonstrate that the antidepressant-like effect of 5-HT6R antagonists in the FST is not related to 5-HT signaling but rather to activation of DA and NA systems.
Figure 3.
Effects of the active compounds 23 and 25 and SB-742457 on the immobility time and climbing of rats in the forced swim test. Data are presented as the mean ± standard error of the mean of N = 7–8 animals per group. *p < 0.05, **p < 0.01, ***p < 0.001 vs vehicle-treated group.
Given the above, the antidepressant-like effect of the tested compounds remains in agreement with the previously published data,23 which showed that noradrenergic system-affecting drugs modify climbing behavior, without any significant changes in the swimming. Notably, the tested compounds, 23 and 25 displayed antidepressant-like activity in relatively low doses (0.3 mg/kg for 23 and 0.1 and 0.3 mg/kg for 25) in the test, while SB-742457 was active in 10–30-fold higher dose (3 mg/kg). The antidepressant-like activity of both compounds and SB-742457 seems specific because they did not affect the rats locomotor activity in the open field paradigm (data not shown). Further, neither 23 nor 25 showed antianxiety properties in the Vogel conflict drinking test. However, SB-742457, which was used as a reference, produced an anticonflict effect at dose of 3 mg/kg (Table 3-SI).
In conclusion, SAR studies around N1-azinylsulfonyl-tetrahydropiridynyl indoles, led to the finding that replacing the naphthyl fragment with quinolinyl or isoquinolinyl moieties increased affinity for 5-HT6 receptors. The most potent compounds 23 and 25 behaved as 5-HT6R antagonists in the cAMP assay and showed good selectivity over a panel of other receptors tested. The lead compound 25 displayed good brain penetration and was active in a NOR test in similar doses to SB-742457 (1 mg/kg), while in FST it was active in 10–30-fold lower dose. In contrast to SB-742457, which was active in the Vogel test (MED = 3 mg/kg), compound 25 displayed no anxiolytic activity. These data further support the potential utility of 5-HT6 receptor antagonists for the treatment of cognitive dysfunction and accompanying affective disorders.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.6b00056.
Characterization data for final compounds and full experimental procedures (PDF)
The study was financially supported by the project “Prokog”, UDA-POIG.01.03.01-12-063/09-00, cofinanced by the European Union from the European Fund of Regional Development (EFRD), by the Polish Ministry of Science and Higher Education (MNiSW), Grant No. NN405 671540, statutory activity from Jagiellonian University Medical College (K/ZDS/004704), and by statutory funds from the Institute of Pharmacology, Polish Academy of Sciences.
The authors declare no competing financial interest.
Supplementary Material
References
- Claeysen S.; Bockaert J.; Giannoni P. Serotonin: a new hope in Alzheimer’s disease?. ACS Chem. Neurosci. 2015, 6, 940–943. 10.1021/acschemneuro.5b00135. [DOI] [PubMed] [Google Scholar]
- Meffre J.; Chaumont-Dubel S.; Mannoury la Cour C.; Loiseau F.; Watson D. J. G.; Dekeyne A.; Séveno M.; Rivet J. M.; Gaven F.; Déléris P.; Hervé D.; Fone K. C. F.; Bockaert J.; Millan M. J.; Marin P. 5-HT6 receptor recruitment of mTOR as a mechanism for perturbed cognition in schizophrenia. EMBO Mol. Med. 2012, 4, 1043–1056. 10.1002/emmm.201201410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhr F.; Déléris P.; Raynaud F.; Séveno M.; Morisset-Lopez S.; Mannoury la Cour C.; Millan M. J.; Bockaert J.; Marin P.; Chaumont-Dubel S. Cdk5 induces constitutive activation of 5-HT6 receptors to promote neurite growth. Nat. Chem. Biol. 2014, 10, 590–597. 10.1038/nchembio.1547. [DOI] [PubMed] [Google Scholar]
- Loiseau F.; Dekeyne A.; Millan M. J. Pro-cognitive effects of 5-HT6 receptor antagonists in the social recognition procedure in rats: implication of the frontal cortex. Psychopharmacology 2008, 196, 93–104. 10.1007/s00213-007-0934-5. [DOI] [PubMed] [Google Scholar]
- Marcos B.; Chuang T. T.; Gil-Bea F. J.; Ramirez M. J. Effects of 5-HT6 receptor antagonism and cholinesterase inhibition in models of cognitive impairment in the rat. Br. J. Pharmacol. 2008, 155, 434–440. 10.1038/bjp.2008.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicke K.; Haupt A.; Bespalov A. Investigational drugs targeting 5-HT6 receptors for the treatment of Alzheimer’s disease. Expert Opin. Invest. Drugs 2015, 24, 1515–1528. 10.1517/13543784.2015.1102884. [DOI] [PubMed] [Google Scholar]
- Codony X.; Vela J. M.; Ramirez M. J. 5-HT(6) receptor and cognition. Curr. Opin. Pharmacol. 2011, 11, 94–100. 10.1016/j.coph.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Johnson C. N.; Ahmed M.; Miller N. D. 5-HT6 receptor antagonists: prospects for the treatment of cognitive disorders including dementia. Curr. Opin. Drug Discovery Devel. 2008, 11, 642–654. [PubMed] [Google Scholar]
- Hirano K.; Piers T. M.; Miller N. D.; Rutter A. R.; Chapman P. F. Procognitive 5-HT6 antagonists in the rat forced swimming test: potential therapeutic utility in mood disorders associated with Alzheimer’s disease. Life Sci. 2009, 84, 558–562. 10.1016/j.lfs.2009.01.019. [DOI] [PubMed] [Google Scholar]
- Ivachtchenko A. V.; Ivanenkov Y. A.; Tkachenko S. E. 5-Hydroxytryptamine subtype 6 receptor modulators: A patent survey. Expert Opin. Ther. Pat. 2010, 20, 1171–1196. 10.1517/13543776.2010.494661. [DOI] [PubMed] [Google Scholar]
- Glennon R. A.; Siripurapu U.; Roth B. L.; Kolanos R.; Bondarev M. L.; Sikazwe D.; Lee M.; Dukat M. The medicinal chemistry of 5-HT6 receptor ligands with a focus on arylsulfonyltryptamine analogs. Curr. Top. Med. Chem. 2010, 10, 579–595. 10.2174/156802610791111542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivachtchenko A. V.; Ivanenkov Y. A.; Skorenko A. V. 5-HT(6) receptor modulators: a patent update. Part 2. Diversity in heterocyclic scaffolds. Expert Opin. Ther. Pat. 2012, 22, 1123–1168. 10.1517/13543776.2012.722205. [DOI] [PubMed] [Google Scholar]
- Cole D. C.; Ellingboe J. W.; Lennox W. J.; Mazandarani H.; Smith D. L.; Stock J. R.; Zhang G.; Zhou P.; Schechter L. E. N1-arylsulfonyl-3-(1,2,3,6-tetrahydropyridin-4-yl)-1H-indole derivatives are potent and selective 5-HT6 receptor antagonists. Bioorg. Med. Chem. Lett. 2005, 15, 379–383. 10.1016/j.bmcl.2004.10.064. [DOI] [PubMed] [Google Scholar]
- Benhamú B.; Martín-Fontecha M.; Vázquez-Villa H.; Pardo L.; López-Rodríguez M. L. Serotonin 5-HT6 receptor antagonists for the treatment of cognitive deficiency in Alzheimer’s disease. J. Med. Chem. 2014, 57, 7160–7181. 10.1021/jm5003952. [DOI] [PubMed] [Google Scholar]
- Rosse G. Quinoline derivatives as 5-HT6 receptor PET ligands. ACS Med. Chem. Lett. 2014, 5, 275–276. 10.1021/ml500045k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajdel P.; Masurier N.; Canale V.; Verdie P.; Amblard M.; Pawłowski M.; Martinez J.; Subra G. The pipecolic linker - An acid-labile handle for derivatization of secondary amines on a solid-support. Part 3. Tetrahedron Lett. 2013, 54, 998–1002. 10.1016/j.tetlet.2012.12.036. [DOI] [Google Scholar]
- Radioligand binding assays were performed according to the previously published procedures:; 17a Bojarski A. J.; Cegla M. T.; Charakchieva-Minol S.; Mokrosz M. J.; Mackowiak M.; Misztal S.; Mokrosz J. L. Structure-activity relationship studies of CNS agents. Part 9:5-HT(1A) and 5-HT2 receptor affinity of some 2- and 3-substituted 1,2,3,4-tetrahydro-ß-carbolines. Pharmazie 1993, 48, 289–294. [PubMed] [Google Scholar]; 17b Paluchowska M. H.; Bugno R.; Duszynska B.; Tatarczynska E.; Nikiforuk A.; Lenda T.; Chojnacka-Wójcik E. The influence of modifications in imide fragment structure on 5-HT1A and 5-HT7 receptor affinity and in vivo pharmacological properties of some new 1-(m-trifluoromethylphenyl)piperazines. Bioorg. Med. Chem. 2007, 15, 7116–7125. 10.1016/j.bmc.2007.07.029. [DOI] [PubMed] [Google Scholar]; 17c Zajdel P.; Marciniec K.; Maslankiewicz A.; Satala G.; Duszynska B.; Bojarski A. J.; Partyka A.; Jastrzbska-Wisek M.; Wróbel D.; Wesolowska A.; Pawlowski M. Quinoline- and isoquinoline-sulfonamide derivatives of LCAP as potent CNS multi-receptor - 5-HT1A/5-HT2A/5-HT7 and D2/D3/D4 - Agents: The synthesis and pharmacological evaluation. Bioorg. Med. Chem. 2012, 20, 1545–1556. 10.1016/j.bmc.2011.12.039. [DOI] [PubMed] [Google Scholar]
- Functional cAMP cellular assays at h5-HT6Rs were performed at Eurofins Cerep according to procedures described online at www.cerep.fr.
- The percentage of inhibition for α1, α2C, H1, M1, M5, 5-HT2B, 5-HT2C, D3, hERG receptors, and SerT for selected compounds 28 and 30 were evaluated at Eurofins Cerep. Experimental conditions for these assays are described online at www.cerep.fr.
- Ennaceur A.; Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav. Brain Res. 1988, 31, 47–59. 10.1016/0166-4328(88)90157-X. [DOI] [PubMed] [Google Scholar]
- Popik P.; Hołuj M.; Nikiforuk A.; Kos T.; Trullas R.; Skolnick P. 1-aminocyclopropanecarboxylic acid (ACPC) produces procognitive but not antipsychotic-like effects in rats. Psychopharmacology 2015, 232, 1025–1038. 10.1007/s00213-014-3738-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt R. D.; Anton G.; Blavet N.; Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur. J. Pharmacol. 1978, 47, 379–391. 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- Detke M. J.; Rickels M.; Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology 1995, 121, 66–72. 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- Vogel J. R.; Beer B.; Clody D. E. A simple and reliable conflict procedure for testing anti-anxiety agents. Psychopharmacology 1971, 21, 1–7. 10.1007/BF00403989. [DOI] [PubMed] [Google Scholar]
- Lacroix L. P.; Dawson L. A.; Hagan J. J.; Heidbreder C. A. 5-HT6 receptor antagonist SB-271046 enhances extracellular levels of monoamines in the rat medial prefrontal cortex. Synapse 2004, 51, 158–164. 10.1002/syn.10288. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.