Abstract
Liver X receptor (LXR), a nuclear hormone receptor, is an essential regulator of immune responses. Activation of LXR-mediated transcription by synthetic agonists, such as T0901317 and GW3965, attenuates progression of inflammatory disease in animal models. However, the adverse effects of these conventional LXR agonists in elevating liver lipids have impeded exploitation of this intriguing mechanism for chronic therapy. Here, we explore the ability of a series of sterol-based LXR agonists to alleviate inflammatory conditions in mice without hepatotoxicity. We show that oral treatment with sterol-based LXR agonists in mice significantly reduces dextran sulfate sodium colitis-induced body weight loss, which is accompanied by reduced expression of inflammatory markers in the large intestine. The anti-inflammatory property of these agonists is recapitulated in vitro in mouse lamina propria mononuclear cells, human colonic epithelial cells, and human peripheral blood mononuclear cells. In addition, treatment with LXR agonists dramatically suppresses inflammatory cytokine expression in a model of traumatic brain injury. Importantly, in both disease models, the sterol-based agonists do not affect the liver, and the conventional agonist T0901317 results in significant liver lipid accumulation and injury. Overall, these results provide evidence for the development of sterol-based LXR agonists as novel therapeutics for chronic inflammatory diseases.—Yu, S., Li, S., Henke, A., Muse, E. D., Cheng, B., Welzel, G., Chatterjee, A. K., Wang, D., Roland, J., Glass, C. K., Tremblay, M. Dissociated sterol-based liver X receptor agonists as therapeutics for chronic inflammatory diseases.
Keywords: LXR, traumatic brain injury, ulcerative colitis, drug discovery
Liver X receptors (LXRs) are proteins that regulate gene expression in the nucleus upon activation by their cognate agonists, typically oxysterols (1). Transactivation of LXR target genes by LXR agonists such as T0901317 and GW3965 mediates numerous cellular processes, including immune modulation and lipid homeostasis (2–4). Additionally, LXR agonists suppress inflammation by transrepression of NF-κB-mediated transcriptional activity (5–8). These agonists have demonstrated preclinical efficacy in treating many diverse types of chronic inflammatory diseases, including atherosclerosis (9), contact dermatitis (10), rheumatoid arthritis (11), and ulcerative colitis (12). However, traditional LXR agonists elevate liver triglycerides via increasing transcriptional levels of genes regulating lipogenesis, such as sterol regulatory element binding transcription factor 1 and Fas (13, 14). This adverse effect leading to hepatotoxicity has impeded exploitation of this promising mechanism for chronic therapy, and to date no therapeutic LXR agonists have progressed beyond phase 1 safety studies (15–17).
Because the liver primarily expresses the LXR-α isoform, amelioration of the lipogenesis side effect can be theoretically surmounted by designing drugs that are selective for LXR-β, the isoform that drives therapeutic effects in immune cells (18). However, the high degree of homology of the binding sites of LXR-α and LXR-β has made the design of isoform-selective agonists challenging (19). Even certain compounds exhibiting LXR-β selectivity still exhibit lipid-driven toxicity in the liver. We have explored multiple alternative mechanisms for achieving tissue-selective LXR activation, including cell type-specific delivery of an LXR agonist via an antibody-drug conjugate (20). Herein, we describe an approach based on previously described sterol-based LXR agonists that exhibit an anti-inflammatory property in leukocytes without inducing liver lipid accumulation.
N,N-dimethyl-3β-hydroxycholenamide (DMHCA) is a sterol-based LXR agonist that has been reported to activate LXR without increasing liver triglycerides (21). Through a small medicinal chemistry effort, we generated methylpiperidinyl-3β-hydroxycholenamide (MePipHCA), an analog of DMHCA that demonstrates improved cellular and in vivo potency. We demonstrate that oral dosing of DMHCA and MePipHCA reduce inflammation in murine dextran sulfate sodium (DSS)-induced colitis and traumatic brain injury, without causing liver injury or lipid accumulation. This phenomenon is associated with increased LXR target gene transcription in the colon and brain. Together, these findings provide evidence for the development of these sterol-based LXR agonists as therapeutics for treating inflammatory diseases, including ulcerative colitis and traumatic brain injury.
MATERIALS AND METHODS
Animal studies
All procedures were carried out in accordance with protocols approved by the California Institute for Biomedical Research (Calibr) Animal Care and Use Committee. C57BL/6 mice (8–10 wk old) were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) and housed in Calibr’s animal facility. For induction of ulcerative colitis, mice were giving drinking water supplemented with 3% DSS (w/v) for 5 d. Recovery in plain water was allowed until the end of the experiment. To study the effect of compounds in DSS-induced colitis progression, compounds were formulated with 0.5% methyl cellulose/0.5% Tween 80, and were orally administered to mice at a dose as indicated in each study. In the end, blood, liver, and colon samples were collected for various tests. For induction of traumatic brain injury, the controlled cortical impact-injury device consists of a spring-controlled impactor with a 3.5 mm diameter tip. Injury was induced by manually pressing down the impactor to extrude the tip with deformation depth of 2 mm. The procedure is as described previously (22). Briefly, mice were anesthetized with isoflurane in a gas mixture containing 30% oxygen/70% nitrous oxide, and administered through a nose mask. Depth of anesthesia was assessed by monitoring respiration rate and pedal withdrawal reflexes. The mouse was placed on a heated pad to maintain its core body temperature. The head surgical site was shaved and cleaned with iodine and ethanol scrubs. A 10 mm midline incision was made over the skull, and a 4 mm craniotomy was made on the central aspect of the left parietal bone. The impounder tip of the injury device was extended to its full stroke distance (44 mm), positioned on the surface of the exposed dura, and reset to impact the cortical surface. This process was repeated 10 times to induce injury. After injury, the incision was closed with sutures, and animal was placed in a heated cage to allow recovery. All animals were monitored carefully for at least 4 h after surgery, and daily thereafter. Sham animals underwent the same procedure as injured mice, except they did not receive an impact.
Colon tissue histology and scoring
The distal colon tissue were collected from the mice and fixed in 10% formalin and sent to Sanford Burnham Prebys Medical Discovery Institute (La Jolla, CA, USA) histology core for hematoxylin and eosin staining. Histology analysis was performed blinded by an investigator on a scale from 0 to 40 as follows: severity of inflammation (0–3: none, slight, moderate, severe), extent of injury (0–3: none, mucosal, mucosal and submucosal, transmural), and crypt damage (0–4: none, basal one third damaged, basal two thirds damaged, only surface epithelium intact, entire crypt and epithelium lost). Each score was then multiplied by a factor equivalent with the percentage of tissue involvement (×1: 0–25, 2: 26–50, 3: 51–75, 4: 76–100%) (23).
Lamina propria mononuclear cell isolation
Lamina propria mononuclear cells (LPMCs) were isolated from 8- to 10-wk-old C57BL/6 mice as previously described (24). Briefly, the colon was removed from dead mice, cut into 4–5 cm pieces, and placed in ice-cold PBS. The colon feces were cleaned with a syringe filled with PBS. After cleaning up the residual mesenteric fat tissue, the colon pieces were opened longitudinally and cut into 1 cm pieces. These pieces were incubated twice with fresh predigestion solution consisting of HBSS with 5 mM EDTA and 1 mM DTT for 20 min at 37 C under slow rotation (40 g). The residual undigested tissue was washed with PBS and cut into 1 mm before incubating with digestion solution [0.05% collagenase D (Roche, Basel, Switzerland), 0.05% DNase I (Sigma-Aldrich, St. Louis, MO, USA), and 0.3% dispase II (Roche) in PBS] for 20 min at 37°C. After incubation, cell solution was vortex intensely and passed through a 40-μm cell strainer. The residual undigested tissue pieces were incubated again or more times with digestion solution until no visible pieces were left. The resulting single cell solution was then centrifuged for 10 min at 500 g at 20 C. The cell pellet was washed with PBS once and resuspended in 10 ml of the 40% Percoll, which is carefully overlaid on top of 5 ml of 80% Percoll. This Percoll gradient was centrifuged for 20 min at 1000 g at 20 C without brakes. The LPMCs were then collected from the interphase of the 2 Percoll solutions. The cells were then washed with PBS and counted and plated with RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), 10 mM penicillin-streptomycin, and 10 mM l-glutamine.
Peripheral blood mononuclear cell isolation
Peripheral blood mononuclear cells (PBMCs) were isolated by carefully overlaying 35 ml 1:1 PBS diluted whole blood on 15 ml Ficoll reagent, followed by centrifugation at room temperature for 30 min at 400 g without brakes. After centrifugation, cells present at the white cell interphase were carefully collected, washed with PBS 3 times, and resuspended in complete PBMC culture medium consisting of RPMI-1640 supplemented with 10% FBS, 10 mM penicillin-streptomycin, and 10 mM l-glutamine.
Kupffer cell isolation
C57BL/6 mice (8–10 wk old) were given an intraperitoneal injection of 10 mg/ml Inactin hydrate (Sigma-Aldrich) at a volume of 10 ml/kg to induce anesthesia. The liver was perfused using 50 ml prewarmed Liver Perfusion Medium (Thermo Fisher Scientific Life Sciences, Waltham, MA, USA) followed by 50 ml prewarmed Liver Digest Medium (Thermo Fisher Scientific Life Sciences) at a rate of 5 ml/min with a butterfly needle inserted into the portal vein. An incision in inferior vena cava was made to allow blood and buffer to flow out of circulation. Liver was carefully removed and placed in the ice-cold sterile transport medium consisting of Hepatozyme (Thermo Fisher Scientific Life Sciences) with 1% bovine serum albumin. The liver cells were gently scraped off from the liver and filtered through a 40 μm nylon cell strainer. The cells were then centrifuged twice at 54 g for 2 min at 4°C to remove hepatocytes. The resulting supernatants were further centrifuged at 1350 g for 10 min at 4°C. The cell pellet was resuspended in 10 ml preservation buffer consisting of Liver Perfusion Medium with 1% bovine serum albumin. This cell suspension was carefully laid on top of a discontinuous gradient of 20 ml 25% Percoll underlain with 20 ml 50% Percoll (Sigma-Aldrich) and centrifuged at 1350 g at 4 C for 30 min brake acceleration and deceleration set to minimum. Kupffer cells were enriched between the interphase of the 2 density cushions. The cells were washed twice with complete Kupffer cell culture medium (RPMI-1640 medium supplemented with 10% FBS, 10 mM penicillin-streptomycin, and 10 mM l-glutamine) before plating.
Cell culture
SW480, THP-1 cells were purchased from American Type Culture Collection (Manassas, VA, USA). SW480 cells were cultured in DMEM medium supplemented with 10% FBS. THP-1 cells were cultured in RPMI (1 mM glucose) with 10% FBS, 0.1% 2-mercaptoethanol, 1 mM sodium pyruvate and 100 U/ml penicillin-streptomycin.
Serum biochemical analysis
Serum cholesterol, alanine transaminase (ALT), alanine phosphatase, and creatinine levels were measured using a Scil Spotchem EZ Chemistry Analyzer (sp-4430; Scil Animal Care, Gurnee, IL, USA)and Spotchem Vital Health panel strips according to manufacturer’s instruction.
Liver triglyceride assay
Livers were weighed and homogenized in cold PBS containing 1% triton X-100 at a concentration of 200 mg/ml. The homogenates were centrifuged at 1000 g for 10 min at 4 C, and the supernatant was used for triglyceride assay using a triglyceride colorimetric assay kit (Cayman Chemical, Ann Arbor, MI, USA) according to the manufacturer’s protocol.
Pharmacokinetics analysis
Liver samples were weighed and homogenized in internal standard (IS) extraction solvent (acetonitrile/water, v/v = 9:1, with 50 ng/ml benztropine as internal control). The standard curve samples were also prepared in the same solvent. The homogenates were centrifuged at 4°C 17,000 g for 30 min. Supernatants were further diluted with IS extraction solvent and HPLC grade 0.1% formic acid before injecting into a liquid chromatography–tandem mass spectrometry column for analysis. Plasma samples were mixed with IS extraction solvent (100% acetonitrile with 10 ng/ml benztropine as internal control), and mixed with 0.1% formic acid before injecting onto the liquid chromatography–tandem mass spectrometry column. The analysis was conducted on a PE Sciex API4000 Q-Trap Mass Spectrometer (Sciex, Framingham, MA, USA).
RT-qPCR assay
Colons were homogenized in 1 ml of Trizol (Invitrogen). RNA was extracted adding 200 μl of chloroform, precipitating the aqueous phase with 400 μl of 70% ethanol and purifying RNA with RNeasy Mini kit (Qiagen, Germantown, MD, USA). Cells were lysed with 400 μl of RLT lysis buffer followed by adding 400 μl of 70% ethanol and purifying RNA with RNeasy Mini kit (Qiagen). RNA was reverse transcribed into cDNA using qScript cDNA synthesis kit (Quanta BioSciences, Gaithersburg, MD, USA). qPCR was performed with PerfeCTa qPCR ToughMix (Quanta BioSciences) on 50 ng cDNA template/reaction using Taqman probes (Thermo Fisher Scientific Life Sciences ), including mouse genes Il1b (Mm00434228_m1), Ccl2 (Mm00441242_m1), Il6 (Mm00446190_m1), Tnf (Mm00443258_m1), Abcg1 (Mm00437390_m1), and human genes IL1B (Hs00174097_m1), chemokine (C-C motif) ligand 2 (CCL2) (Hs00234140_m1), IL6 (Hs00985639_m1), IL8 (Hs00174103_m1), TNF (Hs01113624_g1), ATP-binding cassette transporter G1 (ABCG1) (Hs00245154_m1). Although Thermo Fisher does not disclose the primer sequences used in these proprietary assay reagents, more information about them can be found at www.thermofisher.com.
Cytokine homogeneous time resolved fluorescence assay
For the homogeneous time resolved fluorescence (HTRF) assay with primary human PBMCs, cells were plated at density of 8000 cells/well and were treated with LXR agonists for 6 h before stimulating with 100 ng/ml LPS for 16 h. TNF-α content in the cell culture supernatant was assessed by Human TNFa HTRF assay kit (Cisbio Bioassays, Bedford, MA, USA) according to manufacturer’s protocol. For the HTRF assay with mouse LPMCs, cells were plated at density of 8000 cells/well and were treated with LXR agonists for 16 h before assessing cytokine secretion. The signal was read by a PerkinElmer (Waltham, MA, USA) EnVision Multilabel Reader.
Cytotoxicity assay
After cells were incubated for certain time period as indicated for each experiment, CellTiter-Glo Luminescent cell viability reagent (Promega, Madison, WI, USA) was added at a ratio of 1:5 in volume to cell culture directly. The reagent was mixed with cell culture on a plate shaker. The signal was read on a PerkinElmer EnVision Multilabel Reader after 10 min incubation.
LXR responding element luciferase activity assay
Cignal lenti LXR-α luciferase reporter (catalog no. CLS-7041L) was purchased from Qiagen. Cells were transfected with lenti LXR-α responding element luciferase reporter according to manufacturer’s instruction, and positively transfected cells were selected by adding 1 µg/ml puromycin. For testing compounds’ activity in activating LXR-α, 7000 cells/well were plated in 384-well plate and were incubated with compounds indicated at concentrations as suggested in a total volume of 25 µl. After incubation for 24 h, 5 µl One-Glo luciferase assay reagent (Promega) was added into the cell culture according to manufacturer’s instruction, and the reporter gene activity signal was read by a PerkinElmer EnVision Multilabel Reader.
Statistics
The number of animals used in each study is indicated. Statistics analysis was conducted using Student’s t test or 1-way ANOVA on Prism 5 software. Results are presented as means ± sd or means ± sem.
RESULTS
Sterol-based LXR agonists reduce colitis-induced body weight loss and inflammation in mice
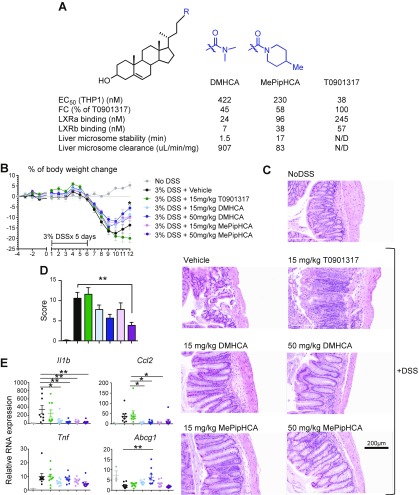
Given that LXR agonists have shown efficacy in treating various types of inflammatory conditions, we sought to improve DMHCA’s potency and efficacy through medicinal chemistry. To assess LXR activation of LXR agonists in macrophages, we generated stable reporter cell lines that express firefly luciferase under the control of an LXR-α response element in the human THP-1 monocyte/macrophage cell line (THP1LXRE-LUC). We treated THP1LXRE-LUC cells with a range of concentrations of sterol-based LXR agonists and the synthetic agonist T0901317 for 24 h before assessing the luminescence intensity. The result shows MePipHCA has improved potency and efficacy compared with DMHCA itself as suggested by EC50 and fold change parameters. Both DMHCA and MePipHCA do not show any appreciable differences in the selectivity of LXR-α/β binding compared with T0901317 (Fig. 1A). The sterol-based LXR agonist DMHCA was shown to attenuate atherosclerotic lesion development in rodent models (9). However, this series of LXR agonists has not been studied in any other chronic inflammatory disease contexts. Ulcerative colitis currently affects approximately 907,000 people in the United States and has a high unmet medical need (25), thus we turned our attention to a rodent model of ulcerative colitis as an ideal chronic inflammatory condition in which to explore the effects of sterol-based LXR agonists. Mice were treated with T0901317, DMHCA, or MePipHCA at a dosage of 15 or 50 mg/kg once daily by oral gavage from 4 d prior to induction of disease until the end of the study. Disease was induced by administering 3% DSS via drinking water. After 5 d of DSS treatment, the water was replaced with regular drinking water to allow recovery. The body weight of mice was monitored daily (Fig. 1B). Mice were euthanized after 16 d and serum and large intestine samples were collected. Although 50 mg/kg DMHCA significantly improved body weight recovery, all DMHCA and MePipHCA treatments showed a trend toward improved body weight recovery. In contrast, T0901317 showed a trend toward exaggerating body weight loss (Fig. 1B). Importantly, histologic analysis of the large intestine showed that both DMHCA and MePipHCA treatments preserved the integrity of the epithelial lining, reduced ulceration, decreased epithelium damage, and diminished immune cell infiltration; in contrast, T0901317-treated mice had significant immune cell infiltration and disrupted epithelial lining (Fig. 1C, D). Gene expression analysis of the large intestine revealed that DMHCA- and MePipHCA-treated mice have significantly reduced expression of inflammatory markers, including Il1b and Ccl2 (Fig. 1E). This reduced inflammation is associated with enhanced expression of Abcg1, an LXR target gene (Fig. 1E), suggesting DMHCA attenuates colitis, at least partially, through LXR activation. However, the lack of LXR target gene induction by MePipHCA—despite its effects on disease parameters—implies that other mechanisms may be involved, or that these effects are highly temporally sensitive. Overall, these results show that this series of sterol-based LXR agonists alleviates inflammation and ulceration in the DSS-induced colitis mouse model.
Figure 1.
Sterol-based LXR agonists attenuate DSS-induced colitis progression in mice. A) EC50 and fold change of LXR responding element (LXRE)-luciferase assay in THP1 cells, and liver microsome stability of DMHCA, MePipHCA, and T0901317. B) Mice were treated with vehicle (0.5% methyl cellulose/0.5% Tween 80) or compounds at doses as indicated for 4 d before administration of 3% DSS drinking water to induce colitis. After 5 d, 3% DSS water was replaced with regular drinking water. Mice were allowed to recover for another 6 d. Body weight of mice was monitored and recorded daily. Percentage of body weight change was calculated. No DSS: n = 3; other groups: n = 9. C) Representative hematoxylin and eosin staining of cross section of colon samples. D) Histology score of hematoxylin and eosin staining of colon samples. E) Gene expression of Il1b, Ccl2, Tnf, and Abcg1 of mouse colon samples. Results are presented as means ± sem. *P < 0.05, **P < 0.01.
Sterol-based LXR agonists reduce inflammation in colonic cells and leukocytes
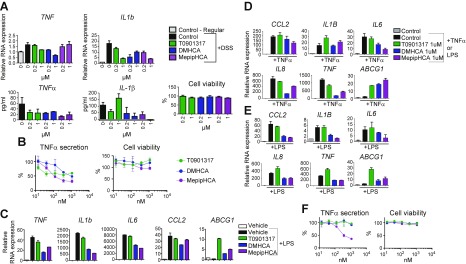
To determine the target cell types of the anti-inflammatory effects of sterol-based LXR agonists, we isolated LPMCs from mice to study direct effects in in vitro culture. LPMCs from mice treated with 3% DSS for 5 d were harvested and treated overnight with DMHCA, MePipHCA, and T0901317 for 16 h before evaluating the induction of Tnf and Il1b mRNA and release of TNF-α and IL-1β into the supernatant. All LXR agonists had a suppressive effect on TNF-α protein but not on induction of Tnf mRNA, but only the sterol-based LXR agonists had a consistent effect on IL-1β protein and Il1b mRNA; importantly, none of these treatments impacted cell viability (Fig. 2A). We also observed anti-inflammatory properties of these LXR agonists in mouse peritoneal macrophages, as they inhibited TNF-α secretion (Fig. 2B) and gene expression of Tnf, Il1b, Il6, and Ccl2 in these cells (Fig. 2C). This inhibition of proinflammatory gene expression and protein secretion is associated with induction of Abcg1 (Fig. 2C).
Figure 2.
Sterol-based LXR agonists reduce inflammation in mouse LPMCs, mouse peritoneal cells, human colonic epithelial cells, and human PBMCs. A) LPMCs were isolated from mice treated with or without DSS water. Freshly isolated LPMCs were treated with 1 μM LXR agonists for 16 h before being collected for RNA expression analysis (upper panel), cytokine secretion analysis, and cell viability by CellTiter Glo assay (lower panel). B) Mouse peritoneal cells were treated with LXR agonists of different concentrations for 6 h before stimulation with LPS for 16 h, followed by HTRF TNF-α secretion assay. C) Mouse peritoneal cells were treated with 1 μM LXR agonists for 6 h before LPS stimulation for 1 h for gene expression analysis. D, E) SW480 cells were pretreated with control or compounds at concentrations as indicated for 16 h, before 10 ng/ml TNF-α (D) or 100 ng/ml LPS (E) stimulation. After 1 h, cells were collected and RNA was isolated. RT-qPCR was performed to determine the gene expression of CCL2, IL1B, IL6, IL8, TNF-α, ABCG1. F) Primary human PBMCs were treated with different LXR agonists for 6 h followed by LPS stimulation for 16 h. TNF-α secretion was assessed by HTRF assay and cell viability by CellTiter Glo assay. Results are presented as means ± sd.
To test whether the anti-inflammatory effects of sterol-based LXR agonists are translatable to human cells, we pretreated the human colonic epithelial cell line SW480 with DMHCA, MePipHCA, or T0901317, followed by stimulation with either LPS or TNFα to induce gene expression associated with inflammation. DMHCA and MePipHCA demonstrated more robust inhibition of CCL2, IL6, IL8, and TNF relative to T0901317 upon TNF-α stimulation (Fig. 2D), as well as more marked inhibition of CCL2, IL1B, IL6, IL8, and TNF upon LPS stimulation (Fig. 2E). Importantly, all 3 compounds strongly induced the LXR target gene ABCG1 under both conditions (Fig. 2D, E). To examine the effects of the compounds in human immune cell types, we treated primary human PBMCs with compounds and stimulated them with LPS. TNF-α secretion in PBMCs is significantly reduced by MePipHCA, with no impact on cell viability (Fig. 2F).
Overall, these results suggest that the anti-inflammatory effects of sterol-based LXR agonists in murine DSS colitis may be driven by effects on both epithelial cells and leukocytes associated with the inflamed colon. Importantly, these effects appear to translate from mouse to human cells.
Sterol-based LXR agonists activate LXR in the brain and reduce inflammation induced by traumatic brain injury
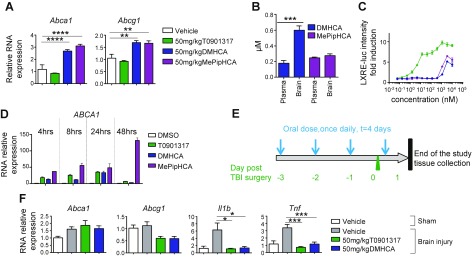
Our preliminary pharmacodynamics experiments also led us to explore the action of sterol-based LXR agonists in the brain. Indeed, both DMHCA and MePipHCA exhibit significant activation of both Abca1 and Abcg1 in mouse whole brain, although T0901317 has no gene induction, even at high doses (Fig. 3A). Single-dose pharmacokinetic studies carried out in parallel showed that both DMHCA and MePipHCA have excellent brain exposure, suggesting these compounds are well distributed in the brain (Fig. 3B). Notably, DMHCA appears to preferentially accumulate in the brain. To study the activity of LXR agonists in neuronal cells, we treated neuroblastoma SHSY5YLXRE-LUC cells with compounds and assessed LXR responding element-luciferase activity after 24 h. All compounds activated LXR in this neuronal cell line (Fig. 3C). To confirm this result, we treated wild-type SHSY5Y cells with 1 μM compounds and observed robust induction of ATP-binding cassette transporter A1 (ABCA1) gene expression (Fig. 3D). To assess the anti-inflammatory function of DMHCA in the brain, we treated mice orally with 50 mg/kg DMHCA or T0901317 for 3 d before induction of traumatic brain injury at the ipsilateral cortex. A fourth dose was given 15 min after the brain injury induction. Mice were sacrificed 24 h after the fourth dose, and gene expression analysis was performed in the ipsilateral brain tissue (Fig. 3E). The result shows both T0901317 and DMHCA significantly reduced traumatic brain injury-induced Il1b and Tnf expression (Fig. 3F). No effect on Abca1 and Abcg1 was observed in these mice, likely due to the analysis being performed 24 h after the last dose, and LXR target genes were induced early. The gene encoding the microglial cell-associated inflammation marker Iba-1 was also unchanged in these experiments. Taken together, these results show that LXR agonists reduce inflammation in traumatic brain injury and that DMHCA may have privileged brain exposure, making it an interesting entry point to a potential therapy for neuroinflammation.
Figure 3.
Sterol-based LXR agonists activate LXR in the brain and reduce inflammation induced by traumatic brain injury (TBI). A) Gene expression analysis of Abca1 and Abcg1 in brain tissues that were collected from mice treated with LXR agonists for 6 d (n = 6). B) Concentrations of LXR agonists in the plasma and brain from mice that were treated with a single dose of LXR agonists. C) LXR agonists activity profile in SHSY5Y cells. D) ABCA1 mRNA expression in SHSY5Y cells that were treated with 1 μM of different LXR agonists for various times. E) TBI experimental design. Compounds or vehicle were given by oral gavage once daily for 3 d before TBI induction. The final dose was given 15 min after injury induction. Tissues were collected 24 h postinjury (n = 5). F) Gene expression analysis of injured brain tissues. Results are presented as means ± sem (A, B, D), and as means ± sd (C). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Unlike T0901317, sterol-based LXR agonists do not induce hepatotoxicity
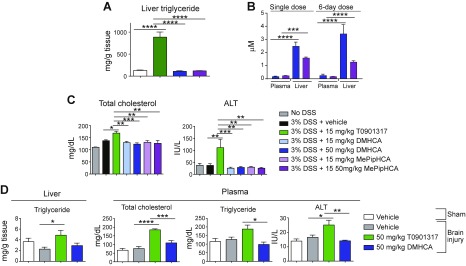
T0901317 causes fatty liver by inducing sterol regulatory element binding protein (SREBP) target genes, which regulate lipogenesis (13) (14). We administered DMHCA, MePipHCA, and T0901317 orally to mice for 6 d and observed a striking induction of triglycerides with T0901317, and neither DMHCA nor MePipHCA showed any change relative to vehicle-treated mice, consistent with previous reports (21) (Fig. 4A). We initially hypothesized that the lack of hepatic effects exhibited by sterol-based LXR agonists may result from poor exposure in the liver; however, pharmacokinetic analysis of whole liver tissue 4 h after the last dose in the 6 d dosing study revealed significantly higher exposure in the liver than in the plasma (Fig. 4B). Thus, lack of exposure cannot explain the absence of a pharmacodynamics effect of sterol-based LXR agonists in the liver. In further support of these experiments, we analyzed plasma samples from the murine DSS colitis model, which showed that in contrast to T0901317, treatment with DMHCA and MePipHCA for 16 d does not lead to increased serum cholesterol or the liver injury biomarker ALT (Fig. 4C). Similarly, in the traumatic brain injury model, treatment of DMHCA for 4 d does not increase liver triglyceride content, plasma cholesterol or triglycerides, or serum ALT levels (Fig. 4D).
Figure 4.
Sterol-based LXR agonists do not induce liver lipid accumulation. A) Liver triglyceride levels of mice treated with 6 d dose of compounds were measured (n = 6). B) Mice were treated with a single dose of 50 mg/kg DMHCA or MePipHCA for 1 or 6 d. The concentration of DMHCA or MePipHCA in plasma and liver was determined by liquid chromatography–mass spectrometry. Single dose: n = 4; 6 d dose: n = 6. C) Total cholesterol and ALT levels were determined in plasma samples of mice from DSS-induced colitis study. Mice were treated with vehicle (0.5% methyl cellulose/0.5% Tween 80) or compounds at doses as indicated for 4 d before administration of 3% DSS drinking water to induce colitis. After 5 d, 3% DSS water was replaced with regular drinking water. Mice were allowed to recover for another 6 d. Body weight of mice was monitored and recorded daily. Percentage of body weight change was calculated. No DSS: n = 3; other groups: n = 9. D) Liver triglyceride, plasma total cholesterol, triglyceride, and ALT of traumatic brain injury experiment samples were assessed (n = 5). Results are presented as means ± sem for all figures. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Overall, these findings suggest these compounds are present but inactive in the liver, as they fail to induce genes that control lipid generation, providing further support for the exploration of sterol-based LXR agonists in the treatment of inflammatory diseases.
DISCUSSION
The immune regulatory function of LXR agonists, such as T0901317 and GW3965, has been characterized in several inflammatory disease contexts (9–12). The mechanism whereby LXR exerts anti-inflammatory functions, at least in part, relies upon association with corepressor complexes that prevent the recruitment of the transcriptional machinery to the promoters of proinflammatory genes (26, 27). However, due to the induction of lipogenesis by activating SREBP targeting genes in the liver, the side effect of these synthetic LXR agonists impeded the development of LXR agonists as inflammatory disease treatments (28, 29). Therefore, we examined anti-inflammatory properties of a series of sterol-based LXR agonists in 2 animal models of inflammation and demonstrated that the efficacy of these compounds in suppressing inflammation is not associated with liver lipid accumulation.
One approach to utilize LXR as a therapeutic target for inflammatory diseases without systemic exposure, and thus avoiding lipogenic effects in the liver, is to use LXR modulators as topical treatments for dermatitis (19). The systemic exposure of these compounds may be restricted to skin through reducing absorption and increasing local metabolism by modulating physicochemical properties. A highly sought-after solution to this problem has been the identification of compounds that are selective for LXR-β, which lacks expression in the liver (30, 31). However, the residual LXR-α activation of these compounds still drives appreciable triglyceride induction in hepatocytes (19). The third approach to spare the liver is to specifically deliver LXR agonists into its target cells or tissues using an antibody–drug conjugate. In this case, a potent LXR agonist was linked to CD11a monoclonal antibody, leading to selective uptake and release of the payload in leukocytes (20). Herein we describe an alternative approach involving identification of therapeutically useful compounds that exhibit tissue- and/or transcription-level selectivity, which leads to an overall dissociated activation profile. Such a compound, DMHCA, was described to have potent transcriptional activity of ABCA1 while exhibiting minimal effects on SREBP both in vitro and in vivo in mice (21). The efficacy of DMHCA in regulating ABCA1/ABCG1-mediated cholesterol efflux without inducing liver lipogenesis was also demonstrated in an atherosclerosis study, in which mice were treated with 8 mg/kg DMHCA for 11 wk (32). This study suggested that the antiatherogenic effect of DMHCA is attributed to the cholesterol efflux genes being up-regulated upon LXR activation. However, whether the attenuated atherosclerosis progression is associated with DMHCA’s anti-inflammatory effect was not assessed in this study. An endogenous LXR agonist, desmosterol—structurally similar to DMHCA—was shown to suppress inflammation and inhibit SREBP processing in mouse macrophage foam cells and human monocyte-derived macrophages (9). These results together suggest this series of dissociated LXR agonists might be good candidates for treating inflammatory diseases. We chose to focus on DMHCA rather than desmosterol, because of the extreme hydrophobic properties of the latter that may hamper cellular uptake, trafficking, and metabolic stability.
Desmosterol accumulation in macrophage foam cells results in a deactivated macrophage phenotype with low-grade inflammation. Previous studies suggest desmosterol and other oxysterols regulate immunity and tumor microenvironment via both LXR-dependent and LXR-independent mechanisms (9, 33). Desmosterol promotes recruitment of LXR and prevents the removal of nuclear receptor corepressor/histone-deacetylase corepressor complexes by inhibiting histone acetylation at inflammatory gene transcriptional regulatory elements (9). However, partial suppressive effects on inflammatory response genes in desmosterol-treated LXR double knockout macrophages are still present (9). Here, we observed a strong suppressive effect of DMHCA (and its analog, MePipHCA) in inflammatory genes in the inflamed tissues of DSS-induced colitis and traumatic brain injury models (Figs. 1 and 3). In in vitro experiments, treatment of SW480 colon cells with different LXR agonists shows the suppressive effect on LPS-induced inflammatory gene expression by sterol-based LXR agonists is stronger than T0901317, despite the fact that T0901317 induces higher ABCG1 expression (Fig. 2D). These observations suggest additional LXR-independent mechanisms are involved in the immune regulatory function of this class of oxysterols. It will be interesting to identify the potential new targets that facilitate the anti-inflammatory action of these sterol-based agonists.
Here, we demonstrate the efficacy of DMHCA and its novel analog MePipHCA in suppressing inflammation in 2 mouse models of inflammatory disease without causing liver lipid accumulation or liver injury. Several hypotheses were put forward to explain the lack of liver toxicity in animals treated with DMHCA or MePipHCA. First, DMHCA might selectively bind to LXR-β and spare the LXR-α activation-induced hepatic lipogenesis. However, the LXR-α and LXR-β binding affinity assay show that there is no appreciable difference in the selectivity of LXR-α/β binding of either DMHCA or MePipHCA (Fig. 1A). Second, these sterol-based agonists might have preferential extrahepatic distribution or rapid hepatic metabolism, such that they do not engage in hepatic transcriptional regulation. However, a PK study in which we compared the distribution of DMHCA and MePipHCA shows these compounds have much higher exposure in the liver than in the serum (Fig. 4B). Third, these agonists might have a suppressive effect on gene transcriptional activity in the liver through molecular modulatory mechanisms. It is possible that these sterol-based LXR agonists exhibit a silent transcriptional activity profile or selectively regulate a subset of LXR target genes excluding ones controlling lipid synthesis and metabolism. This silent hepatic profile might be caused by either recruiting corepressor complexes to the LXR promoter or directly engaging protein targets other than LXR to suppress gene expression. Previously, desmosterol and other cholesterols were suggested to bind to SREBP cleavage activating protein and insulin-induced gene and subsequently prevent SREBP protein processing and maturation in the ER, thus sequestering SREBP from engaging in its transcriptional activity (3). It is likely that DMHCA and its analogs work via a similar mechanism as desmosterol in regulating the biosynthesis of cholesterol and fatty acids. However, follow-up on these speculations are outside the scope of this study. We are working toward molecular-level understanding of DMHCA and its analogs and believe these studies may yield a deeper understanding of DMHCA’s action and putative therapeutic utility, as well as lead to the discovery of novel drug targets.
LXR agonists have shown efficacy in treating various types of inflammatory conditions. However, the side effects of liver lipid toxicity associated with traditional LXR agonists has hindered the development of LXR agonists as therapies in treating inflammatory diseases. Herein, we described a class of novel sterol-based LXR agonists, which reduces inflammation in animal models without inducing liver lipid accumulation or liver injury. Both ulcerative colitis and traumatic brain injury are diseases of unmet medical needs. As many as one-fourth to one-third of ulcerative colitis patients do not respond to available treatments and may need to resort to full or partial removal of their colon. As many as 1.4 million people experience traumatic brain injury with 3.5% death rate and 16.8% hospitalization rate every year in the United States. Therefore, innovative therapies are needed to treat patients with inflammatory diseases who do not actively respond to other treatments and patients who are therefore in need of medicines with better efficacy and lower toxicity. This work contributes provides a basis for developing sterol-based LXR agonists as novel drugs for inflammatory diseases.
Acknowledgments
This work was supported by a Crohn’s and Colitis Foundation of America Research Fellowship Award (368561; to S.Y.), and by a U.S. National Institutes of Health, National Center for Advancing Translational Sciences/Clinical and Translational Science Award (UL1-TR001114; to Scripps Translational Science Institute).
Glossary
- ABCA1
ATP-binding cassette transporter A1
- ABCG1
ATP-binding cassette transporter G1
- ALT
alanine transaminase
- CCL2
chemokine (C-C motif) ligand 2
- DMHCA
N,N-dimethyl-3β-hydroxycholenamide
- DSS
dextran sulfate sodium
- FBS
fetal bovine serum
- HTRF
homogeneous time resolved fluorescence
- IS
internal standard
- LPMC
lamina propria mononuclear cells
- LXR
liver X receptor
- MePipHCA
methylpiperidinyl-3β-hydroxycholenamide
- PBMC
peripheral blood mononuclear cell
- SREBP
sterol regulatory element binding protein
REFERENCES
- 1.Lehmann J. M., Kliewer S. A., Moore L. B., Smith-Oliver T. A., Oliver B. B., Su J. L., Sundseth S. S., Winegar D. A., Blanchard D. E., Spencer T. A., Willson T. M. (1997) Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J. Biol. Chem. 272, 3137–3140 [DOI] [PubMed] [Google Scholar]
- 2.Zelcer N., Tontonoz P. (2006) Liver X receptors as integrators of metabolic and inflammatory signaling. J. Clin. Invest. 116, 607–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spann N. J., Glass C. K. (2013) Sterols and oxysterols in immune cell function. Nat. Immunol. 14, 893–900 [DOI] [PubMed] [Google Scholar]
- 4.Hong C., Tontonoz P. (2014) Liver X receptors in lipid metabolism: opportunities for drug discovery. Nat. Rev. Drug Discov. 13, 433–444 [DOI] [PubMed] [Google Scholar]
- 5.Glass C. K., Saijo K. (2010) Nuclear receptor transrepression pathways that regulate inflammation in macrophages and T cells. Nat. Rev. Immunol. 10, 365–376 [DOI] [PubMed] [Google Scholar]
- 6.Cheng O., Ostrowski R. P., Liu W., Zhang J. H. (2010) Activation of liver X receptor reduces global ischemic brain injury by reduction of nuclear factor-kappaB. Neuroscience 166, 1101–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D., Liu M., Wang Y., Luo M., Wang J., Dai C., Yan P., Zhang X., Wang Y., Tang C., Xiao J. (2011) Synthetic LXR agonist T0901317 attenuates lipopolysaccharide-induced acute lung injury in rats. Int. Immunopharmacol. 11, 2098–2103 [DOI] [PubMed] [Google Scholar]
- 8.Ghisletti S., Huang W., Ogawa S., Pascual G., Lin M. E., Willson T. M., Rosenfeld M. G., Glass C. K. (2007) Parallel SUMOylation-dependent pathways mediate gene- and signal-specific transrepression by LXRs and PPARgamma. Mol. Cell 25, 57–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spann N. J., Garmire L. X., McDonald J. G., Myers D. S., Milne S. B., Shibata N., Reichart D., Fox J. N., Shaked I., Heudobler D., Raetz C. R., Wang E. W., Kelly S. L., Sullards M. C., Murphy R. C., Merrill A. H. Jr., Brown H. A., Dennis E. A., Li A. C., Ley K., Tsimikas S., Fahy E., Subramaniam S., Quehenberger O., Russell D. W., Glass C. K. (2012) Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell 151, 138–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joseph S. B., Castrillo A., Laffitte B. A., Mangelsdorf D. J., Tontonoz P. (2003) Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat. Med. 9, 213–219 [DOI] [PubMed] [Google Scholar]
- 11.Park M. C., Kwon Y. J., Chung S. J., Park Y. B., Lee S. K. (2010) Liver X receptor agonist prevents the evolution of collagen-induced arthritis in mice. Rheumatology (Oxford) 49, 882–890 [DOI] [PubMed] [Google Scholar]
- 12.Jakobsson T., Vedin L. L., Hassan T., Venteclef N., Greco D., D’Amato M., Treuter E., Gustafsson J. A., Steffensen K. R. (2014) The oxysterol receptor LXRβ protects against DSS- and TNBS-induced colitis in mice. Mucosal Immunol. 7, 1416–1428 [DOI] [PubMed] [Google Scholar]
- 13.Repa J. J., Liang G., Ou J., Bashmakov Y., Lobaccaro J. M., Shimomura I., Shan B., Brown M. S., Goldstein J. L., Mangelsdorf D. J. (2000) Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev. 14, 2819–2830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang G., Yang J., Horton J. D., Hammer R. E., Goldstein J. L., Brown M. S. (2002) Diminished hepatic response to fasting/refeeding and liver X receptor agonists in mice with selective deficiency of sterol regulatory element-binding protein-1c. J. Biol. Chem. 277, 9520–9528 [DOI] [PubMed] [Google Scholar]
- 15.Katz A., Udata C., Ott E., Hickey L., Burczynski M. E., Burghart P., Vesterqvist O., Meng X. (2009) Safety, pharmacokinetics, and pharmacodynamics of single doses of LXR-623, a novel liver X-receptor agonist, in healthy participants. J. Clin. Pharmacol. 49, 643–649 [DOI] [PubMed] [Google Scholar]
- 16.Quinet E. M., Basso M. D., Halpern A. R., Yates D. W., Steffan R. J., Clerin V., Resmini C., Keith J. C., Berrodin T. J., Feingold I., Zhong W., Hartman H. B., Evans M. J., Gardell S. J., DiBlasio-Smith E., Mounts W. M., LaVallie E. R., Wrobel J., Nambi P., Vlasuk G. P. (2009) LXR ligand lowers LDL cholesterol in primates, is lipid neutral in hamster, and reduces atherosclerosis in mouse. J. Lipid Res. 50, 2358–2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shih D. M., Shaposhnik Z., Meng Y., Rosales M., Wang X., Wu J., Ratiner B., Zadini F., Zadini G., Lusis A. J. (2013) Hyodeoxycholic acid improves HDL function and inhibits atherosclerotic lesion formation in LDLR-knockout mice. FASEB J. 27, 3805–3817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong C., Tontonoz P. (2008) Coordination of inflammation and metabolism by PPAR and LXR nuclear receptors. Curr. Opin. Genet. Dev. 18, 461–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tice C. M., Noto P. B., Fan K. Y., Zhuang L., Lala D. S., Singh S. B. (2014) The medicinal chemistry of liver X receptor (LXR) modulators. J. Med. Chem. 57, 7182–7205 [DOI] [PubMed] [Google Scholar]
- 20.Lim R. K., Yu S., Cheng B., Li S., Kim N. J., Cao Y., Chi V., Kim J. Y., Chatterjee A. K., Schultz P. G., Tremblay M. S., Kazane S. A. (2015) Targeted delivery of LXR agonist using a site-specific antibody-drug conjugate. Bioconjug. Chem. 26, 2216–2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quinet E. M., Savio D. A., Halpern A. R., Chen L., Miller C. P., Nambi P. (2004) Gene-selective modulation by a synthetic oxysterol ligand of the liver X receptor. J. Lipid Res. 45, 1929–1942 [DOI] [PubMed] [Google Scholar]
- 22.Loane D. J., Washington P. M., Vardanian L., Pocivavsek A., Hoe H. S., Duff K. E., Cernak I., Rebeck G. W., Faden A. I., Burns M. P. (2011) Modulation of ABCA1 by an LXR agonist reduces β-amyloid levels and improves outcome after traumatic brain injury. J. Neurotrauma 28, 225–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vowinkel T., Mori M., Krieglstein C. F., Russell J., Saijo F., Bharwani S., Turnage R. H., Davidson W. S., Tso P., Granger D. N., Kalogeris T. J. (2004) Apolipoprotein A-IV inhibits experimental colitis. J. Clin. Invest. 114, 260–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weigmann B., Tubbe I., Seidel D., Nicolaev A., Becker C., Neurath M. F. (2007) Isolation and subsequent analysis of murine lamina propria mononuclear cells from colonic tissue. Nat. Protoc. 2, 2307–2311 [DOI] [PubMed] [Google Scholar]
- 25.Danese S., Vuitton L., Peyrin-Biroulet L. (2015) Biologic agents for IBD: practical insights. Nat. Rev. Gastroenterol. Hepatol. 12, 537–545 [DOI] [PubMed] [Google Scholar]
- 26.Huang W., Ghisletti S., Perissi V., Rosenfeld M. G., Glass C. K. (2009) Transcriptional integration of TLR2 and TLR4 signaling at the NCoR derepression checkpoint. Mol. Cell 35, 48–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghisletti S., Huang W., Jepsen K., Benner C., Hardiman G., Rosenfeld M. G., Glass C. K. (2009) Cooperative NCoR/SMRT interactions establish a corepressor-based strategy for integration of inflammatory and anti-inflammatory signaling pathways. Genes Dev. 23, 681–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cha J. Y., Repa J. J. (2007) The liver X receptor (LXR) and hepatic lipogenesis. The carbohydrate-response element-binding protein is a target gene of LXR. J. Biol. Chem. 282, 743–751 [DOI] [PubMed] [Google Scholar]
- 29.Chisholm J. W., Hong J., Mills S. A., Lawn R. M. (2003) The LXR ligand T0901317 induces severe lipogenesis in the db/db diabetic mouse. J. Lipid Res. 44, 2039–2048 [DOI] [PubMed] [Google Scholar]
- 30.Hu B., Unwalla R. J., Goljer I., Jetter J. W., Quinet E. M., Berrodin T. J., Basso M. D., Feingold I. B., Nilsson A. G., Wilhelmsson A., Evans M. J., Wrobel J. E. (2010) Identification of phenylsulfone-substituted quinoxaline (WYE-672) as a tissue selective liver X-receptor (LXR) agonist. J. Med. Chem. 53, 3296–3304 [DOI] [PubMed] [Google Scholar]
- 31.Temml V., Voss C. V., Dirsch V. M., Schuster D. (2014) Discovery of new liver X receptor agonists by pharmacophore modeling and shape-based virtual screening. J. Chem. Inf. Model. 54, 367–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kratzer A., Buchebner M., Pfeifer T., Becker T. M., Uray G., Miyazaki M., Miyazaki-Anzai S., Ebner B., Chandak P. G., Kadam R. S., Calayir E., Rathke N., Ahammer H., Radovic B., Trauner M., Hoefler G., Kompella U. B., Fauler G., Levi M., Levak-Frank S., Kostner G. M., Kratky D. (2009) Synthetic LXR agonist attenuates plaque formation in apoE-/- mice without inducing liver steatosis and hypertriglyceridemia. J. Lipid Res. 50, 312–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Traversari C., Sozzani S., Steffensen K. R., Russo V. (2014) LXR-dependent and -independent effects of oxysterols on immunity and tumor growth. Eur. J. Immunol. 44, 1896–1903 [DOI] [PubMed] [Google Scholar]