Abstract
Stenosis is a critical problem in the long-term efficacy of tissue-engineered vascular grafts (TEVGs). We previously showed that host monocyte infiltration and activation within the graft drives stenosis and that TGF-β receptor 1 (TGF-βR1) inhibition can prevent it, but the latter effect was attributed primarily to inhibition of mesenchymal cell expansion. In this study, we assessed the effects of TGF-βR1 inhibition on the host monocytes. Biodegradable TEVGs were implanted as inferior vena cava interposition conduits in 2 groups of C57BL/6 mice (n = 25/group): unseeded grafts and unseeded grafts with TGF-βR1 inhibitor systemic treatment for the first 2 wk. The TGF-βR1 inhibitor treatment effectively improved TEVG patency at 6 mo compared to the untreated control group (91.7 vs. 48%, P < 0.001), which is associated with a reduction in classic activation of mononuclear phagocytes. Consistent with these findings, the addition of rTGF-β to LPS/IFN-γ–stimulated monocytes enhanced secretion of inflammatory cytokines TNF-α, IL-12, and IL-6; this effect was blocked by TGF-βR1 inhibition (P < 0.0001). These findings suggest that the TGF-β signaling pathway contributes to TEVG stenosis by inducing classic activation of host monocytes. Furthermore, blocking monocyte activation by TGF-βR1 inhibition provides a viable strategy for preventing TEVG stenosis while maintaining neotissue formation.—Lee, Y.-U., de Dios Ruiz-Rosado, J., Mahler, N., Best, C. A., Tara, S., Yi, T., Shoji, T., Sugiura, T., Lee, A. Y., Robledo-Avila, F., Hibino, N., Pober, J. S., Shinoka, T., Partida-Sanchez, S., Breuer, C. K. TGF-β receptor 1 inhibition prevents stenosis of tissue-engineered vascular grafts by reducing host mononuclear phagocyte activation.
Keywords: cell seeding, congenital heart defect, Fontan operation, regenerative medicine, inflammation
Previous data from our laboratory demonstrated that bone marrow–derived mononuclear cell (BM-MNC) seeding of tissue-engineered vascular grafts (TEVGs) promoted the process of neovessel formation (1). In contrast to our original expectation that the seeded cells differentiated into mature vascular cells, we found that the seeded BM-MNCs disappeared within a few days of implantation. Instead, the seeded cells exerted their effect by controlling recruitment of host mononuclear phagocytes in early time point, which recruit smooth muscle cells and endothelial cells (∼3 wk) (1). While BM-MNC seeding also significantly improved patency of the TEVGs, both in a clinical study and in mouse models, the occurrence of graft stenosis remained a major complication in 25% of TEVG recipients (2, 3). Our mouse models revealed that the formation of TEVG stenosis is also driven by paracrine functions of infiltrating host mononuclear phagocytes. A significant proportion of cells that form stenotic lesions in the mouse model appear to arise from the endothelial–mesenchymal transition (Endo-MT) (1, 3, 4). Because TGF-β signaling is the key to multiple vascular disorders, including Endo-MT (5–8), we investigated if inhibition of this cytokine could reduce stenosis. As hypothesized, pharmacologic inhibition of TGF-β receptor type 1 (TGF-βR1) decreased Endo-MT and successfully reduced short-term graft stenosis in both systemic treatment and local drug elution (4). These observations left unanswered the question of whether TGF-βR1 inhibition had any effect on the host mononuclear phagocytes that drive the stenotic process. It has been shown that the degree of Endo-MT is regulated by inflammatory cytokines such as TNF-α and IL-1β, which are mainly produced by classically activated monocytes and macrophages in aortic valve stenosis and interstitial fibrosis (9, 10). TGF-β is usually considered to be an anti-inflammatory cytokine, and thus inhibiting its receptor might be anticipated to worsen stenosis. We report here that, contrary to expectation, TGF-βR1 inhibition reduces, rather than enhances, classic inflammatory functions of graft-infiltrating host monocytes. We conclude that the effect of TGF-βR1 inhibition of mononuclear phagocytes may be a major contributory mechanism by which TGF-βR inhibition prevents stenosis. Furthermore, we show the potential role of pharmacologic intervention targeting the TGF-β signaling pathway to replace cell seeding.
MATERIALS AND METHODS
TEVG fabrication
Scaffolds were constructed from a biodegradable polymer mesh with copolymer sealant solution as described previously (11, 12). Briefly, a nonwoven polyglycolic acid mesh (Biomedical Structures, Warwick, RI, USA) and a 50:50 copolymer sealant solution of ε-caprolactone and l-lactide (P(CL/LA); 263,800 Da; Absorbable Polymers International, Birmingham, AL, USA) was assembled around a 19-gauge stainless steel needle in a cylinder. The tubularized scaffold, 1.1 mm outer diameter and 3 mm in length, was snap-frozen at −20°C for 30 min, then lyophilized for 24 h. The scaffolds were stored in a desiccator and sterilized under UV light for 12 h before implantation.
Bone marrow seeding of TEVGs
Syngeneic C57BL/6 mice (n = 5) were euthanized and bone marrow was collected from the femurs and tibias. The BM-MNCs were isolated using a density-gradient centrifugation method. A total of 1.0 × 106 cells/graft were seeded onto the scaffold and then incubated overnight (11).
TEVG implantation
All animal experiments were approved by Nationwide Children’s Hospital institutional guidelines for the use and care of animals. In this experiment, there were 3 groups: untreated control, seeded control, and TGF-βR1 inhibitor-treated grafts (n = 25 each). TEVG scaffolds were implanted into 8 to 10 wk old female C57BL/6 mice as an inferior vena cava (IVC) interposition graft as previously described (11). Briefly, the mouse was anesthetized (anesthesia: ketamine, 100 mg/kg and xylazine 10 mg/kg; analgesia: ketoprofen 5 mg/kg i.p.), and a midline laparotomy incision was performed. The IVC and aorta were bluntly dissected and clamped on both the proximal and distal sides with 2 microclamps. After obtaining vascular control, the IVC was transected, and the TEVG scaffold was implanted as an interposition graft. Postoperatively, the mice were provided pain medication (ibuprofen, 30 mg/kg, in their drinking water) for 48 h. In the group receiving the TGF-βR1 inhibitor, mice were treated systemically with TGF-βR1 kinase inhibitor SB431542 hydrate (Sigma-Aldrich, St. Louis, MO, USA) in DMSO provided by intraperitoneal injection twice daily from postoperative d 0 to postoperative d 14 at a dose of 10 mg/kg.
Ultrasound
To monitor graft patency during the implantation period, mice were imaged at 1, 2, 4, and 6 wk, and 3, 4, 5, and 6 mo with a high-frequency Doppler ultrasound system (Vevo 2100; VisualSonics, Toronto, ON, Canada). After anesthetizing the mice (1.5% isoflurane; Baxter, Deerfield, IL, USA), the abdominal hair was clipped, and ultrasound gel (Aquasonic Clear, Fairfield, NJ, USA) was applied on the abdomen. Long-axis images were acquired in both B mode and color Doppler, and the graft patency was determined by the presence of blood flow through the graft lumen.
Tissue collection and preparation for histology
The grafts were explanted at the end of the 6-mo implantation period. After euthanizing the mice, the grafts were perfusion fixed with 10% formalin, placed in 10% formalin overnight, and then embedded in paraffin as previously described (12).
Immunohistochemistry
The paraffin-embedded sections were stained with hematoxylin and eosin for determining patency and luminal diameter. The sections were incubated with the following primary antibodies: anti-F4/80 (1:1000; AbD Serotec, Oxford, United Kingdom), anti-CD31 (1:50; Abcam, Cambridge, MA, USA), anti–smooth muscle actin (SMA; 1:500; Dako, Glostrup, Denmark), anti-calponin (1:200; Abcam), anti-vimentin (1:1500; Abcam), anti-TGF-β (1:100; Abcam), anti-TGF-βR1 (1:50; Abcam), anti-pSmad2/3 (1:250; Abcam), and anti–latent TGF-β binding protein 1 (LTBP1; 1:100; Abcam). Primary antibody binding was detected with biotinylated secondary antibodies, namely goat anti-rat IgG (1:200; Vector Laboratories, Burlingame, CA, USA), goat anti-rabbit IgG (1:200; Vector Laboratories), and goat anti-rabbit IgG (1:200; Vector Laboratories), respectively, followed by binding of horseradish peroxidase streptavidin (Vector Laboratories) and subsequent chromogenic development with 3,3-diaminobenzidine (Vector Laboratories). Images were obtained with a Zeiss Axio Imager.A2 microscope (Carl Zeiss GmbH, Jena, Germany).
TEVG analysis
Graft luminal diameters were measured from hematoxylin and eosin–stained images by ImageJ software (National Institutes of Health, Bethesda, MD, USA). Patent grafts were defined as those with less than 50% narrowing of the original luminal diameter.
Tissue processing for flow cytometry
After collection, TEVGs were minced with scissors and digested in HBSS solution containing 1 mg/ml collagenase type IV, 0.2 mg/ml DNase I, 200 U/ml hyaluronidase, and 1 mg/ml bovine serum albumin/fraction V (Thermo Fisher Scientific Life Sciences, Waltham, MA, USA). After enzymatic dissociation of TEVGs, cells were stained for flow cytometry.
Monocyte isolation and stimulation
Bone marrow cell suspensions were isolated by flushing femurs and tibias of 8- to 12-wk-old C57BL/6 mice. The BM-MNCs were isolated using a density-gradient centrifugation method (11) and incubated with a mixture of antibody MicroBeads according to the manufacturer’s protocol (Miltenyi Biotec, San Diego, CA, USA). The cells were then run through an LD-negative selection column. The negative fraction was collected (putative monocytes) and cultured in complete RPMI 1640. Cultures of purified monocytes were stimulated overnight with LPS (100 ng/ml; Sigma-Aldrich) and IFN-γ (10 ng/ml; BioLegend, San Diego, CA, USA) and/or rTGF-β (10 ng/ml; R&D Systems, Minneapolis, MN, USA) in the presence or absence of TGF- βR1 inhibitor SB431542 (10 μM; Sigma-Aldrich).
Cytofluorometric analysis
Collected cells from monocyte cultures or TEVGs were incubated in 1 μg/ml of anti-mouse Fc receptor antibody in 100 ml PBS containing 0.5% bovine serum albumin plus 0.02% NaN3 [fluorescence-activated cell sorting (FACS) buffer] for 15 min on ice. After washing, 1 to 3 × 106 cells were stained in FACS buffer for 15 min at 4°C with various fluorescent mAbs combinations and further collected on a LSR II cytofluorometer (Becton Dickinson, Franklin Lakes, NJ, USA). Cells were gated according to size and scatter to eliminate debris (Supplemental Fig. 1). Blue fluorescent reactive dye L23105 (Life Technologies, Carlsbad, CA, USA) was used to exclude dead cells. The following antibody conjugates were purchased from eBioscience (San Diego, CA, USA) and used for extracellular staining: anti-Ly6C (Alexa Fluor 488) anti-CD115 (PE-Cy7, clone AFS98), anti-F480 (Alexa Fluor 488), and anti-Ly6C (eFluor 450). The following antibodies were purchased from BioLegend: anti-CD11b (Alexa Fluor 700), anti-F4/80 (Brilliant Violet 605), anti-Ly6G (PER-CP), and anti-CD45 (Brilliant Violet 510). For intracellular staining, cells were incubated for 6 h in RPMI 1640 5% fetal calf serum with 1 μl/ml Golgi Plug (Becton Dickinson) at 37°C, 5% CO2, washed with FACS buffer, and stained according to the protocol of the cytofix/cytoperm kit (BD Biosciences, San Jose, CA, USA). The following antibodies were purchased from eBioscience and used for intracellular staining: anti-iNOS (PE, clone CXNFT, 12-5920-82) and anti-TGFβ1 (Brilliant Violet 421). The following antibodies were purchased from BioLegend: anti-TNF-α (Brilliant Violet 650) and anti-pSmad2/3 (PE).
Cytokine quantification by CBA
IL-12p70, TNF-α, IFN-γ, monocyte chemotactic protein 1 (MCP-1), IL-10, and IL-6 were quantified in supernatants of bone marrow monocyte cultures using Cytometric Bead Array multiplexed bead-based immunoassays (BD Biosciences). The assay was performed according to the manufacturer’s instructions. Three thousand events were acquired for each sample in a FACS LSR II flow cytometer (Becton Dickinson). Data were analyzed using FCAP array v3 (Soft Flow Hungary, Pécs, Hungary).
Statistical analysis
Statistical comparisons were performed by either Student’s t test or the 1-way ANOVA followed by Tukey’s multiple comparisons for data following normal distribution. The Kruskal-Wallis test was used when the data followed nonnormal distribution. For post hoc tests, a Mann-Whitney U test was used with Bonferroni-Holm correction. Stenosis rates were compared by Fisher’s exact test. Values of P < 0.05 were considered significant. Graphed data are presented as means ± sd or sem.
RESULTS
Cellular and structural changes during neovessel formation in TEVGs
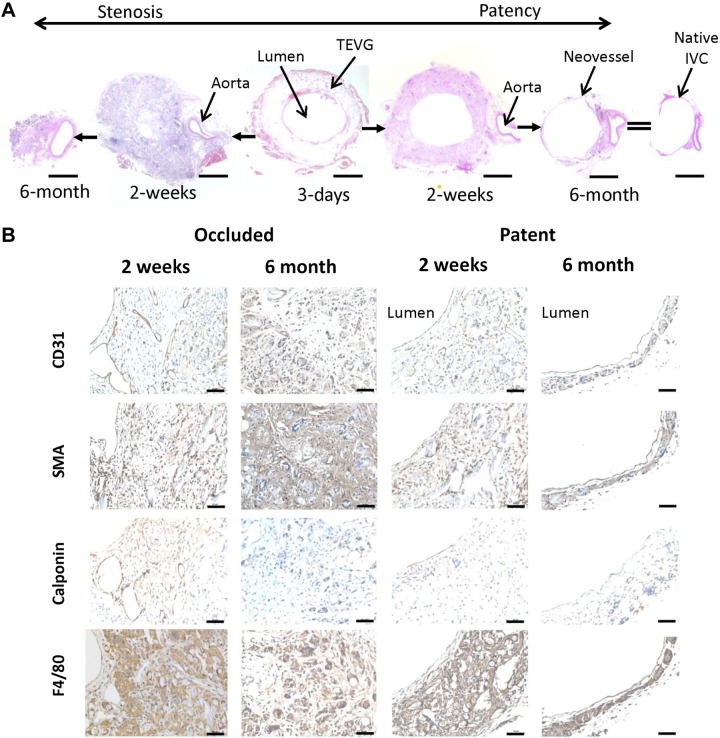
When the TEVG is implanted as an IVC interposition graft, neotissue begins to form as the scaffold degrades. When the scaffold has completely degraded, the resulting structure is called a neovessel. The neovessel resembles the native IVC in both structure and function (1, 13, 14). To examine the cellular changes that occur during neovessel formation, we examined BM-MNC–seeded grafts at 3, 14, and 180 d after implantation (n = 5 each). Three days postoperatively, there was evidence of cellular infiltration into the TEVG scaffold and minimal formation of tissue on the luminal surface of the scaffold (Fig. 1A). By 2 wk after implantation, the stenotic grafts were characterized by extensive monocyte/macrophage infiltration into the scaffold and neotissue formation within the lumen, composed primarily of smooth muscle cells and myofibroblasts. Whereas the patent grafts displayed reduced monocyte/macrophage infiltration into the scaffold with formation of vascular neotissue composed of concentric layers of smooth muscle cells (Fig. 1A, B). Both patent and stenosed grafts showed the presence of endothelial cell lining on the luminal surface of the TEVGs even though the luminal surface of stenosed grafts was much smaller than that of the patent grafts (Fig. 1A). Finally, 6 mo after implantation, the scaffolding had completely degraded, and the stenotic grafts were occluded and had undergone inward remodeling, while the patent grafts formed an intima, media, and adventitia, resembling that of the native IVC (Fig. 1A, B).
Figure 1.
Time course of TEVG development. A) Representative hematoxylin and eosin staining of BM-MNC–seeded TEVGs at different time points after TEVG implantation. Scale bars, 200 μm. B) Immunohistochemical staining of occluded and patent TEVGs at 2 wk and 6 mo time points. CD31 staining is for endothelial cells, α-SMA and calponin for smooth muscle cells, and F4/80 for monocytes/macrophages. Scale bars, 50 μm.
Short-term administration of TGF-βR1 inhibitor provides long-term TEVG patency
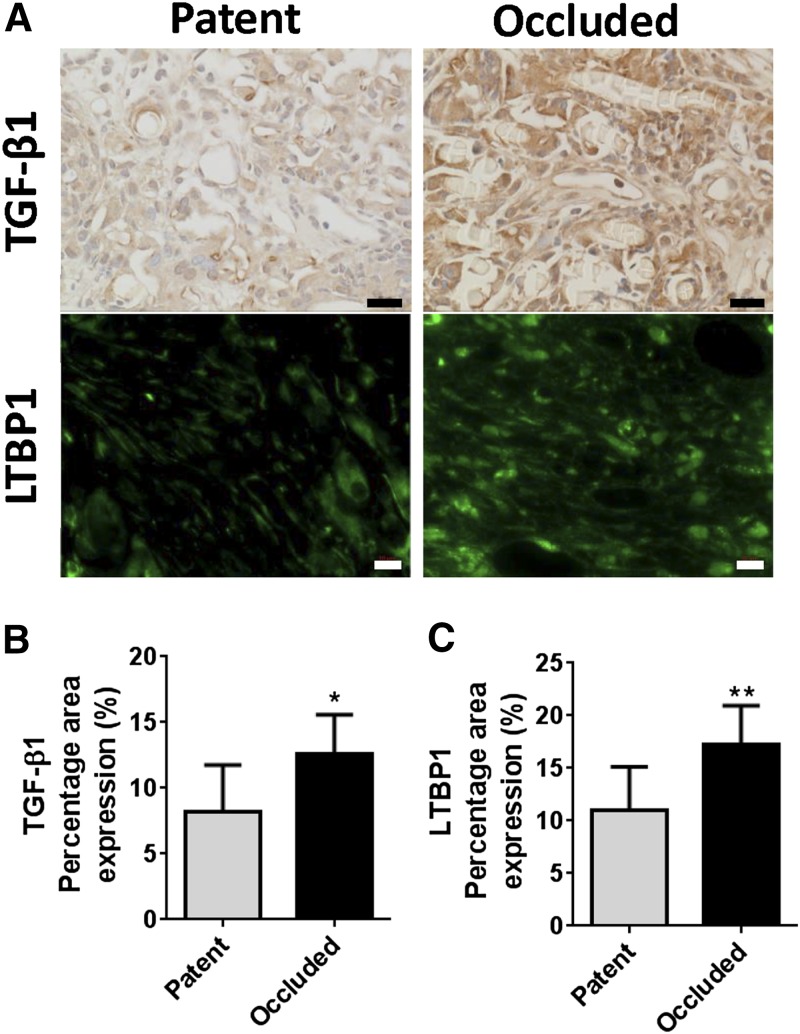
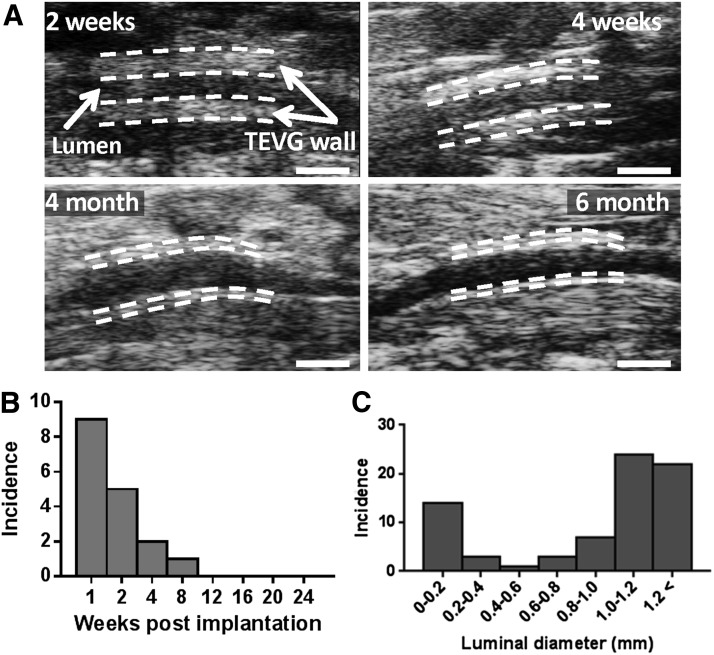
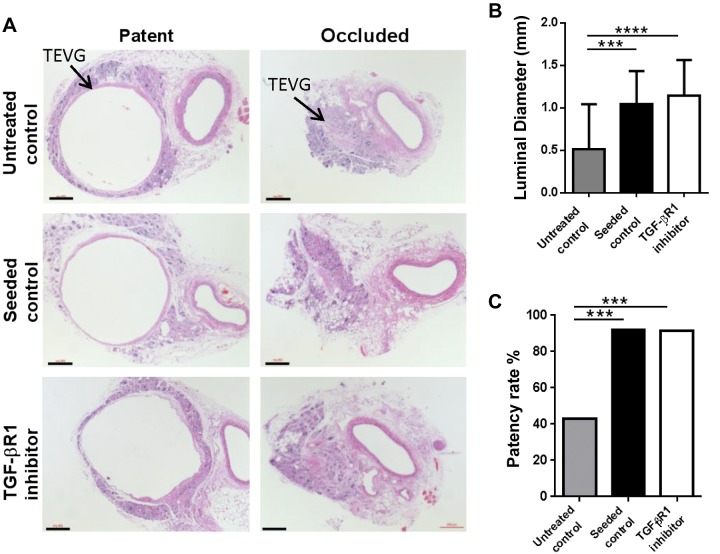
We previously demonstrated that TGF-β signaling affects short-term patency of the TEVGs (4). To study the role of TGF-β signaling in the development of stenosis, we first compared TGF-β1 and the latent TGF-β binding protein 1 (LTBP1) expressions in unseeded grafts at 2 wk (total n = 15, n = 9 patent, n = 6 occluded; Fig. 2A). Both TGF-β1 and LTBP1 expressions in occluded grafts were significantly higher than the patent grafts, showing that high levels of TGF-β expression are associated with TEVG stenosis (P < 0.05 and P < 0.01, respectively; Fig. 2B, C). We then administrated a TGF-βR1 inhibitor (SB431542) to mice for 2 wk after implantation and assessed the effect of this drug on long-term TEVG patency. We implanted 3 groups of TEVGs into C57BL/6 mice: BM-MNC–seeded scaffolds, unseeded scaffolds with TGF-βR1 blocker (SB431542) treatment, and unseeded scaffolds as negative control (n = 25 each). The TEVGs were collected 6 mo after implantation and monitored with serial ultrasound to assess graft morphology and function over the 6 mo time course. Ultrasound interrogation revealed that the wall of the patent TEVGs became progressively thinner as the scaffold degraded, approaching the thickness of the native IVC by 6 mo after implantation (Fig. 3A). Serial ultrasound also revealed that the primary graft–related complication was stenosis, occurring in 17 of 74 TEVGs. There was no evidence of any other graft-related complications such as thrombosis or aneurysmal dilation. Ultrasound analysis also demonstrated that stenosis typically developed within 2 wk of implantation (14 of 17 occluded grafts, combined from all the TEVGs) (Fig. 3B). Upon explantation at 6 mo, graft luminal diameter followed a bimodal distribution, with the grafts developing either critical stenosis (>75% narrowing) or remaining widely patent at explantation at 6 mo (Fig. 3C). The luminal diameter was significantly improved in both the seeded control (positive control) and the TGF-βR1 inhibitor groups compared to the unseeded control group (1.04 ± 0.39 and 1.15 ± 0.42 vs. 0.51 ± 0.53 mm, P < 0.001; Fig. 4A, B). Both the cell-seeded group and the TGF-βR1–treated group showed significantly higher patency rates compared to the untreated group (0.92 and 0.917 vs. 0.48%; P < 0.001; Fig. 4A, C). These results show that early short-term treatment with the TGF-βR1 inhibitor resulted in long-term reduction of TEVG stenosis. Even though the patency and luminal diameter were significantly different at 6 mo after implantation between groups, there was no difference in the number of macrophages and myofibroblasts in the TEVGs in both patent and occluded grafts at that time point (Supplemental Fig. 2). Moreover, TGF-β, TGF-βR1, and pSmad2/3 expressions were also similar between groups, indicating after the scaffold degradation, the inflammation subsides and plays less of a role in neovessel formation (Supplemental Fig. 3).
Figure 2.
TGF-β1 and LTBP1 expressions in TEVGs. Occluded TEVGs showed significantly higher TGF-β1 and LTBP1 expressions compared to patent TEVGs. Scale bars, 20 and 10 μm, respectively. A) Immunohistochemical staining of TGF-β1 and immunofluorescent staining of LTBP1 in patent and occluded TEVGs at 2 wk. B) TGF-β1 percentage area expression. C) LTBP1 percentage area expression. *P < 0.05, **P < 0.01.
Figure 3.
Longitudinal ultrasound interrogation. A) Ultrasound images of TEVGs at various time points. Scale bars, 1 mm. B) Incidence of stenosis measured by serial ultrasound interrogation. C) Luminal diameter distribution of explanted grafts at 6 mo (n = 74 from all implanted TEVGs).
Figure 4.
Effect of short-term TGF-βR1 inhibition on long-term TEVG stenosis. Short-term (2 wk) TGF-βR1 inhibitor treatment effectively prevented long-term (6 mo) TEVG stenosis. A) Representative hematoxylin and eosin staining of patent and occluded TEVGs of untreated control, seeded control, and TGF-βR1 inhibitor-treated mice at 6 mo. Scale bars, 200 μm. B) Luminal diameter of untreated control, seeded control, and TGF-βR1 inhibitor-treated mice at 6 mo. C) Patency rate of untreated control, seeded control, and TGF-βR1 inhibitor-treated mice at 6 mo. Means and sd are shown; n = 25/group. ***P < 0.001, ****P < 0.0001.
Inhibition of TGF-βR1 in mice markedly prevented TEVG stenosis by reducing classic activation of infiltrating monocytes and macrophages
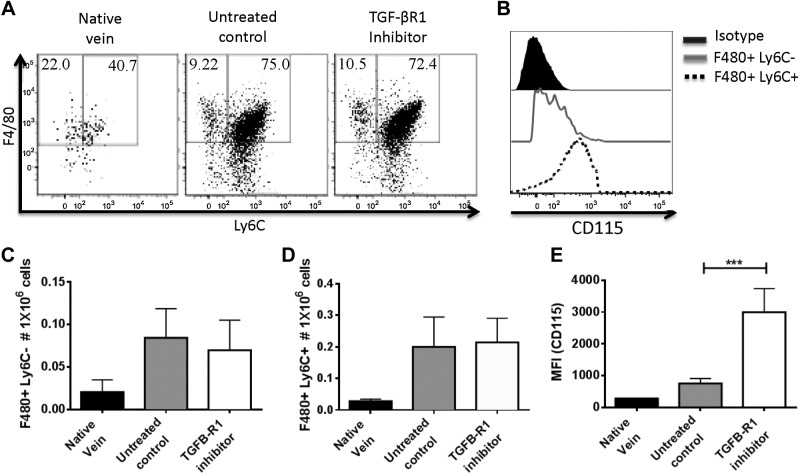
We previously showed that early administration of clodronate after TEVG implantation not only inhibited stenosis but also disrupted neotissue formation in the TEVGs, indicating a dual role for cells of macrophage lineage (3). To assess the effect of TGF-βR1 inhibition on monocyte/macrophage infiltration and function, we implanted TEVGs in C57BL/6 mice and characterized infiltrating monocyte and macrophage populations in TEVGs from untreated and TGF-βR1 inhibitor-treated groups at a 2-wk time point. We found 2 populations of F4/80+ cells in TEVGs, with one population of Ly6C+ expressing high levels of CD115, which indicates that these are newly recruited monocytes, and the other of Ly6C− expressing CD115low, defined here as macrophages (Fig. 5A, B, E). Comparable numbers of infiltrating monocytes and macrophages were observed between untreated and TGF-βR1 inhibitor-treated groups, meaning that TGF-βR1 inhibition does not affect monocyte/macrophage migration or infiltration onto the TEVGs (n = 10; Fig. 5C, D).
Figure 5.
Infiltrating monocytes and macrophages in TEVGs at 14 d after implantation. TGF-βR1 inhibition did not alter monocyte and macrophage infiltration onto TEVGs. A) Representative dot plots of total monocytes and macrophages in TEVGs 14 d after implantation. B) Further characterization of monocytes and macrophages using CD115 antibody. C, D) Comparison between untreated control and TGF-βR1 inhibitor-treated groups in total monocytes (C) and macrophages (D) in TEVGs 14 d after implantation. Means and sem are shown; n = 10/group.
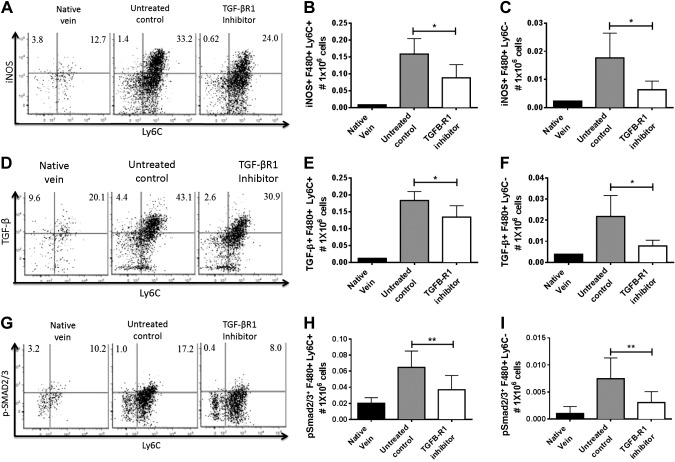
In addition, we further determined iNOS expression in monocytes and macrophages, a prototypical marker for classic activation. Our data demonstrated that administration of TGF-βR1 inhibitor resulted in significantly reduced numbers of iNOS+ monocytes and macrophages at 2 wk after implantation (P < 0.05; Fig. 6A–C). Moreover, TGF-β production in both monocytes and macrophages was also significantly decreased in the TGF-βR1 inhibitor-treated group compared to the untreated group (P < 0.05; Fig. 6D–F). The blockade of TGF-β signaling in monocytes was confirmed by significant reduction in pSmad2/3 in the TGF-βR1 inhibitor group (P < 0.01; Fig. 6H–J). These results suggest that TGF-β signaling enhances classic activation of inflammatory monocytes and macrophages, which may lead to increased inflammation and stenosis in the TEVGs.
Figure 6.
Effect of TGF-βR1 inhibitor on monocyte activation in vivo. TGF-βR1 inhibition significantly reduced activation of monocytes and macrophages in TEVGs. A) Representative dot plots of total iNOS+ monocytes and macrophages in TEVGs 14 d after implantation. Comparison between untreated control and TGF-βR1 inhibitor-treated groups in total iNOS+ (B) monocytes and (C) macrophages in TEVGs 14 d after implantation. D) Representative dot plots of total TGF-β+ monocytes and macrophages in TEVGs 14 d after implantation. Comparison between untreated control and TGF-βR1 inhibitor-treated groups in total TGF-β+ monocytes (E) and macrophages (F) in TEVGs 14 d after implantation. G) Representative dot plots of total pSmad2/3+ monocytes and macrophages in TEVGs 14 d after implantation. Comparison between untreated control and TGF-βR1 inhibitor-treated groups in total pSmad2/3+ monocytes (H) and macrophages (I) in TEVGs 14 d after implantation. Means and sem are shown; n = 10/group. *P < 0.05, **P < 0.01.
TGF-βR1 inhibition reduces classic activation and secretion of key inflammatory cytokines from monocytes in vitro
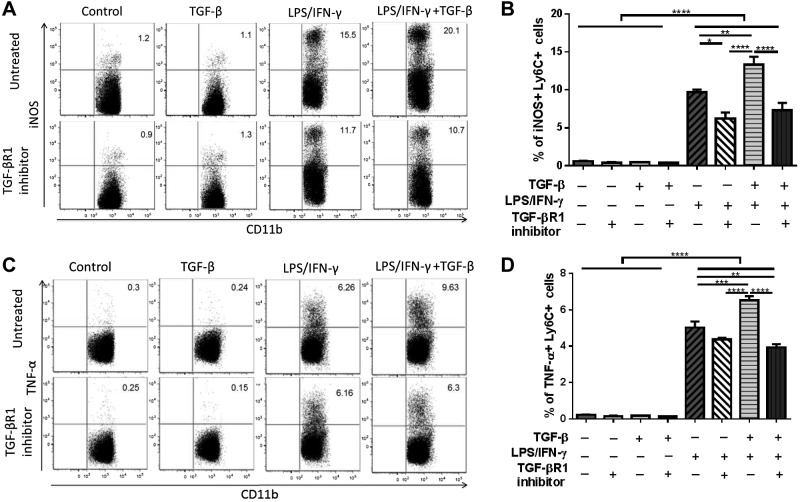
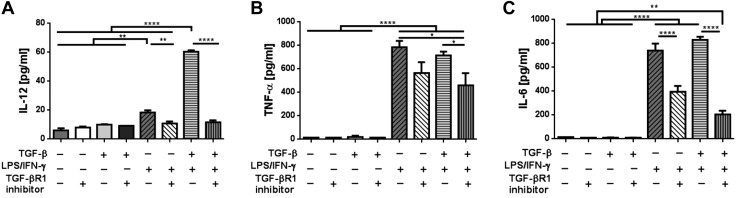
The scaffold that is implanted in vivo initiates a foreign body reaction, which induces an inflammatory response in TEVGs. To mimic the foreign body reaction in vitro and demonstrate the possible role of TGF-β enhancing classic activation of inflammatory monocytes, primary cultured bone marrow monocytes were stimulated in vitro with LPS/IFN-γ and/or TGF-β. After 24 h of culture in vitro, LPS/IFN-γ induced classic activation in bone marrow monocytes, characterized by iNOS and TNF-α production (P < 0.0001). Strikingly, the addition of rTGF-β in LPS/IFN-γ–stimulated monocytes significantly increased both iNOS and TNF-α production compared to the LPS/IFN-γ–stimulated group (P < 0.01 and P < 0.001; Fig. 7). However, the rTGF-β-only stimulation showed a minimal effect on iNOS expression, which suggests that TGF-β synergizes with classic inflammatory stimuli to enhance monocyte activation. This synergistic effect of TGF-β was totally abrogated by the TGF-βR1 inhibitor. (P < 0.0001; Fig. 7). We then further explored the effect of TGF-βR1 inhibition on inflammatory cytokine secretion from activated monocytes using a cytometric bead array on supernatants from the in vitro monocyte culture study. In agreement with these data, LPS/IFN-γ stimulation of primary cultured monocytes also resulted in a significantly increased concentration of TNF-α and other proinflammatory cytokines in the supernatant, including IL-6 and IL-12 (P < 0.0001; Fig. 8). Notably, the addition of rTGF-β to LPS/IFN-γ–stimulated monocytes further increased their secretion of IL-6 and IL-12. Interestingly, the TGF-βR1 inhibitor treatment significantly lowered the secretion of all 3 inflammatory cytokines from activated monocytes, especially IL-12 and IL-6, as these cytokines were lowered close to the unstimulated levels (P < 0.0001 for IL-12 and IL-6; P < 0.05 for TNF- α). Other cytokines tested, MCP-1 and IL-10, did not show detectable differences between groups. These results suggest that the TGF-β signaling pathway affects the production of the main inflammatory cytokines on classically activated monocytes.
Figure 7.
Effect of TGF-βR1 inhibitor on classic activation of monocytes and their TNF-α production in vitro. iNOS and TNF-α expressions on monocytes were suppressed by blocking TGF-βR1 A) Representative dot plots of %iNOS+ monocytes after rTGF-β, LPS/IFN-γ, and/or TGF-βR1 inhibitor treatment in vitro. B) Comparison of iNOS expression on rTGF-β, LPS/IFN-γ, and/or TGF-βR1 inhibitor-treated monocytes in vitro. C) Representative dot plots of percentage TNF-α+ monocytes after rTGF-β, LPS/IFN-γ, and/or TGF-βR1 inhibitor treatment in vitro. D) Comparison of TNF-α expression on rTGF-β, LPS/IFN-γ, and/or TGF-βR1 inhibitor-treated monocytes in vitro. Means and sem are shown; n = 3/group. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Figure 8.
Effect of TGF-βR1 inhibition on inflammatory cytokine secretion from classically activated monocytes using cytometric bead array. Secretion of key inflammatory cytokines IL-12, TNF-α, and IL-6 was significantly decreased with TGF-βR1 inhibitor treatment. IL-12 (A), TNF-α (B), and IL-6 (C) secretion in cell culture supernatant from monocytes treated with rTGF-β, LPS/IFN-γ, and/or TGF-βR1 inhibitor. Means and sem are shown; n = 3/group. *P < 0.05, **P < 0.01, ****P < 0.0001.
DISCUSSION
There is a pressing clinical need for the creation of a vascular graft with growth capacity for use in congenital heart surgery. TEVGs are a promising solution to address this problem, but their long-term efficacy is limited by stenosis. Using mouse models, we have previously shown that stenosis is driven by TEVG infiltration by host monocytes and that an inhibitor of TGF-βR1 signaling could prevent stenosis (3, 4). In the present study, we found that short-term early systemic treatment with the same TGF-βR1 inhibitor limits classic activation of the infiltrating monocytes. Importantly for clinical translation, a relatively brief (2 wk) period of treatment with the inhibitor had clear benefit 6 mo later.
In general, the degree of monocyte infiltration and activation increases in the context of inflammatory diseases (15). In the current study, we showed that classic activation in monocytes and macrophages was significantly reduced by TGF-βR1 inhibition, associated with increased TEVG patency. Reduction of classically activated monocytes/macrophages by blocking TGF-βR1 in the TEVGs suggests that M1 polarization on these cell populations promotes vascular stenosis. It may at first seem paradoxical that TGF-β, a cytokine typically associated with reducing inflammation, is associated with increased recruitment of inflammatory leukocytes into a newly implanted TEVG. However, it is known that TGF-β has dual properties, both pro- and antiinflammatory, depending on the context (16). For example, in the early stages after wounding, TGF-β1 production is increased and triggers the recruitment of additional inflammatory cells that serve to enhance the debridement process by macrophages. However, when the wound is stabilized, TGF-β1 exhibits antiinflammatory roles by decreasing activation of macrophages (17). Our current data are consistent with the early, proinflammatory role of the TGF-β signaling pathway by enhancing monocyte/macrophage activation in TEVGs.
To confirm the role of TGF-β inducing classic activation in monocytes, we stimulated bone marrow monocytes in vitro with LPS/IFN-γ and/or rTGF-β. We observed that combined treatment with LPS/IFN-γ and rTGF-β significantly increased both iNOS and inflammatory cytokine production compared to LPS/IFN-γ–induced monocytes. This synergistic effect was completely abrogated by TGF-β receptor inhibition, indicating that TGF-β synergizes with other inflammatory stimuli to promote classic activation in monocytes. Therefore, it is possible that during neovessel formation in TEVGs, TGF-β collaborates with other inflammatory stimuli to induce classic activation in macrophage lineage cells.
In this study, we focused our attention on classically activated, iNOS-positive monocytes because they are regarded as inflammatory cells and have been shown to play an important role in the early inflammatory process (18). Likewise, our data demonstrate a critical role for these cells during TEVG stenosis; however, we have not fully addressed the potential contributions of alternatively activated monocytes. In reality, there may be many different types of alternatively activated monocytes or macrophages. In our preliminary study, we investigated arginase-1 expression, a widely used marker for so-called M2-activated monocytes and macrophages (19). There was no detectable difference in arginase-1 expression between untreated control and TGF-βR1 inhibitor-treated groups; moreover, the level of expression was close to that of the native vein. This observation elucidated the importance of classically activated monocytes in TEVG stenosis development at early time points. However, it is possible that alternatively activated monocytes/macrophages play an essential role during the neotissue remodeling phase. This hypothesis will be addressed in subsequent studies.
The effects of TGF-β on monocyte activation needs to be considered in the context of effects of TGF-β on other cell types that are already present within the TEVGs in the early stages after implantation. First, a direct effect of TGF-β on endothelial cells may exist. Although generally considered as a prototypical mediator of acute inflammation, TNF-α has also been shown to induce Endo-MT in aortic valve stenosis and intestinal fibrosis (9, 10). In other studies, TNF-α or IL-1β did not induce Endo-MT when used alone but acted synergistically with TGF-β to induce Endo-MT (20, 21). Therefore, in our TEVG model, it is possible that TGF-β synergizes with TNF-α to act on infiltrating endothelial cells, which are a second cellular target to induce Endo-MT and which we have previously shown to be a major contributor to TEVG stenosis. Therefore, blocking Endo-MT with TGF-βR1 inhibitor can significantly reduce TEVG stenosis at early time points (4). Second, TGF-β may directly act on smooth muscle–like cells to induce tissue remodeling in the TEVG, as the TGF-β pathway is a critical mediator of the general tissue remodeling processes (22). During the remodeling period, classically activated monocytes may shut down their proinflammatory activities and give rise to alternatively activated monocytes and possibly further differentiate into macrophages; both of these cell types exhibit profibrotic and antiinflammatory properties (23, 24). Another way by which infiltrating monocytes may contribute to tissue cicatrization and fibrosis is the idea that these cells give rise to fibrocytes, monocyte-derived cells that have a population sharing features of both macrophages and fibroblasts (25, 26). Indeed, the appearance of fibrocytes both in vivo and in vitro is markedly induced by exposure to TGF-β1 and endothelin-1 (25, 27), indicating that TGF-βR in monocytes activates an essential signaling pathway controlling the balance between inflammation and repair in vascular tissue.
In summary, in this study we elucidated the efficacy of short-term anti-TGF-βR1 treatment on long-term graft patency. We were able to modulate monocyte activation by administration of a TGF-βR1 inhibitor and showed that TGF-βR1 inhibition is a viable strategy for preventing TEVG stenosis without blocking neovessel formation. Moreover, we also showed that potential use of anti-TGF-βR1 therapy that can replace cell seeding, which will reduce surgical complications and improve patient safety.
Acknowledgments
The authors acknowledge J. Reinhardt, E. Heuer, A. Singh, E. Clark, and E. Onwuka (Tissue Engineering Program, Nationwide Children’s Hospital) for their contributions to the preparation of histologic samples and surgical procedures. This research was supported by R01-HL098228 (to C.K.B.). S.P.-S. is supported by the U.S National Institutes of Health, National Institute of Allergy and Infectious Diseases (Grant R01AI092117). C.K.B. and T.S. have received grant support from the Pall Corporation and Gunze Ltd. None of the work presented in this article was funded by the Pall Corporation or Gunze Ltd.
Glossary
- BM-MNC
bone marrow–derived mononuclear cell
- Endo-MT
endothelial–mesenchymal transition
- FACS
fluorescence-activated cell sorting
- IVC
inferior vena cava
- LTBP1
latent TGF-β binding protein 1
- MCP-1
monocyte chemotactic protein 1
- SMA
smooth-muscle actin
- TEVG
tissue-engineered vascular graft
- TGF-βR1
TGF-β receptor type 1
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Roh J. D., Sawh-Martinez R., Brennan M. P., Jay S. M., Devine L., Rao D. A., Yi T., Mirensky T. L., Nalbandian A., Udelsman B., Hibino N., Shinoka T., Saltzman W. M., Snyder E., Kyriakides T. R., Pober J. S., Breuer C. K. (2010) Tissue-engineered vascular grafts transform into mature blood vessels via an inflammation-mediated process of vascular remodeling. Proc. Natl. Acad. Sci. USA 107, 4669–4674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hibino N., McGillicuddy E., Matsumura G., Ichihara Y., Naito Y., Breuer C., Shinoka T. (2010) Late-term results of tissue-engineered vascular grafts in humans. J. Thorac. Cardiovasc. Surg. 139, 431–436, 436.e1–436.e2 [DOI] [PubMed] [Google Scholar]
- 3.Hibino N., Yi T., Duncan D. R., Rathore A., Dean E., Naito Y., Dardik A., Kyriakides T., Madri J., Pober J. S., Shinoka T., Breuer C. K. (2011) A critical role for macrophages in neovessel formation and the development of stenosis in tissue-engineered vascular grafts. FASEB J. 25, 4253–4263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duncan D. R., Chen P. Y., Patterson J. T., Lee Y. U., Hibino N., Cleary M., Naito Y., Yi T., Gilliland T., Kurobe H., Church S. N., Shinoka T., Fahmy T. M., Simons M., Breuer C. K. (2015) TGFβR1 inhibition blocks the formation of stenosis in tissue-engineered vascular grafts. J. Am. Coll. Cardiol. 65, 512–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goumans M. J., Lebrin F., Valdimarsdottir G. (2003) Controlling the angiogenic switch: a balance between two distinct TGF-b receptor signaling pathways. Trends Cardiovasc. Med. 13, 301–307 [DOI] [PubMed] [Google Scholar]
- 6.Sridurongrit S., Larsson J., Schwartz R., Ruiz-Lozano P., Kaartinen V. (2008) Signaling via the Tgf-beta type I receptor Alk5 in heart development. Dev. Biol. 322, 208–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vargas R., Rincón J., Pedreañez A., Viera N., Hernández-Fonseca J. P., Peña C., Mosquera J. (2012) Role of angiotensin II in the brain inflammatory events during experimental diabetes in rats. Brain Res. 1453, 64–76 [DOI] [PubMed] [Google Scholar]
- 8.Merino A., Alvarez-Lara M. A., Ramirez R., Carracedo J., Martin-Malo A., Aljama P. (2012) Losartan prevents the development of the pro-inflammatory monocytes CD14+CD16+ in haemodialysis patients. Nephrol. Dial. Transplant. 27, 2907–2912 [DOI] [PubMed] [Google Scholar]
- 9.Farrar E. J., Butcher J. T. (2014) Heterogeneous susceptibility of valve endothelial cells to mesenchymal transformation in response to TNFα. Ann. Biomed. Eng. 42, 149–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahler G. J., Farrar E. J., Butcher J. T. (2013) Inflammatory cytokines promote mesenchymal transformation in embryonic and adult valve endothelial cells. Arterioscler. Thromb. Vasc. Biol. 33, 121–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee Y. U., Yi T., Tara S., Lee A. Y., Hibino N., Shinoka T., Breuer C. K. (2014) Implantation of inferior vena cava interposition graft in mouse model. J. Vis. Exp. 4, 51632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roh J. D., Nelson G. N., Brennan M. P., Mirensky T. L., Yi T., Hazlett T. F., Tellides G., Sinusas A. J., Pober J. S., Saltzman W. M., Kyriakides T. R., Breuer C. K. (2008) Small-diameter biodegradable scaffolds for functional vascular tissue engineering in the mouse model. Biomaterials 29, 1454–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naito Y., Williams-Fritze M., Duncan D. R., Church S. N., Hibino N., Madri J. A., Humphrey J. D., Shinoka T., Breuer C. K. (2012) Characterization of the natural history of extracellular matrix production in tissue-engineered vascular grafts during neovessel formation. Cells Tissues Organs (Print) 195, 60–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naito Y., Lee Y. U., Yi T., Church S. N., Solomon D., Humphrey J. D., Shin’oka T., Breuer C. K. (2014) Beyond burst pressure: initial evaluation of the natural history of the biaxial mechanical properties of tissue-engineered vascular grafts in the venous circulation using a murine model. Tissue Eng. Part A 20, 346–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murray P. J., Wynn T. A. (2011) Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 11, 723–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ashcroft G. S. (1999) Bidirectional regulation of macrophage function by TGF-β. Microbes Infect. 1, 1275–1282 [DOI] [PubMed] [Google Scholar]
- 17.Barrientos S., Stojadinovic O., Golinko M. S., Brem H., Tomic-Canic M. (2008) Growth factors and cytokines in wound healing. Wound Repair Regen. 16, 585–601 [DOI] [PubMed] [Google Scholar]
- 18.Yang Y. P., Dong Q. L., Zhang X. H., Zhang Y. H., Zhu L., Li S. Y., Liu Z. Z., Xu H., Wang N., Jiang H., Liu C. X., Liu X. X., Dong B. (2011) Combination of fluvastatin and losartan relieves atherosclerosis and macrophage infiltration in atherosclerotic plaques in rabbits. Acta Pharmacol. Sin. 32, 1259–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordon S. (2003) Alternative activation of macrophages. Nat. Rev. Immunol. 3, 23–35 [DOI] [PubMed] [Google Scholar]
- 20.Rieder F., Kessler S. P., West G. A., Bhilocha S., de la Motte C., Sadler T. M., Gopalan B., Stylianou E., Fiocchi C. (2011) Inflammation-induced endothelial-to-mesenchymal transition: a novel mechanism of intestinal fibrosis. Am. J. Pathol. 179, 2660–2673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sadler T., Scarpa M., Rieder F., West G., Stylianou E. (2013) Cytokine-induced chromatin modifications of the type I collagen alpha 2 gene during intestinal endothelial-to-mesenchymal transition. Inflamm. Bowel Dis. 19, 1354–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeglinski M. R., Hnatowich M., Jassal D. S., Dixon I. M. (2015) SnoN as a novel negative regulator of TGF-β/Smad signaling: a target for tailoring organ fibrosis. Am. J. Physiol. Heart Circ. Physiol. 308, H75–H82 [DOI] [PubMed] [Google Scholar]
- 23.Nahrendorf M., Swirski F. K., Aikawa E., Stangenberg L., Wurdinger T., Figueiredo J. L., Libby P., Weissleder R., Pittet M. J. (2007) The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J. Exp. Med. 204, 3037–3047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hashimoto D., Chow A., Greter M., Saenger Y., Kwan W. H., Leboeuf M., Ginhoux F., Ochando J. C., Kunisaki Y., van Rooijen N., Liu C., Teshima T., Heeger P. S., Stanley E. R., Frenette P. S., Merad M. (2011) Pretransplant CSF-1 therapy expands recipient macrophages and ameliorates GVHD after allogeneic hematopoietic cell transplantation. J. Exp. Med. 208, 1069–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murray L. A., Chen Q., Kramer M. S., Hesson D. P., Argentieri R. L., Peng X., Gulati M., Homer R. J., Russell T., van Rooijen N., Elias J. A., Hogaboam C. M., Herzog E. L. (2011) TGF-beta driven lung fibrosis is macrophage dependent and blocked by Serum amyloid P. Int. J. Biochem. Cell Biol. 43, 154–162 [DOI] [PubMed] [Google Scholar]
- 26.Reilkoff R. A., Bucala R., Herzog E. L. (2011) Fibrocytes: emerging effector cells in chronic inflammation. Nat. Rev. Immunol. 11, 427–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt M., Sun G., Stacey M. A., Mori L., Mattoli S. (2003) Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J. Immunol. 171, 380–389 [DOI] [PubMed] [Google Scholar]