Abstract
Epidermal growth factor (EGF) is a critical element in dermal repair, but EGF-containing wound dressings have not been successful clinically. However, these dressings have delivered only soluble EGF, and the native environment provides both soluble and matrix-bound EGF. To address our hypothesis that tethered EGF can stimulate cell behaviors not achievable with soluble EGF, we examined single-cell movement and signaling in human immortalized HaCaT keratinocytes treated with soluble or immobilized EGF. Although both EGF treatments increased collective sheet displacement and individual cell speed, only cells treated with immobilized EGF exhibited directed migration, as well as 2-fold greater persistence compared with soluble EGF. Immunofluorescence showed altered EGF receptor (EGFR) trafficking, where EGFR remained membrane-localized in the immobilized EGF condition. Cells treated with soluble EGF demonstrated higher phosphorylated ERK1/2, and cells on immobilized EGF exhibited higher pPLCγ1, which was localized at the leading edge. Treatment with U0126 inhibited migration in both conditions, demonstrating that ERK1/2 activity was necessary but not responsible for the observed differences. In contrast, PLCγ1 inhibition with U73122 significantly decreased persistence on immobilized EGF. Combined, these results suggest that immobilized EGF increases collective keratinocyte displacement via an increase in single-cell migration persistence resulting from altered EGFR trafficking and PLCγ1 activation.—Kim, C. S., Mitchell, I. P., Desotell, A. W., Kreeger, P. K., Masters, K. S. Immobilized epidermal growth factor stimulates persistent, directed keratinocyte migration via activation of PLCγ1.
Keywords: collective migration, EGFR trafficking, PLCγ1 signaling, wound healing
During normal wound healing, keratinocytes are the first dermal cell type to respond to the injury (1, 2), migrating across the wound bed to establish a barrier for immune defense and provide structural and mechanical support for dermal regeneration (3–5). Failure of this re-epithelialization process results in the development of chronic wounds, which affect 6.5 million individuals in the USA and cost $25 billion annually (6). Re-epithelialization is regulated by a number of growth factors (7, 8), including epidermal growth factor (EGF) secreted by macrophages and platelets in the wound bed (7–9). Based on the observation that EGF promotes keratinocyte proliferation and migration, wound dressings that deliver EGF have been widely explored (10, 11). However, these studies have delivered only soluble EGF, despite EGF being present in both soluble and extracellular matrix-bound forms in the native wound bed (12). Moreover, dressings that deliver soluble growth factors have ultimately not provided effective wound-healing therapy.
Application of growth factors in an immobilized format has been an attractive approach in tissue engineering, as this strategy not only provides a means to mimic the extracellular matrix-bound presentation of growth factors often found in vivo but may also allow for a lower dose of growth factor to be used due to improved growth factor stability and reduced diffusion away from the scaffold (13–15). Additionally, there is mounting evidence that presentation of growth factors in a substrate-bound form alters more than simply their stability and diffusion (16–19). Our group previously discovered that immobilized EGF increased the collective movement of keratinocyte sheets relative to treatment with soluble EGF (20). However, to guide the eventual translation of these findings to improve in vivo wound healing, it is essential to identify which single-cell actions (e.g., cell speed, persistence, directionality) are responsible for driving the differences in collective movement and to uncover the mechanisms responsible for this differential response. Our observation that immobilized EGF yielded sustained EGF receptor (EGFR) phosphorylation in a bulk population of keratinocytes (20) provided initial clues that differences in cellular migration likely resulted from differential stimulation of intracellular signaling networks (17, 19, 21). However, examination of single-cell behaviors and specific migration-related signaling pathways is needed to explain the means by which increased collective migration is elicited by immobilized EGF. The current work undertakes a detailed characterization of the migration behavior of individual cells within the collective sheet to identify these signaling mechanisms, thereby yielding novel insight into the role of growth factor presentation in regulating cell behavior, as well as providing guidance for understanding the limitations of existing growth factor-containing wound dressings and designing future wound-healing approaches.
MATERIALS AND METHODS
All materials were purchased from Thermo Fisher Scientific (Waltham, MA, USA) unless noted otherwise.
Cell culture
Immortalized human keratinocytes (HaCaT cells, courtesy of N. Fusenig; DKFZ, Heidelberg, Germany) were maintained at 37°C, 5% CO2 in high-glucose DMEM supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin (Sigma-Aldrich, St. Louis, MO, USA).
Synthesis and characterization of immobilized EGF
EGF (Peprotech, Rocky Hill, NJ, USA) was covalently immobilized to tissue culture polystyrene plates via the heterobifunctional cross-linker sulfo-sulfosuccinimidyl-6-(4′-azido-2′-nitrophenylamino)hexanoate (SS) as previously described (20, 22). Briefly, 2.5 mM EGF in HBSS [115 mM NaCl, 1.2 mM CaCl2 (Sigma-Aldrich), 1.2 mM MgCl2 (Sigma-Aldrich), 2.5 mM K2HPO4, 20 mM HEPES, pH adjusted to 7.6] was reacted with SS in a 1:50 molar ratio for at least 3 h covered in foil. The resulting photoactive EGF conjugated to SS (SS-EGF) solution was then pipetted into a 24 well plate (250 μl/well) and dried at 40°C for 6 h. After drying, SS-EGF was immobilized to the plate via exposure to 365 nm UV light for 120 s using an OmniCure S2000 (Exfo, Inc., Chelmsford, MA, USA), and unreacted EGF was removed by 4 rinses with diH2O on an orbital shaker. For control and soluble EGF conditions, plates were treated with HBSS without the SS-EGF mixture, dried at 40°C for 6 h, and rinsed with diH2O on an orbital shaker.
To quantify the amount of EGF tethered to the plates, fluorescently labeled EGF was generated via reaction with Alexa Fluor 488 C5 maleimide according to manufacturer’s instructions. Briefly, 100 μM EGF was mixed with a 10-fold molar excess of tris-(2-carboxyethyl) phosphine (Sigma-Aldrich) and incubated for 30 min at 50°C to reduce disulfide bonds in the EGF. The sulfhydryl groups in the reduced EGF were then reacted with 1 mM maleimide-conjugated AlexaFluor 488 in HBSS overnight at 4°C. The reaction mixture was purified via centrifugal filtration (Amicon Ultra 3000 MW centrifugal filters; EMD Millipore, Billerica, MA, USA), and AlexaFluor 488 conjugation to EGF was confirmed by measurement of fluorescence intensity at 495/525 nm (Tecan, Morrisville, NC, USA). The fluorescently labeled EGF was then conjugated to SS and immobilized onto tissue culture polystyrene plates as described above. The amount of immobilized EGF was quantified by first cleaving EGF from SS via incubation with trypsin for 2 h at 37°C, followed by collection of the fragmented EGF and measurement of fluorescence intensity at 495/525 nm; known concentrations of AlexaFluor 488-conjugated EGF were used to generate a standard curve. This quantification determined that the immobilization methods resulted in 20 ng of EGF immobilized per well. To deliver an equivalent amount of 20 ng soluble EGF, cells were treated with 500 μl of 40 ng/ml EGF.
Characterization of cell behavior during collective migration
Collective migration of keratinocytes in response to delivery of immobilized vs. soluble EGF was investigated using a modified fence migration assay, in which removable migration barriers were created by placing Ibidi cell culture inserts (catalog no. 80209; Ibidi GmbH, Madison, WI, USA) in the center of each well of a 24 well plate that was prepared as described above. HaCaT cells were harvested by incubation with 0.05% EDTA for 15 min at 37°C and then 0.05% trypsin for 5 min at 37°C. Following neutralization with 0.5 mg/ml soybean trypsin inhibitor, the cells were seeded within the insert area in DMEM supplemented with 0.5% fetal bovine serum and 2 μM AG 1478 (Tocris, Bristol, United Kingdom) at a density of 295,000 cells/cm2. AG 1478 is a reversible EGFR kinase inhibitor (23) used to prevent premature EGFR signaling through immobilized EGF (Supplemental Fig. 1). To facilitate tracking of individual cells within the collective sheet, unstained HaCaT cells were mixed at a 10:1 ratio with HaCaT cells labeled with 5 µM CellTracker green CMFDA dye immediately prior to seeding at 65,000 cells/well. Cells were allowed to attach for 12 h, at which point the Ibidi inserts were removed and the medium was replaced with serum-free medium for the control and immobilized EGF conditions or serum-free medium containing 40 ng/ml EGF for the soluble EGF condition. Cultures were maintained in a microscope-mounted environmental chamber at 37°C, 5% CO2, and images of cell sheets were captured every 15 min for 24 h in both bright field and the 495/517 nm wavelength channel using an Olympus IX81 inverted microscope equipped with a Hamamatsu CCD ORCA/AG camera (Olympus, Center Valley, PA, USA).
For each condition, the x and y coordinates of 75 individual cells were tracked using MetaMorph software (Molecular Devices, Sunnyvale, CA, USA); these data were then analyzed to calculate cell speed, persistence, and directionality, as well as create wind rose plots of cell movement. Individual cell speed (micrometers per minute) was calculated by MetaMorph as the average of the speeds from every 15-min interval. Persistence was calculated as the shortest distance between the start and end points divided by the distance of the traversed cell path (24); as such, this parameter is unitless. Directionality histograms were made using the Directionality plugin in ImageJ (National Institutes of Health, Bethesda, MD, USA), where 0° represents directed cell migration toward the void space and ±90° indicates cell migration along the leading edge. Wind rose plots were created using a self-written MatLab code (MathWorks, Natick, MA, USA) that takes the x and y coordinates from the cell tracking data and draws individual cell paths. Finally, displacement of the collective cell sheet was calculated by overlaying the pictures at 0 and 24 h, and measuring the distance between the leading edge of the 0- and 24-h point using ImageJ at 3 random spots per image.
Inhibition of MEK1/2 and PLCγ1 during migration
The migration of HaCaT cells treated with soluble or immobilized EGF was analyzed following treatment with either 5 μM U0126 (LC Laboratories, Woburn, MA, USA), an inhibitor of MEK1/2, or 5 μM U73122 (Tocris), an inhibitor of PLCγ1-mediated production of inositol trisphosphate (25, 26). HaCaT cells were seeded as described above, and each inhibitor was added to immobilized EGF conditions in serum-free DMEM, or concomitantly with soluble EGF in serum-free DMEM for the soluble EGF condition. Cell cultures were placed in a microscope-mounted environmental chamber, and images were taken every 15 min for 8 h. Migration parameters were calculated as noted previously.
Immunofluorescent staining and analysis
HaCaT cells were fixed at 0 min, 30 min, 2 h, 4 h, and 24 h following treatment with EGF. Samples were fixed by incubation with ice-cold 100% methanol for 15 min on ice for detection of phosphorylated ERK1/2 (pERK1/2), or with 4% paraformaldehyde for 15 min at room temperature for detection of pPLCγ1, EGFR, and early endosome antigen 1 (EEA1). Following fixation, samples were washed with PBS and blocked in 5% goat serum and 0.3% Triton X-100 (Sigma-Aldrich) in PBS for 1 h at room temperature. Antibodies were diluted in 1% goat serum with 0.3% Triton X-100 in PBS for detection of pERK1/2 and pPLCγ1, or 1% BSA with 0.3% Triton X-100 in PBS for detection of EGFR and EEA1. Samples were incubated with the following phospho-specific primary antibodies overnight at 4°C: pERK 1/2 (Thr202/Tyr204, catalog no. 4370, 1:50; Cell Signaling Technology, Beverly, MA, USA), pPLCγ1 (Tyr783, catalog no. 44696G, 1:250; Cell Signaling Technology), EEA1 (catalog no. ab2900, 1:500; Abcam, Cambridge, MA, USA), or EGFR (catalog no. ab30, 1:1000; Abcam). After washing in PBS, corresponding secondary antibodies were applied and incubated for 2 h at room temperature; goat anti-rabbit AlexaFluor488 (1:1000) was used for detection of pERK1/2 and pPLCγ1, goat anti-mouse AlexaFluor488 (1:500) for EGFR, and goat anti-rabbit AlexaFluor647 (1:200; Abcam) for EEA1. Nuclei were counterstained with DAPI (0.333 µg/ml; Sigma-Aldrich) for pERK1/2 and pPLCγ1, and Hoechst 33258 (catalog no. H3569, 1:1000) for EGFR, pERK1/2, and pPLCγ1. Staining was visualized with an Axio Observer inverted microscope A1 (Carl Zeiss, Thornwood, NY, USA) and Leica DMi8 inverted microscope (Leica, Buffalo Grove, IL, USA). EGFR and EEA1 confocal imaging was performed on a Nikon Eclipse Ti inverted microscope (Nikon, Melville, NY, USA). All images were pseudocolored, overlaid, and analyzed using ImageJ. To quantify pERK fluorescent intensity an outline was drawn around the HaCaT sheet, and fluorescent intensity was measured and normalized to the cell area. To correct for background variation between images, the fluorescent intensity per unit area was determined in the wound area and subtracted from the fluorescent readings in the HaCaT sheet. The percentage of cells that were pPLCγ1 positive was determined for the wound leading edge (the first 3 cell layers at the edge of the wound) and the bulk (a 3-cell-wide strip at least 8 cells from the edge).
Statistical analysis
Data were analyzed with GraphPad Prism 6.0 (La Jolla, CA, USA) and are presented as the means ± sd, and all experiments were performed at least twice to ensure reproducibility. Statistical significance was determined using a 1-way ANOVA with post hoc Tukey honest significant difference test, with P < 0.05 considered statistically significant. A χ2 test was performed to determine statistical significance for migration directionality.
RESULTS
Immobilized EGF increases keratinocyte speed, persistence, and directionality
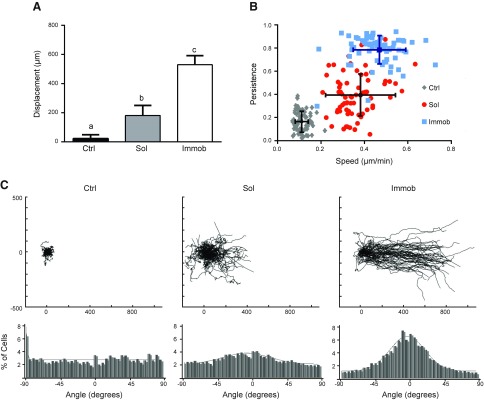
The impact of EGF presentation on collective keratinocyte migration was first assessed via measurement of the displacement of the cell sheet’s leading edge over 24 h. To match the EGF dose across conditions, we determined the amount of EGF tethered per well and delivered an equivalent total amount of soluble EGF based on prior studies demonstrating that the amount of ligand available per cell is a key determinant of cell behavior (27–29). Prior studies examining the effect of soluble and immobilized growth factors have generally not controlled for dose, instead providing saturating, but not equivalent, amounts of growth factor based on theoretical receptor occupancy (21) or experimental measures of pathway activation (30). As expected, control cultures receiving no EGF stimulation exhibited little cell sheet displacement, and displacement was significantly increased by the application of either immobilized or soluble EGF (Fig. 1A). However, the mode of EGF presentation was found to dramatically influence the extent of leading edge displacement, as the leading edge of cells on immobilized EGF traveled 3 times farther than the leading edge receiving the same dose of EGF in soluble form.
Figure 1.
HaCaT cell migration in response to soluble or immobilized EGF. A) Displacement of the leading edge after 24 h. Different letters (a–c) indicate significant difference. P < 0.05. B) Cell speed and persistence of individual cells located on the leading edge for 24 h (lighter color indicates individual cells; darker color indicates population average). C) Wind rose plots demonstrating individual cell tracks (μm) and distribution of migration angles, where 0 indicates movement perpendicular to the leading edge; n = 75 cells for each condition. Ctrl, control; immob, immobilized; sol, soluble.
To determine which individual migration behaviors were responsible for this observed difference in collective migration, we next examined the migration speed, persistence, and directionality of individual HaCaT cells located along the leading edge of the collective sheet. Consistent with the cell sheet displacement outcomes, the lowest cell speed and persistence were found in the control condition, and addition of either immobilized or soluble EGF significantly increased both of these measures (P < 0.05) (Fig. 1B). Additionally, cell speed and persistence were impacted by the mode of EGF presentation, as immobilized EGF was significantly more effective at enhancing cell speed and persistence compared with treatment with soluble EGF (P < 0.05). Presentation of EGF in an immobilized form appeared to influence cell persistence to a greater extent than cell speed, as cells on immobilized EGF exhibited a 2-fold increase in persistence compared with a 1.2-fold increase in speed relative to the soluble EGF condition. The scatter plots in Fig. 1B also suggested differences in the heterogeneity of the migration response within a given condition. Calculation of the coefficients of variation confirmed this observation, as the speed and persistence data for cells treated with soluble EGF had a higher coefficients of variation compared with data for cells treated with immobilized EGF (42 vs. 26% for speed; 46 vs. 17% for persistence).
Although cell speed and persistence are important metrics of cell migration, wound closure also requires directed migration of cells toward the wounded area. To analyze differences in directionality, wind rose plots of individual cell migration paths were prepared for the different conditions (Fig. 1C). Untreated cells exhibited minimal, randomly oriented movement over 24 h, such that when the cell tracks were overlaid to a common origin, there was no substantial migration. Delivery of EGF increased cell migration, and cells in both soluble and immobilized EGF conditions had longer migration tracks than those in the untreated control. Improved persistence relative to all other conditions was evident in cultures treated with immobilized EGF, which had dramatically longer and more linear cell tracks. As the migration tracks for immobilized EGF appeared to predominantly orient toward the direction of the open space on the cell culture substrate, we next quantified migration directionality through measurement of each cell’s migration path angle. The resulting histograms indicated that only 49% of untreated cells and 60% of cells treated with soluble EGF exhibited directional movement (defined as ±45°) toward the void area, but treatment with immobilized EGF resulted in a significant increase in directionality (78%; P < 0.01).
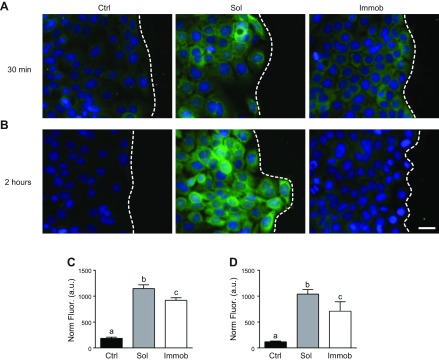
Levels of pERK1/2 are highest in cells treated with soluble EGF
To identify mechanisms responsible for the differential response to soluble vs. immobilized EGF, ERK1/2 activation was investigated. ERK1/2 acts as a major downstream effector of EGFR and is known to drive multiple cellular behaviors, including migration (31). pERK1/2 was not detected at either 30 min or 2 h in the untreated control, indicating that autocrine activity regulating this pathway was minimal (Fig. 2A). Treatment with either soluble or immobilized EGF for 30 min caused a substantial increase in pERK1/2 in HaCaT cells (Fig. 2A; additional representative images available in Supplemental Fig. 2). Quantification of the normalized fluorescence intensity in the cellular area demonstrated that pERK1/2 levels were significantly higher in EGF-treated conditions (P < 0.05) and were further enhanced by application of soluble, rather than immobilized, EGF (Fig. 2C). By 2 h, pERK1/2 appeared diminished in cultures treated with immobilized EGF, and cultures that had received soluble EGF continued to exhibit strong pERK1/2 staining. Quantification of the normalized fluorescence intensity in the cellular area at 2 h confirmed these observations, showing higher pERK1/2 levels in EGF-treated conditions (P < 0.05), with further enhancement by application of soluble, rather than immobilized, EGF (Fig. 2C).
Figure 2.
pERK1/2 levels during collective migration. A, B) Cells were stained for pERK1/2 Thr202/Tyr204 at 30 min (A) or 2 h (B). Green indicates pERK1/2 and blue is nuclear stain; dashed line indicates the leading edge. Scale bar, 25 μm. C, D) Quantification of the fluorescence intensity in the area occupied by cells at 30 min (C) and 2 h (D); n = 3 images per condition. Ctrl, control; immob, immobilized; sol, soluble. Different letters (a–c) indicate significant difference. P < 0.05.
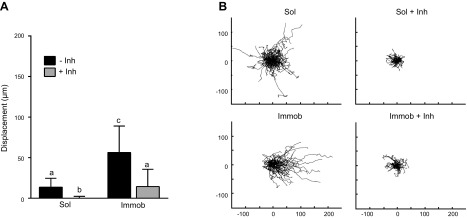
Inhibition of MEK1/2 during collective migration halts cell movement
As shown in Fig. 2, strong and sustained pERK1/2 activation was obtained upon treatment with soluble EGF; this condition was also associated with decreased migration persistence (Fig. 1), potentially suggesting an inhibitory role of pERK1/2 in directed migration. Thus, HaCaT migration was measured in the presence of a MEK1/2 inhibitor, U0126 (32), to assess whether the difference in pERK1/2 activation patterns induced by treatment with soluble vs. immobilized EGF was a possible source of differential HaCaT migration activity. Because U0126 has a short half-life (33), migration behavior was examined during the first 8 h. As shown in Fig. 1, cells that did not receive either immobilized or soluble EGF demonstrated minimal migration and were not impacted by treatment with U0126 (Supplemental Fig. 2). Quantification of leading edge displacement (Fig. 3A) and analysis of wind rose plots of cell migration paths (Fig. 3B) revealed similar cellular responses to MEK1/2 inhibition in both soluble and immobilized EGF conditions. Specifically, MEK1/2 inhibition resulted in near total cessation of cell movement in both EGF treatment conditions, with the cell sheet advancing less than 1 cell length over the course of 8 h. These findings indicated that although ERK1/2 activation was necessary for cell migration, differences in pERK1/2 levels were unlikely to be the source of the observed differences in migration between soluble and immobilized conditions.
Figure 3.
Impact of MEK1/2 inhibitor U0126 on collective cell migration. A) Displacement of the leading edge after 8 h. Different letters (a–c) indicate significant difference. P < 0.05. B) Wind rose plots demonstrating individual cell tracks (μm); n = 75 cells for each condition. Immob, immobilized; inh, inhibitor; sol, soluble.
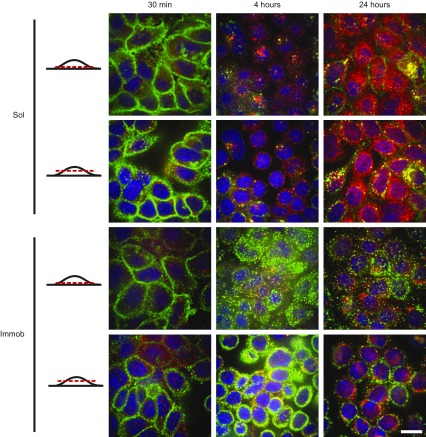
EGFR is maintained at the cell surface in immobilized EGF conditions
Activated EGFR can initiate downstream signaling from both the cell membrane and the endosome after internalization (34). Because EGF was immobilized through covalent attachment by an SS cross-linker, we hypothesized that EGFR trafficking and subsequent downstream signaling may differ between soluble and immobilized EGF conditions. To examine this hypothesis, cells were analyzed by immunofluorescent staining for EGFR and EEA1, which is localized in early endosomes (35). EGFR localization at 30 min posttreatment was similar for both soluble and immobilized EGF conditions (Fig. 4; additional representative images available in Supplemental Fig. 3). Images from different cellular planes revealed that EGFR was localized at the cell membrane in both soluble and immobilized EGF conditions at this early time. By 4 h, however, dramatic differences in EGFR internalization between soluble and immobilized EGF conditions became apparent. In cells treated with soluble EGF, EGFR levels appeared to decrease, with little EGFR remaining at the cell membrane; instead, EGFR was found colocalized with EEA1, indicating endocytosis had occurred. Meanwhile, EGFR expression remained high and localized at the cell membrane at 4 h in cells treated with immobilized EGF. Additionally, these cells displayed a unique localization pattern, with EGFR present in clusters on the cell membrane across the entire cell–substrate interface, rather than just the periphery of the cell body. Similar trends were observed at 24 h, where EGFR was further diminished and no longer membrane-bound in cells treated with soluble EGF; colocalization of EEA-1 with EGFR suggested that the receptor had been endocytosed. In contrast, a portion of EGFR remained localized at the membrane in cells treated with immobilized EGF at 24 h posttreatment, with a similar z axis spatial distribution as noted for the 4-h point.
Figure 4.
Changes in EGFR localization in response to soluble or immobilized EGF. Immunofluorescent staining for EGFR (green) and EEA1 (red); blue is nuclear stain. Red line indicates the cellular plane shown in the panels. Immob, immobilized; sol, soluble. Scale bar, 20 μm.
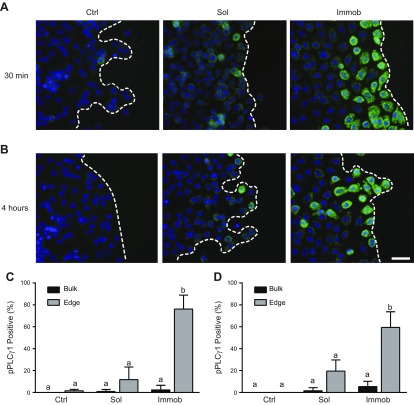
Immobilized EGF induces phosphorylation of PLCγ1 at the leading edge
Given the observed differences in EGFR internalization dynamics, we next investigated the regulation of a downstream effector, PLCγ1, which is preferentially activated by EGFR at the cell surface, to determine whether this pathway was responsible for the observed differences in cell migration (36–38). Of 13 possible phospholipase isoforms, only PLCγ1 and PLCγ2 contain an SRC homology 2 domain that enables interaction with the phosphotyrosine residue of EGFR and can thus be activated by pEGFR (38, 39). Both PLCγ1 and PLCγ2 initiate similar functions and signaling cascades, but only the PLCγ1 isoform is present in HaCaT cells (40). pPLCγ1 was not detected in the untreated control during collective migration at either 30 min or 4 h (Fig. 5A, B; additional representative images available in Supplemental Fig. 4) and was expressed only by isolated cells in the soluble EGF condition. In contrast, and consistent with the differences in EGFR localization (Fig. 4B), HaCaT cells exposed to immobilized EGF exhibited strong pPLCγ1 staining. Intriguingly, this staining appeared to increase with time and was localized to the leading edge of the cell sheet. Quantification of the percentage of pPLCγ1-positive cells in the bulk and leading edge of each condition demonstrated that significantly more cells had activated PLCγ1 in the immobilized condition (P < 0.05), and this altered signaling was specific to the leading edge of the wound (Fig. 4C).
Figure 5.
pPLCγ1 levels during collective migration. A, B) Cells were stained for pPLCγ1 (Tyr783) at 30 min (A) or 4 h (B). Green indicates pPLCγ1 and blue is nuclear stain; dashed line indicates the leading edge. Scale bar, 25 μm. C, D) Quantification of the percentage of pPLCγ1-positive cells in the bulk and at the edge at 30 min (C) and 4 h (D); n = 3 images per condition. Ctrl, control; immob, immobilized; sol, soluble. Different letters (a, b) indicate significant difference. P < 0.05.
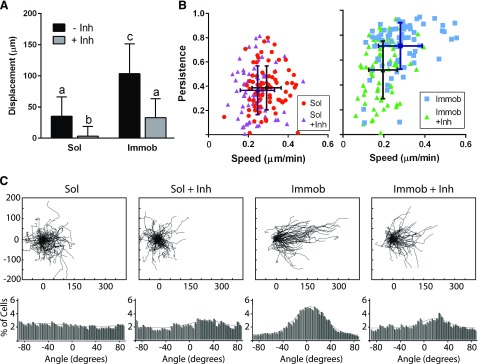
Inhibition of pPLCγ1 decreases speed and persistence in immobilized EGF conditions
To investigate whether the differential activation of PLCγ1 was important for the observed differences in migration between soluble and immobilized EGF conditions, PLCγ1 activity was inhibited during collective migration using U73122 (36). Due to the short half-life of U73122 (41), migration behaviors were again examined over a period of 8 h, which is consistent with the time frame examined for the MEK1/2 inhibition studies described previously. As shown in Fig. 1, cells that did not receive EGF demonstrated minimal migration and were not impacted by treatment with U71322 (Supplemental Fig. 4). Cell sheet displacement was significantly decreased by inhibition of PLCγ1 in cultures treated with either soluble or immobilized EGF (Fig. 6A). Cell migration speed in both soluble and immobilized EGF conditions was also significantly decreased upon treatment with U73122 (Fig. 6B). However, only in the immobilized EGF condition was migration persistence affected by inhibition of PLCγ1. Treatment with U73122 significantly decreased the persistence of cells exposed to immobilized EGF (P < 0.05) but had no effect on the persistence of cells treated with soluble EGF. This observation was also reflected in the wind rose plots and analysis of migration angles for the immobilized EGF condition (Fig. 6C), which showed shortened and less linear cell tracks, as well as loss of directionality upon inhibition of PLCγ1. Specifically, the percentage of cells on immobilized EGF migrating toward the void area (±45°) dropped from 79 to 59% with the application of U73122 (P < 0.01). Meanwhile, the histogram of migration angles revealed no significant differences in directionality between soluble EGF and soluble EGF with U73122 (50 and 55%, respectively), and the wind rose plots for the soluble EGF condition treated with U73122 displayed similar patterns as those for cells treated with soluble EGF alone.
Figure 6.
Impact of pPLCγ1 inhibition on collective migration. A) Cell sheet displacement at 8 h migration. Different letters (a–c) indicate significant difference. P < 0.05. B) Cell speed and persistence of individual cells on the edge of the collective sheet over 8 h (lighter color indicates individual cells; darker color indicates population average). C) Wind rose plots (μm) and migration angles; n = 75 for each condition. Immob, immobilized; inh, inhibitor; sol, soluble.
DISCUSSION
The short half-life of growth factors and their rapid diffusion away from their delivery site have significantly hampered efforts to achieve efficacious growth factor delivery to many in vivo tissues and to dermal wounds in particular (14, 42, 43). These obstacles, combined with the recognition that growth factors in vivo are also present in matrix-bound forms (12), have motivated the development of biomaterial systems that incorporate covalently tethered growth factors (15). In this study, we found that the increased collective movement of keratinocytes treated with immobilized EGF is largely driven by a significant increase in the migration persistence and directionality of individual cells. Moreover, our results indicate that this increased cell migration persistence is due to substantial differences in EGFR trafficking and downstream activation of PLCγ caused by presentation of EGF in an immobilized, rather than soluble, form.
It has been widely suggested that covalent immobilization of growth factors prevents internalization of their cognate receptors (44). To examine this question in our system, we tracked the fate of EGFR under the 2 paradigms. Cells on immobilized EGF maintained higher levels of surface-bound EGFR, which may explain our previous observation that pEGFR levels were elevated for longer times in HaCaT cells exposed to immobilized EGF relative to soluble EGF (20). The characterization of EGFR trafficking also unexpectedly revealed that EGFR localization within the cell membrane was impacted by presentation scheme, with prominent clusters of EGFR found basolaterally on cells on the immobilized EGF condition. Clustering of the EGFR at the plasma membrane has been observed in tumor cells (45) and in response to EGF treatment (46), and results in EGF signal amplification. Although the linker length used in the current work (0.18 nm) is shorter than tethers used in previous studies (47), the dense distribution of immobilized EGF (10 nm spacing between molecules) and the high expression of EGFR by HaCaT cells (approximately 106 receptors/cell) (20, 48) in our system were likely sufficient to enable receptor clustering. This clustering may result in differential activation of signaling pathways to direct cell migration, which could potentially be mimicked through the use of soluble, multivalent EGF complexes such as those developed for other ligands (49).
Although numerous studies have found that an immobilized growth factor can enhance a cellular response (e.g., proliferation, differentiation, angiogenesis) more than the same growth factor in solution (16–20, 30), the implicit assumption in many reports has been that this response was primarily the result of the immobilized format causing sustained activation of the same signaling pathway activated by the soluble factor. However, our results demonstrated that soluble EGF resulted in higher levels of pERK, which is consistent with the observed localization of EGFR in endosomes (34, 50). If this difference in ERK activation were responsible for the observed migration differences, we would hypothesize that treatment with a MEK inhibitor would induce cells treated with soluble EGF to behave more like immobilized EGF with increased persistence. Our experimental results demonstrated instead that ERK activation was necessary for migration in both conditions and not the source of the differences. Although only minimal activation of PLCγ was observed with soluble EGF, keratinocytes on immobilized EGF demonstrated robust levels of pPLCγ on the leading edge, consistent with the localization of the EGFR at the cell membrane in this condition (51). It has been shown that PLCγ activates Rac and induces migrational persistence (52), and treatment with a PLCγ inhibitor in the current work decreased migrational persistence and collective migration exclusively in the immobilized EGF condition. These results clearly show that one can get different signaling pathways activated by changing the growth factor presentation scheme and suggest that, in combination with growth factor identity and dose, presentation method provides an additional design parameter that can be tailored to the target application.
Importantly, our results demonstrate that the differential cellular responses elicited by altering the growth factor presentation scheme may be masked when cells are analyzed only at the population level. For instance, the collective migration analysis in our current and previous (20) work shows that both soluble and immobilized EGF increased wound closure, with immobilized EGF effecting increased closure relative to soluble EGF. However, an analysis of individual cells within the collective sheet revealed that the manner in which the cells acted to close the wound was actually quite different, with both conditions increasing cell speed but only immobilized EGF greatly increasing persistence and directionality. In essence, cells treated with soluble EGF appeared to be engaged in a game of bumper cars, and cells exposed to immobilized EGF traveled on a laned highway and more efficiently closed the wound. Although collective migration into a wound can result from multiple single-cell behaviors, our recent analysis also highlighted the key role of persistence in determining the extent of wound closure when cells are exposed to both mechanical and growth factor cues (53). Thus, the collection of single-cell data may be particularly important in examining differences between soluble and immobilized growth factor delivery, as measurement of only collective outcomes (e.g., wound closure, angiogenic sprouting) may obscure differences in how individual cells are interpreting the delivered growth factor stimuli.
These findings may have particular significance for the design of wound dressings, where very large amounts of soluble growth factor (2000 times the values used in the current work) have been delivered to achieve improved healing outcomes (43), but these doses have also caused side effects meriting a black box warning from the Food and Drug Administration (30). Our findings suggest that delivery of soluble growth factor alone may not be capable of stimulating the range of cell behaviors required to achieve wound healing, particularly in the case of chronic wounds, which are deficient in growth factors (54) that may otherwise be available to stimulate chemotaxis in the wound. The presentation of growth factors in an immobilized form not only provides improved mimicry of the healthy in vivo wound environment, but may be essential in cueing the cell responses that are needed to close a wound.
Supplementary Material
Acknowledgments
The authors acknowledge Min Sung Kim (Department of Biomedical Engineering, University of Wisconsin–Madison) for her assistance with cell culture and fluorescent immunostaining and the University of Wisconsin–Madison Vision Research Core (P30 EY0166650) for use of the confocal microscope. This work was supported by a grant from the U.S. National Institutes of Health, National Institute of General Medical Sciences (R01-GM099031), and a Wisconsin Distinguished Graduate Fellowship Award.
Glossary
- EEA1
early endosome antigen 1
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- pERK1/2
phosphorylated ERK1/2
- SS
sulfo-sulfosuccinimidyl-6-(4′-azido-2′-nitrophenylamino)hexanoate
- SS-EGF
epidermal growth factor conjugated to sulfo-sulfosuccinimidyl-6-(4′-azido-2′-nitrophenylamino)hexanoate
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Stojadinovic O., Brem H., Vouthounis C., Lee B., Fallon J., Stallcup M., Merchant A., Galiano R. D., Tomic-Canic M. (2005) Molecular pathogenesis of chronic wounds: the role of beta-catenin and c-myc in the inhibition of epithelialization and wound healing. Am. J. Pathol. 167, 59–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seeger M. A., Paller A. S. (2015) The roles of growth factors in keratinocyte migration. Adv. Wound Care (New Rochelle) 4, 213–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santoro M. M., Gaudino G. (2005) Cellular and molecular facets of keratinocyte reepithelization during wound healing. Exp. Cell Res. 304, 274–286 [DOI] [PubMed] [Google Scholar]
- 4.Gurtner G. C., Werner S., Barrandon Y., Longaker M. T. (2008) Wound repair and regeneration. Nature 453, 314–321 [DOI] [PubMed] [Google Scholar]
- 5.Moulin V., Auger F. A., Garrel D., Germain L. (2000) Role of wound healing myofibroblasts on re-epithelialization of human skin. Burns 26, 3–12 [DOI] [PubMed] [Google Scholar]
- 6.Sen C. K., Gordillo G. M., Roy S., Kirsner R., Lambert L., Hunt T. K., Gottrup F., Gurtner G. C., Longaker M. T. (2009) Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 17, 763–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhora F. Y., Dunkin B. J., Batzri S., Aly H. M., Bass B. L., Sidawy A. N., Harmon J. W. (1995) Effect of growth factors on cell proliferation and epithelialization in human skin. J. Surg. Res. 59, 236–244 [DOI] [PubMed] [Google Scholar]
- 8.Yu W., Naim J. O., Lanzafame R. J. (1994) Expression of growth factors in early wound healing in rat skin. Lasers Surg. Med. 15, 281–289 [DOI] [PubMed] [Google Scholar]
- 9.Barrientos S., Stojadinovic O., Golinko M. S., Brem H., Tomic-Canic M. (2008) Growth factors and cytokines in wound healing. Wound Repair Regen. 16, 585–601 [DOI] [PubMed] [Google Scholar]
- 10.Brown G. L., Nanney L. B., Griffen J., Cramer A. B., Yancey J. M., Curtsinger L. J. III, Holtzin L., Schultz G. S., Jurkiewicz M. J., Lynch J. B. (1989) Enhancement of wound healing by topical treatment with epidermal growth factor. N. Engl. J. Med. 321, 76–79 [DOI] [PubMed] [Google Scholar]
- 11.Brown G. L., Curtsinger L. III, Brightwell J. R., Ackerman D. M., Tobin G. R., Polk H. C. Jr., George-Nascimento C., Valenzuela P., Schultz G. S. (1986) Enhancement of epidermal regeneration by biosynthetic epidermal growth factor. J. Exp. Med. 163, 1319–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schultz G. S., Wysocki A. (2009) Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 17, 153–162 [DOI] [PubMed] [Google Scholar]
- 13.Doukas J., Chandler L. A., Gonzalez A. M., Gu D., Hoganson D. K., Ma C., Nguyen T., Printz M. A., Nesbit M., Herlyn M., Crombleholme T. M., Aukerman S. L., Sosnowski B. A., Pierce G. F. (2001) Matrix immobilization enhances the tissue repair activity of growth factor gene therapy vectors. Hum. Gene Ther. 12, 783–798 [DOI] [PubMed] [Google Scholar]
- 14.Papanas N., Maltezos E. (2010) Benefit-risk assessment of becaplermin in the treatment of diabetic foot ulcers. Drug Saf. 33, 455–461 [DOI] [PubMed] [Google Scholar]
- 15.Masters K. S. (2011) Covalent growth factor immobilization strategies for tissue repair and regeneration. Macromol. Biosci. 11, 1149–1163 [DOI] [PubMed] [Google Scholar]
- 16.Rodrigues M., Blair H., Stockdale L., Griffith L., Wells A. (2013) Surface tethered epidermal growth factor protects proliferating and differentiating multipotential stromal cells from FasL-induced apoptosis. Stem Cells 31, 104–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehta G., Williams C. M., Alvarez L., Lesniewski M., Kamm R. D., Griffith L. G. (2010) Synergistic effects of tethered growth factors and adhesion ligands on DNA synthesis and function of primary hepatocytes cultured on soft synthetic hydrogels. Biomaterials 31, 4657–4671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCall J. D., Luoma J. E., Anseth K. S. (2012) Covalently tethered transforming growth factor beta in PEG hydrogels promotes chondrogenic differentiation of encapsulated human mesenchymal stem cells. Drug Deliv. Transl. Res. 2, 305–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson S. M., Siegman S. N., Segura T. (2011) The effect of vascular endothelial growth factor (VEGF) presentation within fibrin matrices on endothelial cell branching. Biomaterials 32, 7432–7443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puccinelli T. J., Bertics P. J., Masters K. S. (2010) Regulation of keratinocyte signaling and function via changes in epidermal growth factor presentation. Acta Biomater. 6, 3415–3425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan V. H., Tamama K., Au A., Littrell R., Richardson L. B., Wright J. W., Wells A., Griffith L. G. (2007) Tethered epidermal growth factor provides a survival advantage to mesenchymal stem cells. Stem Cells 25, 1241–1251 [DOI] [PubMed] [Google Scholar]
- 22.Stefonek-Puccinelli T. J., Masters K. S. (2009) Regulation of cell signaling and function via changes in growth factor presentation. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2009, 1167–1171 [DOI] [PubMed] [Google Scholar]
- 23.Gan H. K., Walker F., Burgess A. W., Rigopoulos A., Scott A. M., Johns T. G. (2007) The epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor AG1478 increases the formation of inactive untethered EGFR dimers. Implications for combination therapy with monoclonal antibody 806. J. Biol. Chem. 282, 2840–2850 [DOI] [PubMed] [Google Scholar]
- 24.Ng M. R., Besser A., Danuser G., Brugge J. S. (2012) Substrate stiffness regulates cadherin-dependent collective migration through myosin-II contractility. J. Cell Biol. 199, 545–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bleasdale J. E., Thakur N. R., Gremban R. S., Bundy G. L., Fitzpatrick F. A., Smith R. J., Bunting S. (1990) Selective inhibition of receptor-coupled phospholipase C-dependent processes in human platelets and polymorphonuclear neutrophils. J. Pharmacol. Exp. Ther. 255, 756–768 [PubMed] [Google Scholar]
- 26.Jin W., Lo T. M., Loh H. H., Thayer S. A. (1994) U73122 inhibits phospholipase C-dependent calcium mobilization in neuronal cells. Brain Res. 642, 237–243 [DOI] [PubMed] [Google Scholar]
- 27.Tian D., Kreeger P. K. (2014) Analysis of the quantitative balance between insulin-like growth factor (IGF)-1 ligand, receptor, and binding protein levels to predict cell sensitivity and therapeutic efficacy. BMC Syst. Biol. 8, 98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reddy C. C., Wells A., Lauffenburger D. A. (1996) Receptor-mediated effects on ligand availability influence relative mitogenic potencies of epidermal growth factor and transforming growth factor alpha. J. Cell. Physiol. 166, 512–522 [DOI] [PubMed] [Google Scholar]
- 29.Clarke D. C., Brown M. L., Erickson R. A., Shi Y., Liu X. (2009) Transforming growth factor beta depletion is the primary determinant of Smad signaling kinetics. Mol. Cell. Biol. 29, 2443–2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sridhar B. V., Doyle N. R., Randolph M. A., Anseth K. S. (2014) Covalently tethered TGF-β1 with encapsulated chondrocytes in a PEG hydrogel system enhances extracellular matrix production. J. Biomed. Mater. Res. A 102, 4464–4472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishimoto S., Nishida E. (2006) MAPK signalling: ERK5 versus ERK1/2. EMBO Rep. 7, 782–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davies S. P., Reddy H., Caivano M., Cohen P. (2000) Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 351, 95–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chambon J. P., Nakayama A., Takamura K., McDougall A., Satoh N. (2007) ERK- and JNK-signalling regulate gene networks that stimulate metamorphosis and apoptosis in tail tissues of ascidian tadpoles. Development 134, 1203–1219 [DOI] [PubMed] [Google Scholar]
- 34.Jones S., Rappoport J. Z. (2014) Interdependent epidermal growth factor receptor signalling and trafficking. Int. J. Biochem. Cell Biol. 51, 23–28 [DOI] [PubMed] [Google Scholar]
- 35.Mu F. T., Callaghan J. M., Steele-Mortimer O., Stenmark H., Parton R. G., Campbell P. L., McCluskey J., Yeo J. P., Tock E. P., Toh B. H. (1995) EEA1, an early endosome-associated protein. EEA1 is a conserved alpha-helical peripheral membrane protein flanked by cysteine “fingers” and contains a calmodulin-binding IQ motif. J. Biol. Chem. 270, 13503–13511 [DOI] [PubMed] [Google Scholar]
- 36.Iyer A. K., Tran K. T., Griffith L., Wells A. (2008) Cell surface restriction of EGFR by a tenascin cytotactin-encoded EGF-like repeat is preferential for motility-related signaling. J. Cell. Physiol. 214, 504–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haugh J. M., Schooler K., Wells A., Wiley H. S., Lauffenburger D. A. (1999) Effect of epidermal growth factor receptor internalization on regulation of the phospholipase C-gamma1 signaling pathway. J. Biol. Chem. 274, 8958–8965 [DOI] [PubMed] [Google Scholar]
- 38.Park J. B., Lee C. S., Jang J. H., Ghim J., Kim Y. J., You S., Hwang D., Suh P. G., Ryu S. H. (2012) Phospholipase signalling networks in cancer. Nat. Rev. Cancer 12, 782–792 [DOI] [PubMed] [Google Scholar]
- 39.Ji Q. S., Winnier G. E., Niswender K. D., Horstman D., Wisdom R., Magnuson M. A., Carpenter G. (1997) Essential role of the tyrosine kinase substrate phospholipase C-gamma1 in mammalian growth and development. Proc. Natl. Acad. Sci. USA 94, 2999–3003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haase I., Liesegang C., Binting S., Henz B. M., Rosenbach T. (1997) Phospholipase C-mediated signaling is altered during HaCaT cell proliferation and differentiation. J. Invest. Dermatol. 108, 748–752 [DOI] [PubMed] [Google Scholar]
- 41.Wilsher N. E., Court W. J., Ruddle R., Newbatt Y. M., Aherne W., Sheldrake P. W., Jones N. P., Katan M., Eccles S. A., Raynaud F. I. (2007) The phosphoinositide-specific phospholipase C inhibitor U73122 (1-(6-((17beta-3-methoxyestra-1,3,5(10)-trien-17-yl)amino)hexyl)-1H-pyrrole-2,5-dione) spontaneously forms conjugates with common components of cell culture medium. Drug Metab. Dispos. 35, 1017–1022 [DOI] [PubMed] [Google Scholar]
- 42.Koria P. (2012) Delivery of growth factors for tissue regeneration and wound healing. BioDrugs 26, 163–175 [DOI] [PubMed] [Google Scholar]
- 43.Robson M. C. (1997) The role of growth factors in the healing of chronic wounds. Wound Repair Regen. 5, 12–17 [DOI] [PubMed] [Google Scholar]
- 44.Kuhl P. R., Griffith-Cima L. G. (1996) Tethered epidermal growth factor as a paradigm for growth factor-induced stimulation from the solid phase. Nat. Med. 2, 1022–1027 [DOI] [PubMed] [Google Scholar]
- 45.Wang Y., Gao J., Guo X., Tong T., Shi X., Li L., Qi M., Wang Y., Cai M., Jiang J., Xu C., Ji H., Wang H. (2014) Regulation of EGFR nanocluster formation by ionic protein-lipid interaction. Cell Res. 24, 959–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ichinose J., Murata M., Yanagida T., Sako Y. (2004) EGF signalling amplification induced by dynamic clustering of EGFR. Biochem. Biophys. Res. Commun. 324, 1143–1149 [DOI] [PubMed] [Google Scholar]
- 47.Kuhlman W., Taniguchi I., Griffith L. G., Mayes A. M. (2007) Interplay between PEO tether length and ligand spacing governs cell spreading on RGD-modified PMMA-g-PEO comb copolymers. Biomacromolecules 8, 3206–3213 [DOI] [PubMed] [Google Scholar]
- 48.Game S. M., Huelsen A., Patel V., Donnelly M., Yeudall W. A., Stone A., Fusenig N. E., Prime S. S. (1992) Progressive abrogation of TGF-beta 1 and EGF growth control is associated with tumour progression in ras-transfected human keratinocytes. Int. J. Cancer 52, 461–470 [DOI] [PubMed] [Google Scholar]
- 49.Ashton R. S., Conway A., Pangarkar C., Bergen J., Lim K. I., Shah P., Bissell M., Schaffer D. V. (2012) Astrocytes regulate adult hippocampal neurogenesis through ephrin-B signaling. Nat. Neurosci. 15, 1399–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miaczynska M., Pelkmans L., Zerial M. (2004) Not just a sink: endosomes in control of signal transduction. Curr. Opin. Cell Biol. 16, 400–406 [DOI] [PubMed] [Google Scholar]
- 51.Wang Y., Wang Z. (2003) Regulation of EGF-induced phospholipase C-gamma1 translocation and activation by its SH2 and PH domains. Traffic 4, 618–630 [DOI] [PubMed] [Google Scholar]
- 52.Li S., Wang Q., Wang Y., Chen X., Wang Z. (2009) PLC-gamma1 and Rac1 coregulate EGF-induced cytoskeleton remodeling and cell migration. Mol. Endocrinol. 23, 901–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wickert L. E., Pomerenke S., Mitchell I., Masters K. S. Kreeger P. K. (2016)Hierarchy of cellular decisions in collective behavior: implications for wound healing.Sci. Rep. 6, 20139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Braund R., Hook S., Medlicott N. J. (2007) The role of topical growth factors in chronic wounds. Curr. Drug Deliv. 4, 195–204 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.