Abstract
TGF-β1 induces an increase in paracellular permeability and actin stress fiber formation in lung microvascular endothelial and alveolar epithelial cells via small Rho GTPase. The molecular mechanism involved is not fully understood. Neuronal Wiskott–Aldrich syndrome protein (N-WASP) has an essential role in actin structure dynamics. We hypothesized that N-WASP plays a critical role in these TGF-β1–induced responses. In these cell monolayers, we demonstrated that N-WASP down-regulation by short hairpin RNA prevented TGF-β1–mediated disruption of the cortical actin structure, actin stress filament formation, and increased permeability. Furthermore, N-WASP down-regulation blocked TGF-β1 activation mediated by IL-1β in alveolar epithelial cells, which requires actin stress fiber formation. Control short hairpin RNA had no effect on these TGF-β1–induced responses. TGF-β1–induced phosphorylation of Y256 of N-WASP via activation of small Rho GTPase and focal adhesion kinase mediates TGF-β1–induced paracellular permeability and actin cytoskeleton dynamics. In vivo, compared with controls, N-WASP down-regulation increases survival and prevents lung edema in mice induced by bleomycin exposure—a lung injury model in which TGF-β1 plays a critical role. Our data indicate that N-WASP plays a crucial role in the development of TGF-β1–mediated acute lung injury by promoting pulmonary edema via regulation of actin cytoskeleton dynamics.—Wagener, B. M., Hu, M., Zheng, A., Zhao, X., Che, P., Brandon, A., Anjum, N., Snapper, S., Creighton, J., Guan, J.-L., Han, Q., Cai, G.-Q., Han, X., Pittet, J.-F., Ding, Q. Neuronal Wiskott–Aldrich syndrome protein regulates TGF-β1–mediated lung vascular permeability.
Keywords: acute lung injury, FAK, small Rho GTPases, cytoskeletal dynamics, IL-1β
Acute lung injury (ALI) is a devastating clinical syndrome that is characterized by flooding of alveolar airspaces with protein-rich edema that impairs pulmonary gas exchange and leads to arterial hypoxemia and respiratory failure (1–3). Multiple factors contribute to the respiratory failure and high mortality associated with ALI, including, but not limited to, increased permeability of the alveolar-capillary barrier, decreased surfactant function, and impaired alveolar fluid clearance (1, 2, 4). Increased levels of proinflammatory mediators are found in the bronchoalveolar lavage fluid from patients with ALI that includes TNF-α, IL-1β, and TGF-β1 (5–9). These proinflammatory mediators are known to impair endothelial and epithelial barrier integrity and to cause development of pulmonary edema associated with early-phase ALI (5–9).
Previous studies that evaluated global patterns of gene expression after bleomycin-induced lung injury found that expression levels of several TGF-β–inducible genes were dramatically increased as early as 2 d after the induction of injury (10), a time point that precedes the maximal increase in alveolar flooding. Furthermore, inhibition of the activation of TGF-β1 by pharmacologic blockade or genetic deletion of the αvβ6 integrin prevented the development of pulmonary edema in bleomycin-treated mice (7, 11). In patients with ALI, TGF-β1 expression and activation are elevated in bronchoalveolar lavage fluid (12), and the TGF-β–inducible gene, procollagen III, is one of the earliest predictors of the severity of ALI (13, 14). Adenoviral gene transfer of IL-1β to the lung caused activation of TGF-β1 via a RhoA-αvβ6 integrin–dependent mechanism, and inhibition of active TGF-β1 prevented the development of pulmonary edema induced by IL-1β in mice (11). Finally, adenoviral-mediated transfer of active TGF-β1 to rat lungs induced perivascular and peribronchial edema (15). Taken together, these findings indicate that TGF-β1 is a critical mediator in the development of lung edema associated with ALI.
We and others have previously reported that activation of small Rho GTPase is responsible for actin stress fiber formation and increased paracellular permeability in lung endothelial cell monolayers induced by TGF-β1 or other proinflammatory mediators (11, 16–21). Neuronal Wiskott–Aldrich syndrome protein (N-WASP) is a downstream effector of small Rho GTPases (22–26). The middle region of N-WASP contains the GTPase-binding domain (GBD)/Cdc42 and Rac interactive binding domain, which binds to small Rho GTPases and activates N-WASP (22–26). N-WASP has 3 independent small domains at the C terminus, collectively termed the verprolin homology, central, and acidic regions (VCA) domain. The VCA domain of N-WASP is necessary to activate actin polymerization via an actin-related protein (Arp) 2/3-dependent mechanism (22–26). N-WASP regulates cytoskeletal dynamics and transmits upstream signals to the cellular machinery that is directly involved in modulation of actin filament structures, such as induction of new actin polymerization and actin structures (22, 23, 27–30); however, the role of N-WASP in actin stress fiber formation and increased paracellular permeability induced by a TGF-β1 challenge is still unknown.
In this study, we hypothesized that N-WASP would mediate TGF-β1–induced changes in actin cytoskeleton dynamics in both lung microvascular endothelial cells and alveolar epithelial cells. We determined that changes in actin dynamics were associated with increased paracellular permeability across these cell monolayers. We demonstrate that IL-1β–dependent activation of TGF-β1 via RhoA-αvβ6 integrin is also N-WASP dependent. Furthermore, TGF-β1 induces phosphorylation of Y256 of N-WASP, and this phosphorylation mediates increases in paracellular permeability and actin stress fiber formation. Activation of small Rho GTPase and focal adhesion kinase (FAK) is upstream of N-WASP in the signaling pathway activated by TGF-β1 and, thus, is required for N-WASP activation. Finally, we show that N-WASP deficiency increases survival and protects mice against development of bleomycin-induced lung edema. In summary, we demonstrate that N-WASP is a critical mediator of TGF-β1–mediated lung permeability that is dependent on both small Rho GTPase and FAK.
MATERIALS AND METHODS
Reagents
TGF-β1 and IL-1β were obtained from R&D Systems (Minneapolis, MN, USA). The following purified antibodies were purchased: anti–N-WASP, anti-FAK, anti–phospho-Y256 of N-WASP (Millipore, Billerica, MA, USA); anti–phospho-Y397 of FAK (Cell Signaling Technology, Danvers, MA, USA); anti–glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and anti-myc (Santa Cruz Biotechnology, Santa Cruz, CA, USA); and Alexa Fluor 488 phalloidin (Thermo Fisher Scientific Life Sciences, Waltham, MA, USA). Rho inhibitor I was purchased from Cytoskeleton (Denver, CO, USA) and PF573228 {6-[4-(3-methanesulfonyl-benzylamino)-5-trifluoromethyl-pyrimidin-2-ylamino]-3,4-dihydro-1H-quinolin-2-one; FAK inhibitor)} was purchased from Calbiochem (Billerica, MA, USA). All other reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA) or Thermo Fisher Scientific Life Sciences.
Cell culture
Rat lung microvascular endothelial cells (RMVECs) were derived as previously described (31, 32) and were cultured in DMEM that was supplemented with 10% fetal bovine serum and 100 U/ml penicillin/streptomycin. L2 rat lung alveolar epithelial cells were obtained from American Type Culture Collection (Manassas, VA, USA) and were propagated and maintained in F-12K medium that was supplemented with 10% fetal bovine serum and 100 U/ml penicillin/streptomycin per the manufacturer’s instructions.
Lentiviral and adenoviral vectors
The replication incompetent lentiviral vectors that expressed short hairpin RNA (shRNA) for silencing N-WASP or lentiviral vectors that expressed scrambled, nontargeting shRNA were described previously (33) and were obtained from Thermo Fisher Scientific Life Sciences. RMVECs or L2 alveolar epithelial cells were transfected with lentiviral vectors as previously described (33), and the knockdown effects were confirmed by Western blot analysis (for protein level) and by quantitative RT-PCR (for mRNA level) as previously described (34, 35). The protein concentration of cell lysates was determined by using a bicinchoninic acid kit (Pierce, Rockford, IL, USA) per the manufacturer’s instructions. Generation, amplification, and use of adenoviral vectors were described previously (36–38). In brief, the replication-deficient adenoviral vectors were generated by using the Adeno-X Expression System 2 according to the manufacturer’s instructions (Clontech, Mountain View, CA, USA). The adenoviral vectors were rescued and amplified in 293 cells, and were purified by CsCl gradient centrifugation.
Western blotting
Western blotting assays were performed as previously described (38). In brief, equivalent micrograms of whole-cell lysates were electrophoresed on a disulfide-reduced 7.5% SDS-PAGE, transferred to Immobilon-P membrane (Millipore), probed with indicated antibodies, and developed with ECL system (Pharmacia Biotech, Piscataway, NJ, USA). The expression of GAPDH protein was used as a loading control.
Quantitative real-time RT-PCR analysis
Quantitative RT-PCR was performed as previously described (33). In brief, total RNA was extracted by using RNeasy Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. The following primers were used: N-WASP, sense 5′-CCCCCAAATGGTCCTAATCT-3′ and antisense 5′- ACATGTCCAATGTGCTGGAA-3′; and GAPDH, sense 5′-GAGTCAACGGATTTGGTCGT-3′ and antisense 5′-TTGATTTTGGAGGGATCTCG-3′. Of total RNA, 1–3 μg was reverse transcribed to cDNA with Maloney murine leukemia virus reverse transcriptase (Promega, Madison, WI, USA). Samples were assayed in triplicate and the values were normalized to the relative amounts of GAPDH.
Immunofluorescence microscopy
Immunofluorescence was performed as previously described (39). In brief, cells cultured on glass coverslips were treated as described in relevant figure legends and fixed in 4% buffered paraformaldehyde and were permeabilized. To stain the cytoplasmic filaments, cells were reacted with Alexa Fluor 488 phalloidin (1:100) overnight at 4°C. Cell nuclei were stained with blue Hoechst fluorescence dye to count the total cells per field. Digital fluorescence images were obtained by using a Nikon microscope and software (Nikon, Tokyo, Japan).
Measurement of transcellular resistance
Endothelial and epithelial barrier integrity was measured by using an electric cell-substrate impedance sensing system (Applied Biophysics, Troy, NY, USA) as previously described (31). In brief, RMVECs or L2 cells treated with shRNA as described in the relevant figure legends were plated on 8W10E arrays in normal culture medium and were assayed when resistances reached ± 900 Ω, usually 2–3 d after seeding. Resistance was measured every 10 min for the duration of the experiments. Baseline resistances were measured for 1 h before addition of TGF-β1, and data represent a change in resistance at 24 h normalized to baseline resistance.
Active Rho and active TGF-β1 assays
Rho activation was determined by using a Rho activation kit per the manufacturer’s instructions (Upstate Cell Signaling Solutions, Temecula, CA, USA) as previously described (33). In brief, the level of active Rho (GTP-bound form) in cell lysate was reacted with Rhotekin Rho-binding domain coupled to agarose. The immunoprecipitates were subjected to 10% SDS-PAGE, transferred to Immobilon, and Western blotted with anti-Rho IgG. Active TGF-β1 was measured by adding IL-1β to confluent alveolar epithelial monolayers for 6 h. Thereafter, medium was collected and active TGF-β1 was measured by using an ELISA kit for the measurement of active TGF-β1 (R&D Systems) according to the manufacturer’s instructions. This method of measuring active TGF-β1 has been previously published (40).
In vivo bleomycin-induced ALI model
All animal interventions were approved by the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham. The administration of bleomycin and of adenoviral vectors were previously described (41). In brief, animals (8–12 wk old) were challenged with bleomycin (4 U/kg body weight) or saline by using an intratracheal catheter. Saline with or without recombinant adenoviral vectors [50 μl, 108 plaque-forming units (pfu)] were instilled intratracheally 1 d before bleomycin (or saline) challenge as previously described (41).
Measurement of lung edema
Lung wet-to-dry ratios were determined as previously described (7, 42). In brief, whole lungs were excised and dissected away from the heart and thymus. Lungs were immediately weighed to obtain wet weight, and were then placed and dried in an oven at 75°C for 8 d to obtain dry weight.
Survival
Wild-type or N-WASPflox/flox mice were randomly assigned to groups described in figure legends. Mice were exposed to adenoviral vectors and or bleomycin as described above. Mice were checked every 6 h during the 10 d after the instillation of bleomycin into the lung until death or survival at 10 d. Survival time was defined as the time between instillation and death.
Statistical analyses
All data are summarized as means ± sem. For statistical analysis, we used Statview 5.0 (SAS Institute, Cary, NC, USA) and MedCalc 7.2.0.2 (MedCalc Software, Ostend, Belgium). The normal distribution was verified by using the Kolmogorov–Smirnov test. For normally distributed data, a Student's t test was used to compare 2 experimental groups. Bonferroni correction, which controlled for false positive error rate, was used to adjust for multiple comparisons. A Kaplan–Meier analysis followed by a log rank (Mantel-Cox) test was used to compare the survival between the 2 experimental groups of mice at 10 d. A value of P < 0.05 was considered statistically significant, and all statistical comparison of means was bilateral (2-tailed tests).
RESULTS
N-WASP is involved in TGF-β1–induced actin filament formation and increased paracellular permeability in RMVEC monolayers
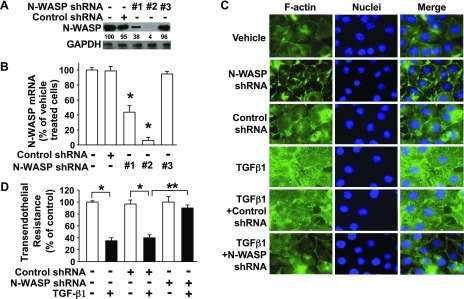
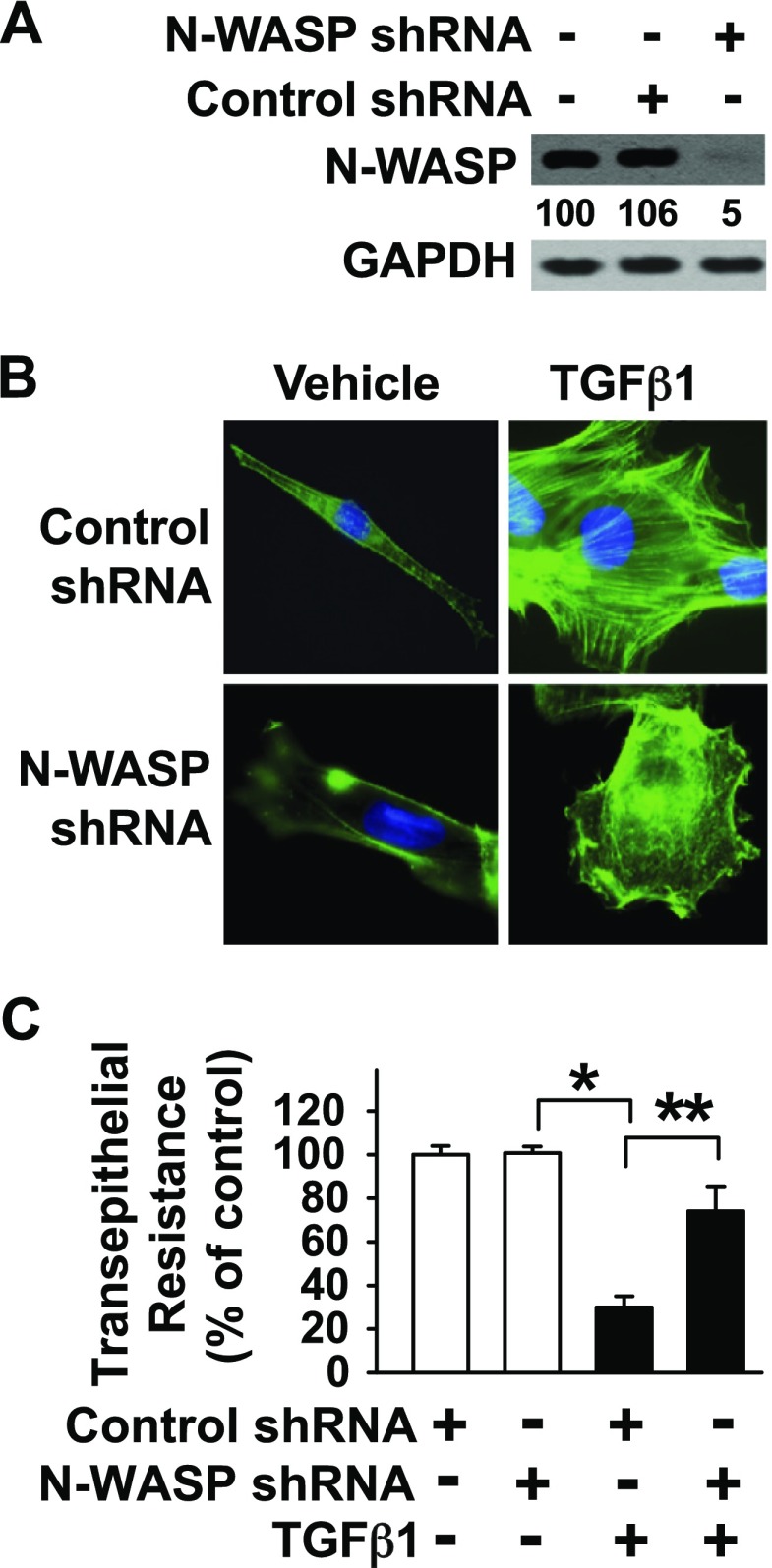
TGF-β1 is a critical mediator of pulmonary edema formation after exposure to bleomycin, Escherichia coli endotoxin, and Pseudomonas Aeruginosa, and it does so by increasing paracellular permeability in lung microvascular endothelial and alveolar epithelial cell monolayers via changes in actin-containing cytoskeleton dynamics (7, 43). To better understand the role of N-WASP in TGF-β1 signaling, we sought to knock down its expression by using a lentiviral vector that contained N-WASP shRNA. N-WASP knockdown was validated at both the protein level (Fig. 1A) and the mRNA level (Fig. 1B) in RMVECs. N-WASP shRNA #2 achieved >90% down-regulation of N-WASP protein expression and has thus been used for subsequent experiments. Unchallenged endothelial cells contain a cortical actin ring structure around the cell edge, which is disrupted by TGF-β1 challenge in endothelial cells (Fig. 1C). Long and thick actin-containing filaments are formed de novo and they are prominent in TGF-β1–challenged endothelial cells (Fig. 1C). N-WASP knockdown inhibited de novo actin stress filament formation in TGF-β1–treated endothelial cells but did not induce significant changes in cortical actin ring structure (Fig. 1C). These results demonstrate an indispensable role for N-WASP in the promotion of actin stress filament formation in lung microvascular endothelial cells in response to TGF-β1.
Figure 1.
N-WASP plays a critical role in TGF-β1–induced de novo actin filament formation and paracellular permeability in RMVECs. A) N-WASP shRNA effectively decreases N-WASP protein levels in RMVECs. Cells were infected with lentiviral vectors that contained N-WASP shRNA #1 (or #2, or #3) or control nontargeting shRNA. Equivalent amounts of whole-cell detergent lysates were Western blotted with antibodies against N-WASP or GAPDH. Densitometry was used to determine down-regulation of N-WASP relative to control cells, and the relative percentage was shown between top and bottom panels. B) N-WASP shRNA effectively decreases N-WASP mRNA levels. Cells were treated as in panel A. N-WASP mRNA level was examined by quantitative real-time RT-PCR using NWASP-specific primers. Data are presented as percent of mRNA in vehicle-treated cells; means ± sem; n = 3. P < 0.001. C) N-WASP knockdown ameliorates TGF-β1–mediated actin filament formation. RMVECs were cultured in serum-free media with 1% bovine serum albumin, treated as in panel A, and stimulated by TGF-β1 (5 ng/ml) for 24 h. Cells were then fixed, stained with Alexa Fluor 488-phalloidin, counterstained with nuclear staining dye (Hoechst), and imaged via confocal fluorescence microscopy. Representative images are shown. D) N-WASP knockdown inhibits TGF-β1–mediated increases in endothelial paracellular permeability. Cells were infected with N-WASP shRNA #2 or control shRNA as in panel A, treated with TGF-β1 (5 ng/ml, or vehicle), and transendothelial resistance was measured via electric cell-substrate impedance sensing. Data are presented as percent of control in vehicle-treated cells; n = 8. P < 0.001. Single asterisk indicates significant difference compared with controls.; Double asterisk indicates significant difference compared with TGF-β1 and control shRNA. Original magnification, ×600.
Previous studies have revealed changes in actin cytoskeleton dynamics that are associated with an increase in paracellular permeability across endothelial cell monolayers (11, 16, 18–21, 43). To examine whether N-WASP promotes TGF-β1–induced increased paracellular permeability across RMVEC monolayers, electric resistance was measured by using electric cell-substrate impedance sensing assays. TGF-β1 significantly decreased transendothelial resistance (Fig. 1D). The decreased transendothelial resistance (electrical resistance) in RMVECs reflects an increase in paracellular permeability of endothelial cells, and N-WASP knockdown rescued the TGF-β1–induced decrease of transendothelial resistance in these cell monolayers. N-WASP knockdown alone had no effect on transendothelial resistance in endothelial cells (vehicle treated). These results support the concept that N-WASP plays a critical role in the signaling pathway that is responsible for TGF-β1–induced increased paracellular permeability in endothelial cells.
Phosphorylation of Y256 of N-WASP is required for TGF-β1–induced increase in paracellular permeability and change in cytoskeletal dynamics
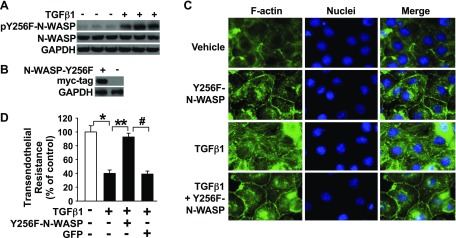
N-WASP stays in an autoinhibitory state until activated by small Rho GTPase, which opens N-WASP structure and exposes Y256 for phosphorylation (22–26, 44–46). We found that TGF-β1 induced phosphorylation of Y256 of N-WASP in RMVECs (Fig. 2A). To understand whether N-WASP Y256 phosphorylation mediates the effect of TGF-β1 on permeability and actin cytoskeleton dynamics of endothelial cell monolayers, an myc-tagged dominant-negative N-WASP mutant, Y256F-NWASP, was constructed and expressed by adenoviral vectors as previously described (33). Y256 of N-WASP was replaced by phenylalanine to create the Y256F-NWASP mutant. Expression of Y256F-NWASP was confirmed by myc-tag expression in endothelial cells (Fig. 2B).
Figure 2.
TGF-β1 induces phosphorylation of Y256 of N-WASP. N-WASP Y256 plays a critical role in TGF-β1–induced paracellular permeability and actin stress fiber formation in RMVECs. A) TGF-β1 induces phosphorylation of Y256 of N-WASP in RMVECs. Phosphorylation of Y256 of N-WASP was examined in lung microvascular endothelial cells that were treated with TGF-β1 (5 ng/ml) by Western blotting. B) Validation of N-WASP Y256F mutant overexpression. Cells were infected with adenoviral vectors that contained myc-tagged Y256F-NWASP mutant. Mutant expression was confirmed by Western blotting against myc. C) Y256F-NWASP mutant overexpression in endothelial cells prevents TGF-β1–mediated actin filament formation. Endothelial cells overexpressing (or not) NWASP-Y256F mutant were fixed, stained with Alexa Fluor 488-phalloidin, counterstained with nuclear staining dye (Hoechst), and imaged via confocal fluorescence microscopy. Representative images are shown. D) Y256F-NWASP mutant overexpression inhibits TGF-β1–mediated increases in endothelial paracellular permeability. Transendothelial resistance was measured in endothelial cells overexpressing (or not) Y256F-NWASP mutant to evaluate paracellular permeability after exposure to TGF-β1 (5 ng/ml, or vehicle) via electric cell-substrate impedance sensing. Data are presented as percent of control in vehicle-treated cells; n = 8. P < 0.001. Single asterisk indicates significant difference compared with control. Double asterisk indicates significant difference compared with TGF-β1 alone. Pound symbol indicatesignificant difference compared with TGF-β1 with overexpressed N-WASP mutant. Original magnification, ×600.
Expression of the dominant-negative Y256F-NWASP mutant prevented TGF-β1–induced actin stress filament formation in endothelial cells (Fig. 2C). Expression of this mutant also significantly attenuated the TGF-β1–induced decrease of transendothelial resistance in lung microvascular endothelial cells (Fig. 2D). Expression of a control vector [green fluorescent protein (GFP)] by adenoviral vector had no significant effect on TGF-β1–induced decrease of transendothelial resistance. Taken together, these results indicate that TGF-β1 increases paracellular permeability and induces actin stress fiber formation via the phosphorylation of N-WASP Y256 in endothelial cell monolayers.
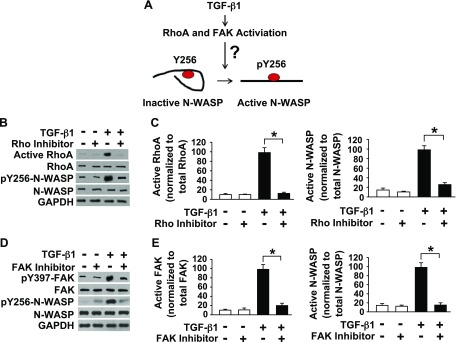
In the next series of experiments, we examined the signaling pathway involved in TGF-β1–induced Y256 phosphorylation of N-WASP. We and others have previously shown that the small Rho GTPase plays a critical role in mediating the increase in lung endothelial permeability induced by multiple proinflammatory mediators (11, 16–21). Furthermore, it is known that activated Rho GTPase can induce N-WASP conformational changes, thereby exposing N-WASP Y256, which is a phenomenon that is referred to as the GBD switch (24–26, 33, 45). This conformational change unfolds N-WASP from its autoinhibitory state, thus allowing Y256 phosphorylation (Fig. 3A) (24–26, 33, 45). To examine whether Rho GTPase is implicated in N-WASP Y256 phosphorylation after exposure to TGF-β1, lung microvascular endothelial cell monolayers were pretreated with a pan Rho inhibitor (1 µg/ml Rho inhibitor 1 or vehicle) and subsequently exposed to TGF-β1. Rho inhibitor I (CT04) inhibits Rho GTPase but does not inhibit other Rho family proteins, such as Rac or Cdc42 (per vendor instructions). Our results demonstrate that TGF-β1–induced Rho activation and phosphorylation of Y256 of N-WASP were inhibited by pretreatment of cell monolayers with a pan Rho inhibitor (Fig. 3B, C). These findings indicate that Rho GTPase activity is necessary for N-WASP Y256 phosphorylation in response to TGF-β1 and that Y256 phosphorylation of N-WASP is downstream of TGF-β1–induced Rho GTPase activation.
Figure 3.
Activation of Rho and FAK is necessary for Y256 phosphorylation of N-WASP induced by TGF-β1 in RMVECs. A) Proposed mechanism of N-WASP activation by TGF-β1. Previous data indicate that Rho GTPase activation unlocks N-WASP from an autoinhibitory, folded structure to an open structure primed for activation; however, the role of TGF-β1–induced RhoA activation in phosphorylation of Y256 of N-WASP is unknown. FAK is also activated by TGF-β1 and we hypothesize that FAK is critical in N-WASP activation (phosphorylation of Y256) in endothelial cells treated with TGF-β1. B, C) Rho inhibition prevents TGF-β1–mediated phosphorylation of Y256 of N-WASP. B) RMVECs were pretreated with a pan Rho inhibitor (1 µg/ml Rho inhibitor I) or vehicle, followed by TGF-β1 treatment (5 ng/ml), and cell lysates were collected. Rho GTPase activation and phosphorylation of Y256 of N-WASP were determined by Western blot by using indicated antibodies. C) Normalized active RhoA/total RhoA or active N-WASP/total N-WASP ratios determined by densitometry analysis from bands in panel B; n = 3. D, E) FAK inhibition prevents TGF-β1–mediated phosphorylation of Y256 of N-WASP. D) Endothelial cells were pretreated with FAK inhibitor (1 µM PF573228) or vehicle, followed by TGF-β1 treatment (5 ng/ml), and cell lysates were collected. FAK and N-WASP phosphorylation was determined by Western blot using indicated antibodies. E) Normalized active FAK/total FAK or active N-WASP/total N-WASP ratios determined by densitometry analysis from bands in panel B; n = 3. For all experiments, the results are presented as means ± sem. *P < 0.05 from TGF-β1 alone.
Our previous report demonstrated that FAK mediates phosphorylation of Y256 of N-WASP in fibroblasts in response to TGF-β1 (33). The potential involvement of FAK in N-WASP activation was thus examined in lung microvascular endothelial cells that were treated with TGF-β1. TGF-β1 induced FAK activation (phosphorylation of Y397 of FAK; Fig. 3D, E). FAK inhibitor (PF573228; 1 µM) blocked TGF-β1–induced FAK activation and TGF-β1–induced phosphorylation of Y256 of N-WASP (Fig. 3D, E). Taken together, these data indicate that TGF-β1 activates small Rho GTPase and that small Rho GTPase opens the N-WASP conformation, thereby allowing FAK to phosphorylate N-WASP at Y256.
N-WASP mediates TGF-β1–induced actin stress fiber formation and increased paracellular permeability in lung epithelial cell monolayers
We have previously reported that TGF-β1 causes an increase in paracellular permeability across alveolar epithelial cell monolayers (16, 47). To understand whether N-WASP mediates TGF-β1–induced increase in paracellular permeability across L2 alveolar epithelial cells (hereafter referred to as L2 cells), a rat alveolar epithelial cell line (48), the expression of N-WASP was knocked down by using our lentiviral vector that contained N-WASP shRNA #2 (Fig. 4A). TGF-β1 induced de novo actin stress filaments in L2 cells and genetic deletion of N-WASP inhibited TGF-β1–mediated actin stress fiber formation (Fig. 4B). In addition, N-WASP deletion alone did not affect the cortical actin ring-like cytoskeletal structure. Finally, N-WASP knockdown attenuated TGF-β1–induced decrease in electric resistance in L2 cell monolayers (Fig. 4C). These results support the idea that N-WASP plays a critical role in TGF-β1–induced change in actin cytoskeleton dynamics and increased paracellular permeability across alveolar epithelial cell monolayers.
Figure 4.
N-WASP plays a critical role in TGF-β1–induced actin stress fiber formation and paracellular permeability in alveolar epithelial cells. A) N-WASP shRNA effectively decreases N-WASP protein levels in L2 (rat lung alveolar epithelial) cells. L2 cells were infected with lentiviral vectors that contained N-WASP shRNA #2 or control nontargeting shRNA. Equivalent amounts of whole-cell detergent lysates were Western blotted with indicated antibodies. Densitometry was used to determine down-regulation of N-WASP relative to control cells, and the relative percentage was shown between top and bottom panels. B) N-WASP knockdown ameliorates TGF-β1–mediated actin filament formation. L2 cells were treated as in panel A, followed by TGF-β1 (5 ng/ml) or vehicle for 24 h. Cells were then fixed, stained with Alexa Fluor 488-phalloidin, counterstained with nuclear staining dye (Hoechst), and imaged via confocal fluorescence microscopy. Representative images are shown. C) N-WASP knockdown inhibits TGF-β1–mediated increases in epithelial paracellular permeability. L2 cells were treated as in panel B and transepithelial resistance was measured via electric cell-stimulated impedance sensing. Data are presented as percent of control in vehicle-treated cells; n = 8. P < 0.01. Single asterisk indicates significant difference compared with control. Double asterisk indicates significant difference compared with TGF-β1 and control shRNA. Original magnification, ×600.
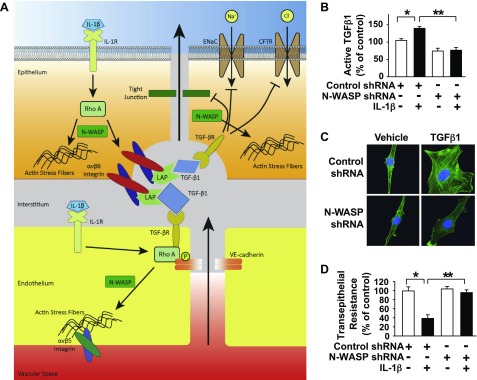
IL-1β induces TGF-β1 activation via an N-WASP–dependent pathway in alveolar epithelial cells
IL-1β is an important inflammatory mediator that contributes to development of pulmonary edema associated with ALI (6, 11, 49). We have previously shown that IL-1β induces TGF-β1 activation and permeability via a RhoA-αvβ6 integrin–mediated pathway in alveolar epithelial cells and also increases lung endothelial permeability via a RhoA-dependent mechanism (Fig. 5A) (11). N-WASP knockdown blocked IL-1β–induced TGF-β1 activation in L2 alveolar epithelial cells (Fig. 5B), which indicates that IL-1β induces TGF-β1 activation through an N-WASP–dependent pathway (bar 1, 1301 ± 72; bar 2, 1733 ± 74; bar 3, 920 ± 102; bar, 951 ± 104 pg/ml active TGF-β1). The amount of active TGF-β1 was measured in the medium of alveolar epithelial cell monolayers at 6 h after IL-1β treatment by ELISA kit (R&D Systems). Of importance, our data also indicated that N-WASP knockdown inhibited IL-1β–induced cytoskeletal reorganization (Fig. 5C) and blocked IL-1β–induced paracellular permeability in L2 cells (Fig. 5D). Taken together, these data support a role for N-WASP in the mediation of IL-1β–induced TGF-β1 activation and a subsequent increase in paracellular permeability across alveolar epithelial cell monolayers.
Figure 5.
N-WASP is required for IL-1β–induced release of active TGF-β1 in alveolar epithelial cells. A) Schematic of epithelial paracellular permeability mediated by IL-1β, TGF-β1, and RhoA (11). The role of N-WASP in this critical signaling cascade is unknown. B) N-WASP knockdown inhibits IL-1β–induced release of active TGF-β1 in alveolar epithelial cells. N-WASP was knocked down in L2 cells via infection with lentiviral N-WASP shRNA #2 or control nontargeting shRNA and followed by treatment with IL-1β (10 ng/ml). Levels of active TGF-β1 released into culture medium were measured by ELISA at 6 h after IL-1β treatment. Bar 1 represents 1300 ± 72 pg/ml active TGF-β1 levels. Data are presented as percent of control in vehicle-treated cells; n = 8. P < 0.01. C) N-WASP knockdown ameliorates IL-1β–mediated actin filament formation in epithelial cells. L2 cells were treated as in panel B, followed by IL-1β (10 ng/ml) or vehicle for 24 h. Cells were then fixed, stained with Alexa Fluor 488-phalloidin, counterstained with nuclear staining dye (Hoechst), and imaged via confocal fluorescence microscopy. Representative images are shown. D) N-WASP knockdown inhibits IL-1β–mediated increases in epithelial paracellular permeability. L2 cells were treated as in panel B and transepithelial resistance was measured via electric cell-stimulated impedance sensing. Data are presented as percent of control in vehicle treated cells; n = 8; P < 0.01. CFTR, cystic fibrosis transmembrane conductance regulator; LAP, latency-associted peptide. Single asterisk indicates significant difference compared with control. Double asterisk indicates sgnificant difference compared with IL-1β and control shRNA. Original magnification, ×600.
N-WASP down-regulation decreases the development of pulmonary edema and increases survival in bleomycin-challenged mice
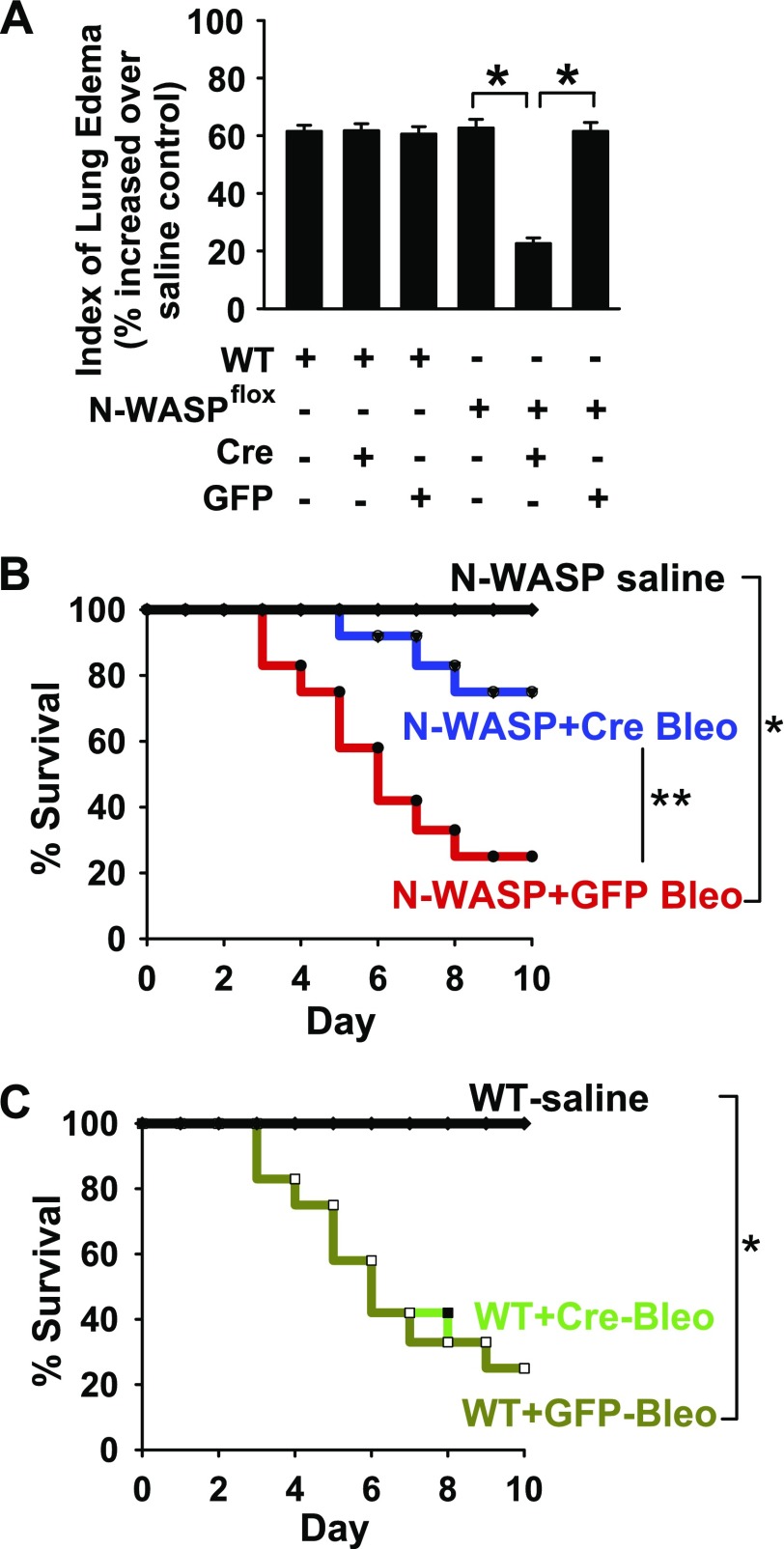
TGF-β1 plays a critical role in lung vascular permeability and fibrosis in response to bleomycin challenge (7, 10, 12–14). To test the effect of N-WASP down-regulation on bleomycin-induced lung injury, recombinant adenoviral Cre vectors (Ad-Cre; 50 μl, 108 pfu) were intratracheally instilled in N-WASPflox/flox mice or wild-type (WT) mice as previously described (38, 41). Adenoviral vectors that expressed GFP were also intratracheally instilled in N-WASPflox/flox mice and WT mice as vector controls. Mice were then intratracheally challenged with bleomycin (4 U/kg; or saline vehicle) as previously described (38, 41). Bleomycin-induced lung edema was significantly decreased in N-WASPflox/flox mice that were treated with Ad-Cre compared with WASPflox/flox mice that were treated with Ad-GFP or WT mice that were treated with either Ad-Cre or Ad-GFP (Fig. 6A; the absolute values of wet/dry: WT+Saline, 3.29 + 0.21; WT+Bleo, 5.32 + 0.27; WT+Cre+Bleo, 5.31 + 0.28; WT+GFP+Bleo, 5.28 + 0.24; N-WASPflox+Saline, 3.23 + 0.25; N-WASPflox+Bleo, 5.25 + 0.19; N-WASPflox +Cre+Bleo, 3.95 + 0.23; N-WASPflox+GFP+Bleo, 5.22 + 0.19). Furthermore, survival was significantly increased in N-WASPflox/flox mice that were treated with Ad-Cre compared with N-WASPflox/flox mice that were treated with Ad-GFP and WT mice that were treated with either Ad-Cre or Ad-GFP (Fig. 6B, C). There were no significant differences in survival among the following bleomycin-challenged groups: WT mice that were treated with bleomycin (WT+Bleo), WT mice that were treated with Ad-Cre and bleomycin (WT+Cre-Bleo), WT mice that were treated with Ad-GFP and bleomycin (WT+GFP-Bleo), and N-WASPflox/flox mice that were treated with Ad-GFP and bleomycin (N-WASP+GFP-Bleo). These results indicate that N-WASP inhibition protects against the development of lung edema and increases survival in mice challenged with bleomycin.
Figure 6.
N-WASP down-regulation significantly decreases pulmonary edema and increases survival in bleomycin-challenged mice. A) N-WASP knockdown in vivo prevents bleomycin-induced lung edema. N-WASP floxed (N-WASPflox/flox) and WT mice were intratracheally instilled with adenoviral vectors that contained Cre recombinase (Cre) or GFP control protein (GFP; 1 × 108 pfu), then challenged with bleomycin (4 U/kg body weight) or saline. Wet/dry ratio was examined at d 6. Data are presented as percent of wet/dry ratio in bleomycin-challenged mice over the wet/dry ratio in same genotype of mice treated with saline. Median wet/dry ratio value for WT mice treated with saline was 3.29 and for NWASP-flox mice treated with saline was 3.23; n = 12 for N-WASPflox/flox with Ad-Cre or Ad-GFP; n = 12–13 for the rest groups. P < 0.01. B, C) N-WASP knockdown in vivo improves bleomycin-induced survival. N-WASPflox/flox (B) and WT (C) mice were treated as in panel A, and survival was evaluated for 10 d; n = 16 per group. P < 0.01. Single asterisk indicates significant difference when compared to control. Double asterisk indicates significant difference when compared to Ad-GFP.
Figure 7.
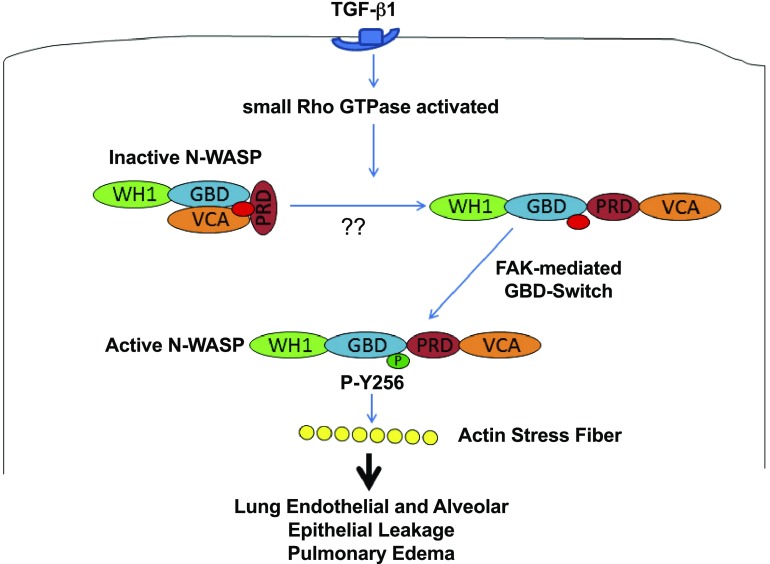
Proposed schematics of N-WASP–mediated actin cytoskeletal dynamics and permeability derangements during lung injury. ALI mediator TGF-β1 causes RhoA activation. We speculate that N-WASP is unlocked by active RhoA GTPase from an autoinhibited conformation to an open molecule structure ready for next step during activation. Data suggest that phosphorylation of Y256 of N-WASP by FAK is a critical switch that activates N-WASP and enhances the ability of N-WASP to promote actin stress fiber formation, actin cytoskeletal dynamics, and increase paracellular permeability during lung injury. PRD, proline-rich domain; WH1, WASH homology domain 1.
DISCUSSION
ALI is a devastating clinical syndrome that is often present in critically ill patients who have been hospitalized for pneumonia, sepsis, trauma, and inhaled irritants (1, 2, 7). ALI causes alveolar epithelial and lung endothelial damage that subsequently leads to impairment of the alveolar capillary-epithelial barrier function, inadequate alveolar fluid clearance, development of protein-rich edema, and inadequate gas exchange (1–3). TGF-β1 is one of the important inflammatory mediators that cause the development of lung edema in patients with ALI (7, 8, 12–14). For example, inhibition of TGF-β1 signaling protects mice from bleomycin-induced lung edema (7). Adenoviral vector–mediated transfer of active TGF-β1 induces perivascular and peribronchial edema in rat lungs (15). Previously studies have reported that TGF-β1–induced endothelial barrier dysfunction involved Smad2 and p38 and the subsequent activation of the small GTPase RhoA (18). Inhibition of RhoA-Rho kinase signaling pathway blunted TGF-β1–induced adherens junction disruption and focal adhesion complex formation (16, 47); however, the downstream mechanisms by which activated Rho GTPases induce actin stress fiber formation and increase paracellular permeability in lung endothelial and alveolar epithelial cells are not fully understood.
N-WASP is a downstream effector of small Rho GTPases (22–26). Rho family GTPases binds to the GBD/Cdc42 and Rac interactive binding region of N-WASP and activates N-WASP (22–26). N-WASP regulates cytoskeletal dynamics and transmits upstream signals to the cellular machinery that is directly involved in modulation of actin filament structures, such as induction of new actin polymerization and actin structures (22, 23, 27–30). Although it is known that N-WASP is essential for actin polymerization and cytoskeletal dynamics during cell migration and differentiation (22–26, 33), the role of N-WASP in actin stress fiber formation and paracellular permeability in the context of TGF-β1 challenge is unknown. Our data demonstrate that N-WASP is directly involved and has a central role in newly formed stress fibers and in the promotion of paracellular permeability in lung microvascular endothelial cells and alveolar epithelial cells in response to TGF-β1 (Figs. 1 and 4). Indeed, the down-regulation of N-WASP expression by shRNA blocked the formation of stress fibers in lung microvascular endothelial cells and alveolar epithelial cells in response to TGF-β1 (Figs. 1 and 4). Furthermore, cell monolayers with decreased N-WASP expression showed less paracellular permeability in response to TGF-β1 (Figs. 1 and 4). Increased intracellular stress fibers presumably lead to increased mechanical tension and contractile force, thereby causing gaps between adjacent cells. Of importance, there was no significant change of cortical actin ring structures in endothelial cells with N-WASP down-regulation (Fig. 1). Data suggest that N-WASP is not required to maintain the cortical actin structure but is required for cytoskeletal dynamics when cells were challenged with TGF-β1. Our data further show that N-WASP is required for pulmonary edema development in vivo. Lung edema is significantly reduced in mice with decreased N-WASP expression compared with that in WT controls after bleomycin challenge (Fig. 6). GFP expression that was mediated by adenoviral vector transfer and used as a control had no significant effect on the development of lung edema in mice. TGF-β1 is a known mediator of bleomycin-induced lung edema in mice (7). As N-WASP is required for TGF-β1–induced paracellular permeability in alveolar epithelial and lung endothelial cell monolayers, the decreased lung edema development in mice with N-WASP down-regulation suggests an attenuated increase in paracellular permeability in response to TGF-β1. Finally, survival was remarkably increased in bleomycin-challenged mice with N-WASP down-regulation compared with bleomycin-challenged mice treated with control (WT or N-WASP-flox mice that received GFP adenoviral vectors; Fig. 6). Our data are consistent with previous findings that inhibition of TGF-β1 protects mice from bleomycin-induced lung edema (7), but demonstrates the critical role of N-WASP in the mediation of TGF-β1 signaling and lung edema development in mice that have been challenged with bleomycin.
N-WASP protein contains 502 aa and has 3 independent small domains at the C terminus that make up the VCA domain (24–26). It is known that the V domain is a G-actin–binding site and is essential for N-WASP–induced formation of microspikes or newly formed branches during F-actin polymerization (30, 50). The CA domains bind the Arp2/3 complex and stimulate it to nucleate and polymerize G-actin (23–25, 50, 51). The VCA domain of N-WASP is necessary to activate actin polymerization through an Arp2/3-dependent mechanism (22–26). During cell migration and differentiation, N-WASP brings G-actin to the branching sites and promotes Arp2/3 complex and other proteins to induce F-actin polymerization (24–26, 33, 46, 52). This can result in a range of dynamic structures, such as lamellipodia, filopodia, stress fibers, and membrane ruffles, at the leading edge of the migrating cell and can generate the force for cell migration and differentiation (24, 33). Our data suggest that N-WASP functions as a key regulator to promote cytoskeletal dynamics in response to TGF-β1 (23–26, 33). It is likely that cell migration and remodeling processes is also a part of body responses in an attempt to repair the TGF-β1–mediated lung injury; therefore, although N-WASP–mediated cell remodeling processes may aim to repair injury, they actually cause an increase in endothelial/epithelial cell permeability. We speculate that N-WASP plays an important role in recruiting, nucleating, and polymerizing G-actin into newly formed F-actin stress fibers via Arp2/3 complex. These possibilities will be investigated in our future studies. Nonetheless, our data demonstrate that N-WASP has an essential role in modulation of TGF-β1–induced cytoskeletal dynamics and paracellular permeability changes in microvascular endothelial and alveolar epithelial cell monolayers.
We found that Y256 of N-WASP is a critical tyrosine residue, and Y256 phosphorylation is required for N-WASP–mediated cytoskeletal dynamics and paracellular permeability (Fig. 2). Transfection of a dominant-negative N-WASP mutant (Y256F) that is unable to be phosphorylated prevented cytoskeletal dynamics and paracellular permeability changes in response to TGF-β1 (Fig. 2). Our data show that activation of both RhoA and FAK are required for Y256 phosphorylation of N-WASP (Fig. 3). It is known that active small Rho GTPases release N-WASP from an autoinhibited conformation for activation (22–27). We have previously shown that TGF-β1–induced FAK activation is essential for the formation of cytoplasmic filaments and N-WASP activation in fibroblasts (33, 38). Our current data again support that FAK activation is an upstream event of N-WASP Y256 phosphorylation (Fig. 3), which is required for TGF-β1–induced changes in cytoskeletal dynamics and paracellular permeability (Fig. 2). Our data are consistent with previous studies that demonstrate that FAK and its signaling partners promote systemic and lung vascular permeability (19–21, 53–62); however, our study, to our knowledge, reports the novel finding that the phosphorylation of N-WASP by FAK is a critical step of TGF-β1–induced cytoskeletal dynamics and paracellular permeability changes in microvascular endothelial cell monolayers.
IL-1β is another important inflammatory mediator in ALI (6, 49). We and others have previously shown that both IL-1β and TGF-β1 increase lung endothelial paracellular permeability via a RhoA-dependent mechanism (Fig. 5A) (11, 18). In addition, we have reported that IL-1β induces TGF-β1 activation via a RhoA-αvβ6–mediated pathway in alveolar epithelial cells and, thus, increase paracellular permeability across these cell monolayers in a TGF-β1–dependent manner (11). The present study supports the new mechanism that IL-1β–induced TGF-β1 activation is mediated through an N-WASP–dependent pathway in alveolar epithelial cells (Fig. 5B). Indeed, NWASP knockdown inhibited IL-1β–induced TGF-β1 activation and blocked IL-1β–induced increase in paracellular permeability in alveolar epithelial cell monolayers (Fig. 5B, D). This process requires the participation of integrins and newly formed actin stress fibers. αvβ6 Integrin is connected to the latency-associated peptide that contains the inactive TGF-β1 (63), and the contraction of the epithelial cells induced by the actin stress fibers may provide the necessary force to open the latency-associated peptide and expose active TGF-β1 (5, 63). Our data support a double effect of N-WASP knockdown on TGF-β1–dependent alveolar epithelial permeability and activation of TGF-β1 by IL-1β. Furthermore, patient with ALI with maximal fluid clearance have better survival than those with impaired fluid clearance (64). Thus, because N-WASP down-regulation inhibits IL-1β–dependent TGF-β1 activation, this effect may also prevent TGF-β1–mediated inhibition of alveolar fluid clearance secondary to a decrease in epithelial sodium channel ENaC (65) and cystic fibrosis transmembrane conductance regulator (66) activity at the cell apical membrane of epithelial cells. Our findings suggest that IL-1β–mediated TGF-β1 activation may contribute to a feed-forward loop to enhance the detrimental effects of TGF-β1 on ALI by enhancing the permeability derangements across the alveolar-capillary barrier during ALI.
Whereas our study focuses on the role of N-WASP in mediating TGF-β1–mediated ALI via activation of actin stress fiber formation, N-WASP likely plays a more integral role in controlling endothelial and alveolar epithelial barrier function. For example, a previous report demonstrated that plasminogen activator inhibitor 1 is critical for survival and eradication of P. aeruginosa (67). Furthermore, it was revealed that plasminogen activator inhibitor 1 controlled early development of ALI by disruption of the endothelial barrier but was also necessary for activation of the later innate immune response. This process was Toll-like receptor 4/p38/RhoA/NF-κB dependent. In addition, another study demonstrated that phosphodiesterase 4 inhibition was able to inhibit neutrophil migration through transepithelial barriers and prevented lipopolysaccharide-induced changes to the actin cytoskeleton (69). Although these studies do not directly implicate N-WASP in their results, they indicate that, via Rho A and changes in the actin cytoskeleton, a role for N-WASP may be found in more precise regulation of barrier integrity that allows influx of immune cells to fight infection and, at the same time, to prevent inappropriate barrier disruption or enhance barrier repair.
The limitations of this study include focusing on a single in vitro modeling system (TGF-β1, a critical mediator of ALI) and a single in vivo animal model of ALI (bleomycin-induced ALI in which TGF-β1 has a critical role). We will expand our study in the future by studying other critical mediators of ALI and by using additional animal models of ALI. Nonetheless, the findings clearly demonstrate the important role of N-WASP in TGF-β1–induced cytoskeletal dynamics and paracellular permeability in lung microvascular endothelial and alveolar epithelial cells and the lung edema development in bleomycin-challenged mice.
In summary, we demonstrate in this study that N-WASP plays an essential role in actin cytoskeleton dynamics and paracellular permeability changes induced by TGF-β1. TGF-β1 induces the phosphorylation of Y256 of N-WASP that is mediated by small Rho GTPases and FAK. Phosphorylation of Y256 of N-WASP mediates paracellular permeability and actin cytoskeleton dynamics in response to TGF-β1 challenge. N-WASP also plays an essential role in TGF-β1 activation in IL-1β–treated alveolar epithelial cells. N-WASP down-regulation increases survival and protects mice against bleomycin-induced lung edema, an effect mediated by TGF-β1. Taken together, our data indicate that N-WASP plays an important role in ALI in a bleomycin model of ALI by promoting the development of pulmonary edema via the regulation of actin cytoskeleton dynamics.
Acknowledgments
This work was supported by U.S. National Institutes of Health, National Heart, Lung, and Blood Institute Grant R01-HL085324, the Flight Attendant Medical Research Institute (to Q.D.), and by a Mentored Research Training Grant from the Foundation for Anesthesia Education and Research (to B.M.W.).
Glossary
- ALI
acute lung injury
- Arp
actin-related protein
- FAK
focal adhesion kinase
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GBD
GTPase-binding domain
- GFP
green fluorescent protein
- N-WASP
neuronal Wiskott–Aldrich syndrome protein
- pfu
plaque-forming units
- RMVEC
rat lung microvascular endothelial cell
- shRNA
short hairpin RNA
- VCA
verprolin homology, central, and acidic regions
REFERENCES
- 1.Ware L. B., Matthay M. A. (2000) The acute respiratory distress syndrome. N. Engl. J. Med. 342, 1334–1349 [DOI] [PubMed] [Google Scholar]
- 2.Rubenfeld G. D., Caldwell E., Peabody E., Weaver J., Martin D. P., Neff M., Stern E. J., Hudson L. D. (2005) Incidence and outcomes of acute lung injury. N. Engl. J. Med. 353, 1685–1693 [DOI] [PubMed] [Google Scholar]
- 3.Wagener B. M., Roux J., Carles M., Pittet J. F. (2015) Synergistic inhibition of β2-adrenergic receptor-mediated alveolar epithelial fluid transport by interleukin-8 and transforming growth factor-β. Anesthesiology 122, 1084–1092 [DOI] [PubMed] [Google Scholar]
- 4.Lewis J. F., Jobe A. H. (1993) Surfactant and the adult respiratory distress syndrome. Am. Rev. Respir. Dis. 147, 218–233 [DOI] [PubMed] [Google Scholar]
- 5.Pittet J. F., Koh H., Fang X., Iles K., Christiaans S., Anjun N., Wagener B. M., Park D. W., Zmijewski J. W., Matthay M. A., Roux J. (2013) HMGB1 accelerates alveolar epithelial repair via an IL-1β- and αvβ6 integrin-dependent activation of TGF-β1. PLoS One 8, e63907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pugin J., Ricou B., Steinberg K. P., Suter P. M., Martin T. R. (1996) Proinflammatory activity in bronchoalveolar lavage fluids from patients with ARDS, a prominent role for interleukin-1. Am. J. Respir. Crit. Care Med. 153, 1850–1856 [DOI] [PubMed] [Google Scholar]
- 7.Pittet J. F., Griffiths M. J., Geiser T., Kaminski N., Dalton S. L., Huang X., Brown L. A., Gotwals P. J., Koteliansky V. E., Matthay M. A., Sheppard D. (2001) TGF-beta is a critical mediator of acute lung injury. J. Clin. Invest. 107, 1537–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pugin J., Verghese G., Widmer M. C., Matthay M. A. (1999) The alveolar space is the site of intense inflammatory and profibrotic reactions in the early phase of acute respiratory distress syndrome. Crit. Care Med. 27, 304–312 [DOI] [PubMed] [Google Scholar]
- 9.Olman M. A., White K. E., Ware L. B., Simmons W. L., Benveniste E. N., Zhu S., Pugin J., Matthay M. A. (2004) Pulmonary edema fluid from patients with early lung injury stimulates fibroblast proliferation through IL-1 beta-induced IL-6 expression. J. Immunol. 172, 2668–2677 [DOI] [PubMed] [Google Scholar]
- 10.Kaminski N., Allard J. D., Pittet J. F., Zuo F., Griffiths M. J., Morris D., Huang X., Sheppard D., Heller R. A. (2000) Global analysis of gene expression in pulmonary fibrosis reveals distinct programs regulating lung inflammation and fibrosis. Proc. Natl. Acad. Sci. USA 97, 1778–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ganter M. T., Roux J., Miyazawa B., Howard M., Frank J. A., Su G., Sheppard D., Violette S. M., Weinreb P. H., Horan G. S., Matthay M. A., Pittet J. F. (2008) Interleukin-1beta causes acute lung injury via alphavbeta5 and alphavbeta6 integrin-dependent mechanisms. Circ. Res. 102, 804–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fahy R. J., Lichtenberger F., McKeegan C. B., Nuovo G. J., Marsh C. B., Wewers M. D. (2003) The acute respiratory distress syndrome: a role for transforming growth factor-beta 1. Am. J. Respir. Cell Mol. Biol. 28, 499–503 [DOI] [PubMed] [Google Scholar]
- 13.Pittet J. F., Mackersie R. C., Martin T. R., Matthay M. A. (1997) Biological markers of acute lung injury: prognostic and pathogenetic significance. Am. J. Respir. Crit. Care Med. 155, 1187–1205 [DOI] [PubMed] [Google Scholar]
- 14.Chesnutt A. N., Matthay M. A., Tibayan F. A., Clark J. G. (1997) Early detection of type III procollagen peptide in acute lung injury. Pathogenetic and prognostic significance. Am. J. Respir. Crit. Care Med. 156, 840–845 [DOI] [PubMed] [Google Scholar]
- 15.Sime P. J., Xing Z., Graham F. L., Csaky K. G., Gauldie J. (1997) Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J. Clin. Invest. 100, 768–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ganter M. T., Roux J., Su G., Lynch S. V., Deutschman C. S., Weiss Y. G., Christiaans S. C., Myazawa B., Kipnis E., Wiener-Kronish J. P., Howard M., Pittet J. F. (2009) Role of small GTPases and alphavbeta5 integrin in Pseudomonas aeruginosa-induced increase in lung endothelial permeability. Am. J. Respir. Cell Mol. Biol. 40, 108–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duluc L., Wojciak-Stothard B. (2014) Rho GTPases in the regulation of pulmonary vascular barrier function. Cell Tissue Res. 355, 675–685 [DOI] [PubMed] [Google Scholar]
- 18.Lu Q., Harrington E. O., Jackson H., Morin N., Shannon C., Rounds S. (2006) Transforming growth factor-beta1-induced endothelial barrier dysfunction involves Smad2-dependent p38 activation and subsequent RhoA activation. J. Appl. Physiol. 101, 375–384 [DOI] [PubMed] [Google Scholar]
- 19.Chen X. L., Nam J. O., Jean C., Lawson C., Walsh C. T., Goka E., Lim S. T., Tomar A., Tancioni I., Uryu S., Guan J. L., Acevedo L. M., Weis S. M., Cheresh D. A., Schlaepfer D. D. (2012) VEGF-induced vascular permeability is mediated by FAK. Dev. Cell 22, 146–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weis S., Shintani S., Weber A., Kirchmair R., Wood M., Cravens A., McSharry H., Iwakura A., Yoon Y. S., Himes N., Burstein D., Doukas J., Soll R., Losordo D., Cheresh D. (2004) Src blockade stabilizes a Flk/cadherin complex, reducing edema and tissue injury following myocardial infarction. J. Clin. Invest. 113, 885–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birukova A. A., Cokic I., Moldobaeva N., Birukov K. G. (2009) Paxillin is involved in the differential regulation of endothelial barrier by HGF and VEGF. Am. J. Respir. Cell Mol. Biol. 40, 99–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rotty J. D., Wu C., Bear J. E. (2013) New insights into the regulation and cellular functions of the ARP2/3 complex. Nat. Rev. Mol. Cell Biol. 14, 7–12 [DOI] [PubMed] [Google Scholar]
- 23.Rohatgi R., Ma L., Miki H., Lopez M., Kirchhausen T., Takenawa T., Kirschner M. W. (1999) The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell 97, 221–231 [DOI] [PubMed] [Google Scholar]
- 24.Pollard T. D., Borisy G. G. (2003) Cellular motility driven by assembly and disassembly of actin filaments. Cell 112, 453–465 [DOI] [PubMed] [Google Scholar]
- 25.Dominguez R. (2009) Actin filament nucleation and elongation factors--structure-function relationships. Crit. Rev. Biochem. Mol. Biol. 44, 351–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takenawa T., Miki H. (2001) WASP and WAVE family proteins: key molecules for rapid rearrangement of cortical actin filaments and cell movement. J. Cell Sci. 114, 1801–1809 [DOI] [PubMed] [Google Scholar]
- 27.Sturge J., Hamelin J., Jones G. E. (2002) N-WASP activation by a beta1-integrin-dependent mechanism supports PI3K-independent chemotaxis stimulated by urokinase-type plasminogen activator. J. Cell Sci. 115, 699–711 [DOI] [PubMed] [Google Scholar]
- 28.Snapper S. B., Meelu P., Nguyen D., Stockton B. M., Bozza P., Alt F. W., Rosen F. S., von Andrian U. H., Klein C. (2005) WASP deficiency leads to global defects of directed leukocyte migration in vitro and in vivo. J. Leukoc. Biol. 77, 993–998 [DOI] [PubMed] [Google Scholar]
- 29.Suetsugu S., Hattori M., Miki H., Tezuka T., Yamamoto T., Mikoshiba K., Takenawa T. (2002) Sustained activation of N-WASP through phosphorylation is essential for neurite extension. Dev. Cell 3, 645–658 [DOI] [PubMed] [Google Scholar]
- 30.Takenawa T., Suetsugu S. (2007) The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat. Rev. Mol. Cell Biol. 8, 37–48 [DOI] [PubMed] [Google Scholar]
- 31.Jian M. Y., Alexeyev M. F., Wolkowicz P. E., Zmijewski J. W., Creighton J. R. (2013) Metformin-stimulated AMPK-α1 promotes microvascular repair in acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 305, L844–L855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Creighton J., Jian M., Sayner S., Alexeyev M., Insel P. A. (2011) Adenosine monophosphate-activated kinase alpha1 promotes endothelial barrier repair. FASEB J. 25, 3356–3365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cai G. Q., Chou C. F., Hu M., Zheng A., Reichardt L. F., Guan J. L., Fang H., Luckhardt T. R., Zhou Y., Thannickal V. J., Ding Q. (2012) Neuronal Wiskott-Aldrich syndrome protein (N-WASP) is critical for formation of α-smooth muscle actin filaments during myofibroblast differentiation. Am. J. Physiol. Lung Cell. Mol. Physiol. 303, L692–L702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding Q., Stewart J. Jr., Olman M. A., Klobe M. R., Gladson C. L. (2003) The pattern of enhancement of Src kinase activity on platelet-derived growth factor stimulation of glioblastoma cells is affected by the integrin engaged. J. Biol. Chem. 278, 39882–39891 [DOI] [PubMed] [Google Scholar]
- 35.Che P., Yang Y., Han X., Hu M., Sellers J. C., Londono-Joshi A. I., Cai G. Q., Buchsbaum D. J., Christein J. D., Tang Q., Chen D., Li Q., Grizzle W. E., Lu Y. Y., Ding Q. (2015) S100A4 promotes pancreatic cancer progression through a dual signaling pathway mediated by Src and focal adhesion kinase. Sci. Rep. 5, 8453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schlaepfer D. D., Jones K. C., Hunter T. (1998) Multiple Grb2-mediated integrin-stimulated signaling pathways to ERK2/mitogen-activated protein kinase: summation of both c-Src- and focal adhesion kinase-initiated tyrosine phosphorylation events. Mol. Cell. Biol. 18, 2571–2585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cai G. Q., Zheng A., Tang Q., White E. S., Chou C. F., Gladson C. L., Olman M. A., Ding Q. (2010) Downregulation of FAK-related non-kinase mediates the migratory phenotype of human fibrotic lung fibroblasts. Exp. Cell Res. 316, 1600–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ding Q., Gladson C. L., Wu H., Hayasaka H., Olman M. A. (2008) Focal adhesion kinase (FAK)-related non-kinase inhibits myofibroblast differentiation through differential MAPK activation in a FAK-dependent manner. J. Biol. Chem. 283, 26839–26849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ding Q., Stewart J. Jr., Prince C. W., Chang P. L., Trikha M., Han X., Grammer J. R., Gladson C. L. (2002) Promotion of malignant astrocytoma cell migration by osteopontin expressed in the normal brain: differences in integrin signaling during cell adhesion to osteopontin versus vitronectin. Cancer Res. 62, 5336–5343 [DOI] [PubMed] [Google Scholar]
- 40.Bechara R. I., Brown L. A., Roman J., Joshi P. C., Guidot D. M. (2004) Transforming growth factor beta1 expression and activation is increased in the alcoholic rat lung. Am. J. Respir. Crit. Care Med. 170, 188–194 [DOI] [PubMed] [Google Scholar]
- 41.Ding Q., Cai G. Q., Hu M., Yang Y., Zheng A., Tang Q., Gladson C. L., Hayasaka H., Wu H., You Z., Southern B. D., Grove L. M., Rahaman S. O., Fang H., Olman M. A. (2013) FAK-related nonkinase is a multifunctional negative regulator of pulmonary fibrosis. Am. J. Pathol. 182, 1572–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaner R. J., Ladetto J. V., Singh R., Fukuda N., Matthay M. A., Crystal R. G. (2000) Lung overexpression of the vascular endothelial growth factor gene induces pulmonary edema. Am. J. Respir. Cell Mol. Biol. 22, 657–664 [DOI] [PubMed] [Google Scholar]
- 43.Goldberg P. L., MacNaughton D. E., Clements R. T., Minnear F. L., Vincent P. A. (2002) p38 MAPK activation by TGF-beta1 increases MLC phosphorylation and endothelial monolayer permeability. Am. J. Physiol. Lung Cell. Mol. Physiol. 282, L146–L154 [DOI] [PubMed] [Google Scholar]
- 44.Misra A., Lim R. P., Wu Z., Thanabalu T. (2007) N-WASP plays a critical role in fibroblast adhesion and spreading. Biochem. Biophys. Res. Commun. 364, 908–912 [DOI] [PubMed] [Google Scholar]
- 45.Peterson J. R., Bickford L. C., Morgan D., Kim A. S., Ouerfelli O., Kirschner M. W., Rosen M. K. (2004) Chemical inhibition of N-WASP by stabilization of a native autoinhibited conformation. Nat. Struct. Mol. Biol. 11, 747–755 [DOI] [PubMed] [Google Scholar]
- 46.Wu X., Suetsugu S., Cooper L. A., Takenawa T., Guan J. L. (2004) Focal adhesion kinase regulation of N-WASP subcellular localization and function. J. Biol. Chem. 279, 9565–9576 [DOI] [PubMed] [Google Scholar]
- 47.Carles M., Lafargue M., Goolaerts A., Roux J., Song Y., Howard M., Weston D., Swindle J. T., Hedgpeth J., Burel-Vandenbos F., Pittet J. F. (2010) Critical role of the small GTPase RhoA in the development of pulmonary edema induced by Pseudomonas aeruginosa in mice. Anesthesiology 113, 1134–1143 [DOI] [PubMed] [Google Scholar]
- 48.Greenlee M. M., Mitzelfelt J. D., Yu L., Yue Q., Duke B. J., Harrell C. S., Neigh G. N., Eaton D. C. (2013) Estradiol activates epithelial sodium channels in rat alveolar cells through the G protein-coupled estrogen receptor. Am. J. Physiol. Lung Cell. Mol. Physiol. 305, L878–L889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cohen T. S., Prince A. S. (2013) Activation of inflammasome signaling mediates pathology of acute P. aeruginosa pneumonia. J. Clin. Invest. 123, 1630–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu R., Abreu-Blanco M. T., Barry K. C., Linardopoulou E. V., Osborn G. E., Parkhurst S. M. (2009) Wash functions downstream of Rho and links linear and branched actin nucleation factors. Development 136, 2849–2860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Welch M. D. (1999) The world according to Arp: regulation of actin nucleation by the Arp2/3 complex. Trends Cell Biol. 9, 423–427 [DOI] [PubMed] [Google Scholar]
- 52.Parsons J. T. (2003) Focal adhesion kinase: the first ten years. J. Cell Sci. 116, 1409–1416 [DOI] [PubMed] [Google Scholar]
- 53.Jean C., Chen X. L., Nam J. O., Tancioni I., Uryu S., Lawson C., Ward K. K., Walsh C. T., Miller N. L., Ghassemian M., Turowski P., Dejana E., Weis S., Cheresh D. A., Schlaepfer D. D. (2014) Inhibition of endothelial FAK activity prevents tumor metastasis by enhancing barrier function. J. Cell Biol. 204, 247–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu M. H., Guo M., Yuan S. Y., Granger H. J. (2003) Focal adhesion kinase mediates porcine venular hyperpermeability elicited by vascular endothelial growth factor. J. Physiol. 552, 691–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Infusino G. A., Jacobson J. R. (2012) Endothelial FAK as a therapeutic target in disease. Microvasc. Res. 83, 89–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Criscuoli M. L., Nguyen M., Eliceiri B. P. (2005) Tumor metastasis but not tumor growth is dependent on Src-mediated vascular permeability. Blood 105, 1508–1514 [DOI] [PubMed] [Google Scholar]
- 57.Eliceiri B. P., Paul R., Schwartzberg P. L., Hood J. D., Leng J., Cheresh D. A. (1999) Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol. Cell 4, 915–924 [DOI] [PubMed] [Google Scholar]
- 58.Bhattacharya S., Sen N., Yiming M. T., Patel R., Parthasarathi K., Quadri S., Issekutz A. C., Bhattacharya J. (2003) High tidal volume ventilation induces proinflammatory signaling in rat lung endothelium. Am. J. Respir. Cell Mol. Biol. 28, 218–224 [DOI] [PubMed] [Google Scholar]
- 59.Turner C. E. (1998) Paxillin. Int. J. Biochem. Cell Biol. 30, 955–959 [DOI] [PubMed] [Google Scholar]
- 60.Bai Y., Xu G., Xu M., Li Q., Qin X. (2014) Inhibition of Src phosphorylation reduces damage to the blood-brain barrier following transient focal cerebral ischemia in rats. Int. J. Mol. Med. 34, 1473–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Usatyuk P. V., Natarajan V. (2005) Regulation of reactive oxygen species-induced endothelial cell-cell and cell-matrix contacts by focal adhesion kinase and adherens junction proteins. Am. J. Physiol. Lung Cell. Mol. Physiol. 289, L999–L1010 [DOI] [PubMed] [Google Scholar]
- 62.Infante J. R., Camidge D. R., Mileshkin L. R., Chen E. X., Hicks R. J., Rischin D., Fingert H., Pierce K. J., Xu H., Roberts W. G., Shreeve S. M., Burris H. A., Siu L. L. (2012) Safety, pharmacokinetic, and pharmacodynamic phase I dose-escalation trial of PF-00562271, an inhibitor of focal adhesion kinase, in advanced solid tumors. J. Clin. Oncol. 30, 1527–1533 [DOI] [PubMed] [Google Scholar]
- 63.Jenkins R. G., Su X., Su G., Scotton C. J., Camerer E., Laurent G. J., Davis G. E., Chambers R. C., Matthay M. A., Sheppard D. (2006) Ligation of protease-activated receptor 1 enhances alpha(v)beta6 integrin-dependent TGF-beta activation and promotes acute lung injury. J. Clin. Invest. 116, 1606–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ware L. B., Matthay M. A. (2001) Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 163, 1376–1383 [DOI] [PubMed] [Google Scholar]
- 65.Frank J., Roux J., Kawakatsu H., Su G., Dagenais A., Berthiaume Y., Howard M., Canessa C. M., Fang X., Sheppard D., Matthay M. A., Pittet J. F. (2003) Transforming growth factor-beta1 decreases expression of the epithelial sodium channel alphaENaC and alveolar epithelial vectorial sodium and fluid transport via an ERK1/2-dependent mechanism. J. Biol. Chem. 278, 43939–43950 [DOI] [PubMed] [Google Scholar]
- 66.Roux J., Carles M., Koh H., Goolaerts A., Ganter M. T., Chesebro B. B., Howard M., Houseman B. T., Finkbeiner W., Shokat K. M., Paquet A. C., Matthay M. A., Pittet J. F. (2010) Transforming growth factor beta1 inhibits cystic fibrosis transmembrane conductance regulator-dependent cAMP-stimulated alveolar epithelial fluid transport via a phosphatidylinositol 3-kinase-dependent mechanism. J. Biol. Chem. 285, 4278–4290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goolaerts A., Lafargue M., Song Y., Miyazawa B., Arjomandi M., Carlès M., Roux J., Howard M., Parks D. A., Iles K. E., Pittet J. F. (2011) PAI-1 is an essential component of the pulmonary host response during Pseudomonas aeruginosa pneumonia in mice. Thorax 66, 788–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Konrad F. M., Bury A., Schick M. A., Ngamsri K. C., Reutershan J. (2015) The unrecognized effects of phosphodiesterase 4 on epithelial cells in pulmonary inflammation. PLoS One 10, e0121725 [DOI] [PMC free article] [PubMed] [Google Scholar]