Abstract
Obesity and nonalcoholic fatty liver disease (NAFLD) can increase susceptibility to hepatotoxicity induced by some xenobiotics including drugs, but the involved mechanisms are poorly understood. For acetaminophen (APAP), a role of hepatic cytochrome P450 2E1 (CYP2E1) is suspected since the activity of this enzyme is consistently enhanced during NAFLD. The first aim of our study was to set up a cellular model of NAFLD characterized not only by triglyceride accumulation but also by higher CYP2E1 activity. To this end, human HepaRG cells were incubated for one week with stearic acid or oleic acid, in the presence of different concentrations of insulin. Although cellular triglycerides and the expression of lipid-responsive genes were similar with both fatty acids, CYP2E1 activity was significantly increased only by stearic acid. CYP2E1 activity was reduced by insulin and this effect was reproduced in cultured primary human hepatocytes. Next, APAP cytotoxicity was assessed in HepaRG cells with or without lipid accretion and CYP2E1 induction. Experiments with a large range of APAP concentrations showed that the loss of ATP and glutathione was almost always greater in the presence of stearic acid. In cells pretreated with the CYP2E1 inhibitor chlormethiazole, recovery of ATP was significantly higher in the presence of stearate with low (2.5 mM) or high (20 mM) concentrations of APAP. Levels of APAP-glucuronide were significantly enhanced by insulin. Hence, HepaRG cells can be used as a valuable model of NAFLD to unveil important metabolic and hormonal factors which can increase susceptibility to drug-induced hepatotoxicity.
Keywords: NAFLD, obesity, liver, hepatotoxicity, acetaminophen, CYP2E1
Introduction
Liver injury can be induced by numerous drugs, herbals and industrial toxicants (Biour et al., 2004; Seeff et al., 2015; Wahlang et al., 2013). In the most severe cases, xenobiotic-induced liver injury can require hospitalization and eventually lead to death of the patient (Björnsson, 2009). Among the different predisposing factors increasing the risk of liver injury, there is growing evidence that nonalcoholic fatty liver disease (NAFLD) could play a significant role (Fromenty, 2013; Robin et al., 2005a; Tarantino et al., 2007). NAFLD is often associated with obesity and type 2 diabetes and encompasses a large spectrum of liver lesions including fatty liver, nonalcoholic steatohepatitis (NASH) and cirrhosis (Michelotti et al., 2013).
Greater hepatotoxicity in the context of obesity and NAFLD has been documented in rodents and humans with some drugs, including acetaminophen (APAP), halothane and methotrexate, as well as other xenobiotics such as ethanol and carbon tetrachloride (Donthamsetty et al., 2007; Fromenty, 2013; Michaut et al., 2014; Robin et al., 2005a; Tarantino et al., 2007). However, the mechanisms involved in this higher susceptibility are poorly understood, although different hypotheses have been put forward (Fromenty, 2013). Furthermore, these mechanisms could be complex and different from one compound to another (Carmiel-Haggai et al., 2003; Fromenty, 2013; Robin et al., 2005a). Notably, obesity and NAFLD in rodents and humans are associated with different alterations in the activity of hepatic enzymes involved in drug metabolism including cytochromes P450 (CYPs), UDP-glucuronosyltransferases and transporters (Brill et al., 2012; Canet et al., 2015). More specifically, these dysmetabolic disorders are associated with higher CYP2E1 activity, lower CYP3A4 activity and increased capacity of glucuronide conjugation for different drugs such as APAP and lorazepam (Aubert et al., 2011; Brill et al., 2012; Chalasani et al., 2003; Emery et al., 2003; Kolwankar et al., 2007; Woolsey et al., 2015).
Deciphering the mechanisms whereby some drugs and environmental toxins are more hepatotoxic in the context of obesity and NAFLD requires appropriate experimental models. Although obese mice and rats can be useful (Aubert et al., 2011; Carmiel-Haggai et al., 2003; Massart et al., 2012; Robin et al., 2005a), there are numerous differences between rodents and humans regarding hepatic drug metabolism (Chu et al., 2013; Martignoni et al., 2006). In addition, investigations in animals are cumbersome and ethically problematic. Thus, a relevant human cellular model could be helpful in order to study hepatotoxicity in NAFLD.
In the past few years, the metabolically competent human hepatoma HepaRG cell line has been shown to be a valuable model to study the mechanism of hepatotoxicity induced by drugs and toxicants (Anthérieu et al., 2011; McGill et al., 2011; Savary et al., 2014; Sharanek et al., 2014; Tobwala et al., 2015). Indeed, HepaRG cells express most of the enzymes and transcription factors involved in xenobiotic biotransformation and transport (Andersson et al., 2012; Aninat et al., 2006; Anthérieu et al., 2012). In addition, HepaRG cells have been successfully used to study the effects of nutrients, hormones and drugs on the expression of various enzymes involved in carbohydrate and lipid metabolism (Anthérieu et al., 2011; Madec et al., 2011; Nagasawa et al., 2007; Samanez et al., 2012).
By using HepaRG cells, the aim of the present study was two-fold. First, we sought to set up a cell model of NAFLD, in particular regarding the alterations of CYP2E1 and CYP3A4 activity that are observed in this hepatic disease. To this end, differentiated HepaRG cells were treated for one week with stearic acid (C18:0) or oleic acid (C18:1) in the presence of different concentrations of insulin. The insulin effect was investigated because previous studies suggested that this hormone could modulate CYP2E1 expression and activity (Ioannides et al., 1998; Moncion et al., 2002; Woodcroft and Novak, 1999). Second, we used this cell model of NAFLD in order to determine the cytotoxic effects of the painkiller APAP. Indeed, some data in rodents and humans suggested that NALFD could be associated with more severe APAP-induced hepatotoxicity, especially after an overdose (Aubert et al., 2012; Kon et al., 2010; Michaut et al., 2014; Myers and Shaheen, 2008; NGuyen et al., 2008). One of these rodent studies proposed that higher APAP hepatotoxicity in NAFLD could be attributed to greater activity of CYP2E1 (Aubert et al., 2012), the primary enzyme responsible for the biotransformation of APAP to the highly toxic metabolite N-acetyl-p-benzoquinone imine (NAPQI) (Hinson et al., 2010; McGill and Jaeschke, 2013). Overall, our results indicate that HepaRG cells loaded with stearic acid can be used as a valuable model to study the mechanisms whereby APAP is more toxic in the context of NAFLD.
Materials and methods
Chemicals
5-Aminoimidazole-4-carboxyamide ribonucleoside (AICAR), APAP, APAP-β-D-glucuronide, APAP-sulfate, testosterone, 6β-hydroxytestosterone, chlormethiazole (CMZ), chlorzoxazone (CZX), metformin, dimethyl sulfoxide (DMSO), oleic acid, stearic acid, insulin and oil Red O were purchased from Sigma Aldrich (Saint-Quentin-Fallavier, France). William’s E medium was obtained from Eurobio laboratories (Les Ulis, France). Fetal Bovine Serum (FBS) was supplied by Lonza (Levallois-Perret, France). Glutamine, penicillin and streptomycin were obtained from Invitrogen (Cergy Pontoise, France). Hydrocortisone hemisuccinate was purchased from Upjohn Pharmacia (Guancourt, France). Protease and phosphatase inhibitors were purchased from Roche Diagnostics (Indianapolis, IN).
Cell cultures and treatments
Native HepaRG cells were cultured as previously described (Aninat et al., 2006). Briefly, HepaRG cells were seeded at a density of 2.6 × 104 cells/cm2 and were first incubated in a William’s E medium supplemented with 10% FBS, 100 units/ml penicillin, 100 μg/ml streptomycin, 2 mM glutamine, 5 μg/ml insulin and 50 μM hydrocortisone hemisuccinate. After 2 weeks, cell differentiation was induced by the same culture medium supplemented with 2% DMSO (differentiation medium) for 2 additional weeks. Cells were subsequently treated for 1 week with different concentrations of insulin (0, 0.01 or 5 μg/ml), stearic acid (0 or 100 µM), or oleic acid (0 or 100 µM). In some experiments, lower or higher concentrations of fatty acids were used. For the APAP experiments, cells were incubated with different concentrations of this drug (0 to 20 mM) during the last 6, 24 or 48 h of the 7-day treatment, so that untreated and APAP-treated cells were investigated at the same time point (i.e. at day 7). Stearic acid, oleic acid and APAP were dissolved in DMSO whose final concentration in cultures was always set at 2%. For the investigations performed with the prototypical CYP2E1 inhibitor CMZ, cells were incubated 1 week with 150 µM of CMZ after the end of differentiation. Whatever the treatments, the culture medium was renewed every 2 or 3 days during the 7-day experiments. For the experiments carried out with the AMP-activated protein kinase (AMPK) activators AICAR and metformin, cells were first preincubated for 6 h with or without 500 µM of each activator. Cells were then treated for 24 h with 20 mM of APAP, with or without each AMPK activator (500 µM). HepaRG cells were used at passages 11 to 15.
Primary human hepatocytes (PHH) from adult donors were prepared at Biopredic International (Saint-Grégoire, France) by perfusion of liver fragments provided by the Centre de Ressources Biologiques (CRB) Santé de Rennes. Information about the liver donors is provided in the Supplementary Table 1. Hepatocytes were then immediately seeded at a density of 11 × 104 cells/cm2 in a William’s E medium supplemented with 10% FBS, 100 units/ml penicillin, 100 μg/ml streptomycin, 2 mM glutamine, 5 μg/ml insulin and 5 μM hydrocortisone hemisuccinate. The medium was discarded 12 h after seeding and cells were then maintained in the same differentiation medium used for HepaRG cells, which was renewed every other day. Two days after seeding, PHH were then treated or not with oleic acid, stearic acid and insulin for one week.
Oil red O staining, cellular triglycerides and apolipoprotein B in culture medium
For staining of neutral lipids, live cells were washed with phosphate-buffered saline, incubated for 45 min at room temperature with an oil red O-saturated solution, washed again, and observed under a phase-contrast microscope. Triglyceride quantification (nmol/mg of proteins) was measured with a colorimetric kit purchased from Biovision (Milpitas, CA), using the manufacturer’s recommendations. Apolipoprotein B (apoB) levels (µg/ml) in culture medium were determined with the human ELISA kit purchased from Abcam (Cambridge, UK), according to the manufacturer’s instructions.
Determination of CYP2E1 and CYP3A4 activities
In order to measure CYP2E1 and CYP3A4 activities, HepaRG cells or PHH were incubated for 6 and 2 h in phenol red-free and DMSO-free William’s E medium containing 300 μM CZX or 200 μM testosterone, respectively. At the end of the incubation, aliquots of culture media were collected and stored at −80°C until analysis. 6-Hydroxychlorzoxazone was then quantified by high-performance liquid chromatography (HPLC)-tandem mass spectrometry (Xenoblis, Rennes, France), whereas 6β-hydroxytestosterone was measured by HPLC analysis, as previously described (Aninat et al., 2006).
Cellular ATP and GSH
Cellular ATP was measured with the CellTiter-Glo® assay purchased from Promega (Charbonnieres, France), using the manufacturer’s recommendations. Briefly, HepaRG cells were incubated with the reagent 10 min at 37°C and the luminescent signal was quantified using a POLARstar Omega microplate reader (BMG Labtech, Ortenberg, Germany). Results were expressed in comparison to untreated cells. Total glutathione (GSH) was quantified by fluorescence with the Glutathione assay kit purchased from Cayman Chemical (Ann Arbor, USA), using the manufacturer’s recommendations.
Assessment of APAP metabolites and APAP-protein adducts
The quantification of APAP-β-D-glucuronide and APAP-sulfate in the cell supernatants was performed on a Thermo Scientific Q Exactive mass spectrometer (San Jose, CA). An HESI-II ion source was used for the electrospray ionization of the target compounds. Chromatographic separation of the analytes was performed with an Accela pump (Thermo Scientific) equipped with a Thermo Fisher Hypersil Gold C18 column (3.0 mm, 2.1, 100 mm) as previously described (Gicquel et al., 2013). Data were acquired in Targeted SIM (Single Ion Monitoring) mode. For the assessment of APAP-protein adducts in treated HepaRG cells, APAP-cysteine was measured by using HPLC separation and electrochemical detection, as previously described for cultured cells (McGill et al., 2013). APAP-protein adducts result from the covalent binding of NAPQI to different cellular proteins, in particular at the mitochondrial level (McGill and Jaeschke, 2013; McGill et al., 2013).
Isolation of RNA and real-time quantitative PCR analysis
Total RNA was extracted from ca. 106 HepaRG cells with the SV total RNA isolation system purchased from Promega (Charbonnièresles-Bains, France) and from ca. 600.000 PHH with RNeasy Micro kit purchased from Qiagen (Courtaboeuf, France). Each kit included a DNase treatment step. RNAs were reverse-transcribed into cDNA using the High-Capacity cDNA Archive kit purchased from Life technologies (Saint-Aubin, France). Real-time quantitative PCR (RT-qPCR) was then performed using the SYBR Green PCR Master Mix on an Applied Biosystems 7900HT Fast Real-Time PCR System (Applied Biosystem, Woolston, UK). Expression of the human TATA box binding protein (TBP) was used as reference, and the 2–ΔΔCt method was used to express the relative expression of each selected gene. The sequences of the primers used in this study are presented in the Supplementary Table 2. Amplification of specific transcripts was confirmed by melting curve profiles generated at the end of each run.
Western blot analysis
To assess the protein expression of CYP2E1 and CYP3A4 in HepaRG cells, proteins underwent SDS-polyacrylamide electrophoresis. Briefly, cells were lysed in a RIPA buffer (25 mM Tris-HCL pH 7.6, 150 mM NaCl, 1% NP40, 1% sodium deoxycholate and 0.1% sodium dodecyl sulfate) supplemented with protease and phosphatase inhibitors. Fifteen μg of proteins were then separated by electrophoresis on 4–12% gradient Bis-Tris gels (Invitrogen, Cergy-Pontoise, France), transferred to 0.2-µm nitrocellulose membranes (Bio-Rad, Hercules, CA), which were saturated in 2% BSA/TBS-T (0.2% tween in TBS) for 2 h at room temperature. Proteins were then immunoblotted with antibodies against CYP2E1 (Oxford Biomedical Research, Rochester Hills, MI), CYP3A4 (Oxford Biomedical Research), or heat-shock cognate 70 (HSC70) (Tebu-bio, Le Perray-en-Yvelines, France). Finally, blots were incubated with appropriate secondary antibodies, and protein bands were revealed by enhanced chemiluminescence in a Chemi-smart imager (Fisher Scientific, Illkirch, France). HSC70 was used to normalize protein loadings, and quantification was performed with the BIO-1D software.
Statistical analysis
All results are expressed as mean ± SEM (standard error of mean). Comparisons between multiple groups were performed with two-way analysis of variance (ANOVA). When ANOVA provided significant differences, individual means were compared with the post-hoc Bonferroni test. The Mann-Whitney test was used for the experiments carried out in cultured PHH.
Results
Effects of stearate, oleate and insulin on lipid content, CYP2E1 and CYP3A4
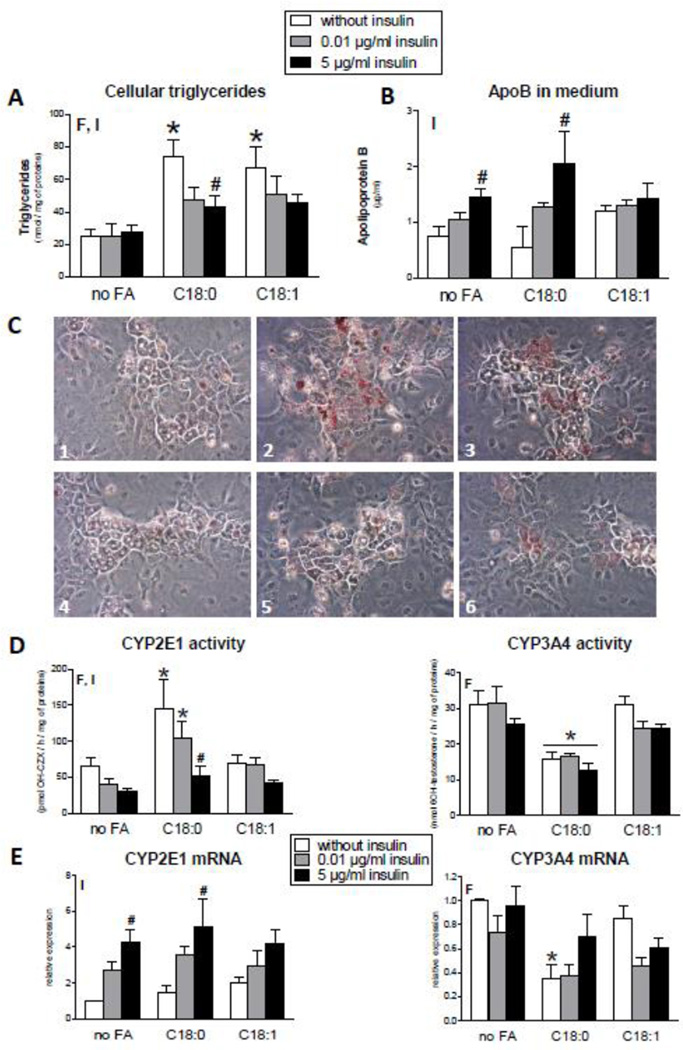
HepaRG cells were incubated without or with stearic acid, oleic acid and insulin for 1 week, as described in the Methods section. Because insulin was studied at three different concentrations (0, 0.01 and 5 µg/ml), nine different experimental conditions were initially studied. As expected, incubation of these cells with each fatty acid (100 µM) was associated with significantly higher cellular triglycerides (Fig. 1A). In addition, insulin significantly decreased triglycerides in cells loaded with each fatty acid (Fig. 1A) and concomitantly enhanced apoB levels in the culture medium, although this effect was less marked in the presence of oleic acid (Fig. 1B). Hence, lower accumulation of cellular triglycerides induced by insulin in steatotic HepaRG cells could be due, at least in part, to a greater secretion of VLDL triglycerides. This is in keeping with previous data in rat and human indicating that insulin favors hepatic triglyceride secretion in the long-term, contrary to its acute effect (Bartlett and Gibbons, 1988; Reaven et al., 1967; Zammit et al., 2001). Assessment of total neutral lipids (Fig. 1C) was in keeping with the measurement of cellular triglycerides (Fig. 1A).
Figure 1.
Effects of 1 week of treatment with 100 µM stearic acid (C18:0), 100 µM oleic acid (C18:1) and different concentrations of insulin on cellular triglycerides, neutral lipid deposition, apoB levels in culture medium and mRNA activity and expression of CYP2E1 and CYP3A4 in HepaRG cells. (A) Cellular triglycerides. Results are means ± SEM for 5 independent cultures, with data in duplicates for each culture. (B) ApoB levels in culture medium. Results are means ± SEM for 3 independent cultures. (C) Cellular lipids stained with oil red O. The pictures 1 to 6 (magnification × 200) are representative of the following conditions: 1) HepaRG cells incubated without insulin and fatty acids; 2) HepaRG cells incubated without insulin and with stearic acid; 3) HepaRG cells incubated without insulin and with oleic acid; 4) HepaRG cells incubated with 5 µg/ml insulin and without fatty acids; 5) HepaRG cells incubated with 5 µg/ml insulin and with stearic acid; 6) HepaRG cells incubated with 5 µg/ml insulin and with oleic acid. (D) CYP2E1 and CYP3A4 activity. Results are means ± SEM for 5 independent cultures, with data in duplicates for each culture. (E) mRNA levels of CYP2E1 and CYP3A4. Results are means ± SEM for 5 independent cultures, with data in duplicates for each culture. In panels showing bar graphs, letters F and I indicate a significant effect (P<0.05) of fatty acids and insulin, respectively. *Significantly different from HepaRG cells incubated without fatty acids and with the same condition of insulin treatment (P<0.05). #Significantly different from HepaRG cells incubated without insulin and with the same condition of fatty acid treatment (P<0.05).
Next, the activity and expression of CYP2E1 and CYP3A4 were studied in all the experimental conditions. CYP2E1 activity was increased by stearic acid but not by oleic acid (Fig. 1D). Moreover, insulin reduced CYP2E1 activity in a concentration-dependent manner in HepaRG cells (Fig. 1D) and this was associated with an increase of its mRNA (Fig. 1E). Insulin also augmented the protein levels of CYP2E1 in a concentration-dependent manner (Supplementary Fig. 1). In contrast, CYP3A4 activity was significantly reduced by stearic acid, without significant effect of insulin (Fig. 1D). The mRNA of CYP3A4 was decreased by stearic acid, especially in the absence of insulin (Fig. 1E). CYP3A4 protein was reduced by stearate but also by oleate in the absence of insulin (Supplementary Fig. 1). Notably, the activities of both CYP2E1 and CYP3A4 were not significantly changed after 1 week of incubation with 50 µM of each fatty acid, or after 48 h of incubation with 100 µM of these fatty acids (Supplementary Fig. 2).
Effects of stearate, oleate and insulin on the expression of genes responsive to lipids and insulin in HepaRG cells
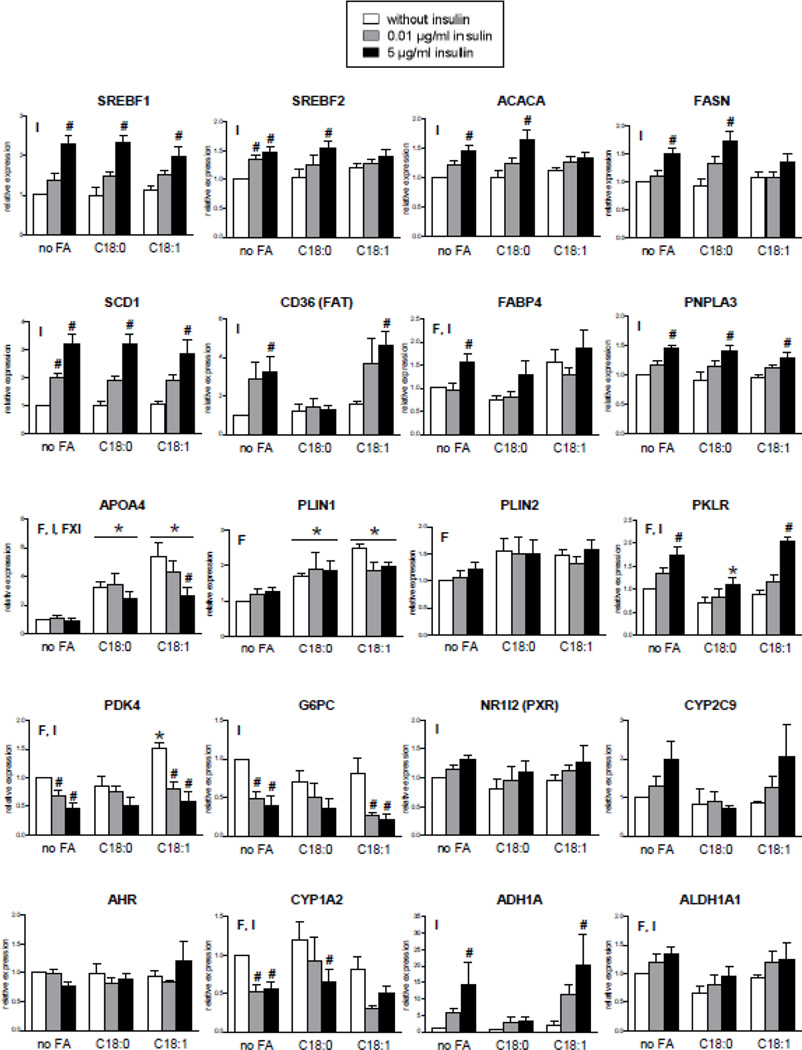
We also assessed in normal and steatotic HepaRG cells the mRNA expression of different genes known to be regulated by lipids, insulin or both factors as well as genes that are of potential interest in the context of NAFLD (Baker et al., 2010; Begriche et al., 2013; Lu et al., 2015; Miquilena-Colina et al., 2011; Postic and Girard, 2008; Sookoian et al., 2010; Xie et al., 2009). As expected, the expression of sterol regulatory element binding transcription factor 1 (SREBF1, also known as SREBP-1c), SREBF2, acetyl-CoA carboxylase alpha (ACACA), fatty acid synthase (FASN), fatty acid translocase (FAT/CD36), stearoyl-Coenzyme A desaturase 1 (SCD1) and glucose-6-phosphatase (G6PC) was regulated by insulin in a concentration-dependent manner, whereas each fatty acid had no effect (Fig. 2). Insulin also enhanced the expression of patatin-like phospholipase domain containing 3 (PNPLA3) (Fig. 2), in keeping with previous studies (Dubuquoy et al., 2011; Huang et al., 2010). Expression of pyruvate dehydrogenase kinase 4 (PDK4), liver pyruvate kinase (PKLR), fatty acid binding protein 4 (FABP4) and apolipoprotein A-IV (APOA4) was regulated by fatty acids and insulin, while that of perilipin 1 (PLIN1) and 2 (PLIN2, also known as ADFP) was regulated only by the fatty acids (Fig. 2). Whereas expression of aryl hydrocarbon receptor (AHR) was unchanged in the different tested conditions, CYP1A2 expression was negatively regulated by insulin (Fig. 2), in keeping with previous investigations (Barker et al., 1994; Martinez-Jiménez et al., 2006). CYP1A2 mRNA levels were also significantly modulated by fatty acids but this effect was less pronounced compared to insulin action and apparently opposite between stearate and oleate (Fig. 2). Previous investigations in obese individuals showed that CYP1A2 expression and activity were not significantly changed in simple steatosis, whereas these parameters were significantly reduced in NASH (Fisher et al., 2009; Lake et al., 2011). Expression of pregnane X receptor (PXR also referred to as NR1I2) was positively regulated by insulin, and this hormone tended to enhance CYP2C9 expression (Fig. 2). There was no effect of fatty acids on CYP2C9 mRNA levels, in keeping with the data reported by Fisher et al. (2009) for simple fatty liver. Finally, expression of alcohol dehydrogenase 1A (ADH1A) and aldehyde dehydrogenase 1A1 (ALDH1A1) was positively regulated by insulin (Fig. 2). This suggested that higher expression of these enzymes in NAFLD (Baker et al., 2010) could be related, at least in part, to hyperinsulinemia.
Figure 2.
Effects in HepaRG cells of 1 week of treatment with 100 µM stearic acid (C18:0), 100 µM oleic acid (C18:1) and different concentrations of insulin on the expression of 20 different genes. Results are means ± SEM for 5 independent cultures, with data in duplicates for each culture. Letters F and I indicate a significant effect (P<0.05) of fatty acids and insulin, respectively. FxI indicates a significant interaction between the effects of fatty acids and insulin. *Significantly different from HepaRG cells incubated without fatty acids and with the same condition of insulin treatment (P<0.05). #Significantly different from HepaRG cells incubated without insulin and with the same condition of fatty acid treatment (P<0.05).
Effects of stearate, oleate and insulin on CYP2E1 and CYP3A4 activities and gene expression in cultured PHH
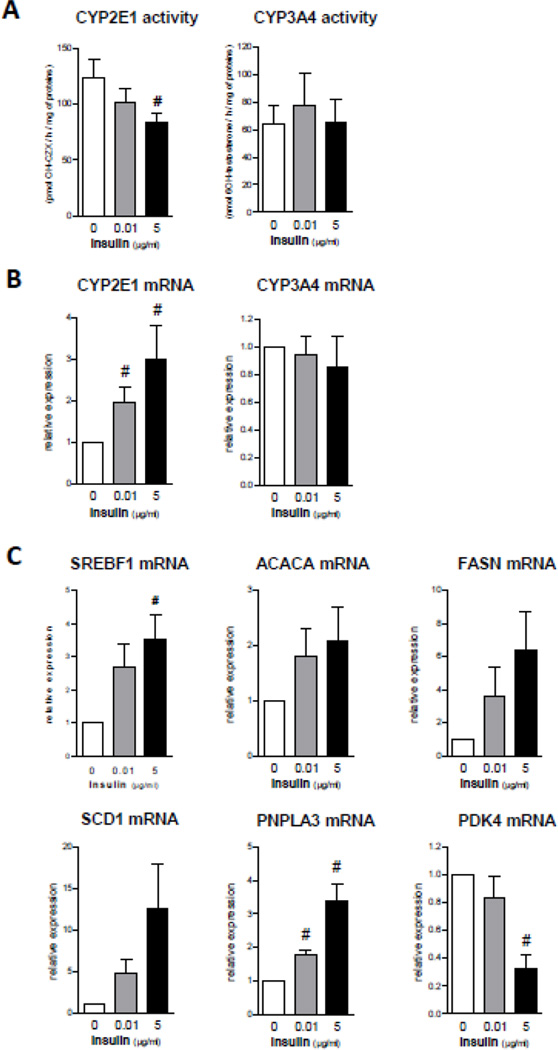
In another series of experiments, the effects of stearic acid, oleic acid and insulin were studied in PHH cultured for 1 week. In the absence of fatty acids, insulin (5 µg/ml) significantly decreased CYP2E1 activity with a concomitant increase in CYP2E1 mRNA levels (Fig. 3). Insulin also enhanced the protein levels of CYP2E1 in a concentration-dependent manner in cultured PHH of 3 different donors (Supplementary Fig. 3). In contrast, insulin did not change CYP3A4 mRNA expression and activity (Fig. 3). Importantly, insulin increased the mRNA expression of SREBF1, ACACA, FASN, SCD1 and PNPLA3 and reduced that of PDK4 (Fig. 3). Thus, when incubated without fatty acid, the effects induced by insulin were similar between cultured PHH and HepaRG cells. In contrast, treatment with 100 µM of stearic acid did not increase CYP2E1 activity (data not shown). However, the PHH used to assess CYP2E1 activity (Supplementary Table 1) presented varying degrees of steatosis before their incubation with stearic acid (Supplementary Fig. 4) and thus CYP2E1 activity might have already been augmented in these cells.
Figure 3.
Effects of 1 week of treatment with different concentrations of insulin in cultured PHH. (A) CYP2E1 and CYP3A4 activity. Results are means ± SEM for 5 and 4 independent cultures, respectively for the activity of CYP2E1 and CYP3A4. #Significantly different from PHH incubated without insulin (P<0.05). (B) mRNA levels of CYP2E1 and CYP3A4. Results are means ± SEM for 4 independent cultures. #Significantly different from PHH incubated without insulin (P<0.05). (C) mRNA expression of genes responsive to insulin. Results are means ± SEM for 4 independent cultures. #Significantly different from PHH incubated without insulin (P<0.05).
APAP-induced cytotoxicity and GSH depletion in normal and steatotic HepaRG cells
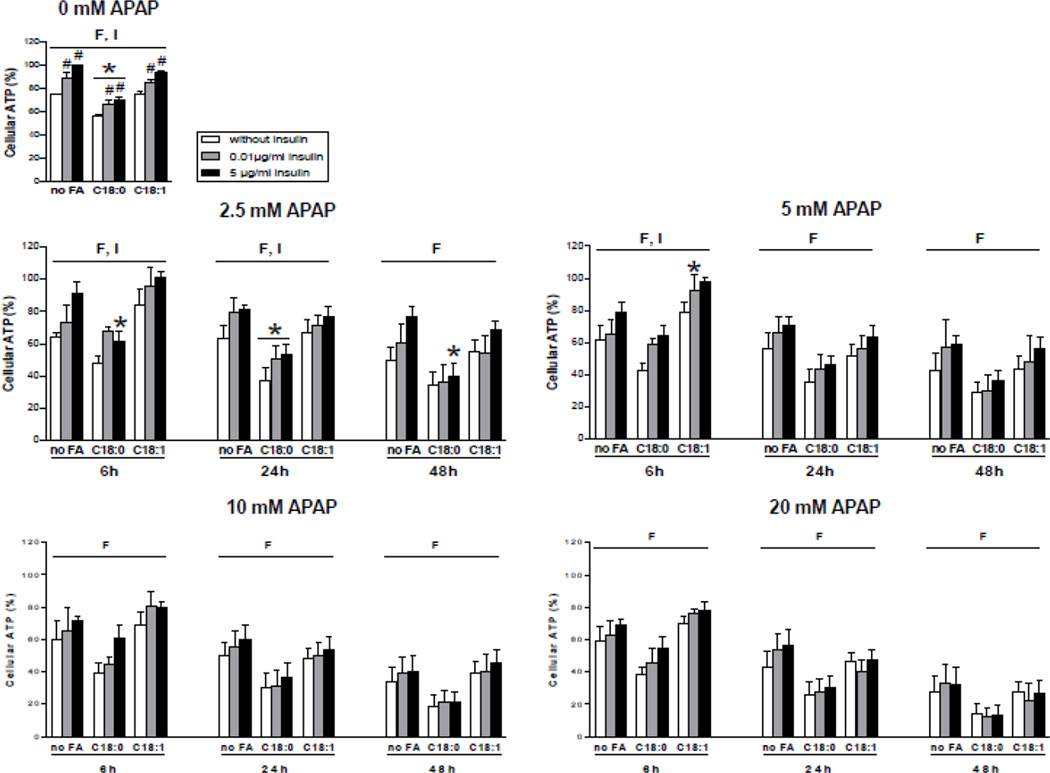
In order to determine APAP cytotoxicity in normal and steatotic HepaRG cells, a large range of APAP concentrations was selected and cellular ATP was measured after 6, 24 and 48 h of treatment. Whereas no significant cytotoxicity was observed for 1 mM APAP, reduced ATP levels were already observed with 2.5 mM although the effects greatly depended of the culture conditions (Supplementary Fig. 5). Indeed, the extent of APAP-induced ATP depletion was consistently greater in the presence of stearic acid (Fig. 4 and Supplementary Fig. 5). Moreover, incubation without insulin was associated with a significantly greater ATP depletion after 6 and 24 h for 2.5 mM APAP and after 6 h for 5 mM of this drug (Fig. 4). It was noteworthy that ATP levels in the basal state (i.e. without APAP treatment) were significantly lower in the absence of insulin and were particularly reduced with stearic acid (Fig. 4). However, when the basal ATP levels were equally set at 100 % for the three different conditions of fatty acid incubation, APAP cytotoxicity remained greater in the presence of stearic acid, except in the presence of 5 µg/ml of insulin after 6 h of APAP treatment (Supplementary Fig. 5). Thus, these results indicated that the higher ATP depletion induced by APAP in the presence of stearic acid was not a mere consequence of the basal cytotoxicity induced by this fatty acid.
Figure 4.
Levels of ATP in HepaRG cells treated or not with APAP. HepaRG cells were incubated for 1 week with different conditions of incubation with 100 µM stearic acid (C18:0), 100 µM oleic acid (C18:1) and different concentrations of insulin and subsequently treated or not with 2.5, 5, 10 or 20 mM of APAP. ATP levels were measured 6, 24 or 48 h after APAP treatment. Cellular ATP levels were set at 100% in HepaRG cells incubated for 1 week without fatty acids and with 5 µg/ml of insulin and not treated with APAP. Results are means ± SEM for 3 to 6 independent cultures, with data in duplicates for each culture. Letters F and I indicate a significant effect (P<0.05) of fatty acids and insulin, respectively. *Significantly different from HepaRG cells incubated without fatty acids and with the same condition of insulin treatment (P<0.05). #Significantly different from HepaRG cells incubated without insulin and with the same condition of fatty acid treatment (P<0.05).
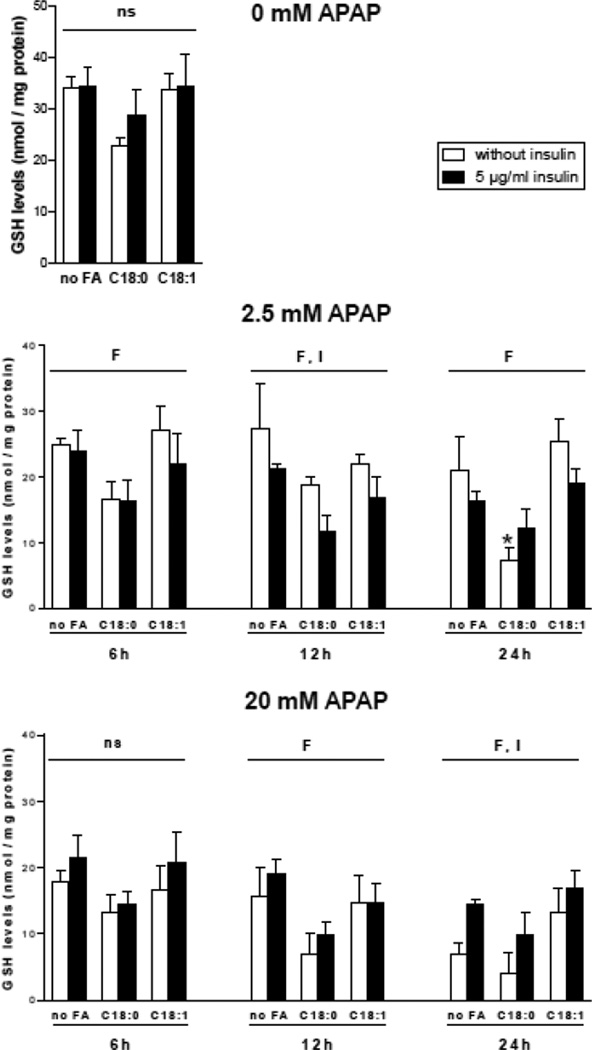
In the next series of investigations, cellular GSH levels were measured after a treatment with low (2.5 mM) and high (20 mM) APAP concentrations. These investigations were carried out with the three conditions of fatty acid incubation but with only two concentrations of insulin (i.e. 0 and 5 µg/ml). After APAP treatment, the extent of GSH depletion was generally greater in the presence of stearic acid (Fig. 5). A significant effect of insulin was also observed under some conditions. Indeed, GSH depletion after 12 h of 2.5 mM APAP was greater in the presence of insulin, whereas GSH depletion after 24 h of 20 mM APAP was greater in the absence of insulin (Fig. 5). Basal GSH levels were not significantly different between the 6 different conditions, although there was a trend towards lower GSH in the presence of stearic acid (P=0.11) (Fig. 5).
Figure 5.
Levels of total GSH in HepaRG cells treated or not with APAP. HepaRG cells were incubated for 1 week with different conditions of incubation with 100 µM stearic acid (C18:0), 100 µM oleic acid (C18:1) and two concentrations of insulin and subsequently treated or not with 2.5 or 20 mM of APAP. Total GSH levels were measured 6, 12 or 24 h after APAP treatment. Results are means ± SEM for 5 independent cultures. Letters F and I indicate a significant effect (P<0.05) of fatty acids and insulin, respectively and ns, not significant with a two-way ANOVA analysis. *Significantly different from HepaRG cells incubated without fatty acids and with the same condition of insulin treatment (P<0.05).
APAP-induced expression of oxidative stress genes in normal and steatotic HepaRG cells
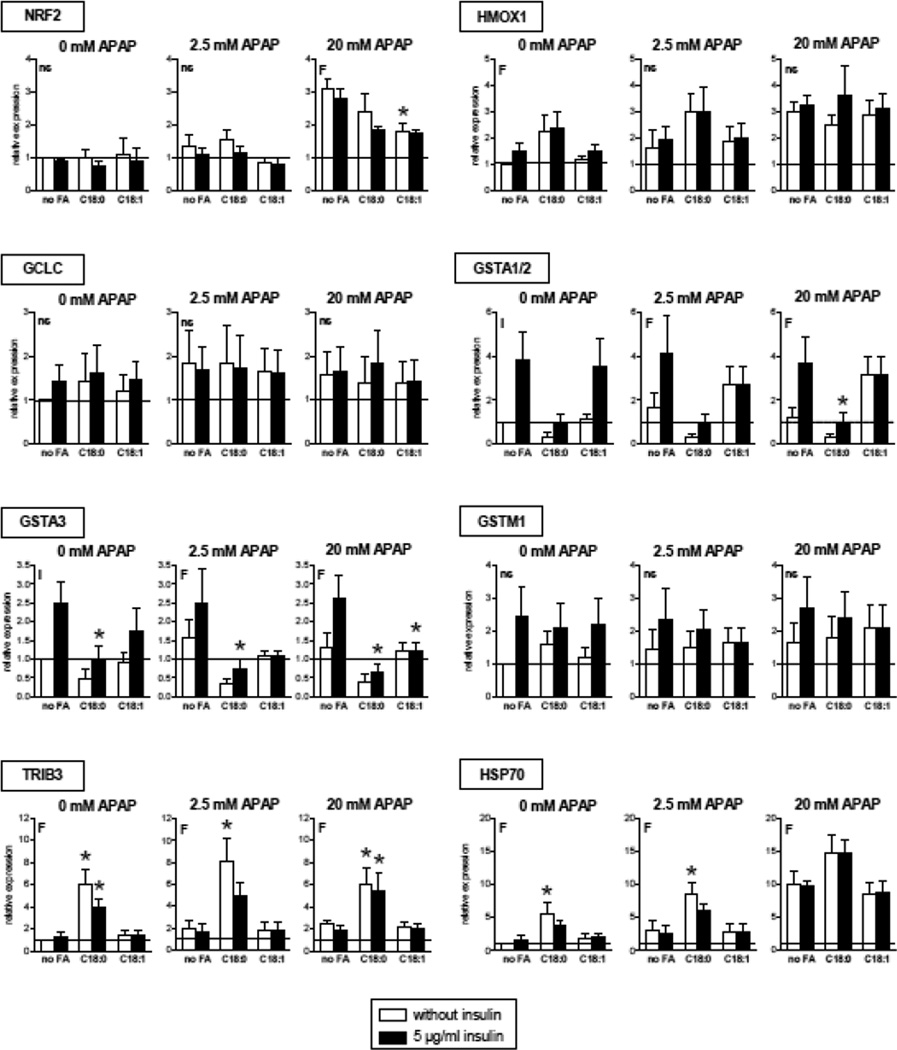
Oxidative stress, including induced by APAP, can secondarily increase the expression of different genes such as heme oxygenase 1, γ-glutamylcysteine synthase, different glutathione S-transferases, heat shock protein 70 and tribbles pseudokinase 3 (Aubert et al., 2012; Chan et al., 2001). Importantly, nuclear factor, erythroid 2-like 2 (NRF2, or NFE2L2) is a transcription factor playing a major role in the regulation of most of these genes (Chan et al., 2001; Ma and He, 2012). Hence, the mRNA expression of these genes was assessed in HepaRG cells treated with 2.5 or 20 mM APAP (Fig. 6). Expression of NRF2, HMOX1, TRIB3 and HSP70 was increased by APAP, sometimes in a dose-dependent manner. However, it was noteworthy that the expression of some oxidative stress genes was already induced in the basal state (i.e. in cells not treated with APAP), in particular when HepaRG cells were incubated with stearic acid. This was observed for instance with HMOX1, TRIB3 and HSP70. Moreover, we found that in the basal state insulin significantly increased the expression of GSTA1/2 and GSTA3. Previous studies in primary cultured rat hepatocytes also showed that insulin enhanced the expression of GSTA1/2 and GSTA3/5 (Kim et al., 2006). Interestingly, the expression of these genes was significantly decreased after APAP treatment in cells incubated with stearic acid. The lack of clear correlations between mRNA levels of several NRF2 targets was probably due to the different effects of APAP, fatty acids and insulin on their expression, as well as possible complex interactions between these factors.
Figure 6.
Expression of different genes involved in oxidative stress in HepaRG cells treated or not with APAP. HepaRG cells were incubated for 1 week with different conditions of incubation with 100 µM stearic acid (C18:0), 100 µM oleic acid (C18:1) and two concentrations of insulin and subsequently treated with 2.5 or 20 mM of APAP. Gene expression was measured 6 h after APAP treatment. Results are means ± SEM for 3–4 independent cultures, with data in duplicates for each culture. Letters F and I indicate a significant effect (P<0.05) of fatty acids and insulin, respectively and ns, not significant with a two-way ANOVA analysis. *Significantly different from HepaRG cells incubated without fatty acids and with the same condition of insulin treatment (P<0.05).
APAP-induced cytotoxicity, GSH depletion and APAP-protein adducts in normal and steatotic HepaRG cells treated or not with the CYP2E1 inhibitor CMZ
Another series of experiments was subsequently designed in order to determine the exact role of CYP2E1 in APAP-induced toxicity in HepaRG cells. For this purpose, HepaRG cells were incubated with 150 µM of CMZ for 1 week before APAP treatment. This protocol of CMZ treatment was selected because preliminary experiments showed a maximal inhibitory effect of CYP2E1 activity without significant cytotoxicity (data not shown).
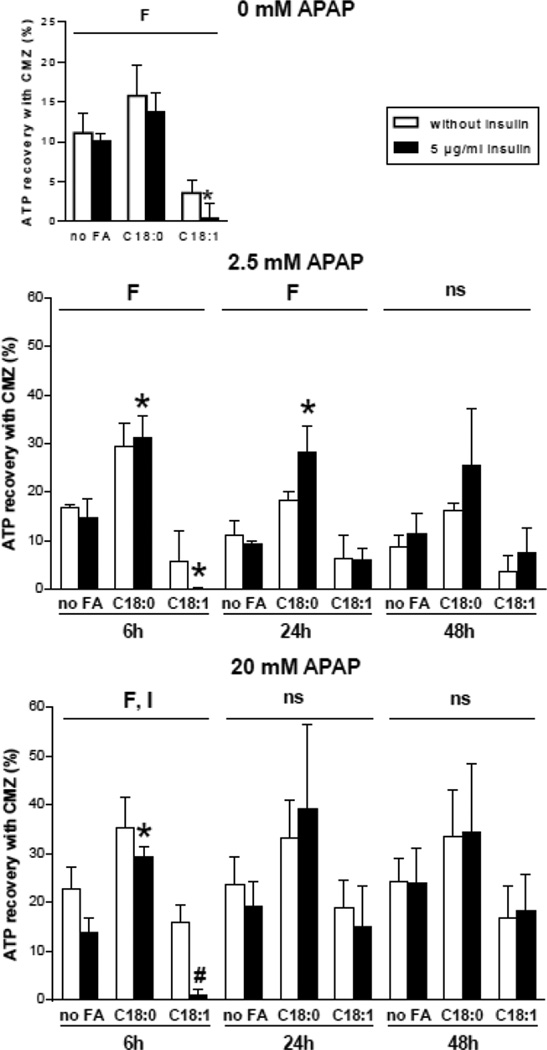
Because our experiments were carried out simultaneously with and without CMZ, the recovery of ATP in the presence of CMZ could be calculated for these experiments. ATP recovery was almost always observed in the different tested conditions (Fig. 7). With 2.5 mM of APAP, ATP recovery with CMZ was significantly higher in the presence of stearic acid after 6 and 24 h of APAP treatment, but no significant effect was observed regarding insulin (Fig. 7). With 20 mM of APAP, ATP recovery was also higher in the presence of stearic acid but only after 6 h of APAP treatment (Fig. 7). Moreover, ATP recovery at this time point was significantly lower in the presence of insulin (Fig. 7). Notably, ATP recovery in the basal state was significantly changed in the presence of fatty acids. In particular, ATP recovery was higher with stearic acid and lower with oleic acid (Fig. 7). Taken together, these results indicated that CYP2E1 was involved in the loss of ATP levels after APAP treatment, but also at a lesser degree in the basal state. Moreover, ATP loss related to CYP2E1 activity was usually higher in the presence of stearic acid.
Figure 7.
Recovery of ATP in HepaRG cells pretreated with the CYP2E1 inhibitor CMZ and subsequently treated with APAP. HepaRG cells were incubated for 1 week with different conditions of incubation with 100 µM stearic acid (C18:0), 100 µM oleic acid (C18:1), two concentrations of insulin and 150 µM CMZ and subsequently treated or not with 2.5 or 20 mM of APAP. ATP levels were measured 6, 24 or 48 h after APAP treatment. ATP recovery was determined by calculating the difference of ATP levels measured with and without CMZ. Results are means ± SEM for 3 independent cultures, with data in triplicates for each culture. Letters F and I indicate a significant effect (P<0.05) of fatty acids and insulin, respectively and ns, not significant with a two-way ANOVA analysis. *Significantly different from HepaRG cells incubated without fatty acids and with the same condition of insulin treatment (P<0.05). #Significantly different from HepaRG cells incubated without insulin and with the same condition of fatty acid treatment (P<0.05).
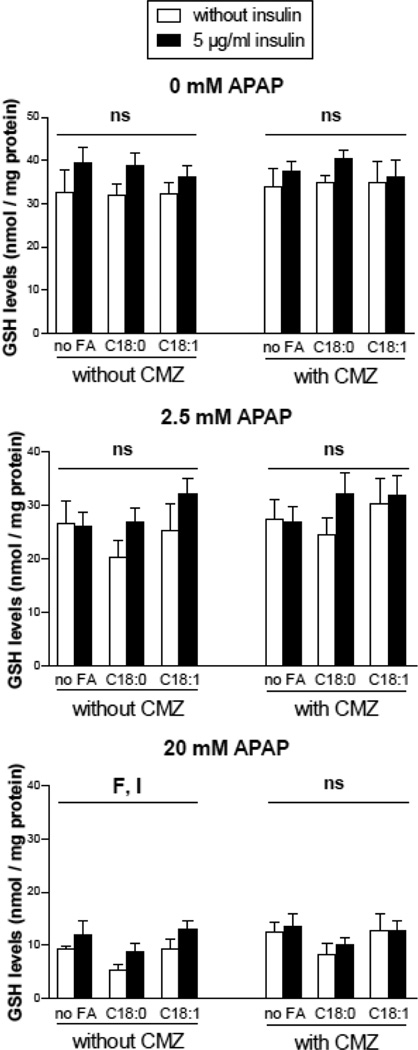
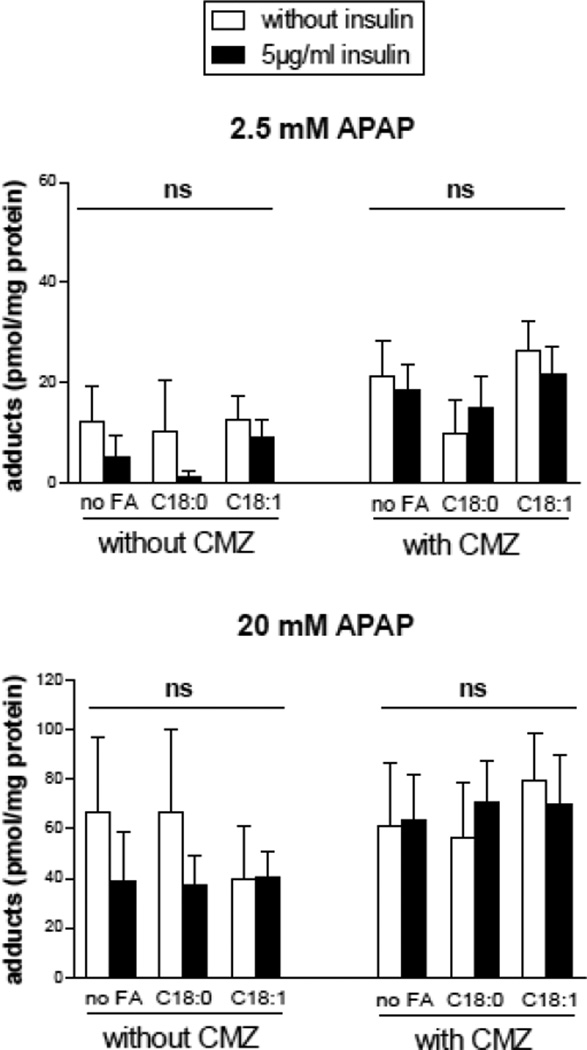
The effect of CMZ pretreatment on total GSH levels and APAP-protein adducts was also assessed in different conditions of fatty acids, insulin and APAP treatment (2.5 or 20 mM). Surprisingly, CMZ did not prevent the loss of total GSH after 24 h (Fig. 8), thus indicating that CYP2E1 was not primarily involved in GSH depletion after APAP treatment in HepaRG cells. Moreover, although APAP induced the formation of APAP-protein adducts in a concentration dependent manner, CMZ did not prevent the generation of these adducts 6 h after APAP treatment (Fig. 9).
Figure 8.
Total GSH levels in HepaRG cells pretreated or not with the CYP2E1 inhibitor CMZ and subsequently treated with APAP. HepaRG cells were incubated for 1 week with different conditions of incubation with 100 mM stearic acid (C18:0), 100 mM oleic acid (C18:1), two concentrations of insulin and 150 mM CMZ and subsequently treated or not with 2.5 or 20 mM APAP. GSH levels were measured 24 hours after APAP treatment. Results are means ± SEM for 5 independent cultures. Letters F and I indicate a significant effect (P<0.05) of fatty acids and insulin, respectively and ns, not significant with a two-way ANOVA analysis.
Figure 9.
APAP-protein adducts in HepaRG cells pretreated or not with the CYP2E1 inhibitor CMZ and subsequently treated with APAP. HepaRG cells were incubated for 1 week with different conditions of incubation with 100 mM stearic acid (C18:0), 100 mM oleic acid (C18:1), two concentrations of insulin and 150 mM CMZ and subsequently treated or not with 2.5 or 20 mM APAP. APAP-protein adducts were measured 6 hours after APAP treatment. Results are means ± SEM for 3 independent cultures. The letters ns indicate that there was no significant effect of fatty acids or insulin with a two-way ANOVA analysis.
APAP-glucuronide and APAP-sulfate in normal and steatotic HepaRG cells
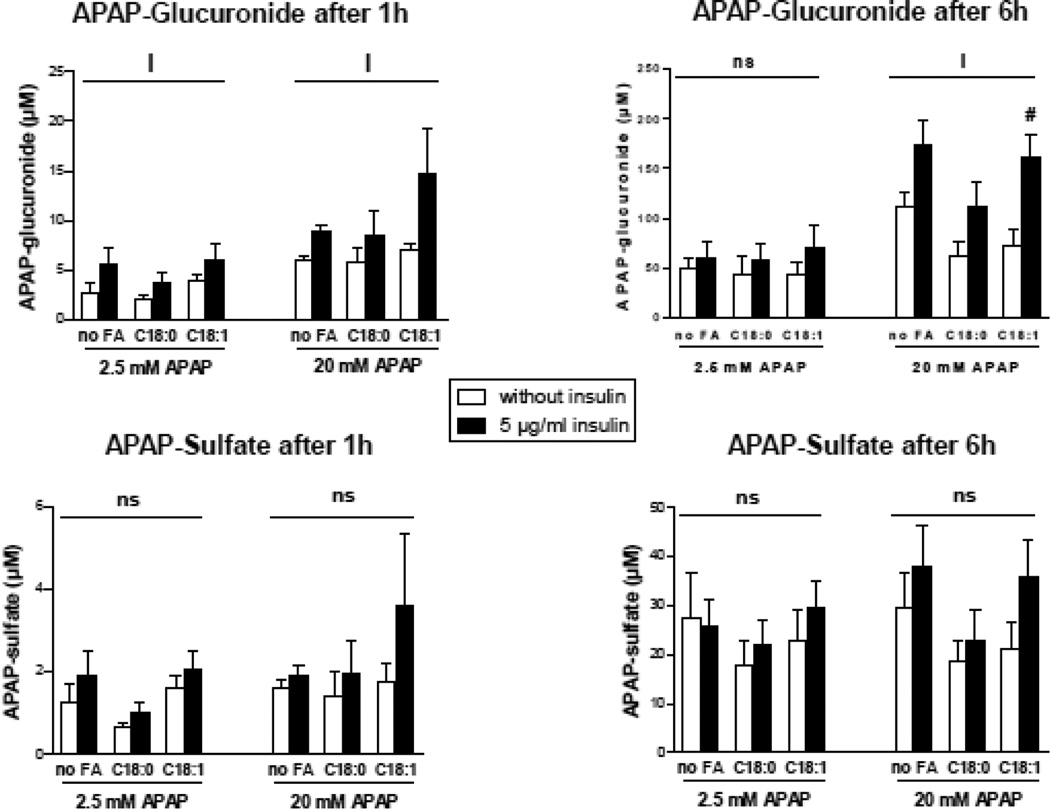
APAP-glucuronide and APAP-sulfate were measured in the culture media of normal or steatotic HepaRG cells treated for 1 or 6 h with 2.5 or 20 mM of APAP. Whereas APAP-sulfate levels were unchanged in the different conditions of culture, APAP-glucuronide levels were consistently higher in the presence of insulin (Fig. 10). In contrast, there was no effect of stearate or oleate on APAP-glucuronide levels (Fig. 10).
Figure 10.
Concentrations of APAP-glucuronide and APAP-sulfate in the culture media of HepaRG cells treated with 2.5 or 20 mM of APAP. HepaRG cells were incubated for 1 week with different conditions of incubation with 100 µM stearic acid (C18:0), 100 µM oleic acid (C18:1) and two concentrations of insulin and subsequently treated with 2.5 or 20 mM of APAP. APAP-glucuronide and APAP-sulfate were measured 1 and 6 h after APAP treatment. Results are means ± SEM for 4 independent cultures. The letter I indicates a significant effect (P<0.05) of insulin, and ns, not significant with a two-way ANOVA analysis. #Significantly different from HepaRG cells incubated without insulin and with the same condition of fatty acid treatment (P<0.05).
Lack of APAP-induced activation of c-Jun N-Terminal Kinase in HepaRG cells
A recent study reported that APAP-induced cell death in HepaRG cells was not associated with c-Jun N-terminal kinase (JNK) activation, which was assessed from 1 to 24 h after treatment (Xie et al., 2014). In this study, we confirmed these data and also verified that JNK was not phosphorylated at earlier time (i.e.15 min) after APAP treatment (data not shown). In contrast, JNK was found significantly phosphorylated in HepaRG cells treated by other stimuli such as hydrogen peroxide, tumor necrosis factor-α and glycochenodeoxycholic acid (data not shown). Thus, the lack of JNK activation in HepaRG cells after APAP treatment cannot be explained by a defect in the JNK signaling pathway.
Prevention of APAP-induced cytotoxicity in HepaRG cells by activators of AMP-activated protein kinase
AMP-activated protein kinase (AMPK) is a master regulator of energy metabolism in liver, especially by enhancing mitochondrial fatty acid oxidation and biogenesis (Foretz and Viollet, 2011; Hasenour et al., 2013). Interestingly, recent investigations in primary mouse hepatocytes showed that APAP-induced necrosis was prevented by the AMPK activator III (DHPO), thus suggesting a role of AMPK impairment in APAP cytotoxicity (Saberi et al., 2014). Thus, in a last series of investigations, we determined the effect of the prototypical AMPK activators AICAR and metformin on APAP-induced cytotoxicity in non-steatotic and steatotic HepaRG cells. Preliminary experiments allowed us to show that the expression of both phospho-AMPK and phospho-acetyl-CoA carboxylase (phospho-ACC) was efficiently enhanced with 500 µM of each prototypical AMPK activator (data not shown). Using this concentration, both AMPK activators prevented the ATP depletion measured 24 h after 20 mM of APAP (Supplementary Fig. 6), although the ATP recovery was in general lower compared to CMZ in the same conditions of APAP treatment (Fig. 7). In contrast, there was almost no GSH recovery with both AMPK activators, excepted in HepaRG cells incubated without fatty acid and without insulin (Supplementary Fig. 6).
Discussion
There is growing evidence that NAFLD can increase the risk and the severity of hepatotoxicity induced by some drugs and toxins (Fromenty, 2013; Robin et al., 2005a; Tarantino et al., 2007). However, little is known regarding the involved mechanisms, although some hypotheses have been proposed (Fromenty, 2013). Thus, relevant experimental models are needed to determine which compounds can pose a specific risk in NAFLD and to understand why they are more hepatotoxic in this dysmetabolic context. Since the HepaRG cell line has been shown to be a valuable model to study hepatotoxicity induced by drugs and toxins (Anthérieu et al., 2011; McGill et al., 2011; Savary et al., 2014; Sharanek et al., 2014; Tobwala et al., 2015), a first goal of our study was to determine whether this cell line could also be used as a pertinent model of human NALFD. To this end, HepaRG cells were incubated for one week in different conditions of incubation with stearic acid, oleic acid and insulin. The most relevant data regarding our cellular model of NAFLD can be summarized as followed:
Incubation of HepaRG cells for one week with stearic acid was associated with higher CYP2E1 activity and lower CYP3A4 activity, thus reproducing data collected in patients with obesity and NAFLD (Aubert et al., 2011; Brill et al., 2012; Chalasani et al., 2003; Emery et al., 2003; Kolwankar et al., 2007; Woolsey et al., 2015). In contrast, oleic acid had no effect on CYP2E1 and CYP3A4 activity.
The slight to moderate loss of cellular ATP observed in some conditions in HepaRG cells not treated with APAP was, at least in part, secondary to basal CYP2E1 activity. Increased activity of CYP2E1 in HepaRG cells incubated with stearic acid was associated with altered expression of several genes responsive to oxidative stress.
Our data with different concentrations of insulin showed that CYP2E1 activity in HepaRG cells was negatively regulated by this hormone in a concentration-dependent manner. However, insulin was responsible for an increased CYP2E1 mRNA and protein expression in a concentration-dependent way. Notably, experiments in cultured PHH indicated that insulin also reduced CYP2E1 activity with a concomitant increase in CYP2E1 mRNA levels.
Our results regarding the effects of stearic acid, oleic acid and insulin on the activity of CYP2E1 and CYP3A4 in HepaRG cells deserve further discussion.
First, although different investigations consistently reported higher hepatic CYP2E1 activity in human NAFLD (Aubert et al., 2011; Brill et al., 2012; Chalasani et al., 2003; Emery et al., 2003), the exact mechanism of this induction is still unknown. CYP2E1 induction in obese individuals has been associated with several dysmetabolic parameters such as the degree of steatosis, hyperketonemia, hyperlipidemia, insulin resistance and type 2 diabetes (Chalasani et al., 2003; Chtioui et al., 2007; Emery et al., 2003; Lucas et al., 1998; Wang et al., 2003). Importantly, our study showed that despite the similar amount of cellular triglycerides, CYP2E1 activity was selectively enhanced when steatosis was induced by stearic acid, but not by oleic acid. Altogether, these data suggest that higher CYP2E1 activity in human NAFLD could depend not only on the degree of steatosis but also on the composition of the deposited lipids.
Second, an important observation in this study was that insulin significantly reduced CYP2E1 activity in HepaRG cells and PHH in a concentration-dependent manner. Hence, low insulin was associated with higher CYP2E1 activity. Interestingly, insulin deficiency in rodents treated with streptozotocin was previously associated with higher hepatic CYP2E1 activity and this induction was corrected by insulin therapy (Ioannides et al., 1998; Raza et al., 2004). Thus, our results could also explain why higher CYP2E1 activity has been associated with insulin resistance (Chalasani et al., 2003), which is frequently linked to NAFLD (Begriche et al., 2013; Tilg and Moschen 2014).
Third, insulin reduced CYP2E1 activity but increased the mRNA and protein levels of this CYP in both HepaRG cells and PHH. Thus, CYP2E1 activity could be regulated post-translationally by this hormone in hepatocytes, as suggested by previous investigations (Oesch-Bartlomovicz et al., 1998; Wauthier et al., 2006). It is also noteworthy that the positive effect of insulin on CYP2E1 mRNA expression in human hepatocytes is in sharp contrast with previous investigations carried out in rat hepatocytes (Moncion et al., 2002; Woodcroft and Novak, 1999). However, CYP2E1 activity was not reported in these studies. Hence, CYP2E1 expression could be differentially regulated by insulin between rats and humans. Further investigations in human hepatocytes will be needed in order to determine the transcriptional, translational and post-translational regulation of CYP2E1 by insulin. HepaRG cells could be helpful for this purpose.
Fourth, different studies reported lower hepatic CYP3A4 activity during NAFLFD (Brill et al., 2012; Kolwankar et al., 2007; Patoine et al., 2013; Woolsey et al., 2015), but no mechanism has been proposed so far. In this study, CYP3A4 activity was reduced in HepaRG cells incubated with stearic acid but not with oleic acid. Interestingly, a previous study performed in PHH showed that a mixture of palmitic acid (C16:0) and oleic acid reduced CYP3A4 activity (Donato et al., 2006). Thus, CYP3A4 activity could be specifically reduced by long-chain saturated fatty acids. It will be interesting to determine whether oxidative stress is involved in this effect, as suggested by previous studies (Anthérieu et al., 2013; Gallagher et al., 1995).
The painkiller APAP is an interesting example of a drug that could be particularly hepatotoxic in the context of NAFLD. Indeed, several investigations in rodents and humans reported that NALFD is associated with more severe APAP-induced hepatotoxicity, especially after an overdose (Aubert et al., 2012; Kon et al., 2010; Michaut et al., 2014; Myers and Shaheen, 2008; NGuyen et al., 2008). Moreover, in vitro investigations showed that steatotic rat hepatocytes in primary culture were more susceptible to APAP-induced acute cytotoxicity (Kucera et al., 2012). Although the mechanism is still poorly understood, some data suggest a significant role of CYP2E1 whose activity is consistently increased in NAFLD (Aubert et al., 2011, 2012; Michaut et al., 2014). Thus, the second objective of our study was to assess APAP-induced toxicity in non-steatotic and steatotic HepaRG cells.
The most relevant data regarding APAP toxicity can be summarized as followed:
Experiments with a large range of APAP concentrations (2.5 to 20 mM) showed that APAP-induced loss of cellular ATP was almost always greater in the presence of stearic acid. In contrast, APAP cytotoxicity in HepaRG cells incubated with oleic acid was similar (and sometimes lower) to non-steatotic cells.
Investigations with the prototypical CYP2E1 inhibitor CMZ indicated that the stronger cytotoxicity observed with 2.5 or 20 mM APAP in the presence of stearic acid could be attributed, at least in part, to higher CYP2E1 activity. However, the involvement of CYP2E1 in the higher APAP cytotoxicity observed without insulin was significant only with 20 mM APAP.
Incubation of HepaRG cells without insulin was associated with lower generation of APAP-glucuronide for low or high APAP concentrations.
Some of these results also deserve further discussion. First, our data suggest that the risk of APAP-induced hepatotoxicity in NAFLD patients could depend not only on the composition of the deposited lipids but also on hepatic insulin signaling. Indeed, accumulation of stearic acid could favor APAP cytotoxicity by enhancing CYP2E1 activity, although this fatty acid could also increase APAP toxicity via its deleterious effects on JNK signaling and mitochondrial function (Malhi et al., 2006). In addition, low (i.e. impaired) insulin signaling could increase the risk of APAP toxicity by reducing the generation of APAP-glucuronide and enhancing the CYP2E1-mediated biotransformation of APAP to NAPQI. Further investigations will be needed to determine whether this greater risk can occur not only after APAP overdose but also with therapeutic doses.
Second, it was noteworthy that pretreatment of HepaRG cells with CMZ was associated with significant cellular ATP recovery but this CYP2E1 inhibitor did not afford a significant protection regarding total GSH and APAP-protein adducts. Notably, APAP-induced cytotoxicity could be the consequence of the covalent binding of NAPQI to specific cellular proteins, in particular within the mitochondrial compartment (McGill and Jaeschke, 2013; Tirmenstein and Nelson, 1989). In addition, several studies reported the presence of CYP2E1 in liver mitochondria, in particular in humans (Bansal et al., 2013; Knockaert et al., 2011). Thus, it is conceivable that inhibition of CYP2E1 by CMZ could have prevented APAP-induced ATP depletion by inhibiting the covalent binding of this drug to a small number of key mitochondrial proteins but without modifying the total GSH levels since the mitochondrial pool of this antioxidant represents 10 to 15% of the whole cellular GSH reserve (Fernandez-Checa and Kaplowitz, 2005; Robin et al., 2005b). Finally, CMZ pretreatment of HepaRG cells may not have been able to prevent the generation of most APAP-protein adducts and the depletion of total GSH because NAPQI is also generated by CYP3A4, a microsomal enzyme that has not been reported to be located in the mitochondrial compartment.
Although some investigations reported that patients with NAFLD had an overall higher risk of severe APAP-induced liver injury (Myers and Shaheen, 2008; NGuyen et al., 2008), such risk may actually vary from one obese individual to another. Indeed, while some factors linked to obesity and fatty liver could increase the risk of APAP hepatotoxicity (e.g. higher CYP2E1 activity, lower GSH stores and mitochondrial dysfunction), other factors could be protective (e.g. increased volume of distribution, lower CYP3A4 activity and higher APAP glucuronidation) (Michaut et al., 2014). Furthermore, the occurrence of hepatic insulin resistance during the course of NAFLD might play an additional role on CYP2E1 activity and APAP glucuronidation, as previously discussed. Hence, the occurrence and outcome of APAP-induced liver injury in an obese individual with NAFLD might depend on the delicate balance between metabolic factors that favor hepatic cytolysis and others that are directly, or indirectly, hepatoprotective (Michaut et al., 2014).
Our cellular model has some limitations. First, HepaRG cells present low basal expression and activity of CYP1A2 compared to primary human hepatocytes, although this CYP is highly inducible in the HepaRG cell line by prototypical inducers such as 3-methylcholanthrene and omeprazole (Anthérieu et al., 2010; Guillouzo et al., 2007). Notably, previous investigations suggested that CYP1A2 could play a role in APAP bioactivation and hepatotoxicity (Laine et al., 2009; Zaher et al., 1998), although some investigations did not support a significant role for this CYP in vivo (Tonge et al., 1998). Hence, low basal CYP1A2 activity might modify the toxicity profile of APAP in HepaRG cells. Second, our cellular model does not take into account the involvement of non-parenchymal cells (e.g. Kupffer and stellate cells) and factors (e.g. proinflammatory cytokines and adiponectin) that have a key pathophysiological role in the development of both NAFLD and drug-induced hepatotoxicity (Begriche et al., 2013; Hinson et al., 2010; Teranishi et al., 2015). Thus, rodent models of obesity can also be helpful to determine the mechanisms whereby some xenobiotics are more hepatotoxic in NAFLD (Aubert et al., 2012; Carmiel-Haggai et al., 2003; Donthamsetty et al., 2007; Kon et al., 2010).
In conclusion, we set up a cellular model of human NAFLD which can be a valuable tool to gain insight regarding the mechanisms whereby this frequent hepatic disease appears to favor APAP-induced acute liver failure in some patients, in particular after an overdose (Michaut et al., 2014; Myers and Shaheen, 2009; NGuyen et al., 2008). This model can also be used to test the effect of other drugs or toxins suspected to be more hepatotoxic in the context of obesity and NAFLD, especially as a consequence of CYP2E1 induction (Fromenty, 2013).
Supplementary Material
Acknowledgments
Harmut Jaeschke reports grants from National Institutes of Health, during the conduct of the study. Bernard Fromenty reports personal fees from Sigma-Tau and Novo Nordisk, outside the submitted work.
This work was mostly supported by INSERM (Institut National de la Recherche et de la Santé Médicale). Part of the work was also supported by the Fonds d’Innovation pour la Recherche of the CHU of Rennes and the Conseil Régional de Bretagne (project call from ID2Santé -formerly known as CRITT Santé Bretagne- project LipoHepaRG n° R13113NN), as well as by National Institutes of Health (grant n° R01 DK102142). Anaïs Michaut was a recipient of a 6-month fellowship from the Fondation pour la Recherche Médicale (fellowship n° FDT20140931112). Additional support to Mitchell R. McGill came from the "Training Program in Environmental Toxicology" from the National Institute of Environmental Health Sciences (program n° T32 ES007079-26A2). We are also grateful to Mireille Desille-Dugast and Dr Bruno Turlin from the Centre de Ressources Biologiques (CRB) Santé de Rennes for their involvement in the preparation of the primary human hepatocytes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
Anaïs Michaut, Dounia Le Guillou, Caroline Moreau, Simon Bucher, Mitchell McGill, Sophie Martinais, Thomas Gicquel, Isabelle Morel and Marie-Anne Robin have nothing to disclose.
Supplementary data
Supplementary data are available in a separate file.
References
- Andersson TB, Kanebratt KP, Kenna JG. The HepaRG cell line: a unique in vitro tool for understanding drug metabolism and toxicology in human. Expert Opin Drug Metab Toxicol. 2012;8:909–920. doi: 10.1517/17425255.2012.685159. [DOI] [PubMed] [Google Scholar]
- Aninat C, Piton A, Glaise D, Le Charpentier T, Langouët S, Morel F, Guguen-Guillouzo C, Guillouzo A. Expression of cytochromes P450, conjugating enzymes and nuclear receptors in human hepatoma HepaRG cells. Drug Metab. Dispos. 2006;34:75–83. doi: 10.1124/dmd.105.006759. [DOI] [PubMed] [Google Scholar]
- Anthérieu S, Chesné C, Li R, Camus S, Lahoz A, Picazo L, Turpeinen M, Tolonen A, Uusitalo J, Guguen-Guillouzo C, Guillouzo A. Stable expression, activity, and inducibility of cytochromes P450 in differentiated HepaRG cells. Drug Metab. Dispos. 2010;38:516–525. doi: 10.1124/dmd.109.030197. [DOI] [PubMed] [Google Scholar]
- Anthérieu S, Rogue A, Fromenty B, Guillouzo A, Robin MA. Induction of vesicular steatosis by amiodarone and tetracycline is associated with up-regulation of lipogenic genes in HepaRG cells. Hepatology. 2011;53:1895–1905. doi: 10.1002/hep.24290. [DOI] [PubMed] [Google Scholar]
- Anthérieu S, Chesné C, Li R, Guguen-Guillouzo C, Guillouzo A. Optimization of the HepaRG cell model for drug metabolism and toxicity studies. Toxicol. In Vitro. 2012;26:1278–1285. doi: 10.1016/j.tiv.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Anthérieu S, Bachour-El Azzi P, Dumont J, Abdel-Razzak Z, Guguen-Guillouzo C, Fromenty B, Robin MA, Guillouzo A. Oxidative stress plays a major role in chlorpromazine-induced cholestasis in human HepaRG cells. Hepatology. 2013;57:1518–1529. doi: 10.1002/hep.26160. [DOI] [PubMed] [Google Scholar]
- Aubert J, Begriche K, Knockaert L, Robin MA, Fromenty B. Increased expression of cytochrome P450 2E1 in nonalcoholic fatty liver disease: mechanisms and pathophysiological role. Clin. Res. Hepatol. Gastroenterol. 2011;35:630–637. doi: 10.1016/j.clinre.2011.04.015. [DOI] [PubMed] [Google Scholar]
- Aubert J, Begriche K, Delannoy M, Morel I, Pajaud J, Ribault C, Lepage S, McGill MR, Lucas-Clerc C, Turlin B, Robin MA, Jaeschke H, Fromenty B. Differences in early acetaminophen hepatotoxicity between obese ob/ob and db/db mice. J. Pharmacol. Exp. Ther. 2012;342:676–687. doi: 10.1124/jpet.112.193813. [DOI] [PubMed] [Google Scholar]
- Baker SS, Baker RD, Liu W, Nowak NJ, Zhu L. Role of alcohol metabolism in non-alcoholic steatohepatitis. PLoS One. 2010;5:e9570. doi: 10.1371/journal.pone.0009570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal S, Anandatheerthavarada HK, Prabu GK, Milne GL, Martin MV, Guengerich FP, Avadhani NG. Human cytochrome P450 2E1 mutations that alter mitochondrial targeting efficiency and susceptibility to ethanol-induced toxicity in cellular models. J. Biol. Chem. 2013;288:12627–12644. doi: 10.1074/jbc.M113.452367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker CW, Fagan JB, Pasco DS. Down-regulation of P4501A1 and P4501A2 mRNA expression in isolated hepatocytes by oxidative stress. J. Biol. Chem. 1994;269:3985–3990. [PubMed] [Google Scholar]
- Bartlett SM, Gibbons GF. Short- and longer-term regulation of very-low-density lipoprotein secretion by insulin, dexamethasone and lipogenic substrates in cultured hepatocytes. A biphasic effect of insulin. Biochem. J. 1988;249:37–43. doi: 10.1042/bj2490037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begriche K, Massart J, Robin MA, Bonnet F, Fromenty B. Mitochondrial adaptations and dysfunctions in nonalcoholic fatty liver disease. Hepatology. 2013;58:1497–1507. doi: 10.1002/hep.26226. [DOI] [PubMed] [Google Scholar]
- Biour M, Ben Salem C, Chazouillères O, Grangé JD, Serfaty L, Poupon R. Drug-induced liver injury; fourteenth updated edition of the bibliographic database of liver injuries and related drugs. Gastroenterol. Clin. Biol. 2004;28:720–759. doi: 10.1016/s0399-8320(04)95062-2. [DOI] [PubMed] [Google Scholar]
- Björnsson E. The natural history of drug-induced liver injury. Semin. Liver Dis. 2009;29:357–363. doi: 10.1055/s-0029-1240004. [DOI] [PubMed] [Google Scholar]
- Brill MJ, Diepstraten J, van Rongen A, van Kralingen S, van den Anker JN, Knibbe CA. Impact of obesity on drug metabolism and elimination in adults and children. Clin. Pharmacokinet. 2012;51:277–304. doi: 10.2165/11599410-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Canet MJ, Merrell MD, Hardwick RN, Bataille AM, Campion SN, Ferreira DW, Xanthakos SA, Manautou JE, Hesham A-Kader H, Erickson RP, Cherrington NJ. Altered regulation of hepatic efflux transporters disrupts acetaminophen disposition in pediatric nonalcoholic steatohepatitis. Drug Metab. Dispos. 2015;43:829–835. doi: 10.1124/dmd.114.062703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmiel-Haggai M, Cederbaum AI, Nieto N. Binge ethanol exposure increases liver injury in obese rats. Gastroenterology. 2003;125:1818–1833. doi: 10.1053/j.gastro.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Chalasani N, Gorski JC, Asghar MS, Asghar A, Foresman B, Hall SD, Crabb DW. Hepatic cytochrome P450 2E1 activity in nondiabetic patients with nonalcoholic steatohepatitis. Hepatology. 2003;37:544–550. doi: 10.1053/jhep.2003.50095. [DOI] [PubMed] [Google Scholar]
- Chan K, Han XD, Kan YW. An important function of Nrf2 in combating oxidative stress: detoxification of acetaminophen. Proc. Natl. Acad. Sci. U. S. A. 2001;98:4611–4616. doi: 10.1073/pnas.081082098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chtioui H, Semela D, Ledermann M, Zimmermann A, Dufour JF. Expression and activity of the cytochrome P450 2E1 in patients with nonalcoholic steatosis and steatohepatitis. Liver Int. 2007;27:764–771. doi: 10.1111/j.1478-3231.2007.01524.x. [DOI] [PubMed] [Google Scholar]
- Chu X, Bleasby K, Evers R. Species differences in drug transporters and implications for translating preclinical findings to humans. Expert Opin. Drug Metab. Toxicol. 2013;9:237–252. doi: 10.1517/17425255.2013.741589. [DOI] [PubMed] [Google Scholar]
- Donato MT, Lahoz A, Jimenez N, Pérez G, Serralta A, Mir J, Castell JV, Gomez-Lechon MJ. Potential impact of steatosis on cytochrome P450 enzymes of human hepatocytes isolated from fatty liver grafts. Drug Metab. Dispos. 2006;34:1556–1562. doi: 10.1124/dmd.106.009670. [DOI] [PubMed] [Google Scholar]
- Donthamsetty S, Bhave VS, Mitra MS, Latendresse JR, Mehendale HM. Nonalcoholic fatty liver sensitizes rats to carbon tetrachloride hepatotoxicity. Hepatology. 2007;45:391–403. doi: 10.1002/hep.21530. [DOI] [PubMed] [Google Scholar]
- Dubuquoy C, Robichon C, Lasnier F, Langlois C, Dugail I, Foufelle F, Girard J, Burnol AF, Postic C, Moldes M. Distinct regulation of adiponutrin/PNPLA3 gene expression by the transcription factors ChREBP and SREBP1c in mouse and human hepatocytes. J. Hepatol. 2011;55:145–153. doi: 10.1016/j.jhep.2010.10.024. [DOI] [PubMed] [Google Scholar]
- Emery MG, Fisher JM, Chien JY, Kharasch ED, Dellinger EP, Kowdley KV, Thummel KE. CYP2E1 activity before and after weight loss in morbidly obese subjects with nonalcoholic fatty liver disease. Hepatology. 2003;38:428–435. doi: 10.1053/jhep.2003.50342. [DOI] [PubMed] [Google Scholar]
- Fisher CD, Lickteig AJ, Augustine LM, Ranger-Moore J, Jackson JP, Ferguson SS, Cherrington NJ. Hepatic cytochrome P450 enzyme alterations in humans with progressive stages of nonalcoholic fatty liver disease. Drug Metab. Dispos. 2009;37:2087–2094. doi: 10.1124/dmd.109.027466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foretz M, Viollet B. Regulation of hepatic metabolism by AMPK. J. Hepatol. 2011;54:827–829. doi: 10.1016/j.jhep.2010.09.014. [DOI] [PubMed] [Google Scholar]
- Fromenty B. Drug-induced liver injury in obesity. J. Hepatol. 2013;58:824–826. doi: 10.1016/j.jhep.2012.12.018. [DOI] [PubMed] [Google Scholar]
- Gallagher EP, Buetler TM, Stapleton PL, Wang C, Stahl DL, Eaton DL. The effects of diquat and ciprofibrate on mRNA expression and catalytic activities of hepatic xenobiotic metabolizing and antioxidant enzymes in rat liver. Toxicol. Appl. Pharmacol. 1995;134:81–91. doi: 10.1006/taap.1995.1171. [DOI] [PubMed] [Google Scholar]
- Gicquel T, Aubert J, Lepage S, Fromenty B, Morel I. Quantitative analysis of acetaminophen and its primary metabolites in small plasma volumes by liquid chromatography-tandem mass spectrometry. J. Anal. Toxicol. 2013;37:110–116. doi: 10.1093/jat/bks139. [DOI] [PubMed] [Google Scholar]
- Guillouzo A, Corlu A, Aninat C, Glaise D, Morel F, Guguen-Guillouzo C. The human hepatoma HepaRG cells: a highly differentiated model for studies of liver metabolism and toxicity of xenobiotics. Chem. Biol. Interact. 2007;168:66–73. doi: 10.1016/j.cbi.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Hasenour CM, Berglund ED, Wasserman DH. Emerging role of AMP-activated protein kinase in endocrine control of metabolism in the liver. Mol. Cell. Endocrinol. 2013;366:152–162. doi: 10.1016/j.mce.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinson JA, Roberts DW, James LP. Mechanisms of acetaminophen-induced liver necrosis. Handb. Exp. Pharmacol. 2010;196:369–405. doi: 10.1007/978-3-642-00663-0_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, He S, Li JZ, Seo YK, Osborne TF, Cohen JC, Hobbs HH. A feed-forward loop amplifies nutritional regulation of PNPLA3. Proc. Natl. Acad. Sci. U. S. A. 2010;107:7892–7897. doi: 10.1073/pnas.1003585107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannides C, Bass SL, Ayrton AD, Trinick J, Walker R, Flatt PR. Streptozotocin-induced diabetes modulates the metabolic activation of chemical carcinogens. Chem. Biol. Interact. 1988;68:189–202. doi: 10.1016/0009-2797(88)90016-6. [DOI] [PubMed] [Google Scholar]
- Kim SK, Abdelmegeed MA, Novak RF. Identification of the insulin signaling cascade in the regulation of alpha-class glutathione S-transferase expression in primary cultured rat hepatocytes. J. Pharmacol. Exp.Ther. 2006;316:1255–1261. doi: 10.1124/jpet.105.096065. [DOI] [PubMed] [Google Scholar]
- Knockaert L, Fromenty B, Robin MA. Mechanisms of mitochondrial targeting of cytochrome P450 2E1: physiopathological role in liver injury and obesity. FEBS. J. 2011;278:4252–4260. doi: 10.1111/j.1742-4658.2011.08357.x. [DOI] [PubMed] [Google Scholar]
- Kolwankar D, Vuppalanchi R, Ethell B, Jones DR, Wrighton SA, Hall SD, Chalasani N. Association between nonalcoholic hepatic steatosis and hepatic cytochrome P-450 3A activity. Clin. Gastroenterol. Hepatol. 2007;5:388–393. doi: 10.1016/j.cgh.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Kon K, Ikejima K, Okumura K, Arai K, Aoyama T, Watanabe S. Diabetic KK-A(y) mice are highly susceptible to oxidative hepatocellular damage induced by acetaminophen. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;299:G329–G337. doi: 10.1152/ajpgi.00361.2009. [DOI] [PubMed] [Google Scholar]
- Kucera O, Al-Dury S, Lotkova H, Rousar T, Rychtrmoc D, Cervinkova Z. Steatotic rat hepatocytes in primary culture are more susceptible to the acute toxic effect of acetaminophen. Physiol. Res. 2012;61(Suppl 2):S93–S101. doi: 10.33549/physiolres.932395. [DOI] [PubMed] [Google Scholar]
- Lake AD, Novak P, Fisher CD, Jackson JP, Hardwick RN, Billheimer DD, Klimecki WT, Cherrington NJ. Analysis of global and absorption, distribution, metabolism, and elimination gene expression in the progressive stages of human nonalcoholic fatty liver disease. Drug Metab. Dispos. 2011;39:1954–1960. doi: 10.1124/dmd.111.040592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine JE, Auriola S, Pasanen M, Juvonen RO. Acetaminophen bioactivation by human cytochrome P450 enzymes and animal microsomes. Xenobiotica. 2009;39:11–21. doi: 10.1080/00498250802512830. [DOI] [PubMed] [Google Scholar]
- Lu P, Yan J, Liu K, Garbacz WG, Wang P, Xu M, Ma X, Xie W. Activation of aryl hydrocarbon receptor dissociates fatty liver from insulin resistance by inducing fibroblast growth factor 21. Hepatology. 2015;61:1908–1919. doi: 10.1002/hep.27719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas D, Farez C, Bardou LG, Vaisse J, Attali JR, Valensi P. Cytochrome P450 2E1 activity in diabetic and obese patients as assessed by chlorzoxazone hydroxylation. Fundam. Clin. Pharmacol. 1998;12:553–558. doi: 10.1111/j.1472-8206.1998.tb00985.x. [DOI] [PubMed] [Google Scholar]
- Ma Q, He X. Molecular basis of electrophilic and oxidative defense: promises and perils of Nrf2. Pharmacol. Rev. 2012;64:1055–1081. doi: 10.1124/pr.110.004333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madec S, Cerec V, Plée-Gautier E, Antoun J, Glaise D, Salaun JP, Guguen-Guillouzo C, Corlu A. CYP4F3B expression is associated with differentiation of HepaRG human hepatocytes and unaffected by fatty acid overload. Drug Metab. Dispos. 2011;39:1987–1996. doi: 10.1124/dmd.110.036848. [DOI] [PubMed] [Google Scholar]
- Malhi H, Bronk SF, Werneburg NW, Gores GJ. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J. Biol. Chem. 2006;281:12093–12101. doi: 10.1074/jbc.M510660200. [DOI] [PubMed] [Google Scholar]
- Martignoni M, Groothuis GM, de Kanter R. Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin. Drug Metab. Toxicol. 2006;2:875–894. doi: 10.1517/17425255.2.6.875. [DOI] [PubMed] [Google Scholar]
- Martinez-Jiménez CP, Castell JV, Gomez-Lechon MJ, Jover R. Transcriptional activation of CYP2C9, CYP1A1, and CYP1A2 by hepatocyte nuclear factor 4alpha requires coactivators peroxisomal proliferator activated receptor-gamma coactivator 1alpha and steroid receptor coactivator 1. Mol. Pharmacol. 2006;70:1681–1692. doi: 10.1124/mol.106.025403. [DOI] [PubMed] [Google Scholar]
- Massart J, Robin MA, Noury F, Fautrel A, Lettéron P, Bado A, Eliat PA, Fromenty B. Pentoxifylline aggravates fatty liver in obese and diabetic ob/ob mice by increasing intestinal glucose absorption and activating hepatic lipogenesis. Br. J. Pharmacol. 2012;165:1361–1374. doi: 10.1111/j.1476-5381.2011.01580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Jaeschke H. Metabolism and disposition of acetaminophen: recent advances in relation to hepatotoxicity and diagnosis. Pharm. Res. 2013;30:2174–2187. doi: 10.1007/s11095-013-1007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Yan HM, Ramachandran A, Murray GJ, Rollins DE, Jaeschke H. HepaRG cells: a human model to study mechanisms of acetaminophen hepatotoxicity. Hepatology. 2011;53:974–982. doi: 10.1002/hep.24132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Lebofsky M, Norris HR, Slawson MH, Bajt ML, Xie Y, Williams CD, Wilkins DG, Rollins DE, Jaeschke H. Plasma and liver acetaminophen-protein adduct levels in mice after acetaminophen treatment: dose-response, mechanisms, and clinical implications. Toxicol. Appl. Pharmacol. 2013;269:240–249. doi: 10.1016/j.taap.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaut A, Moreau C, Robin MA, Fromenty B. Acetaminophen-induced liver injury in obesity and nonalcoholic fatty liver disease. Liver Int. 2014;34:e171–e179. doi: 10.1111/liv.12514. [DOI] [PubMed] [Google Scholar]
- Michelotti GA, Machado MV, Diehl AM. NAFLD, NASH and liver cancer. Nat. Rev. Gastroenterol. Hepatol. 2013;10:656–665. doi: 10.1038/nrgastro.2013.183. [DOI] [PubMed] [Google Scholar]
- Miquilena-Colina ME, Lima-Cabello E, Sanchez-Campos S, Garcia-Mediavilla MV, Fernandez-Bermejo M, Lozano-Rodriguez T, Vargas-Castrillon J, Buqué X, Ochoa B, Aspichueta P, Gonzalez-Gallego J, Garcia-Monzon C. Hepatic fatty acid translocase CD36 upregulation is associated with insulin resistance, hyperinsulinaemia and increased steatosis in non-alcoholic steatohepatitis and chronic hepatitis C. Gut. 2011;60:1394–1402. doi: 10.1136/gut.2010.222844. [DOI] [PubMed] [Google Scholar]
- Moncion A, Truong NT, Garrone A, Beaune P, Barouki R, De Waziers I. Identification of a 16-nucleotide sequence that mediates post-transcriptional regulation of rat CYP2E1 by insulin. J. Biol. Chem. 2002;277:45904–45910. doi: 10.1074/jbc.M207841200. [DOI] [PubMed] [Google Scholar]
- Myers RP, Shaheen AA. Hepatitis C, alcohol abuse, and unintentional overdoses are risk factors for acetaminophen-related hepatotoxicity. Hepatology. 2009;49:1399–1400. doi: 10.1002/hep.22798. [DOI] [PubMed] [Google Scholar]
- Nagasaw M, Akasaka Y, Ide T, Hara T, Kobayashi N, Utsumi M, Murakami K. Highly sensitive upregulation of apolipoprotein A-IV by peroxisome proliferator-activated receptor alpha (PPARα) agonist in human hepatoma cells. Biochem. Pharmacol. 2007;74:1738–1746. doi: 10.1016/j.bcp.2007.08.020. [DOI] [PubMed] [Google Scholar]
- Nguyen GC, Sam J, Thuluvath PJ. Hepatitis C is a predictor of acute liver injury among hospitalizations for acetaminophen overdose in the United States: a nationwide analysis. Hepatology. 2008;48:1336–1341. doi: 10.1002/hep.22536. [DOI] [PubMed] [Google Scholar]
- Oesch-Bartlomowicz B, Padma PR, Becker R, Richter B, Hengstler JG, Freeman JE, Wolf CR, Oesch F. Differential modulation of CYP2E1 activity by cAMP-dependent protein kinase upon Ser129 replacement. Exp. Cell Res. 1998;242:294–302. doi: 10.1006/excr.1998.4120. [DOI] [PubMed] [Google Scholar]
- Patoine D, Levac X, Pilote S, Drolet B, Simard C. Decreased CYP3A expression and activity in guinea pig models of diet-induced metabolic syndrome: is fatty liver infiltration involved? Drug Metab. Dispos. 2013;41:952–957. doi: 10.1124/dmd.112.050641. [DOI] [PubMed] [Google Scholar]
- Postic C, Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J. Clin. Invest. 2008;118:829–838. doi: 10.1172/JCI34275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza H, Prabu SK, Robin MA, Avadhani NG. Elevated mitochondrial cytochrome P450 2E1 and glutathione S-transferase A4-4 in streptozotocin-induced diabetic rats: tissue-specific variations and roles in oxidative stress. Diabetes. 2004;53:185–194. doi: 10.2337/diabetes.53.1.185. [DOI] [PubMed] [Google Scholar]
- Reaven GM, Lerner RL, Stern MP, Farquhar JW. Role of insulin in endogenous hypertriglyceridemia. J. Clin. Invest. 1967;46:1756–1767. doi: 10.1172/JCI105666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin MA, Demeilliers C, Sutton A, Paradis V, Maisonneuve C, Dubois S, Poirel O, Lettéron P, Pessayre D, Fromenty B. Alcohol increases tumor necrosis factor alpha and decreases nuclear factor-κB to activate hepatic apoptosis in genetically obese mice. Hepatology. 2005a;42:1280–1290. doi: 10.1002/hep.20949. [DOI] [PubMed] [Google Scholar]
- Robin MA, Sauvage I, Grandperret T, Descatoire V, Pessayre D, Fromenty B. Ethanol increases mitochondrial cytochrome P450 2E1 in mouse liver and rat hepatocytes. FEBS Lett. 2005b;579:6895–6902. doi: 10.1016/j.febslet.2005.11.029. [DOI] [PubMed] [Google Scholar]
- Saberi B, Ybanez MD, Johnson HS, Gaarde WA, Han D, Kaplowitz N. Protein kinase C (PKC) participates in acetaminophen hepatotoxicity through c-jun-N-terminal kinase (JNK)-dependent and -independent signaling pathways. Hepatology. 2014;59:1543–1554. doi: 10.1002/hep.26625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez CH, Caron S, Briand O, Dehondt H, Duplan I, Kuipers F, Hennuyer N, Clavey V, Staels B. The human hepatocyte cell lines IHH and HepaRG: models to study glucose, lipid and lipoprotein metabolism. Arch. Physiol. Biochem. 2012;118:102–111. doi: 10.3109/13813455.2012.683442. [DOI] [PubMed] [Google Scholar]
- Savary CC, Jossé R, Bruyère A, Guillet F, Robin MA, Guillouzo A. Interactions of endosulfan and methoxychlor involving CYP3A4 and CYP2B6 in human HepaRG cells. Drug Metab. Dispos. 2014;42:1235–1240. doi: 10.1124/dmd.114.057786. [DOI] [PubMed] [Google Scholar]
- Seeff LB, Bonkovsky HL, Navarro VJ, Wang G. Herbals and the liver. A review of adverse effects and mechanisms. Gastroenterology. 2015;148:517–532. doi: 10.1053/j.gastro.2014.12.004. [DOI] [PubMed] [Google Scholar]
- Sharanek A, Azzi PB, Al-Attrache H, Savary CC, Humbert L, Rainteau D, Guguen-Guillouzo C, Guillouzo A. Different dose-dependent mechanisms are involved in early cyclosporine A-induced cholestatic effects in HepaRG cells. Toxicol. Sci. 2014;141:244–253. doi: 10.1093/toxsci/kfu122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sookoian S, Castano GO, Burgueno AL, Gianotti TF, Rosselli MS, Pirola CJ. The nuclear receptor PXR gene variants are associated with liver injury in nonalcoholic fatty liver disease. Pharmacogenet. Genomics. 2010;20:1–8. doi: 10.1097/FPC.0b013e328333a1dd. [DOI] [PubMed] [Google Scholar]
- Tarantino G, Conca P, Basile V, Gentile A, Capone D, Polichetti G, Leo E. A prospective study of acute drug-induced liver injury in patients suffering from non-alcoholic fatty liver disease. Hepatol. Res. 2007;37:410–415. doi: 10.1111/j.1872-034X.2007.00072.x. [DOI] [PubMed] [Google Scholar]
- Teranishi Y, Matsubara T, Krausz KW, Le TT, Gonzalez FJ, Yoshizato K, Ikeda K, Kawada N. Involvement of hepatic stellate cell cytoglobin in acute hepatocyte damage through the regulation of CYP2E1-mediated xenobiotic metabolism. Lab. Invest. 2015;95:515–524. doi: 10.1038/labinvest.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilg H, Moschen AR. Evolving therapies for non-alcoholic steatohepatitis. Expert Opin. Drug Discov. 2014;9:687–696. doi: 10.1517/17460441.2014.911283. [DOI] [PubMed] [Google Scholar]
- Tirmenstein MA, Nelson SD. Subcellular binding and effects on calcium homeostasis produced by acetaminophen and a nonhepatotoxic regioisomer, 3'-hydroxyacetanilide, in mouse liver. J. Biol. Chem. 1989;264:9814–9819. [PubMed] [Google Scholar]
- Tobwala S, Khayyat A, Fan W, Ercal N. Comparative evaluation of N-acetylcysteine and N-acetylcysteineamide in acetaminophen-induced hepatotoxicity in human hepatoma HepaRG cells. Exp. Biol. Med. 2015;240:261–272. doi: 10.1177/1535370214549520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonge RP, Kelly EJ, Bruschi SA, Kalhorn T, Eaton DL, Nebert DW, Nelson SD. Role of CYP1A2 in the hepatotoxicity of acetaminophen: investigations using Cyp1a2 null mice. Toxicol. Appl. Pharmacol. 1998;153:102–108. doi: 10.1006/taap.1998.8543. [DOI] [PubMed] [Google Scholar]
- Wahlang B, Beier JI, Clair HB, Bellis-Jones HJ, Falkner KC, McClain CJ, Cave MC. Toxicant-associated steatohepatitis. Toxicol. Pathol. 2013;41:343–360. doi: 10.1177/0192623312468517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Hall SD, Maya JF, Li L, Asghar A, Gorski JC. Diabetes mellitus increases the in vivo activity of cytochrome P450 2E1 in humans. Br. J. Clin. Pharmacol. 2003;55:77–85. doi: 10.1046/j.1365-2125.2003.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wauthier V, Schenten V, Verbeeck RK, Calderon PB. Ageing is associated with increased expression but decreased activity of CYP2E1 in male Wistar rats. Life Sci. 2006;79:1913–1920. doi: 10.1016/j.lfs.2006.06.046. [DOI] [PubMed] [Google Scholar]
- Woodcroft KJ, Novak RF. The role of phosphatidylinositol 3-kinase, Src kinase, and protein kinase A signaling pathways in insulin and glucagon regulation of CYP2E1 expression. Biochem. Biophys. Res. Commun. 1999;266:304–307. doi: 10.1006/bbrc.1999.1817. [DOI] [PubMed] [Google Scholar]
- Woolsey SJ, Mansell SE, Kim RB, Tirona RG, Beaton MD. CYP3A activity and expression in nonalcoholic fatty liver disease. Drug Metab. Dispos. 2015;43:1484–1490. doi: 10.1124/dmd.115.065979. [DOI] [PubMed] [Google Scholar]
- Xie X, Liao H, Dang H, Pang W, Guan Y, Wang X, Shyy JY, Zhu Y, Sladek FM. Down-regulation of hepatic HNF4α gene expression during hyperinsulinemia via SREBPs. Mol. Endocrinol. 2009;23:434–443. doi: 10.1210/me.2007-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, McGill MR, Dorko K, Kumer SC, Schmitt TM, Forster J, Jaeschke H. Mechanisms of acetaminophen-induced cell death in primary human hepatocytes. Toxicol. Appl. Pharmacol. 2014;279:266–274. doi: 10.1016/j.taap.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaher H, Buters JT, Ward JM, Bruno MK, Lucas AM, Stern ST, Cohen SD, Gonzalez FJ. Protection against acetaminophen toxicity in CYP1A2 and CYP2E1 double-null mice. Toxicol. Appl. Pharmacol. 1998;152:193–199. doi: 10.1006/taap.1998.8501. [DOI] [PubMed] [Google Scholar]
- Zammit VA, Waterman IJ, Topping D, McKay G. Insulin stimulation of hepatic triacylglycerol secretion and the etiology of insulin resistance. J. Nutr. 2001;131:2074–2077. doi: 10.1093/jn/131.8.2074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.