Abstract
A variety of neuromodulation approaches have been described for the management of pelvic neuropathies, including interstitial cystitis, pudendal neuralgia and persistent genital arousal disorder. The benefits of a combined sacral and pudendal nerve neuromodulator has yet to be explored for these patients. In this report, we describe the case of a 35-year-old woman with a complex pelvic neuropathy resulting in urinary, sexual and gastro-intestinal dysfunction. She presented with an established diagnosis of interstitial cystitis; however, she also fulfilled diagnostic criteria for pudendal neuralgia and persistent genital arousal disorder. The patient underwent implantation of a combined sacral and pudendal nerve neuoromodulation device at the time of surgical decompression of the pudendal nerves. An impressive clinical response followed. This case demonstrates a unique clinical presentation and highlights the value of a combined surgical and neuromodulatory approach in the management of patients with complex pelvic neuropathies.
Background
Since its innovative use in the 1960s, neuromodulation has become a successful adjuvant treatment for patients with intractable interstitial cystitis, anorectal pain and chronic pelvic pain. Numerous techniques have been described, including transforaminal sacral nerve stimulation (SNS) and percutaneous peripheral nerve stimulation (PNS), with varying effects.1 SNS has provided symptomatic improvement in up to 87% of patients with refractory interstitial cystitis; a urological diagnosis of exclusion characterised by urinary urgency, frequency, nocturia and pelvic pain. The condition is typically managed with antidepressant or anticholinergic medications, cystoscopic hydrodistension and ultimately with cystectomy and urinary diversion. Research suggests IC is triggered by neurovascular dysfunction resulting in vasomotor-induced ischaemia of the bladder epithelium. This concept supports the use of neuromodulator therapies in these patients.2
PNS has been applied to patients with pudendal neuralgia (PN), defined as chronic pain in the sensory distribution of the pudendal nerve. The pudendal nerve is derived from sacral S2–4 nerve roots and provides motor control of the anal and urethral sphincters and pelvic floor muscles; sensory innervation to the anal, perineal and genital regions; and sympathetic innervation to the visceral organs of the pelvis. Conventional treatments for PN include pharmacotherapy, pudendal nerve block and nerve decompression surgery. In a recent study of chronic pudendal neuromodulation, 16 out of 19 patients with pudendal neuralgia had a significant improvement in symptomatology.3 Recently, Buffenois et al4 published a prospective series of patients who had failed to improve after pudendal nerve decompression surgery and who responded well to spinal cord stimulation at the level of the conus medullaris.
Less frequently discussed in the realm of neuromodulation is persistent genital arousal disorder (PGAD); a pelvic neuropathy characterised by unwelcome and intrusive episodes of genital arousal without corresponding desire or stimulation. The proposed pathophysiology includes small fibre-sensory neuropathy, vascular abnormalities, sacral cysts and pudendal nerve entrapment. One PGAD case report demonstrated a 90% reduction in spontaneous orgasms through the use of transcutaneous electrical nerve stimulation (TENS).5 There have been no documented reports of implantable sacral or pudendal neuromodulators for this condition.
Case presentation
A 35-year-old female presented with a 15-year history of urinary, gastro-intestinal and sexual dysfunction associated with pain in all three domains. The symptoms began after her first vaginal delivery and were initially limited to urinary frequency, urgency and nocturia, associated with a sensation of incomplete emptying and exquisite pain at the external urethral meatus. She later described experiencing a distressing and persistent arousal of the genitals which was unrelated to sexual desire or stimuli and unrelieved by orgasm. In addition, she described vulval paresthesia and shooting pains in the perineum with associated dyspareunia on vaginal penetration. Bowel symptoms included perceived constipation and a need to strain despite soft bowel motions. Symptoms were exacerbated by sitting, bladder or rectal filling, voiding and defecation. Pain scores ranged from 4/10 to 9/10 on a daily basis with significant impact on her activities of daily living (ADLs).
Past obstetric history revealed two vaginal deliveries, the first of which included an episiotomy and extensive vaginal tears. She described an otherwise uncomplicated menstrual and gynaecological history. The patient denied a history of sexual abuse or mental health disturbance. The patient's medical record demonstrated repeatedly normal urine microbiology, urodynamic studies, pelvic CT and ultrasounds. She had undergone a previous appendectomy and multiple cystoscopic procedures, including diathermy to a Hunner's ulcer. Current medications included the contraceptive pill, gabapentin and pentosan polysulfate sodium. The patient was a non-smoker and denied opiate or illicit drug use.
Physical examination revealed a Caucasian female with normal BMI, mild right-sided sacro-iliac joint tenderness and a non-tender abdomen. The external genitalia were unremarkable with hyperesthesia of the perineum and vestibule. Vaginal examination demonstrated a wide hiatus with myalgia of the levator ani muscles and exquisitely tenderness in the region of Alcock's Canal (ischial spine) bilaterally.
Despite fulfilling diagnostic criteria for PGAD6 and IC,7 the history and clinical examination is highly suggestive of bilateral pudendal neuralgia with potential nerve entrapment. This diagnosis could account for the persistent genital arousal symptoms and perhaps exacerbate the symptoms of interstitial cystitis. Clinical assessment by an experienced pelvic floor physiotherapist was also in agreement with this diagnosis. Psychologist review and the K10 assessment tool also demonstrated a very high level of psychological distress associated with her condition. The patient was started on duloxetine and amitriptyline as part of multimodal therapy. Additional analgesics included regular paracetamol and as required oxycodone and diazepam.
Investigations
The patient underwent pelvic MRI, which reported bilateral narrowing of the perineural space surrounding the pudendal nerve above the level of the ischial spine with a thick interligamentous band tethering the sacrospinous and sacrotuberous ligaments bilaterally. To confirm the diagnosis, an unguided bilateral pudendal nerve block was performed under general anaesthesia. The patient did not respond as expected to neural blockade with maintained perineal sensation and no reduction in symptoms. This suggested an unsuccessful infiltration of the nerve secondary to either anatomical variation or incorrect needle placement at the time of the procedure.
Differential diagnosis
See the Background section.
Treatment
Given the convincing clinical presentation and MRI findings, the patient was offered surgery in form of bilateral pudendal nerve release with neuromodulator insertion under general anaesthesia.
Operative findings included thickened ligaments which were adherent to the somewhat flattened pudendal nerves. The nerves were transposed medially. A four-lead neurostimulator was simultaneously implanted with two leads placed at the sacral hiatus and two leads placed adjacent to the pudendal nerves (figure 1). The sacral leads were programmed for the treatment of the pelvic and perineal pain, whereas the pudendal leads were programmed to address the urinary and arousal symptomatology.
Figure 1.
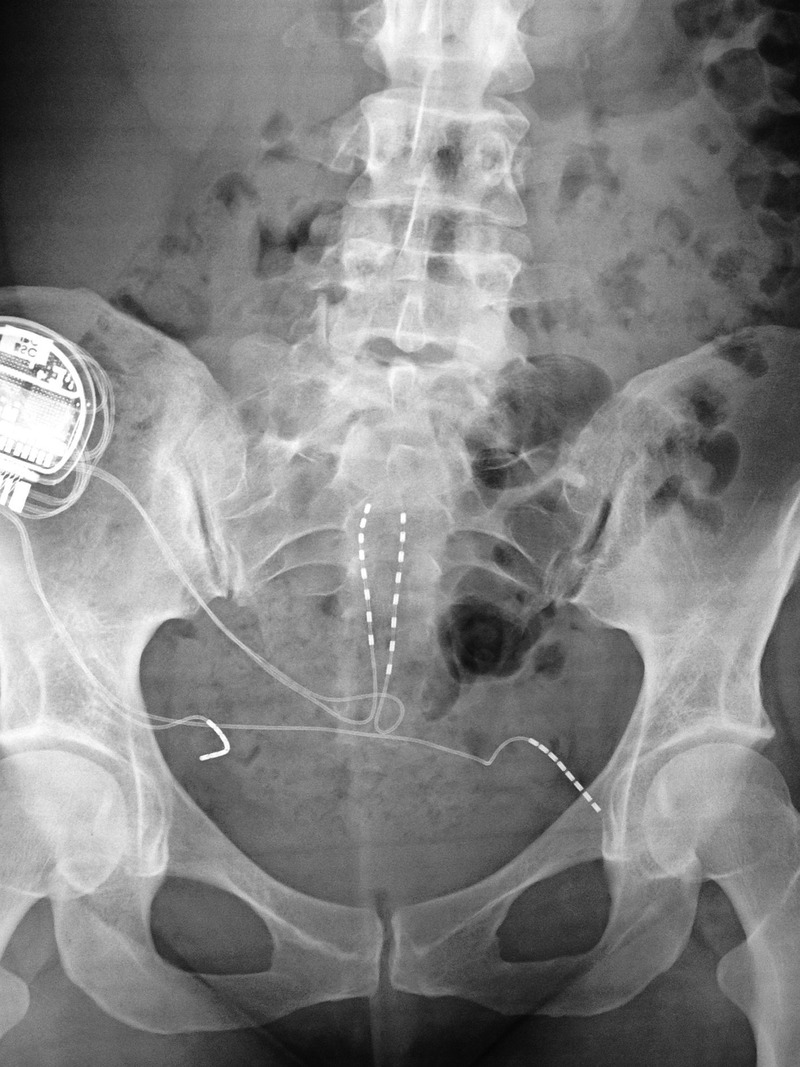
Intraoperative X-ray following insertion of the four-lead neurostimulator.
Outcome and follow-up
Three months postoperatively, a pelvic X-ray showed well-placed stimulator leads with minimal migration of the right pudendal lead. The patient described a moderate improvement in symptoms with normal bowel function, a substantial reduction in urinary symptoms and mild improvement in perineal pain. At 6-month review, the patient was free of all pelvic pain except after strenuous physical activity and regained her independence with ADLs. Her sensation of arousal had abated completely with only mild ongoing urinary urgency and frequency.
Discussion
PN, IC and PGAD are pelvic pain syndromes with emerging evidence of symptomatic improvement with neuromodulation therapy. Frequently, these patients experience a delay in diagnosis and receive inadequate therapies in the interim. These diagnoses have a significant clinical intersection, which could reflect the presence of a common derivation. Specifically, the pudendal nerve may play a more important role than that is currently recognised.
Direct PNS may magnify afferent pathway recruitment, permitting a greater response to therapy. PNS could allow for activation of somatic and autonomic pathways within the spinal cord and higher centres as opposed to a predominantly somatic response with a transforaminal approach. One pelvic pain study comparing SNS versus PNS of the pudendal nerve reported a 20% between-group difference in favour of PNS.1 Combined sacral and pudendal nerve stimulation was used in this case to (1) remove the abnormal irritation of the pudendal nerve resulting from the compression and (2) to globally modulate the complex somatic and autonomic pathways in a patient with urological, sexual and gastro-intestinal dysfunction through simultaneous stimulation at different levels with different parameters. We hope this case study may incite further interest in combined surgery and site-specific neuro-modulation for patients with complex pelvic neuropathies.
Learning points.
PN, IC and PGAD are complex pelvic neuropathies with significant clinical intersection.
Neuromodulation therapies have an emerging role in the treatment of these conditions.
Combining SNS and PNS may provide superior outcomes when compared to SNS or PNS alone.
Footnotes
Contributors: TGV provided the patient evaluation, treatment and follow-up. GLA performed the case review, literature review, manuscript writing and editing with assistance from TGV. All authors contributed equally to the drafting and review process and agreed for the submitted version.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Yang CC. Neuromodulation in male chronic pelvic pain syndrome: rationale and practice. World J Urol 2013;31:767–72. 10.1007/s00345-013-1066-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Comiter CV. Sacral neuromodulation for the symptomatic treatment of refractory interstitial cystitis: a prospective study. J Urol 2003;169:1369–73. 10.1097/01.ju.0000053863.96967.5a [DOI] [PubMed] [Google Scholar]
- 3.Peters KM, Killinger KA, Jaeger C et al. . Pilot study exploring chronic pudendal neuromodulation as a treatment option for pain associated with pudendal neuralgia. Low Urin Tract Symptoms 2015;7:138–42. 10.1111/luts.12066 [DOI] [PubMed] [Google Scholar]
- 4.Buffenoir K, Rioult B, Hamel O et al. . Spinal cord stimulation of the conus medullaris for refractory pudendal neuralgia: a prospective study of 27 consecutive cases. Neurourol Urodyn 2015;34:177–82. 10.1002/nau.22525 [DOI] [PubMed] [Google Scholar]
- 5.Waldinger MD, de Lint GJ, Venema PL et al. . Successful transcutaneous electrical nerve stimulation in two women with restless genital syndrome: the role of adelta- and C-nerve fibers. J Sex Med 2010;7:1190–9. 10.1111/j.1743-6109.2009.01578.x [DOI] [PubMed] [Google Scholar]
- 6.Basson R, Leiblum S, Brotto L, Definitions of women's sexual dysfunctions reconsidered: advocating expansion and revision. J Psychosom Obstet Gynaecol 2003;24:221–9. 10.3109/01674820309074686 [DOI] [PubMed] [Google Scholar]
- 7.Hanno PM, Burks DA, Clemens JQ et al. , Interstitial Cystitis Guidelines Panel of the American Urological Association Education and Research, Inc. AUA guideline for the diagnosis and treatment of interstitial cystitis/bladder pain syndrome. J Urol 2011;185:2162–70. 10.1016/j.juro.2011.03.064 [DOI] [PMC free article] [PubMed] [Google Scholar]


