Abstract
Human T-cell lymphotropic virus type-1 (HTLV-1) is endemic in Japan, the Caribbean and in South American countries such as Ecuador. This virus is the cause of HTLV-1-associated myelopathy or tropical spastic paraparesis (HAM/TSP), a myelopathy characterised by chronic progressive paraparesis, spasticity and urinary symptoms. We report the case of a 40-year-old man who received a kidney transplant from a living donor and developed HAM/TSP, 24 months after transplant. The diagnosis was confirmed by detection of HTLV-1 in blood and cerebrospinal fluid by the ELISA and Western Blot tests. For myelopathy, the patient was treated with pulse methylprednisolone, but had poor response to treatment. We recommend that all patients receiving transplants and their donors who come from endemic countries be given a mandatory screening for HTLV-1 through an ELISA test, in an effort to inform candidates for renal transplantation of the potential risk of infection and the development of this disease.
Background
The human T-cell lymphotropic virus type-1 (HTLV-1), is a δ-retrovirus that, along with HTLV-2, represents an oncogenic virus that infects the CD4, and CD45 RO T-lymphocytes.1 2 HTLV-1 is associated with adult T-cells leukaemia/lymphoma and HTLV-1-associated myelopathy or tropical spastic paraparesis (HAM/TSP). It has been estimated that about 15–20 million people worldwide have been infected by this virus. HTLV-1 is endemic in Japan, the Caribbean and countries in South America (Brazil, Peru, Ecuador and Venezuela).2 On the other hand, the seroprevalence of HTLV-1 in the USA and Europe is low (1%). The majority of affected patients come from endemic regions.3 4
The principal modes of transmission of HTLV-1 include prolonged breast feeding, sexual intercourse with seropositive individuals, intravenous drug injection, solid organ transplant (SOT) and transfusion of infected blood cells.5 6 However, few cases have been reported in SOT recipients, particularly after a kidney transplant. Once the individual is infected by the virus, the risk of it progressing to HAM/TSP is 1–2%, affecting females more frequently than males. The male/female ratio is 1:2.3 7
HAM/TSP is a chronic progressive myelopathy characterised by paraparesis, spasticity and urinary symptoms. Sensory involvement is occasional and usually mild.7–11 Most patients have an insidiously progressive course ranging from months to years. Nevertheless, about 10–20% of patients’ infections progress to severe gait impairment over a period of 1–3 months.12 In SOT recipients, the evolution of the disease is different because the latency period between infection and presentation of disease is shorter and characterised by a rapid clinical course with significant physical disability. The factors that facilitate the development of disease are an increased proviral load, immunodeficiency by administration of immunosuppressive drugs, association with host human leukocyte antigen (HLA) subtypes (B*5401, DRB1*0101), and non-HLA-related genetic factors involved in the transcription of cytokines such as tumour necrosis factor-α and interleukins 10, 15 and 28β; however, the mechanism is not well understood.7 13–16
Very few case reports have been described in the literature regarding HAM/TSP associated with SOT, specifically after kidney transplantation. In view of the high degree of disability caused by such circumstances, we have presented the following case report.
Case presentation
A 40-year-old man of Mestizo ethnicity, with a medical history of testicular cancer diagnosed at 20 years of age, had at that time been treated with right-side orchiectomy and chemotherapy. He had complete remission of the tumour. In addition, the patient had a history of hypertension from 30 years of age and chronic renal disease of unknown aetiology that began at 33 years of age and that was treated over 4 years with peritoneal dialysis up until kidney transplantation. At 37 years of age, he had undergone living donor renal transplantation from his niece. The patient received induction therapy based on methylprednisolone, basiliximab and mycophenolate mofetil. During transplant surgery, he had a complication involving hypovolemic shock, which required blood transfusion. Three months after transplantation, the patient presented acute humoural and cellular rejection requiring five sessions of plasmapheresis. Intravenous immunoglobulin treatment was not administered to this patient. After 4 months, he was diagnosed with an infection from polyomavirus with positive cytology for decoy cells, and is currently being treated with sirolimus, prednisone and mycophenolate sodium.
On this diagnosis, the patient was hospitalised due to weakness of the lower limbs, which had progressed over a period of <2 months, along with gait impairment, urinary retention and erectile dysfunction, which began occurring 1 week prior to entry.
General examination was normal. Neurological examination showed a lucid patient with weakness and spasticity of the lower extremities. A manual muscle strength test showed a weakness grade of 2 in both legs, and deep tendon reflexes were hyperactive with clonus. In addition, the patient presented bilateral Babinski's sign. He presented no loss of sensory involvement. The patient also had symptoms of urinary retention and erectile dysfunction indicating dysfunction of the autonomic nervous system. The disability measured by the Expanded Disability Status Scale (EDSS) was 7.
Investigations
The blood test on admission to the hospital showed normal blood count, blood chemistry, and CD4 and CD8 T-cell counts. Serum antibodies for HTLV-1 by the ELISA and Western Blot tests were positive. There was no evidence of co-infection with HIV, or hepatitis B or C. Regarding the tests of cerebrospinal fluid (CSF), antibodies for HTLV-1 were detected by the ELISA and Western Blot tests. The CSF protein content was 87 mg/dL (range 10–40) and leucocyte levels of 120 cells/mm3 (range 0–10), of which 100% were mononuclear with otherwise normal glucose content. In addition, the CSF Gram staining and bacterial culture, cryptococcal antigen, fungal and acid bacillus stains and cultures, cytology and PCR for herpes viruses (herpes simplex virus, cytomegalovirus, Epstein-Barr virus) were all negative.
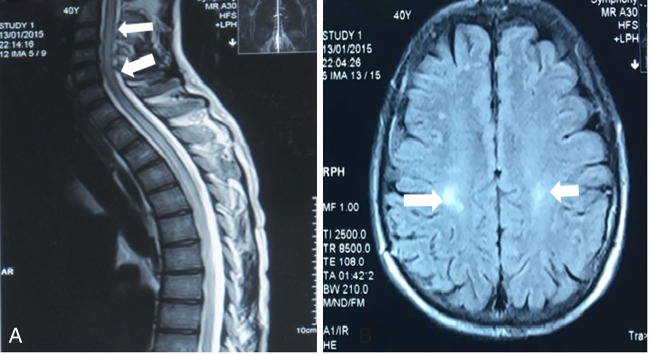
MRI of the brain showed multiple patchy foci of signal abnormality in periventricular white matter. Moreover, medullary MRI showed signs of hyperintensity in the cervical spinal cord up to the thoracic level in addition to spinal cord oedema (figure 1). Urodynamic study showed a hypotonic urinary bladder with retention. The serostatus of HTLV-1 of the patient and donor before transplantation was unknown.
Figure 1.
MRI findings. (A) High-intensity lesion in T2-weighted images of the cervical and thoracic spinal cord. (B) Axial fluid-attenuated inversion recovery image showed high-intensity lesions in the periventricular white matter.
Treatment
For myelopathy, the patient was treated with pulse methylprednisolone, with poor response to treatment.
Discussion
The patient met the WHO's diagnostic criteria for HAM/TSP. Clinical diagnosis was confirmed by the detection of HTLV-1 in blood and CSF by the ELISA and Western Blot tests (box 1).17 The pretransplant serology to HTLV-1 in the patient and the donor was unknown. It was not possible to conduct new serological studies for HTLV-1 with the donor because she had died more than a year prior by suicide, as a result of severe depression. Therefore, it was not possible to determine whether the patient had acquired the disease as a result of the donated organ, the blood transfusion given during kidney transplant surgery, or if the patient had already been an asymptomatic carrier of the virus before transplantation.
Box 1. Guidelines for the diagnosis of human T-cell lymphotropic virus type-1 (HTLV-1)-associated myelopathy/tropical spastic paraparesis (HAM/TSP).
Age and sex
▸ Mostly sporadic, sometimes familial. Adult females predominate, occasionally in childhood
Onset
▸ Usually insidious
Main neurological manifestations
▸ Chronic spastic paraparesis, which usually progresses slowly, but may remain static after initial progression
▸ Weakness of lower limbs, more marked proximally
▸ Bladder disturbance usually an early feature; constipation usually occurs later; impotence and decreased libido are common
▸ Sensory symptoms are more prominent than objective physical signs
▸ Low lumbar pain with radiation to the legs is common
▸ Vibration sense is frequently impaired
▸ Hyperreflexia of lower limbs, often with clonus and Babinski's sign
▸ Hyperreflexia of upper limbs; positive Hoffman's and Tromner's signs are frequent; weakness may be absent
▸ Exaggerated jaw jerk in some patients
Less frequent neurological findings
▸ Cerebellar signs, optic atrophy, deafness, nystagmus, other cranial nerve deficits, hand tremor, absent or depressed ankle jerk
Other neurological manifestations that may be associated with HAM/TSP
▸ Muscular atrophy, fasciculations, polymyositis, peripheral neuropathy, polyradiculopathy, cranial neuropathy, meningitis, encephalopathy
Systemic non-neurological manifestations that may be associated with HAM/TSP
▸ Pulmonary alveolitis, uveitis, Sjögren's syndrome, arthropathy, vasculitis, ichthyosis, cryoglobulinaemia, monoclonal gammopathy, adult T-cell leukaemia/lymphoma
Laboratory criteria
▸ Presence of HTLV-1 antibodies or antigens in blood and cerebrospinal fluid
▸ Cerebrospinal fluid may show mild lymphocytic pleocytosis
▸ Lobulated lymphocytes may be present in blood or cerebrospinal fluid, or in both
▸ Mild-to-moderate increase of protein may be present in cerebrospinal fluid
▸ Viral isolation from blood and/or cerebrospinal fluid when possible
*Adapted from Verdonck, et al.17
HAM/TSP is a disease that affects the spinal cord, with a predilection for the medial thoracic segments, but the entire spinal cord may be affected, including fibres that regulate muscle tone; areas such as the cerebellum, the brainstem and the vestibulospinal tract have also been affected.18 19 Postmortem studies have shown areas of demyelination and axonal loss in the anterior, lateral and posterior columns of the spinal cord with perivascular and parenchymal lymphocytic infiltration, and with the presence of foamy macrophages, proliferation of astrocytes and fibrillar gliosis.20
There are two theories to explain the mechanisms by which HTLV-1 causes myelopathy. One is the autoimmune theory, which suggests that HTLV-1 triggers the autoreactive T-cells, which in turn cause destructive changes in the central nervous system. The other theory is the cytotoxic model, which states that the HTLV-1 virus infects glial cells, initiating a cytotoxic immune response to these cells, thereby causing demyelination of white matter of the spinal cord.21 22
In reality, the development of HAM/TSP after kidney transplantation is rare and has been identified in very few reports in the literature, most of them from Japan (table 1).3 7 9 23–27
Table 1.
Cases of HAM/TSP after kidney transplantation
| Reference | Age | Gender | HLA typing | Donor | Period after transplantation (months) | Immunosuppressants | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | 58 | M | None reported | Cadaveric | 24 | CsA/MMF/prednisone | mPSL/IFN-α | Not effective |
| 51 | F | None reported | Cadaveric | 18 | CsA/MMF/prednisone | mPSL | Not effective | |
| 3 | 54 | F | A 2 | Cadaveric | 24 | CsA/MZR/mPSL | Oral prednisolone | Not effective |
| 57 | M | A 2 | Cadaveric | 24 | CsA/mPSL | Oral prednisolone | Not reported | |
| 7 | 59 | F | None reported | Living | 11 | TAC/MMF/prednisone | Raltegravir | Not effective |
| 9 | 38 | F | A 2 A 11 B 61 DR 11 DR 15 | Living | 2 | TAC/MMF | IFN-α | Improved |
| 21 | 32 | M | A 24 | Living | 11 | CsA/MZR /AZA | IFN-α | Temporarily improved |
| 22 | 54 | M | None reported | Cadaveric | 48 | CsA/MZR | None reported | Not reported |
| 23 | 56 | M | None reported | Cadaveric | 84 | CsA/MZR/mPSL | PE | Not reported |
| 24 | 54 | M | DR 4 DR 9 | Living | 10 | CsA/mPSL | IFN-α | Improved |
| 25 | 50 | M | None reported | Living | 5 | TAC/MMF/prednisone | Prednisone, zidovudine, lamivudine | Temporarily improved EDSS 7 |
| Present case | 40 | M | None reported | Living | 24 | MMF/sirolimus/prednisone | mPSL | Not effective |
Adapted from Nagamine et al.9
AZA, azathioprine; CsA, ciclosporin A; EDSS, Expanded Disability Status Scale; F, female; HAM/TSP, human T-cell lymphotropic virus type-1 (HTLV-1)-associated myelopathy or tropical spastic paraparesis; IFN-α, interferon-α; M, male; MMF, mycophenolate mofetil; mPSL, methylprednisolone; MZR, mizoribine; PE, plasma exchange; TAC, tacrolimus.
On the basis of a series of case studies, it has been shown that the average development time of HAM/TSP is longer in individuals who received a kidney transplant from a deceased donor (from 17 months to 7 years, average 3.3 years) when compared with patients who received a transplant from a living donor (as in the case of our patient). The average development time of HAM/TSP of patients was shorter (10–11 months) when the organ came from a living donor. The reason for this is unknown.3 7 If we assume that our patient's infection was acquired through the transplanted kidney, the period of time in which symptoms developed was longer than what has been described in the literature.
Another aspect to consider in the case of our patient is the possibility that HTLV-1 was acquired from blood transfusions. It is known that 40% of patients who developed a HAM/TSP received one or more blood transfusions. It is also noteworthy that the seroconversion rate (40–60%) and the development of the disease is higher in patients who acquired the virus through contaminated blood products than it is in patients who were infected by other means such as breast milk or sexual intercourse.3 It is estimated that 5% of patients infected with the virus will have symptoms and 0.3% will develop myelopathy.3 28 As a pretransplant screening for HTLV-1 was never conducted in our patient’s case, it is impossible to know if the infection was acquired during the administration of blood transfusions required to treat hypovolemic shock.
Kidney transplant alone is not considered a risk factor for the occurrence of HAM/TSP.29 30 However, the Infectious Diseases Society of America (IDSA) and the Transplantation Community of Practice recommend screening HTLV-1 in populations at high risk, such as the people in our country, followed by disease monitoring in SOT recipients in whom it is suspected that the transplanted organ is seropositive and also in those who have received an infected organ for transplantation. The degree to which this practice is implemented worldwide is currently unknown2 and, unfortunately, in our country, prerenal transplant screening is not a policy.
The ELISA screens for HTLV-1/2, but cannot distinguish between the two types. For this reason, diagnosis of HTLV-1 requires confirmatory tests, which include the Western Blot and PCR. Unfortunately, due to high costs, it is not possible to provide these confirmatory tests in developing countries. The ELISA is highly sensitive and specific (98% and 100%, respectively). However, it lacks a high positive predictive value when applied to populations of low prevalence.2 31 A study conducted in Peru showed that, in this population of high prevalence, the positive predictive value of the ELISA test reached 100%.32 That study suggests that, in areas of high prevalence, confirmation with Western Blot or PCR would not be necessary.
The imaging study of choice for the brain and spinal cord is MRI. In most cases of patients infected with HTLV-1, spinal cord MRIs are normal (58%). Atrophy of the cervical and thoracic segments are present in 34% of cases, and only 8% of cases show hyperintense lesions on the spinal cord on T2-weighted sequences, as presented in our patient.33 The incidence of brain MRI abnormalities in patients with HAM/TSP is common (25–80%). When present, they appear in deep periventricular, subcortical brain regions.34
As can be seen in our patient's case, the effect of HAM/TSP on the pyramidal tracts can result in serious disability. According to the epidemiological risk, screening for this virus should be performed in high-risk populations, such as the people in our country, in an effort to inform candidates for renal transplantation of the potential risk of infection and the development of this disease.35
With regard to treatment, there is no standard therapy for HAM/TSP and, so far, no treatment has proven to improve the prognosis of this disease. Several studies have reported clinical improvement with the use of anti-inflammatories and immunomodulators. Glucocorticoids are the most commonly used drugs. The explanation for their apparent effectiveness is attributed to their ability to reduce the expression of proinflammatory genes, resulting in several benefits, including: reduction of the migration of inflammatory cells, regulation of Th1/Th2 cytokines and reduction in the proviral load.36 However, there are no reliable data to guide clinicians regarding the initiation, dose or duration of treatment, due to a lack of well-designed controlled trials. Very little evidence based on controlled case studies can suggest the use of steroid treatments.36 37 Our patient received intravenous methylprednisolone after serological diagnosis. However, we did not see evidence of clinical improvement in EDSS.
There are also limited data on the use of plasmapheresis. In a number of cases, transient clinical improvement (2–4 weeks) was demonstrated in more than half of the patients treated with plasmapheresis, but these were an uncontrolled series of cases. Anti-HTLV-I-antibody titres were lowered in serum but not in the CSF following plasmapheresis. Future studies analysing the relationship between changes to the titres of antineuronal antibody implicated in HAM/TSP and clinical response following plasmapheresis may further our understanding of the relative pathogenic contribution of anti-HTLV-I humoural response. It is unknown whether treatment with periodic plasmapheresis or immunoglobulin could lead to sustained clinical benefits for patients.33
Daclizumab reduces the number of CD4+CD25+ cells and reduces the HTLV-1 proviral load, achieving a partial inhibition of immune activation of the virus. However, there is a lack of clinical trials in patients with HAM/TSP available to us.37 Interferon-α is considered a standard treatment of HAM/TSP in Japan, and has shown a clinical benefit in patients in a double-blind trial. The drug has antiviral and cytostatic properties, but its long-term benefit is still unknown.36–38 However, a meta-analysis showed that treatment with interferon-α for hepatitis C in patients with renal transplantation was poorly tolerated and resulted in diminished safety. Therefore, administration of interferon-α is not recommended in patients with renal transplantation.39
Although observational studies suggest a clinical benefit and a reduction in the proviral load of HTLV-1 with lamivudine and zidovudine, a double-blind placebo-controlled study showed neither benefit nor reduction in the proviral load of HTLV-1.40 An open clinical trial in Nagasaki, Japan, showed that using prosultiamine can safely improve motor dysfunction of the lower extremities, as well as improve bladder function and reduce the proviral load of HTLV-1 in peripheral blood. Therefore, this medicine is considered by many to be the latest therapeutic tool for patients with HAM/TSP.41
In conclusion, this case report allows us to demonstrate that HAM/TSP after renal transplantation is a rare, yet serious condition that leads to a high degree of disability. For this reason, we recommend a mandatory screening for HTLV-1 through the ELISA in endemic countries such as ours, because, as in the case of this particular patient, there could have been multiple ways of acquiring the virus and the source of infection cannot be determined. Moreover, as we have seen, the disease is rapidly evolving in the context of organ transplant recipients receiving immunosuppressants.
Learning points.
Human T-cell lymphotropic virus type-1 (HTLV-1) is associated with adult T-cell leukaemia/lymphoma and HTLV-1 associated myelopathy or tropical spastic paraparesis (HAM/TSP).
The principal modes of transmission of HTLV-1 include prolonged breast feeding, sexual intercourse with seropositive individuals, intravenous drug injection, solid organ transplant and transfusion of infected blood cells.
HAM/TSP is a chronic progressive myelopathy characterised by paraparesis, spasticity and urinary symptoms.
The Infectious Diseases Society of America (IDSA) and the Transplantation Community of Practice recommend pretransplant screening of HTLV-1 in populations at high risk.
Footnotes
Contributors: MJMA and EPCD contributed to the analysis of article and acquisition of data. MEB contributed to the acquisition of data.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Villafruela Mateos A, Arruza Echevarría A, Martín Bazaco J et al. [HTLV infection after renal transplant]. Arch Esp Urol 2005;58:1064–8. [DOI] [PubMed] [Google Scholar]
- 2.Kaul DR, Davis JA, AST Infectious Diseases Community of Practice. Human T cell lymphotropic virus 1/2 in solid organ transplantation. Am J Transplant 2013;13(Suppl 4):355–60. 10.1111/ajt.12127 [DOI] [PubMed] [Google Scholar]
- 3.Zarranz Imirizaldu JJ, Gomez Esteban JC, Rouco Axpe I et al. Post-transplantation HTLV-1 myelopathy in three recipients from a single donor. J Neurol Neurosurg Psychiatry 2003;74:1080–4. 10.1136/jnnp.74.8.1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toro C, Benito R, Aguilera A et al. Infection with human T lymphotropic virus type I in organ transplant donors and recipients in Spain. J Med Virol 2005;76:268–70. 10.1002/jmv.20331 [DOI] [PubMed] [Google Scholar]
- 5.Gotuzzo Herencia E, González Lagos E, Verdonck Bosteels K et al. Veinte años de investigación sobre HTLV-1 y sus complicaciones médicas en el Perú: Perspectivas generales. Artículo de revisión. Acta Med Per 2010;27:196–203. [Google Scholar]
- 6.Khameneh ZR, Sepehrvand N, Masudi S et al. Seroprevalence of HTLV-1 among kidney graft recipients: a single-center study. Exp Clin Transplant 2010;8:146–9. [PubMed] [Google Scholar]
- 7.Torres JA, Taimur S. Postrenal transplant human T-cell lymphotropic virus type I-associated myelopathy/tropical spastic paraparesis: a case report and review of the literature. Transplant Direct 2015;1:e3 10.1097/TXD.0000000000000512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsukasaki K, Tobinai K. Human T-cell lymphotropic virus type I-associated adult T-cell leukemia-lymphoma: new directions in clinical research. Clin Cancer Res 2014;20:5217–25. 10.1158/1078-0432.CCR-14-0572 [DOI] [PubMed] [Google Scholar]
- 9.Nagamine Y, Hayashi T, Kato Y et al. Human T lymphotropic virus type-1-associated myelopathy manifesting shortly after living-donor renal transplantation. Intern Med 2015;54:75–8. 10.2169/internalmedicine.54.2950 [DOI] [PubMed] [Google Scholar]
- 10.Román GC, Navarro-Román LI. The discovery of HTLV-1 myelitis: 21 years later. Lancet Neurol 2007;6:104–5. 10.1016/S1474-4422(07)70012-1 [DOI] [PubMed] [Google Scholar]
- 11.Kendall EA, González E, Espinoza I et al. Early neurologic abnormalities associated with human T-cell lymphotropic virus type 1 infection in a cohort of Peruvian children. J Pediatr 2009;155:700–6. 10.1016/j.jpeds.2009.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olindo S, Cabre P, Lézin A et al. Natural history of human T-lymphotropic virus 1-associated myelopathy: a 14-year follow-up study. Arch Neurol 2006;63:1560–6. 10.1001/archneur.63.11.1560 [DOI] [PubMed] [Google Scholar]
- 13.Ghaffari J, Ebrahimi M, Makhlough A et al. Seroepidemiology of human T-cell lymphotropic virus 1 infection in hemodialysis patients: should we be concerned about it? Iran J Kidney Dis 2013;7:187–90. [PubMed] [Google Scholar]
- 14.Zarranz JJ, Rouco I, Gómez-Esteban JC. Human T lymphotropic virus type I (HTLV-1) associated myelopathy acquired through a liver transplant. J Neurol Neurosurg Psychiatry 2001;71:818 10.1136/jnnp.71.6.818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sabouri AH, Saito M, Lloyd AL et al. Polymorphism in the interleukin-10 promoter affects both provirus load and the risk of human T lymphotropic virus type I-associated myelopathy/tropical spastic paraparesis. J Infect Dis 2004;190:1279–85. 10.1086/423942 [DOI] [PubMed] [Google Scholar]
- 16.Treviño A, Lopez M, Vispo E et al. Development of tropical spastic paraparesis in human T-lymphotropic virus type 1 carriers is influenced by interleukin 28B gene polymorphisms. Clin Infect Dis 2012;55:e1–4. 10.1093/cid/cis343 [DOI] [PubMed] [Google Scholar]
- 17.Verdonck K, González E, Van Dooren S et al. Human T-lymphotropic virus 1: recent knowledge about an ancient infection. Lancet Infect Dis 2007;7:266–81. 10.1016/S1473-3099(07)70081-6 [DOI] [PubMed] [Google Scholar]
- 18.Labanca L, Borges Starling AL, de Sousa-Pereira SR et al. Electrophysiological analysis shows dizziness as the first symptom in human T cell lymphotropic virus type-associated myelopathy/tropical spastic paraparesis. AIDS Res Hum Retroviruses 2015;31:649–54. 10.1089/AID.2014.0153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dixon PS, Bodner AJ, Okihiro M et al. Human T-lymphotropic virus type I (HTLV-l) and tropical spastic paraparesis or HTLV-1-associated myelopathy in Hawaii. West J Med 1990;152:261–7. [PMC free article] [PubMed] [Google Scholar]
- 20.Saito M, Bangham CR. Immunopathogenesis of human T-cell leukemia virus type-1-associated myelopathy/tropical spastic paraparesis: recent perspectives. Leuk Res Treatment 2012;2012:259045 10.1155/2012/259045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.IlIum S, Washburn R. Human T-cell lymphotropic virus type myelopathy/tropical spastic paraparesis in a 65-year-old man. Hosp Physician 2006;42:66–71. [Google Scholar]
- 22.Gessain A, Mahieux R. Tropical spastic paraparesis and HTLV-1 associated myelopathy: clinical, epidemiological, virological and therapeutic aspects. Rev Neurol (Paris) 2012;168:257–69. 10.1016/j.neurol.2011.12.006 [DOI] [PubMed] [Google Scholar]
- 23.Kuroda Y, Takashima H, Yukitake M et al. Development of HTLV-1 associated myelopathy after blood transfusion in a patient with aplastic anemia and a recipient of renal transplant. J Neurol Sci 1992;109:196–9. 10.1016/0022-510X(92)90168-K [DOI] [PubMed] [Google Scholar]
- 24.Nakatsuji Y, Sugai F, Watanabe S et al. HTLV-1-associated myelopathy manifested after renal transplantation. J Neurol Sci 2000;177:154–6. 10.1016/S0022-510X(00)00332-4 [DOI] [PubMed] [Google Scholar]
- 25.Shinntani Y, Hirano A, Inagaki T et al. A case of HTLV-1 associated myelopathy manifested after cadaveric renal transplantation. Ishoku (Japanese Journal of Transplantation) 2002;37:85–7. [Google Scholar]
- 26.Inose Y, Akiyama S, Mochizuki A et al. A case report of HTLV-1 associated myelopathy (HAM) manifested after renal transplantation. Clin Neurol 2010;50:241–5. [DOI] [PubMed] [Google Scholar]
- 27.Gonzales L, Jorge L, Mario P et al. HTLV-1 associated myelopathy of rapid onset and progression following living-donor kidney transplant: clinical description and initial response to empirical treatment. Retrovirology 2011;8(Suppl 1):A56 10.1186/1742-4690-8-S1-A56 [DOI] [Google Scholar]
- 28.Manns A, Wilks RJ, Murphy EL et al. A prospective study of transmission by transfusion of HTLV-I and risk factors associated with seroconversion. Int J Cancer 1992;51:886–91. 10.1002/ijc.2910510609 [DOI] [PubMed] [Google Scholar]
- 29.Naghibi O, Nazemian F, Naghibi M et al. Prognosis of HTLV-1 positive renal transplant recipients in Iran. Saudi J Kidney Dis Transpl 2011;22:670–4. [PubMed] [Google Scholar]
- 30.Armstrong MJ, Corbett C, Rowe IA et al. HTLV-1 in solid-organ transplantation: current challenges and future management strategies. Transplantation 2012;94:1075–84. 10.1097/TP.0b013e318263ad7a [DOI] [PubMed] [Google Scholar]
- 31.Kaul DR, Taranto S, Alexander C et al. , HTLV Donor Screening Advisory Group. Donor screening for human T-cell lymphotropic virus 1/2: changing paradigms for changing testing capacity. Am J Transplant 2010;10:207–13. 10.1111/j.1600-6143.2009.02867.x [DOI] [PubMed] [Google Scholar]
- 32.Verdonck K, González E, Maldonado F et al. Comparison of three ELISAs for the routine diagnosis of human T-lymphotropic virus infection in a high-prevalence setting in Peru. Trans R Soc Trop Med Hyg 2009;103:420–2. 10.1016/j.trstmh.2008.12.002 [DOI] [PubMed] [Google Scholar]
- 33.Yukitake M, Takase Y, Nanri Y et al. Incidence and clinical significances of human T-cell lymphotropic virus type I-associated myelopathy with T2 hyperintensity on spinal magnetic resonance images. Intern Med 2008;47:1881–6. 10.2169/internalmedicine.47.1284 [DOI] [PubMed] [Google Scholar]
- 34.Morgan DJ, Caskey MF, Abbehusen C et al. Brain magnetic resonance imaging white matter lesions are frequent in HTLV-I carriers and do not discriminate from HAM/TSP. AIDS Res Hum Retroviruses 2007;23:1499–504. 10.1089/aid.2007.0077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramanan P, Deziel PJ, Norby SM et al. Donor-derived HTLV-1 associated myelopathy after transplantation: a call for targeted screening. Am J Transplant 2015;15:1125 10.1111/ajt.13146 [DOI] [PubMed] [Google Scholar]
- 36.Pillat MM, Bauer ME, de Oliveira AC et al. HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP): still an obscure disease. Cent Nerv Syst Agents Med Chem 2011;11:239–45. 10.2174/1871524911106040239 [DOI] [PubMed] [Google Scholar]
- 37.Oh U, Jacobson S. Treatment of HTLV-I-associated myelopathy/tropical spastic paraparesis: towards rational targeted therapy. Neurol Clin 2008;26:781–97. 10.1016/j.ncl.2008.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Izumo S, Goto I, Itoyama Y et al. Interferon-alpha is effective in HTLV-I-associated myelopathy: a multicenter, randomized, double-blind, controlled trial. Neurology 1996;46:1016–21. 10.1212/WNL.46.4.1016 [DOI] [PubMed] [Google Scholar]
- 39.Fabrizi F, Lunghi G, Dixit V et al. Meta-analysis: anti-viral therapy of hepatitis C virus-related liver disease in renal transplant patients. Aliment Pharmacol Ther 2006; 24:1413–22. 10.1111/j.1365-2036.2006.03151.x [DOI] [PubMed] [Google Scholar]
- 40.Taylor GP, Goon P, Furukawa Y et al. Zidovudine plus lamivudine in human T-lymphotropic virus type-I-associated myelopathy: a randomised trial. Retrovirology 2006;3:63 10.1186/1742-4690-3-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakamura T, Matsuo T, Fukuda T et al. Efficacy of prosultiamine treatment in patients with human T lymphotropic virus type I-associated myelopathy/tropical spastic paraparesis: results from an open-label clinical trial. BMC Med 2013;11:182 10.1186/1741-7015-11-182 [DOI] [PMC free article] [PubMed] [Google Scholar]