Abstract
A 6½ years Indian boy was brought by his parents, who were anxious about the excessive increase in the size of the boy's phallus, from the age of 2 years. On physical examination, the child had a penis length greater than the 97th centile for age, a sexual maturity rating of gonads at stage 2 and pubic hair at stage 3, with height in the high normal range (90–97th centile). The bone age was 12 years. Laboratory evaluation showed pubertal levels of testosterone and pubertal gonadotropin response to stimulation, confirming central precocious puberty (CPP). Incidentally, the hormonal profile also suggested congenital adrenal hyperplasia (CAH). This case report depicts a case of CPP probably caused by CAH in boys, which is rare.
Background
The aetiology of precocious or premature puberty needs to be localised to a Gonadotropin-releasing hormone (GnRH)-dependent (central) or GnRH-independent cause. The delay in management can lead to permanent short stature, sexual anxiety and sexual abuse. Although central precocious puberty (CPP) is usually caused by central nervous system (CNS) lesions or idiopathic reasons, rarely, CAH can be a peripheral cause of CPP. There are very few case reports of this aetiology.1 2 The management should be focused on CAH as well as on arresting puberty. This case report adds to the knowledge of paediatricians and general practitioners of the aetiology of sexual precocity.
Case presentation
A 6½ years Indian boy (figure 1) was brought by his parents due to their anxiety about the boy's continuing growth in phallic length since the age of 2 years. There was an increase in pubic hair for the past 6 months. He had no history of headache, seizures, visual problems, behavioural changes or neurological deficits. There was no history of head trauma or surgery or prolonged drug intake. Family history and perinatal history was unremarkable. Physical examination revealed a blood pressure of 98/62 mm Hg (<90th centile), with no postural drop. The rest of his vitals were normal. Anthropometric measurements were: weight 23 kg (75–97th centile), height 127 cm (90–97th centile);3 height SD score (SDS) ‘+1.67’; midparental height 156 cm, target height SDS ‘−2.04’; upper segment to lower segment ratio 1.01. Genital examination revealed tanner stage 2 of scrotum, and testicular volume of 4 mL on the right and 3 mL on the left.4 Pubic hair was tanner stage 3 (figures 2 and 3). The stretched penile length was 8 cm, longer than normal for age.5 There were no skin or bone lesions suggestive of McCune Albright syndrome. Systemic examination was unremarkable. A clinical diagnosis of precocious puberty was provisionally made.
Figure 1.

Profile picture of patient.
Figure 2.

Sexual maturity rating: tanner score of pubic hair stage 3, genitalia stage 2.
Figure 3.

Testicular volume 4 mL on right side, with the left side being 3 mL.
Investigations
Complete blood count, renal and liver function tests were normal. Serum Na was 138 meq/L and K was 4.2 meq/L. Urine routine was normal.
Hand X-ray bone age was 12 years (figure 4). Chest X-ray has shown normal study.
Figure 4.
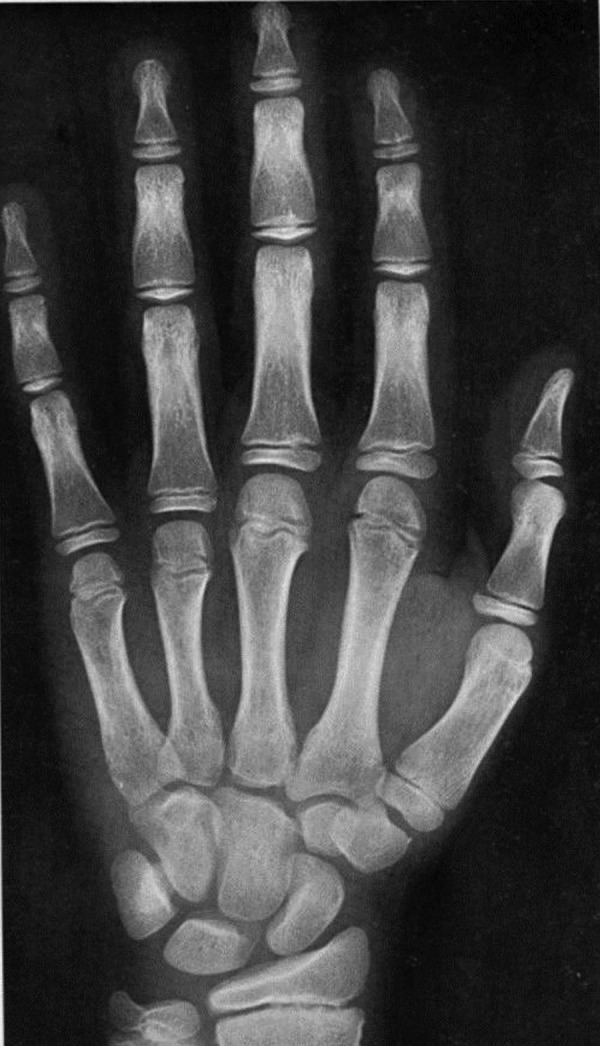
Bone age 12 years.
Ultrasound sonography (USG) of the scrotum showed volume of 3.6 and 4.2 mL in the left and right testis, respectively. USG of the whole abdomen was unremarkable, with no enlargement of adrenals.
MRI of the hypothalamus-pituitary region revealed mild hyperplasia of the anterior pituitary gland (11×7×6 mm), which was concurred with the radiologist to be physiological pituitary hyperplasia (figure 5); and brain screening was normal.
Figure 5.
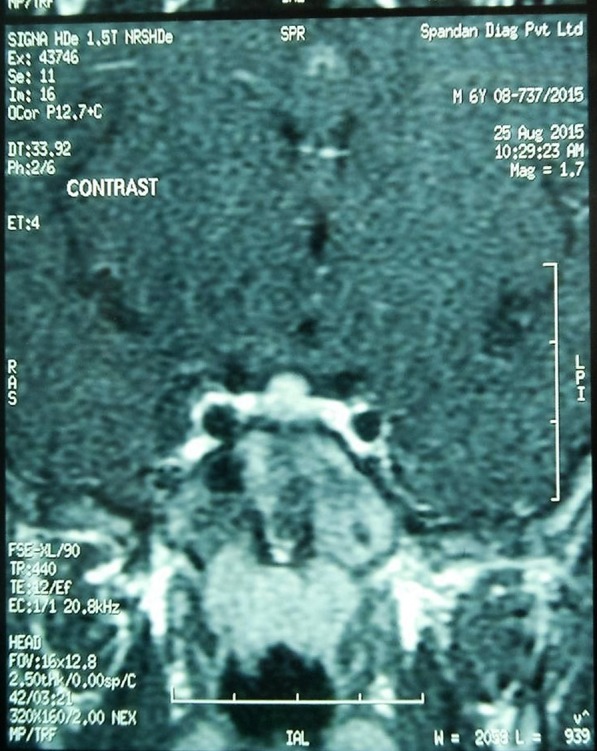
MRI of the hypothalamus-pituitary region showing mild hyperplasia of the anterior pituitary gland.
Diagnosis
This 6½ years young boy had clinical features of accelerated bone age, and signs of puberty as indicated by testicular volume, pubic hair and penile length. His serum testosterone was in pubertal range with pubertal levels of gonadotropins ((luteinising hormone (LH), follicle stimulating hormone (FSH); table 1). A GnRH stimulation test was carried out, which showed a pubertal response further confirming puberty (table 1). These features confirmed gonadotropin-dependent CPP. MRI did not show abnormality of the hypothalamus-pituitary region except for anterior pituitary hyperplasia, as in physiological puberty (figure 5), and β-human chorionic gonadotropin (HCG) levels were normal (table 1), ruling out CNS lesions and HCG secreting tumours, respectively.6
Table 1.
Hormonal evaluation
| Test (serum) | Value | Normal range |
|---|---|---|
| Free T4, ng/dL | 0.898 | 0.58–1.64 |
| Thyroid stimulating hormone (TSH), micro IU/mL | 1.88 | 0.34–4.2 |
| Testosterone, ng/dL | 910 | 290–1000 ( adult) |
| Follicle stimulating hormone (FSH), mIU/mL | 1.36 | 1.79–8.78 (adult) |
| FSH (40 min post-100 µg triptorelin injection s.c), mIU/mL | 2.27 | |
| Luteinising hormone (LH), mIU/mL | 1.20 | 2.12–10.89 (pubertal range) |
| LH (40 min post-100 µg triptorelin injection s.c) mIU/mL | 12.49 | |
| Human chorionic gonadotropin (β subunit) (β-HCG), mIU/mL | <0.60 | Up to 2.6 |
| 17-hydroxyprogesterone (17-OHP), ng/mL | 20.1 | 0.05–1.6 |
| Cortisol (8:00), mcg/dL | 4.9 | 3.7–19.4 |
| Adrenocorticotrophic hormone (ACTH), pg/mL | 188 | 7–63 |
| Post-cosyntropin stimulation cortisol, mcg/dL | 15.1 | |
| Dihydroepiandrosterone sulfate (DHEA-S), mcg/dL | 343.5 | 2.8–85.2 For a 6-year-old male |
| Plasma renin activity, ng/mL/hour | 2.53 | Supine 0.50–1.90 |
| Serum aldosterone, pg/mL | 455.70 | Age 3–16 years Range=12–340 |
The penis had started enlarging at 2 years, before scrotal enlargement. The testicular volumes on both sides were mildly asymmetrical. These are features of a peripheral cause of puberty.7
Further laboratory evaluation revealed the 17-hydroxy (OH)progesterone to be very high, with low cortisol levels (table 1). Serum dehydroepiandrosterone sulfate was also elevated for age (table 1). A short synacthen test showed adequate cortisol reserve and normal aldosterone and renin levels (table 1). These laboratory parameters were indicative of a diagnosis of classic non-salt wasting CAH. The final diagnosis was a case of GnRH-dependent CPP probably due to untreated classic non-salt wasting CAH.1 2
Treatment
Oral hydrocortisone at 15 mg/m2 per day was initiated to suppress the adrenocorticotropic hormone (ACTH) feedback response and thereby to decrease the androgen production. Blood pressure and serum potassium were monitored monthly. The dose of hydrocortisone was titrated to reduce the serum 17-OH progesterone at the upper limit of normal. Luteinising hormone releasing hormone (LHRH) analogue ( 3.75 mg SC leuprolide injection) monthly depot injection was suggested to the patient's parents as an adjunct treatment to hydrocortisone, for preventing final height reduction in this case of precocious puberty.
Outcome and follow-up
The patient is being followed up with assessment of height, height velocity, weight and secondary sexual characteristics, initially monthly for the first few months and thereafter after every three months. Bone age by hand X-ray will be carried out every six months, to observe slowing of acceleration of bone growth. Lifelong hydrocortisone therapy is indicated; LHRH analogue therapy for 1 or 2 years will also be required, to suppress the pubertal cyclical surge of LH and FSH hormones, thereby arresting the increasing penile length and testicular growth and, most importantly, preventing premature closure of the epiphyseal growth plates, thereby preventing short stature.
Discussion
Precocious puberty in a boy is characterised by the appearance of any sign of secondary sexual maturation before the age of 9 years. True or CPP is due to premature reactivation of the hypothalamic GnRH pulse generator; the changes are GnRH and gonadotropin dependent. The hormonal profile shows pubertal levels of LH, FSH and testosterone. The causes are either idiopathic or CNS lesions or, rarely, due to late treatment of CAH.6 Incomplete or GnRH-independent precocious puberty is due to HCG secreting tumours and autonomous androgen secretion by the adrenal gland or by the testis. The hormonal profile shows pubertal levels of testosterone but suppressed levels of LH and FSH. CAH usually presents as GnRH-independent precocity in boys with suppressed LH levels, elevated androgens and prepubertal testicular size.8
Rarely, CAH can present with GnRH-dependent precocious puberty in males. The mechanism is due to chronic elevated levels of androgen precursors causing premature reactivation of the GnRH pulse generator of the hypothalamus, and there is a direct correlation with advanced bone age.1 2
Even though the pubertal response to GnRH stimulation was typical of a central source in this patient, the increase in penis length even at 2 years, long before testicular enlargement, denotes a peripheral source of androgens too. The 17-0H progesterone levels were elevated, indicative of CAH.
The treatment of CAH is by glucocorticoids, to reduce the ACTH-driven androgen production using negative feedback, as in this case. The goal is to arrest puberty and delay final height reduction, which can occur in precocious puberty due to premature epiphyseal fusion. Treatment with LHRH analogue for 2–3 years and supplementation with hydrocortisone have demonstrated decreased height loss in CAH with CPP .1 2
Learning points.
Congenital adrenal hyperplasia (CAH) can present as GnRH-dependent central precocious puberty in males.
Early identification is necessary to prevent short stature, social anxiety and child abuse.
Lifelong hydrocortisone for CAH and luteinising hormone releasing hormone analogue to arrest puberty is used for treatment.
Footnotes
Contributors: PKS and KSGS contributed to the clinical diagnosis, initial laboratory workup and treatment. NS and KC contributed to the discussion regarding differential diagnosis and in parental counselling, and helped in editing this article.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Pescovitz OH, Comite F, Cassorla F et al. True precocious puberty complicating congenital adrenal hyperplasia: treatment with a luteinizing hormone-releasing hormone analog. J Clin Endocrinol Metab 1984;58:857–61. 10.1210/jcem-58-5-857 [DOI] [PubMed] [Google Scholar]
- 2.Soliman AT, AILamki M, AISalmi I et al. Congenital adrenal hyperplasia complicated by central precocious puberty: linear growth during infancy and treatment with gonadotropin-releasing hormone analog. Metabolism 1997;6:513–17. [DOI] [PubMed] [Google Scholar]
- 3.Khadilkar V, Yadav S, Agrawal KK, et al. , Indian Academy of Pediatrics Growth Charts Committee. Revised IAP growth charts for height, weight and body mass index for 5- to 18-year-old Indian children. Indian Pediatr 2015;52:47–55. 10.1007/s13312-015-0566-5 [DOI] [PubMed] [Google Scholar]
- 4.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child 1969;44:291–303. 10.1136/adc.44.235.291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee PA, Mazur T, Danish R et al. Micropenis. I. Criteria, etiologies and classification. Johns Hopkins Med J 1980;146:156–63. [PubMed] [Google Scholar]
- 6.Grumbach MM. True or central precocious puberty. In: Kreiger DT, Bardin CW, eds. Current therapy in endocrinology and metabolism. Toronto, BC: Decker, 1985:4–8. [Google Scholar]
- 7.Melmed S, Polonsky SK, Larsen RP et al. Flow chart for diagnosing sexual precocity in a phenotypic male. Figure 25-72. In: Melmed S, Polonsky SK, Larsen R et al. eds. WILLIAMS textbook of endocrinology. 12th edn Elsevier Saunders, 2012:25–72. [Google Scholar]
- 8.Melmed S, Polonsky SK, Larsen RP et al. Differential diagnosis of sexual precocity. Table 25-4. In: Melmed S, Polonsky SK, Larsen R et al. eds. WILLIAMS textbook of endocrinology. 12th edn Elsevier Saunders, 2012:1175. [Google Scholar]


