Abstract
A 36-year-old man presented to hospital with gross haematuria and evidence of severe, refractory thrombotic thrombocytopenic purpura. Initial treatment with high-volume plasma exchange therapy and early administration of rituximab failed to achieve a sustained clinical response. His clinical course was complicated by left hemianopsia and despite an urgent splenectomy he developed a large right-sided stroke with malignant cerebral oedema that required an emergent decompressive craniotomy. He also had numerous infectious complications as a consequence of an aggressive immunosuppressive strategy. While the patient did not respond to cyclophosphamide, cyclosporine, N-acetylcysteine, and one course of bortezomib, he eventually responded to a second course of bortezomib. One year later, the patient remains in remission and maintains excellent cognitive function. However, he has not completely recovered from his stroke and continues to participate in rehabilitation for his residual physical deficits.
Background
Refractory thrombotic thrombocytopenic purpura (TTP) is defined for a patient whose ADAMTS13 at presentation is less than 10%, with no or transient response to plasma exchange for less or up to 30 days of therapy; platelet count remains below 100×109/L; lactate dehydrogenase (LDH) level is above 1.5 times the upper limit of normal, red cell fragments present in the peripheral blood; and the presence of either worsening neurological or renal function without an identifiable cause. Refractory TTP can associate with severe, rapidly evolving complications. Clinicians are often challenged to balance the timing of and need for aggressive immunosuppressive therapy with the risk of life-threatening infections. Here, we present a case of severe, rapidly evolving refractory TTP, and discuss an early and aggressive approach with immunosuppressive therapy.
Case presentation
A 36-year-old man presented with a 1-day history of gross haematuria without other systemic symptoms. He had a medical history of psoriasis and a previous history of TTP, in 2012, attributed to ustekinumab.1 His only medication was acitretin.
Investigations
This patient's initial platelet count was 20×109/L (normal 150–400×109/L), LDH level was 520 U/L (normal less than or equal to 225 U/L), ADAMTS13 activity was less than 1% (normal 41–130%), and inhibitor level (to ADAMTS13) was 49 U/mL (figure 1A). Red blood cell fragmentation was present on peripheral blood smear.
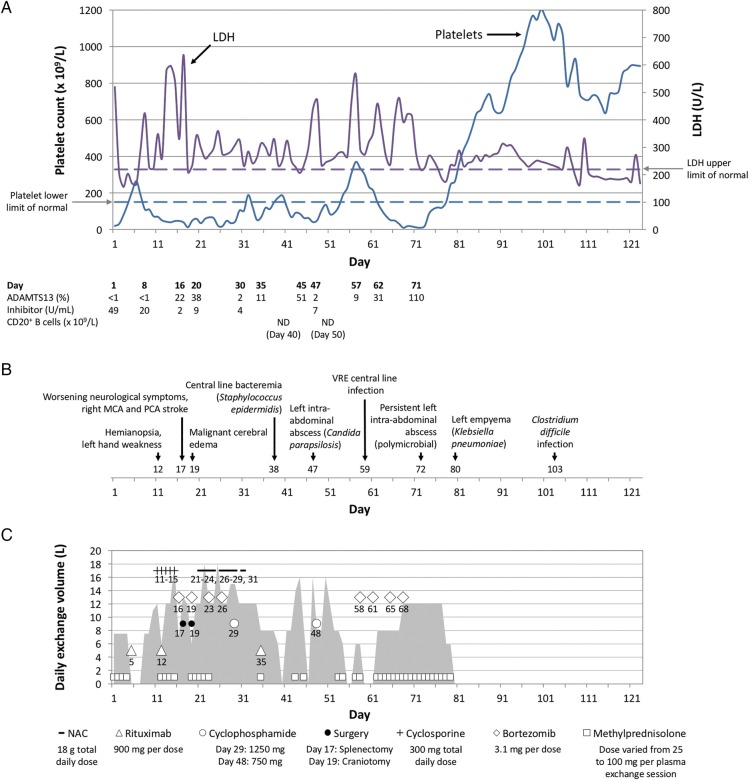
Figure 1.
Clinical course, major events, and therapeutic interventions. (A) shows the timeline of platelet counts (×109/L, depicted by the solid purple line with the dashed purple line indicating the lower limit of normal) and serum lactate dehydrogenase levels (U/L, depicted by the solid blue line with the dashed blue line indicating the upper limit of normal). The laboratory results for ADAMTS13, inhibitory autoantibodies against ADAMTS13 (U/mL), and CD20+ B cells by flow cytometry are shown below the graph. (B) shows the timeline of complications from thrombotic thrombocytopenic purpura and aggressive immunosuppression. (C) shows the timeline of total daily volume (L) of plasma exchange therapy, indicated by the grey-shaded area. The frequency of plasma exchange therapy can be determined by the following: 8L or less, daily; 10L to 16L, twice daily; more than 16L, three times daily. The graph in (C) also shows all treatments the patient received for the thrombotic thrombocytopenic purpura and its neurological complications. Except for methylprednisolone, the graph includes the specific day for each therapy or intervention. LDH, lactate dehydrogenase; NAC, N-acetylcysteine; ND, not detectable; MCA, middle cerebral artery; PCA, posterior cerebral artery; VRE, vancomycin-resistant Enterococcus.
Treatment
This patient was promptly started on daily high-volume plasma exchange and methylprednisolone followed by two weekly injections of rituximab (375 mg/m2) on day 5 and 12 (figure 1C).2 However, his TMA remained active despite no detectable circulating B cells and a trial of oral cyclosporine (300 mg/day).3 He developed a hemianopsia and left hand weakness on day 12 (figure 1B). Following a failure to detect a response to rituximab and cyclosporine, we started a course of bortezomib on day 16, which consisted of four doses (1.3 mg/m2 per dose) given intravenously over 2 weeks (day 16, 19, 23 and 26).4 Unfortunately, his neurological symptoms persisted, and he underwent a laparoscopic emergent splenectomy on day 17.3 Following his splenectomy, he developed a right posterior and middle cerebral artery territory infarct with malignant cerebral oedema that required an emergent decompressive craniotomy on day 19. We did not observe a clinical response despite twice daily plasma exchanges more than 75% of the time, a trial of intravenous N-acetylcysteine,5 two doses of intravenous cyclophosphamide6 and one additional dose of rituximab.
We elected to administer a second cycle of bortezomib on day 58, and a clinical response was finally observed on day 80. During this time, the patient was concurrently treated for a fungal and polymicrobial intra-abdominal abscess, empyema and line sepsis (figure 1B).
Outcome and follow-up
One year following the patient's clinical response to a second cycle of bortezomib, he remains in remission. He has excellent cognitive function. Unfortunately, he has not completely recovered from his stroke and suffers from difficulties with balance and walking endurance. He continues to participate in stroke rehabilitation.
Discussion
The case described above highlights the severity of ‘typical’ refractory TTP (defined as an ADAMTS13 activity level of <10% and ADAMTS13 inhibitor greater or equal to 50%).7 This young patient progressed rapidly in spite of twice daily large volume plasma exchanges with daily high-dose methylprednisolone therapy and weekly rituximab infusions. He eventually remitted his rapidly deteriorating neurological course after emergent splenectomy and decompressive craniotomy. We suspect that his sustained clinical response observed on day 80 was due to the second cycle of bortezomib. Interestingly, pathological examination of his spleen revealed a large number of B cells despite no circulating B cells at the time.
Early, aggressive immunosuppressive therapy offered to this patient was thought to have ensured his survival. However, this patient also showed that serious infections must be monitored closely in hospital. In a recently published prospective case series in Canada, 3 of 40 patients treated for refractory or relapsing TTP died, with two of three deaths occurring in the those with ‘typical’ refractory TTP.7 These patients died in the presence of septicaemia (fungaemia), active TTP or complications of TTP. These situations are not uncommon, and the clinician is challenged with how to treat severe, active refractory TTP with immunosuppression when patients do not respond to intense plasma exchange therapy. Concurrent treatment of both the infections and refractory TTP is often required.
Our approach to treatment is to promptly administer rituximab after confirmation of severe, ‘typical’ refractory TTP. If there is no clinical response to rituximab and there is no evidence of circulating CD20-positive B cells on flow cytometry, we suggest starting a course of bortezomib over N-acetylcysteine or cyclophosphamide. An emergent splenectomy remains an option for severe cases of TTP with rapidly deteriorating neurological symptoms. However, it must be noted that our recommendations are based on published clinical cases and anecdotal evidence as there is a lack of higher quality therapeutic evidence.
Learning points.
Patients with ‘typical’ refractory thrombotic thrombocytopenic purpura (TTP) have severe, active disease.
Aggressive high-volume plasma exchange therapy and prompt administration of additional immunosuppressive therapy (with or without splenectomy) for rapid evolution of neurological signs may help minimise morbidity and mortality.
Given our prior experience with fatal cases of rapidly evolving refractory TTP, we suggest adopting an aggressive, immunosuppressive treatment approach to achieve a meaningful clinical response.
However, one must maintain a high index of suspicion for infections and aggressively investigate, control and rapidly treat when identified.
Footnotes
Contributors: RRA wrote the manuscript. WFC is an expert in thrombotic microangiopathies and supervisor. MG and AK contributed to the manuscript. All authors were healthcare providers for the patient when admitted to hospital.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Huang SH, Xenocostas A, Moist LM et al. Ustekinumab associated thrombotic thrombocytopenic purpura. Transfus Apher Sci 2012;47:185–8. 10.1016/j.transci.2012.06.024 [DOI] [PubMed] [Google Scholar]
- 2.Elliott MA, Heit JA, Pruthi RK et al. Rituximab for refractory and or relapsing thrombotic thrombocytopenic purpura related to immune-mediated severe ADAMTS13-deficiency: a report of four cases and a systematic review of the literature. Eur J Haematol 2009;83:365–72. 10.1111/j.1600-0609.2009.01292.x [DOI] [PubMed] [Google Scholar]
- 3.Jhaveri KD, Scheuer A, Cohen J et al. Treatment of refractory thrombotic thrombocytopenic purpura using multimodality therapy including splenectomy and cyclosporine. Transfus Apher Sci 2009;41:19–22. 10.1016/j.transci.2009.05.007 [DOI] [PubMed] [Google Scholar]
- 4.Shortt J, Oh DH, Opat SS. ADAMTS13 antibody depletion by bortezomib in thrombotic thrombocytopenic purpura. N Engl J Med 2013;368:90–2. 10.1056/NEJMc1213206 [DOI] [PubMed] [Google Scholar]
- 5.George JN, López JA, Konkle BA. N-Acetylcysteine: an old drug, a new insight, a potentially effective treatment for thrombotic thrombocytopenic purpura. Transfusion 2014;54:1205–7. 10.1111/trf.12561 [DOI] [PubMed] [Google Scholar]
- 6.Allan DS, Kovacs MJ, Clark WF. Frequently relapsing thrombotic thrombocytopenic purpura treated with cytotoxic immunosuppressive therapy. Haematologica 2001;86:844–50. [PubMed] [Google Scholar]
- 7.Clark WF, Rock G, Barth D et al. A phase-II sequential case-series study of all patients presenting to four plasma exchange centres with presumed relapsed/refractory thrombotic thrombocytopenic purpura treated with rituximab. Br J Haematol 2015;170:208–17. 10.1111/bjh.13408 [DOI] [PubMed] [Google Scholar]