Abstract
Prostate leiomyosarcoma is an extremely rare and highly aggressive neoplasm that accounts for >0.1% of all primary prostate malignancies. We report a case of a patient, presenting with recurrent episodes of dysuria, who had been diagnosed and operated for benign prostatic hyperplasia 1 month earlier, and now presented with similar symptoms postoperatively. Trans-rectal biopsy of the prostate was carried out and histopathology revealed leiomyosarcoma of the prostate.
Background
The majority of prostate malignancies are adenocarcinoma in nature. We report this case as the patient's histopathology revealed a very rare form of prostatic malignancy, requiring immediate aggressive management.
Case presentation
A 49-year-old man presented with lower urinary tract symptoms and pain in the perineal region for the past 7 months. His International Prostate Symptom Score (IPSS) was 30 (severely symptomatic).1 He had no history of haematuria. He was not a known case of diabetes, hypertension or tuberculosis, and had no significant medical or surgical history. He had no history of malignancies (prostate, ovary, breast) in family members. He was a primary school teacher. He did not have any addictions. On digital rectal examination, his prostate was non-tender, lobulated and asymmetrically enlarged, with variable consistency, abutting the rectum.
Investigations
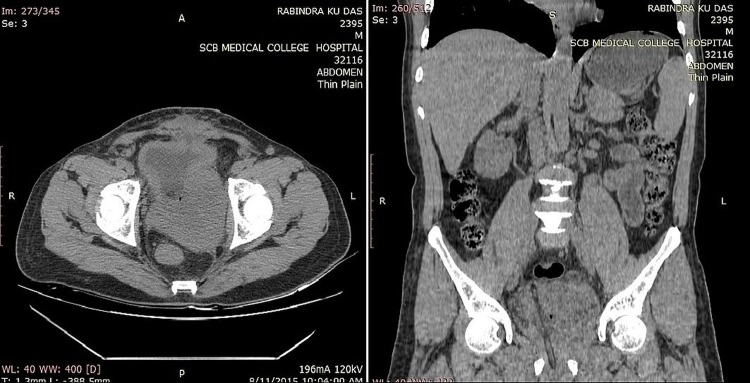
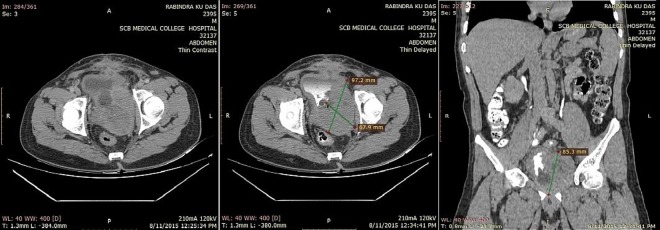
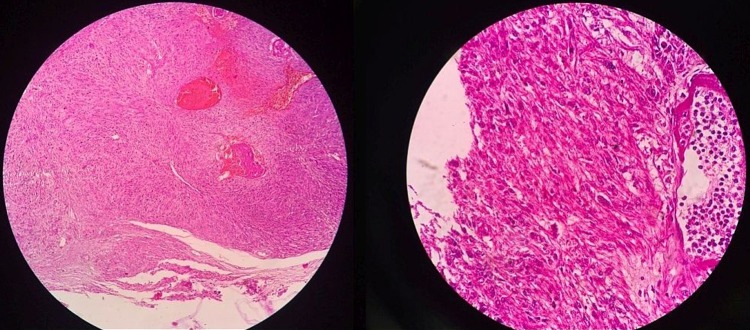
Ultrasound of the pelvis showed a grossly enlarged prostate with heterogeneous parenchyma (volume 184 cc) and high postvoid residual urine (129 cc) noted. Serum prostate-specific antigen (PSA) measurement performed using the electrochemiluminescence immune assay method was 1.03 ng/mL (<4 ng/mL). A clinical diagnosis of benign hyperplasia of the prostate was made and the patient advised to undergo open prostatectomy, considering the large prostate volume. However, the patient declined open prostatectomy and, alternatively, bipolar trans-urethral resection of the prostate (TURP) was planned. Though holmium laser enucleation of the prostate is a suitable alternative to open prostatectomy, it is not available in our institute. Histopathological examination of TURP chips revealed benign hyperplasia of the prostate with evidence of neither prostatic intraepithelial neoplasia nor carcinoma. The patient was apparently normal for 1 month, after which he presented with urinary tract obstruction, when his serum PSA was found to be 1.19 ng/mL (<4ng/mL). On pelvis ultrasound, a grossly enlarged prostate with heterogeneous parenchyma and an empty catheterised urinary bladder was noted (figure 1). Non-contrast CT of the pelvis showed a grossly enlarged prostate (∼462 cc; figure 2) with contrast-enhanced CT of the pelvis showing a heterogeneously enhancing enlarged prostate with invasion of periprostatic fat plane, B/L seminal vesicles, posterior wall of the bladder and intravesical extension (figure 3). Trans-rectal biopsy was advised. HPE of biopsy samples revealed proliferating fascicles of spindle-shaped cells having elongated blunt end nuclei and fibrillar eosinophilic cytoplasm with increased cellularity, mitotic activity and moderate atypia (figure 4). Immunohistochemistry (IHC) revealed cytokeratin, desmin, smooth muscle actin and progesterone receptor positivity in lesional cells. IHC was negative for PSA, CD34, CD117 and S-100. We histopathologically reviewed the TURP chip samples; these did not reveal any evidence of malignancy. Whole body CT scan did not reveal metastasis in any organ. Diagnosis of leiomyosarcoma of the prostate was made (stage IV: T4N1M1a). The patient was planned for neoadjuvant chemotherapy, after which there was a significant reduction in tumour size, followed by external beam radiotherapy (EBRT). Follow-up CT scan was carried out to monitor the volume of the prostate; currently, he is waiting for radical cystoprostatectomy, as decided by our tumour board. He was fully cooperative throughout the treatment course.
Figure 1.
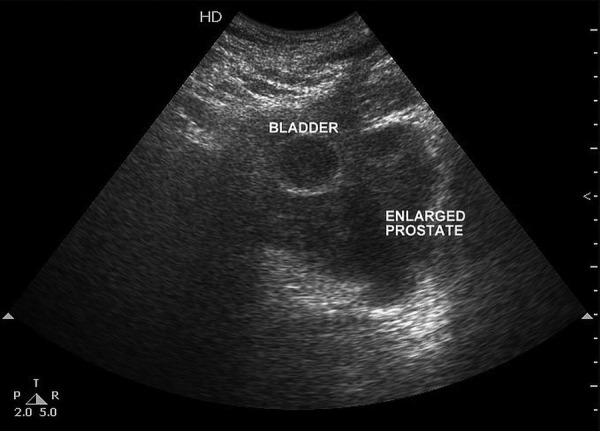
Ultrasound of the pelvis in transverse view shows a heterogeneous enlarged prostate with loss of fat plane between prostate and posterior wall of the bladder, suggesting invasion of the bladder. (Bladder is empty with Foley's bulb in situ).
Figure 2.
Non-contrast CT of the abdomen and pelvis in axial and coronal images show an asymmetrically enlarged prostate invading posterior wall of the bladder, bilateral seminal vesicles and periprostatic tissues.
Figure 3.
Contrast-enhanced CT of the abdomen and pelvis in portal venous phase axial, delayed axial and delayed coronal images, show an enlarged heterogeneously enhancing prostate with internal necrotic areas.
Figure 4.
Microscopic HPE images of the trans-rectal biopsy sample show proliferating fascicles of spindle-shaped cells with elongated blunt end nuclei and fibrillar eosinophilic cytoplasm with increased cellularity, mitotic activity and moderate atypia.
The sudden drastic increase in the size of the tumour from 184 to 462 cc is quite unusual, and can be attributed to the very highly malignant nature of the disease; this makes our case unique.
Differential diagnosis
Adenocarcinoma of the prostate.
Treatment
The patient received two cycles of neoadjuvant chemotherapy with ifosfamide (500 mg/m2) and epirubicin (60 mg/m2). EBRT was given with a total dose of 6000 cGy/30# over 45 days using three-dimensional conformal radiation therapy. The patient tolerated radiotherapy well with no significant toxicity. After chemoradiotherapy, he showed partial symptomatic improvement (IPSS score 16). A radical cystoprostatectomy has been planned.
Outcome and follow-up
Depending on the clinical and radiological improvement, the patient will be taken up for radical surgery after completion of radiotherapy.
The patient has survived and has had symptomatic improvement after neoadjuvant chemoradiotherapy; he is currently awaiting radical surgery.
Discussion
The majority of prostatic malignancies (more than 95%) are adenocarcinoma in nature. However, in past few decades, many unusual tumours have been described in the literature. These tumours have had almost similar clinical and imaging features but varying prognoses.2 They may arise from the prostatic epithelium, stroma or ectopically located cells within the prostate.3
Sarcomas of the prostate are rare malignancies accounting for <0.1% of primary prostatic neoplasms originating from non-epithelial mesenchymal components. Leiomyosarcoma is the most common histological type in adults (38% to 52% of primary prostatic sarcomas), while rhabdomyosarcoma is the most common in paediatric patients.4–6 Globally, <200 cases have been reported in the literature and <100 cases have been reported in the English literature.7
Patients commonly present in the age group of 41–78 years (mean age 61 years), with signs and symptoms of urinary obstruction, as in our case. Other symptoms include perineal/rectal pain, haematuria, painful ejaculation, problems in defaecation and constitutional symptoms such as weight loss.6–9 Urinary obstruction was present in 89% of cases, as reported by Vandoros et al.5 The lack of specific symptoms led to delayed presentation with up to 25% of cases presenting with metastasis at the time of diagnosis, mostly to the lungs, followed by the liver and bone.6–8 The disease tends to travel via lymphatics and blood vessels, causing widespread regional and distant metastases.10
Physical examination reveals non-specific enlargement of the prostate as in benign hyperplasia of the prostate, with a normal serum PSA level (because of the non-epithelial origin of the tumour). Ultrasound is a useful preliminary investigation, but CT scan or MRI is very important for evaluating local extent as well as determining the presence or absence of metastasis and assessment of response to treatment. Diagnosis is made by ultrasound-guided trans-rectal needle biopsy, TURP or open surgical procedures.5 7 11
The vast majority of leiomyosarcoma of the prostate are high grade, showing necrosis, cystic degeneration, severe nuclear atypia and increased mitosis. Low-grade leiomyosarcoma are rare. They show moderate atypia, scattered mitoses and a focally infiltrative growth pattern around benign prostate glands.4 8 11 12 Neoplastic cells commonly express vimentin, smooth muscle antigen, desmin and cytokeratin. Some tumour cells express progesterone receptor. PSA, S-100, CD 117 and CD 34 are negative in all tumours.5 7 Our case was a WHO grade 1 leiomyosarcoma. The Ki-67 proliferative labelling index was 20%.
Owing to rarity of this tumour, no definite treatment protocols have been established. Multimodality therapy using a combination of surgery, chemotherapy and radiotherapy has yielded better results. Surgical procedures with curative intent usually involve radical retropubic prostatectomy, radical cystoprostatectomy, suprapubic prostatectomy and pelvic exenteration.11 13 14 Neoadjuvant therapy including chemotherapy or chemoradiation should be considered if the tumour is bulky with local invasion on preoperative staging.13 15 There are various chemotherapeutic regimens, using anthracycline (doxorubicin or epirubicin)-based combinations with alkylating agents (cyclophosphamide, ifosfamide, or dacarbazine) and/or vinca alkaloids (vinblastine or vincristine). A platinum-based combination (cisplatin), and methotrexate and etoposide, have been used with acceptable efficacy.6 15–17 A radiation dose of at least 60 Gy is required to achieve local control. However, treatment failure is common, and locoregional spread by lymphatics and distant haematogenous metastasis does occur.11 18
Prostatic leiomyosarcomas are aggressive tumours with a poorer clinical outcome than adenocarcinoma. Median survival is about 15–18 months (range 5–95 months). Tumour diameter <5 cm, low histological grade, paratesticular or bladder tumour site, complete surgical excision and absence of distant metastasis are good prognostic factors.6 18 19
Patient's perspective.
I thought that I just had a big prostate and that after the operation I would be fine. But I later came to know that I had a rare form of cancer and could die soon. I am taking tablets and being treated with some sort of radiation. I am very depressed. Life is not fair.
Learning points.
Leiomyosarcoma of the prostate, though very rare, should be suspected in patients having recurrent enlargement of the prostate post-trans-urethral resection of the prostate, over a short duration of time and with normal serum PSA levels.
Approximately 25% of cases present with metastasis at the time of diagnosis, mostly to the lungs.
These tumours are positive for vimentin, smooth muscle antigen, desmin and cytokeratin, in the immunohistochemistry.
A relatively good outcome of this devastating malignancy is seen with aggressive multimodality treatment involving radical surgery, chemotherapy and radiation therapy in carefully selective patients.
Footnotes
Twitter: Follow Dinesh Raj at @harveymbbs.1987
Contributors: PKD contributed to the conception or design of the work, or the acquisition, analysis or interpretation of data. PKS was involved in drafting the work or revising it critically for important intellectual content. JM and DHR were involved in final approval of the version published.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Barry MJ, Fowler FJ Jr, O'Leary MP et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol 1992;148:1549–57; discussion 1564. [DOI] [PubMed] [Google Scholar]
- 2.Varghese SL, Grossfeld GD. The prostatic gland: malignancies other than adenocarcinomas. Radiol Clin North Am 2000;38:179–202. 10.1016/S0033-8389(05)70155-X [DOI] [PubMed] [Google Scholar]
- 3.Chang JM, Lee HJ, Lee SE. Pictorial review: unusual tumours involving the prostate: radiological–pathological findings. Br J Radiol 2008;81:907–15. 10.1259/bjr/68294775 [DOI] [PubMed] [Google Scholar]
- 4.Miedler JD, MacLennan GT. Leiomyosarcoma of the prostate. J Urol 2007; 178:668 10.1016/j.juro.2007.05.036 [DOI] [PubMed] [Google Scholar]
- 5.Vandoros GP, Theodoros M, Karamouzis MV et al. Leiomyosarcoma of the prostate: case report and review of 54 previously published cases. [DOI] [PMC free article] [PubMed]
- 6.Sexton WJ, Lance RE, Reyes AO et al. Adult prostate sarcoma, the M.D Anderson Cancer center's experience. J Urol 2001;166:521–5. 10.1016/S0022-5347(05)65974-5 [DOI] [PubMed] [Google Scholar]
- 7.Cheville JC, Dundore PA, Nascimento AG et al. Leiomyosarcoma of the prostate: report of 23 cases. Cancer 1995;76:1422–7. [DOI] [PubMed] [Google Scholar]
- 8.Singh JP, Chakraborty D, Bera MK et al. Leiomyosarcoma of prostate: a rare, aggressive tumor. J Cancer Res Ther 2013;9:743–5. 10.4103/0973-1482.126482 [DOI] [PubMed] [Google Scholar]
- 9.Hansel DE, Herawi M, Montgomery E et al. Spindle cell lesions of the adult prostate. Mod Pathol 2007;20:148–58. 10.1038/modpathol.3800676 [DOI] [PubMed] [Google Scholar]
- 10.Perez C. Principles and practice of radiation oncology. 1998;1:1605–6. [Google Scholar]
- 11.El-Sharkawi S, Vaughton K. Prostatic leiomyosarcoma: the case for combined modality therapy. Sarcoma 1997;1:59–60. 10.1080/13577149778498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubey A, Sivananthan G, Bradel T et al. Prostatic leiomyosarcoma—a rare case report with review of literature. Internet J Oncol 2010;8. [Google Scholar]
- 13.Talapatra K, Nemade B, Bhutani R et al. Recurrent episodes of hematuria: a rare presentation of leiomyosarcoma of prostate. J Cancer Res Ther 2006;2:212–14. 10.4103/0973-1482.29837 [DOI] [PubMed] [Google Scholar]
- 14.Osca García JM, Alfaro Ferredes L, Ruíz Cerda JL et al. Leiomyosarcoma of the prostate. Arch Esp Urol 1993;46:831–3. [PubMed] [Google Scholar]
- 15.Suppiah R, Wood L, Elson P et al. Phase I/II study of docetaxel, ifosfamide, and doxorubicin in advanced, recurrent, or metastatic soft tissue sarcoma (STS). Invest New Drugs 2006;24:509–14. 10.1007/s10637-006-9035-2 [DOI] [PubMed] [Google Scholar]
- 16.Sakano Y, Yonese J, Okubo Y et al. Leiomyosarcoma of the prostate: a case report of remission for 9 years by radiotherapy. Hinyokika Kiyo 1995;41:629–32. [PubMed] [Google Scholar]
- 17.Serrone L, Zeuli M, Gamucci T et al. A phase II study of dose-intense ifosfamide plus epirubicin with hematopoietic growth factors for the treatment of patients with advanced soft tissue sarcomas; a novel sequential schedule. Cancer Chemother Pharmacol 2001;47:206–10. [DOI] [PubMed] [Google Scholar]
- 18.Vakilha M, Dadgar FN, Tirgari T. Leiomyosarcoma of prostate; report of a case Iran. J Radiat Res 2004;1:229–31. [Google Scholar]
- 19.Russo P, Brady MS, Conlon K et al. Adult urological sarcoma. J Urol 1992;147:1032–6. [DOI] [PubMed] [Google Scholar]