Deep brain stimulation (DBS) is a well established electrophysiological treatment initially applied to treat medication-refractory motor symptoms in Parkinson's disease (PD), and is now being explored for several neurological and psychiatric disorders. The specific physiological mechanisms underlying the effectiveness of DBS are not fully understood, although some hypothesized general mechanisms may be acceptable (Wichmann and DeLong, 2016). Early hypotheses suggested that stimulation of the subthalamic nucleus (STN) in PD produced the same clinical effect as a lesion. In other words, DBS was initially considered to suppress or modulate the abnormal bursting discharge patterns that occur in STN neurons in parkinsonian patients. Several mechanisms have been proposed to explain this effect, invoking what would happen at the site of stimulation and/or in the neuronal circuitry to which the targeted region for stimulation is functionally connected. These mechanisms include depolarization block caused by increase of potassium currents, inactivation of sodium channels, presynaptic depression of excitatory afferents, and stimulation-induced activation of inhibitory afferents. However, evidence in favor of activation of STN neurons was also provided, possibly mediated by stimulation-induced activation of excitatory projections from the motor cortex, or by a direct effect of stimulation directly on STN neurons. Moreover, neuronal activity may be phase locked to the pulse train, with following frequencies dictated by the stimulus interval.
Major challenges in understanding the actions of DBS are also provided by the different cronaxie of neuronal somata and axons. This means that by using stimulation parameters such as those usually employed for STN DBS using standard quadripolar electrodes (130 Hz, pulse duration of 60 µs, and voltage amplitudes of approximately 3 V), it is likely that fibers of passage rather than neuronal cell bodies are stimulated. This issue is especially critical when stimulation is applied in brainstem structures in PD and atypical parkinsonism, in which degeneration of neuronal cell bodies may occur to a variable extent or not at all in every targeted region, contrary to what happens when stimulation is applied to the STN, whose neurons are still present in PD. When applied in brainstem structures, such as the pedunculopontine tegmental nucleus (PPTg), DBS must be applied at low frequency of 25–40 Hz, and the benefits may be attributable to a modulation of neuronal intrinsic membrane properties and circuits through activation of fiber tracts rather than to the stimulation of the decreasing neuronal population that characterizes the PPTg in PD and atypical parkinsonism. Although discordant data have been reported about the effectiveness of PPTg DBS in PD and atypical parkinsonism, freezing of gait and postural instability have been consistently reported by several authors in PD patients. These motor deficits are resistant to dopaminergic medications, thus it is likely that the effect may be not be dependent on the dopaminergic nigrostriatal system. Descending fibers to the spinal cord arising from the PPTg, which is part of the so-called mesencephalic locomotor region, and increased arousal due to ascending projections of the PPTg to thalamic nuclei (Garcia-Rill, 2015), may contribute to improving motor performance in implanted patients. Whether the cerebellum may participate in these effects has not been explored.
We addressed this issue in a recent article (Vitale et al., 2016), taking into account three major lines of evidence. First, the basal ganglia communicate with the cerebellum through pathways linking, respectively, the dentate nucleus to the striatum by way of thalamic nuclei, and the STN to the cerebellar cortex by way of the pontine nuclei (Bostan et al., 2013). Second, tractography studies have shown the existence of a PPTg cerebellum projection in the human brain (Aravamuthan et al., 2007), and PPTg fibers directed at the cerebellum have been reported in animal studies. Third, changes of functional connectivity involving the striatum, the cortex, and the cerebellum have been also found in PD patients (Wu et al., 2011).
In our study, we report for the first time that microstimulation of the PPTg in intact rats as well as in rats in which loss of acetylcholine (ACh)-containing neurons was induced (Figure 1), evoked a short latency and brief activation of neurons in fastigial, interpositus and dentate nuclei. This activation could follow short trains of stimuli delivered up to 200 Hz. In animals in which cholinergic neurons had degenerated, the percentage of neurons activated from the PPTg significantly decreased, thus supporting the idea that the response was mainly due to activation of PPTg neurons, rather to activation of passing fibers. Given that dentate nucleus neurons were the most responsive among the cerebellar nuclei to PPTg stimulation (76.2% for dentate vs. 55.2% and 7.0% for interpositus and fastigial, respectively), we further investigated the properties of the PPTg-evoked response in dentate neurons. We concluded that the activation of dentate neurons could be mediated by ACh since iontophoretic application of the ACh antagonists, atropine or mecamylamine, onto dentate neurons while stimulating the PPTg, reduced, if not completely abolished, the evoked responses. A low number of antidromic responses were recorded in cerebellar nuclei following PPTg stimulation. This is in agreement with anatomical studies which have not reported cerebellar nuclei projections to PPTg (see Vitale et al., 2016 for complete references). This does not seem to occur in monkeys in which fibers from deep cerebellar nuclei directed at thalamic nuclei have been reported to innervate the PPTg. Orthodromic or antidromic responses could theoretically also arise from activation of fibers travelling in the superior cerebellar peduncle. However, this possibility is unlikely since we stimulated the caudal part of the PPTg, which is located in the most lateral edge of the superior cerebellar peduncle. This region is relatively distant from the stimulated site, and being also smaller compared to the core of the cerebellar peduncle should also contain a lower number of fibers.
Figure 1.
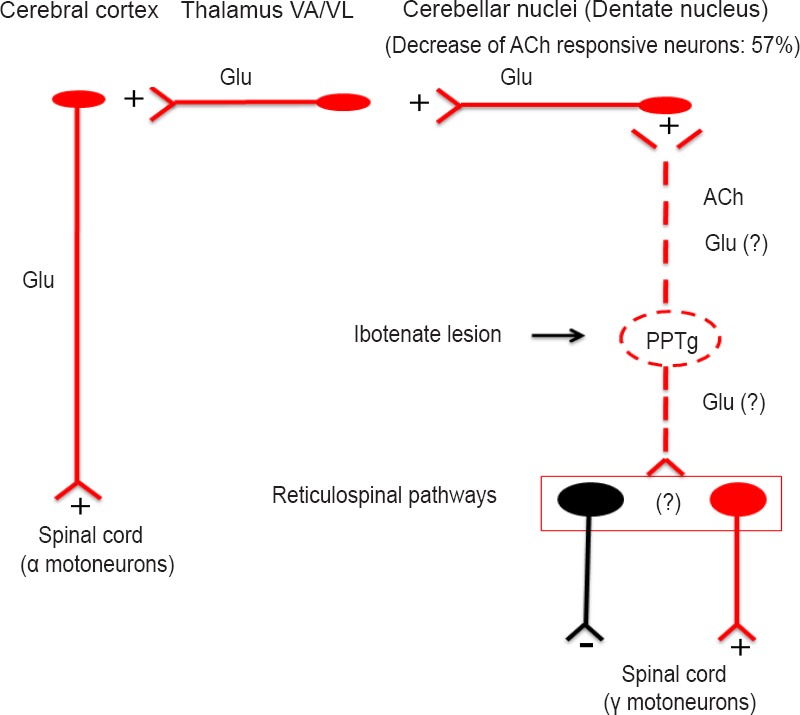
Summary of the neural circuitry involved in our data.
Electrical stimulation of the PPTg activated cerebellar nuclei, likely via direct fibers. When neuronal somata in the PPTg were destroyed by ibotenic acid, a 57% decrease of activatory responses occurred in the dentate nucleus. This means that in normal condition stimulation of the PPTg would activate cerebral cortex via multiple activatory neuronal tracts. It is reasonable to hypothesize that when cholinergic neurons in the PPTg degenerate, as it occurs Parkinson's disease, the cerebral cortex becomes less active, likely involving both motor and non motor cortical areas since all the cerebellar nuclei were activated by PPTg stimulation, although at a variable extent. The loss of the PPTg input to the cerebellum would also have effects on cerebellar mechanisms involved in postural control. Finally, degeneration of PPTg neurons would also affect descending fibers to brainstem nuclei from which reticulospinal pathways arise, thus disrupting spinal cord control of muscle tone. Neuronal components: excitatory in red, inhibitory in black.
In the future, intracellular investigation might provide insights into the monosynaptic nature of this pathway and explore the participation of other possible neurotransmitters in the evoked response such as glutamate.
Our study reveals a number of interesting perspectives in understanding the functions of the basal ganglia and DBS applied in the brainstem. Functional changes in PD are traditionally viewed as the consequences of neuronal degeneration of DA neurons and disruption of basal ganglia outputs. These deficits have been related to the major motor deficits that characterize PD, i.e., akinesia, rigidity, tremor, gait disturbance, and dyskynesia. The role of the cerebellum in these motor deficits is poorly known. In the 6-hydroxydopamine and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) rodent and primate models of parkinsonism, reduction of metabolic activity or neuronal degeneration has been observed in the cerebellum (Rolland et al., 2007). Furthermore, hyperactivation of Purkinje cells occurs in MPTP-treated monkeys, and increased activation of the cerebellum has been reported by several authors during motor execution and motor learning in PD patients. Changes of functional connectivity involving the striatum, the cortex, and the cerebellum were also found in PD patients (Wu et al., 2011). The severity of axial instability and gait has also been correlated with a decrease of acetylcholinesterase activity in the midbrain and cerebellum (Gilman et al., 2010).
Thus, the above findings together with those reviewed by other authors (Wu and Hallett, 2013), suggest that the cerebellum is involved in the pathophysiology of gait and axial disturbances in PD, in agreement with the critical gateway of the cortico-pontine-cerebello-thalamo-cortical pathway. The nature of hyperactivation or strengthened connectivity in the cerebellum of PD patients is not yet understood, although at present, it has been considered as a compensatory effect of the hypoactivity that occurs in pathways involving other cortical regions, including the supplementary motor area. In our opinion, the PPTg now assumes a major role in driving basal ganglia signals to the cerebellum and integrating sensory signals that are important for correct gait and postural control to which PPTg neurons are responsive. A surprising effect revealed by our study was that, among the cerebellar nuclei, the dentate nucleus was the most responsive to PPTg stimulation. This would indicate that the PPTg, through its projections to the dentate nucleus, may also participate in higher functions, such as motor learning and programming of movements, which are typically carried out through the cerebro-cerebellar pathway. Moreover, PPTg stimulation may have a more effective facilitatory action on motor functions compared to STN stimulation, owing to its descending projections to brainstem and spinal cord.
Another perspective arises from the evidence that the PPTg is surrounded by three major ascending sensory pathways, i.e., the medial lemniscus, the spinothalamic tract, and the superior cerebellar peduncle. Thus, it would also be reasonable to hypothesize a role for ascending sensory signals travelling in these pathways. Such a possibility is also supported by the fact that impaired functional integration of postural sensory signals occurs in patients with PD (Muller et al., 2013). Since PPTg neurons receive proprioceptive signals, it is reasonable to hypothesize that, in such circumstances, a disruption of proprioceptive integration at the level of both the PPTg and cerebellum may be involved in the postural instability of PD patients refractory to drug treatment.
Finally, as far as neuronal regeneration and plasticity is concerned, it is worth noting that long term PPTg DBS suppresses late cortical components of somatosensory potentials evoked by tibial nerve stimulation, which reappear if stimulation ceases (Mazzone et al., 2016). This on the one hand suggests that cortical integration of sensory signals may be modulated by PPTg DBS, and on the other is indicative of changes in synaptic plasticity that may occur after release of acetylcholine from pedunculopontine fibers innervating both thalamic nuclei and cerebellum. Such observations should greatly stimulate clinical neurophysiologists for further investigations directed at understanding the details of the consequences of brainstem stimulation on sensorimotor integration in the cerebral cortex.
References
- Aravamuthan BR, Muthusamy KA, Stein JF, Aziz TZ, Johansen-Berg H. Topography of cortical and subcortical connections of the human pedunculopontine and subthalamic nuclei. Neuroimage. 2007;37:694–705. doi: 10.1016/j.neuroimage.2007.05.050. [DOI] [PubMed] [Google Scholar]
- Bostan AC, Dum RP, Strick PL. Cerebellar networks with the cerebral cortex and basal ganglia. Trends Cogn Sci. 2013;17:241–254. doi: 10.1016/j.tics.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rill E. Waking and the reticular activating system in health and disease. Academic Press; 2015. [Google Scholar]
- Gilman S, Koeppe RA, Nan B, Wang CN, Wang X, Junck L, Chervin RD, Consens F, Bhaumik A. Cerebral cortical and subcortical cholinergic deficits in parkinsonian syndromes. Neurology. 2010;74:1416–1423. doi: 10.1212/WNL.0b013e3181dc1a55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzone P, Vilela FO, Viselli F, Insola A, Sposato S, Vitale F, Scarnati E. Our first decade of experience in deep brain stimulation of the brainstem: elucidating the mechanism of action of stimulation of the ventrolateral pontine tegmentum. J Neural Transm (Vienna) 2016 doi: 10.1007/s00702-016-1518-5. doi: 10.1007/s00702-016-1518-5. [DOI] [PubMed] [Google Scholar]
- Muller ML, Albin RL, Kotagal V, Koeppe RA, Scott PJ, Frey KA, Bohnen NI. Thalamic cholinergic innervation and postural sensory integration function in Parkinson's disease. Brain. 2013;136:3282–3289. doi: 10.1093/brain/awt247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland AS, Herrero MT, Garcia-Martinez V, Ruberg M, Hirsch EC, Francois C. Metabolic activity of cerebellar and basal ganglia-thalamic neurons is reduced in parkinsonism. Brain. 2007;130:265–275. doi: 10.1093/brain/awl337. [DOI] [PubMed] [Google Scholar]
- Vitale F, Mattei C, Capozzo A, Pietrantoni I, Mazzone P, Scarnati E. Cholinergic excitation from the pedunculopontine tegmental nucleus to the dentate nucleus in the rat. Neuroscience. 2016;317:12–22. doi: 10.1016/j.neuroscience.2015.12.055. [DOI] [PubMed] [Google Scholar]
- Wichmann T, DeLong MR. Deep brain stimulation for movement disorders of basal ganglia origin: restoring function or functionality? Neurotherapeutics. 2016;13:264–283. doi: 10.1007/s13311-016-0426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Hallett M. The cerebellum in Parkinson's disease. Brain. 2013;136:696–709. doi: 10.1093/brain/aws360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Wang LA, Hallett M, Chen Y, Li KC, Chan P. Effective connectivity of brain networks during self-initiated movement in Parkinson's disease. Neuroimage. 2011;55:204–215. doi: 10.1016/j.neuroimage.2010.11.074. [DOI] [PubMed] [Google Scholar]


