Multiple sclerosis (MS) is a chronic, severe and complex disease of still uncertain etiopathogenesis, with lesions in the cerebral white matter and spinal cord. The disease is heterogeneous, but is characterized by neuroinflammatory and neurodegenerative processes, usually associated with altered activation of the immune system following presumable stimulation by still unknown autoantigens. Several data confirm that MS is a systemic disease involving the central and peripheral nervous systems (Macchi et al., 2015). The role of adaptive immunity, sustained by T and B cells, in MS has been studied for decades. More recently, however, increasing attention has been paid to the role of innate immunity in MS progression. Our understanding of the molecular and cellular bases of the innate immune responses, showing heterogeneity of their genotypic and phenotypic features, has been improved in recent years. Non-cellular components of the innate response include cellular sensors of microbial components including pattern recognition receptors (PRRs), mediators of the intracellular signaling triggered by PRRs, cytokines, and other effector molecules. Signaling pathways of programmed cell death (PCD) play important roles in the innate response. In addition, cellular components of the innate response include innate lymphoid cells (ILCs) exerting natural killer (NK) activity and interferon (IFN)γ production, plasmacytoid dendritic cells involved in IFNγ production, and monocytes/macrophages. Innate response effectors have also been recently recognized to exhibit memory of previous responses, and related phenomena have been termed “trained immunity” (Quintin et al., 2014).
An overall analysis of the role of innate responses in MS is beyond the aim of this perspective, and it has been extensively reported in a recent review (Fernandez-Paredes et al., 2016). This perspective focuses on two selected components of innate immunity, namely, PCD and NK cells, as potential therapeutic targets in MS. These two components of the innate response share the eventual aim of their functional roles: the death of targeted cells. Processes that regulate survival/death within the central nervous system (CNS) and in peripheral blood mononuclear cells (PBMCs) of MS patients are involved in the progression of the disease.
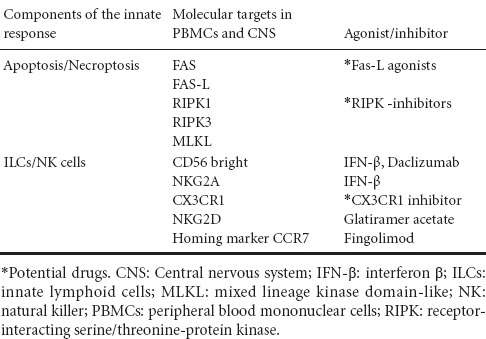
Apoptosis is the best known form of PCD. PBMCs from MS patients affected by relapsing-remitting MS and the secondary-progressive form of the disease are less susceptible to apoptosis induced by activation with phytohemagglutinin, in comparison with age-matched controls, despite high expression of the Fas receptor (Macchi et al., 1999). Failure to trigger activation-induced apoptosis in PBMCs of MS patients, rather than an intrinsic defect in the apoptotic machinery, is attributed to defective Fas-ligand production that is negatively correlated with disability score (Macchi et al., 2001). A recent study reported impaired activation-induced cell death in T helper (Th)17 compared with Th1 clones from healthy individuals and MS patients that was associated with reduced levels of Fas-ligand transcription in the same clones (Cencioni et al., 2015). Considering the higher Th17/Th1 cell ratios in PBMCs from MS patients with respect to healthy individuals, this could explain previous results. Moreover, a preferential transcription of apoptosis-related genes has been recently observed in clinically isolated syndrome (CIS) with early demyelination switching to MS, in comparison with non-converting CIS patients (Hagman et al., 2015). All these data depict a sort of discrepancy between expression of apoptotic markers and failure to undergo PCD in PBMCs from MS patients, with a profile of resistance to apoptosis and favoring immune activation of potentially autoreactive lymphocytes. Regarding the CNS, although the origin of the plaques, a major pathognomonic event of MS, are heterogeneous, it seems that early lesions are characterized by oligodendrocyte apoptosis and microglial activation with limited lymphocyte infiltration. In preactive lesions from autopsy specimens from MS patients, recruitment of cytokines and oxidative stress mediators are observed and considered to be the main causative stimulus for apoptotic cell death in the brain during MS (Haider et al., 2011). The interpretation of neural cell death in MS has been recently revised. Ofengeim et al. (2015) provided evidence that necroptosis, a newly recognized form of PCD, plays a role in MS. In particular, they showed defective expression of the activated form of caspase-8 in microglial cells that was associated with activation of the characteristic markers of necroptosis receptor-interacting serine/threonine-protein kinase (RIPK)1, RIPK3 and mixed lineage kinase domain-like (MLKL) in cortical lesions from MS brain. This evidence could add an important piece to the puzzle of death of neural cells in MS brain, suggesting that microglia make oligodendrocytes more susceptible to necroptosis (Ofengeim et al., 2015). Thus, results in humans seem to contrast, at least partially, with those obtained in animal models of MS in which oligodendrocyte PCD is caspase and death receptor dependent.
This emerging picture of PCD in MS could contribute to define the immunopathological aspects of MS, while suggesting novel strategies for therapeutic intervention. For example, administration to MS patients of specific, exogenous apoptotic stimuli, such as Fas-ligand or Fas-agonist antibodies, could kill dangerous immune-activated cells at the peripheral level without causing PCD-related damage in the brain. In addition, the simultaneous administration of pharmacological agents capable of reducing inflammatory and/or oxidative stress or negatively interfering with necroptosis could be beneficial against CNS damage during MS, without hindering attempts to eliminate detrimental activated lymphocytes at the peripheral level.
Another important role for innate immunity during MS is played by NK cells. Following the initial recognition and definition of NK cells according to morphological and functional criteria, recently, a more complex scenario for these cells, endowed with a fundamental immunoregulatory role, has been revealed. NK cells are now recognized to belong to group 1 of ILCs. They can exhibit a phenotype that can be distinct or in common with other lymphocytes and, once activated, can secrete immunoregulatory cytokines and chemokines, regulate the immune and the inflammatory response, and kill target cells. The NK cells, distributed in blood, secondary lymphoid organs, and in peripheral organs exhibit a CD3− phenotype and are subdivided in CD56dim (90%) mostly cytotoxic, and CD56bright (10%) mainly regulatory (Poli et al., 2009). Several studies have highlighted the role of NK cells in MS at the CNS as well as peripheral level. Initial studies have shown failure of NK activity in PBMCs of MS patients. Further studies have revealed that NK cells mature in the CNS and engage different ligands on neural target cells, which suggests the mechanisms underlying the regulatory role of NK cytotoxicity in the CNS of MS patients. A decrease in peripheral NK activity was correlated with the relapsing phase of MS, which preceded onset of clinical attacks, as well as with the emergence of active plaques and exacerbation of the disease (Chanvillard et al., 2013). Subsequent studies in an expanded cohort of MS patients found that the frequency of NK cells expressing the chemokine receptor, CX3CR1, in peripheral blood, was decreased in the stable phase of the disease, while it increased during relapse. NK cells are also present in cerebrospinal fluid of MS patients with an increase in regulatory/effector (CD56bright/CD56dim) ratio, in comparison with other inflammatory neurological diseases. This suggests that expansion of the regulatory subset of NK cells reflects an attempt to contrast the immune activation typical of MS (Rodriguez-Martin et al., 2015). Other data suggest that the engagement of the NKG2D receptor on target cells is important in the regulation of the autoimmune response by some NK cells in the CNS. Alteration in the expression of NKG2D receptors in autoreactive T cells could lead to their activation, aimed at interacting with NKG2D ligands present on CNS cells such as oligodendrocytes or astrocytes (Saikali et al., 2007). The immunoregulatory activity of NK cells in MS seems to involve an NKG2D receptor/ligand interaction. How NK cells eliminate target autoreactive cells remains to be elucidated. Nevertheless, it seems that activated CD56bright NK cells eliminate activated autoreactive T cells through a caspase-independent cytotoxic mechanism based on the release of granzyme A and granzyme K (GRK), accompanied by mitochondrial failure and release of intracellular reactive oxygen species. Involvement of GRK was supported by GRK gene silencing with siRNA, which abolished the ability of human CD56bright expressing NK cells to kill autologous activated T cells. ILCs and NK cells recently gained attention in MS because they were found to be modulated and/or redistributed following therapy with disease-modifying drugs. The approved first-line therapy for MS consists of interferon β (IFN-β) and glatirameracetate (GA). Following treatment with IFN-β, an expansion of CD56bright NK cells occurs in the peripheral blood of responders, and this is associated with an increase of NKG2A NK+ in comparison with non-responders (Martinez-Rodriguez et al., 2011). GA increases the cytotoxic activity of NK cells from MS patients compared with mature and immature autologous dendritic cells. NK cells from patients showed increased expression of the NK-activating cytotoxic receptors NKp30, NKp44, NKp46 and NKG2D (Hoglund et al., 2013). Among the options for second-line therapy, natalizumab has an effect on NK cells. The phenotypical analysis of lymphocytes from MS patients after 1 year treatment with natalizumab showed a decrease of regulatory CD56bright NK cells and a simultaneous increase of cytotoxic CD56dim NK cells. Selective targeting was ascribed to high expression of the adhesion molecule very late antigen-4. This effect was followed by functional restoration of T-cell responsiveness versus specific antigen or mitogens at the peripheral level. Treatment with natalizumab led to sequestration of cytotoxic NK cellsin the blood, prevention of their migration into the CNS, and amelioration of the inflammatory process (Mellergard et al., 2013). Redistribution of NK cells was also observed in PBMCs of MS patients treated with FTY720 (fingolimod), a sphingosine 1-phosphate receptor agonist, which aimed to decrease inflammation in the CNS. Egression of CD56bright CD62L+ CCR7+ NK cells from the periphery to secondary lymphoid organs in treated MS patients, in comparison with untreated patients and healthy donors, was detected (Johnson et al., 2011). Patients enrolled in clinical trials with daclizumab exhibited a decreased number of activated and regulatory T cells, likely following inhibition of interleukin (IL)-2 stimulation through the receptor. Conversely, IL-2-driven stimulation of cytotoxic activity and expansion of CD56bright NK cells from MS treated patients was observed (Martin et al., 2010). Recent studies have confirmed that the individual variability of CD56bright cells in MS patients under treatment with daclizumab could partly provide prognostic information (Elkins et al., 2015). The potential targets of drugs in MS are summarized in Table 1. These data show that ILCs/NK cells can be efficiently targeted by drugs that, at least partially, reverse the autoimmune, inflammatory and neurodegenerative responses in MS patients. This suggests that death of selected target cells induced by NK cells could be one of the mechanisms involved in the beneficial effects of these drugs.
Table 1.
Ascertained or potential molecular targets of therapeutic interest for innate responses in multiple sclerosis
Recognition of the regulatory roles of PCD and NK cells, two major components of innate immunity, towards the inflammatory and adaptive immune responses has improved investigation of their pharmacological targeting in diseases associated with dysfunction in these responses. Both the restoration of an apoptotic response and improvement of NK cell activity could lead to cell death-dependent inhibition of autoreactive T-cell activation and the restoration of specific T-cell responses in MS. Great efforts have been already made to find reliable therapeutic agents and corresponding markers of response to therapy in MS. However, results await clarification, and side effects can reduce the utilization of some drugs. In this scenario, PCD and NK cells seem to represent intriguing and promising targets for the development of novel therapeutic strategies to control the progression of MS.
References
- Cencioni MT, Santini S, Ruocco G, Borsellino G, De Bardi M, Grasso MG, Ruggieri S, Gasperini C, Centonze D, Barila D, Battistini L, Volpe E. FAS-ligand regulates differential activation-induced cell death of human T-helper 1 and 17 cells in healthy donors and multiple sclerosis patients. Cell Death Dis. 2015;6:e1741. doi: 10.1038/cddis.2015.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanvillard C, Jacolik RF, Infante-Duarte C, Nayak RC. The role of natural killer cells in multiple sclerosis and their therapeutic implications. Front Immunol. 2013;4:63. doi: 10.3389/fimmu.2013.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins J, Sheridan J, Amaravadi L, Riester K, Selmaj K, Bielekova B, Parr E, Giovannoni G. CD56(bright) natural killer cells and response to daclizumab HYP in relapsing-remitting MS. Neurol Neuroimmunol Neuroinflamm. 2015;2:e65. doi: 10.1212/NXI.0000000000000065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Paredes L, de Diego RP, de Andres C, Sanchez-Ramon S. Close encounters of the first kind: innate sensors and multiple sclerosis. Mol Neurobiol. 2016 doi: 10.1007/s12035-015-9665-5. doi:10.1007/s12035-015-9665-5. [DOI] [PubMed] [Google Scholar]
- Hagman S, Kolasa M, Basnyat P, Helminen M, Kahonen M, Dastidar P, Lehtimaki T, Elovaara I. Analysis of apoptosis-related genes in patients with clinically isolated syndrome and their association with conversion to multiple sclerosis. J Neuroimmunol. 2015;280:43–48. doi: 10.1016/j.jneuroim.2015.02.006. [DOI] [PubMed] [Google Scholar]
- Haider L, Fischer MT, Frischer JM, Bauer J, Hoeftberger R, Botond G, Esterbauer H, Binder CJ, Witztum JL, Lassmann H. Oxidative damage in multiple sclerosis lesions. Brain. 2011;134:1914–1924. doi: 10.1093/brain/awr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoglund RA, Holmoy T, Harbo HF, Maghazachi AA. A one year follow-up study of natural killer and dendritic cells activities in multiple sclerosis patients receiving glatiramer acetate (GA) PLoS One. 2013;8:e62237. doi: 10.1371/journal.pone.0062237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TA, Evans BL, Durafourt BA, Blain M, Lapierre Y, Bar-Or A, Antel JP. Reduction of the peripheral blood CD56(bright) NK lymphocyte subset in FTY720-treated multiple sclerosis patients. J Immunol. 2011;187:570–579. doi: 10.4049/jimmunol.1003823. [DOI] [PubMed] [Google Scholar]
- Macchi B, Matteucci C, Nocentini U, Caltagirone C, Mastino A. Impaired apoptosis in mitogen-stimulated lymphocytes of patients with multiple sclerosis. Neuroreport. 1999;10:399–402. doi: 10.1097/00001756-199902050-00034. [DOI] [PubMed] [Google Scholar]
- Macchi B, Matteucci C, Nocentini U, Tacconi S, Pagnini V, Mastino A, Caltagirone C. Defective Fas ligand productions in lymphocytes from MS patients. Neuroreport. 2001;12:4113–4116. doi: 10.1097/00001756-200112210-00050. [DOI] [PubMed] [Google Scholar]
- Macchi B, Marino-Merlo F, Nocentini U, Pisani V, Cuzzocrea S, Grelli S, Mastino A. Role of inflammation and apoptosis in multiple sclerosis: Comparative analysis between the periphery and the central nervous system. J Neuroimmunol. 2015;287:80–87. doi: 10.1016/j.jneuroim.2015.08.016. [DOI] [PubMed] [Google Scholar]
- Martin JF, Perry JS, Jakhete NR, Wang X, Bielekova B. An IL-2 paradox: blocking CD25 on T cells induces IL-2-driven activation of CD56(bright) NK cells. J Immunol. 2010;185:1311–1320. doi: 10.4049/jimmunol.0902238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Rodriguez JE, Lopez-Botet M, Munteis E, Rio J, Roquer J, Montalban X, Comabella M. Natural killer cell phenotype and clinical response to interferon-beta therapy in multiple sclerosis. Clin Immunol. 2011;141:348–356. doi: 10.1016/j.clim.2011.09.006. [DOI] [PubMed] [Google Scholar]
- Mellergard J, Edstrom M, Jenmalm MC, Dahle C, Vrethem M, Ernerudh J. Increased B cell and cytotoxic NK cell proportions and increased T cell responsiveness in blood of natalizumab-treated multiple sclerosis patients. PLoS One. 2013;8:e81685. doi: 10.1371/journal.pone.0081685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofengeim D, Ito Y, Najafov A, Zhang Y, Shan B, DeWitt JP, Ye J, Zhang X, Chang A, Vakifahmetoglu-Norberg H, Geng J, Py B, Zhou W, Amin P, Berlink Lima J, Qi C, Yu Q, Trapp B, Yuan J. Activation of necroptosis in multiple sclerosis. Cell Rep. 2015;10:1836–1849. doi: 10.1016/j.celrep.2015.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli A, Michel T, Theresine M, Andres E, Hentges F, Zimmer J. CD56bright natural killer (NK) cells: an important NK cell subset. Immunology. 2009;126:458–465. doi: 10.1111/j.1365-2567.2008.03027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintin J, Cheng SC, van der Meer JW, Netea MG. Innate immune memory: towards a better understanding of host defense mechanisms. Curr Opin Immunol. 2014;29:1–7. doi: 10.1016/j.coi.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Martin E, Picon C, Costa-Frossard L, Alenda R, Sainz de la Maza S, Roldan E, Espino M, Villar LM, Alvarez-Cermeno JC. Natural killer cell subsets in cerebrospinal fluid of patients with multiple sclerosis. Clin Exp Immunol. 2015;180:243–249. doi: 10.1111/cei.12580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikali P, Antel JP, Newcombe J, Chen Z, Freedman M, Blain M, Cayrol R, Prat A, Hall JA, Arbour N. NKG2D-mediated cytotoxicity toward oligodendrocytes suggests a mechanism for tissue injury in multiple sclerosis. J Neurosci. 2007;27:1220–1228. doi: 10.1523/JNEUROSCI.4402-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]


